Abstract
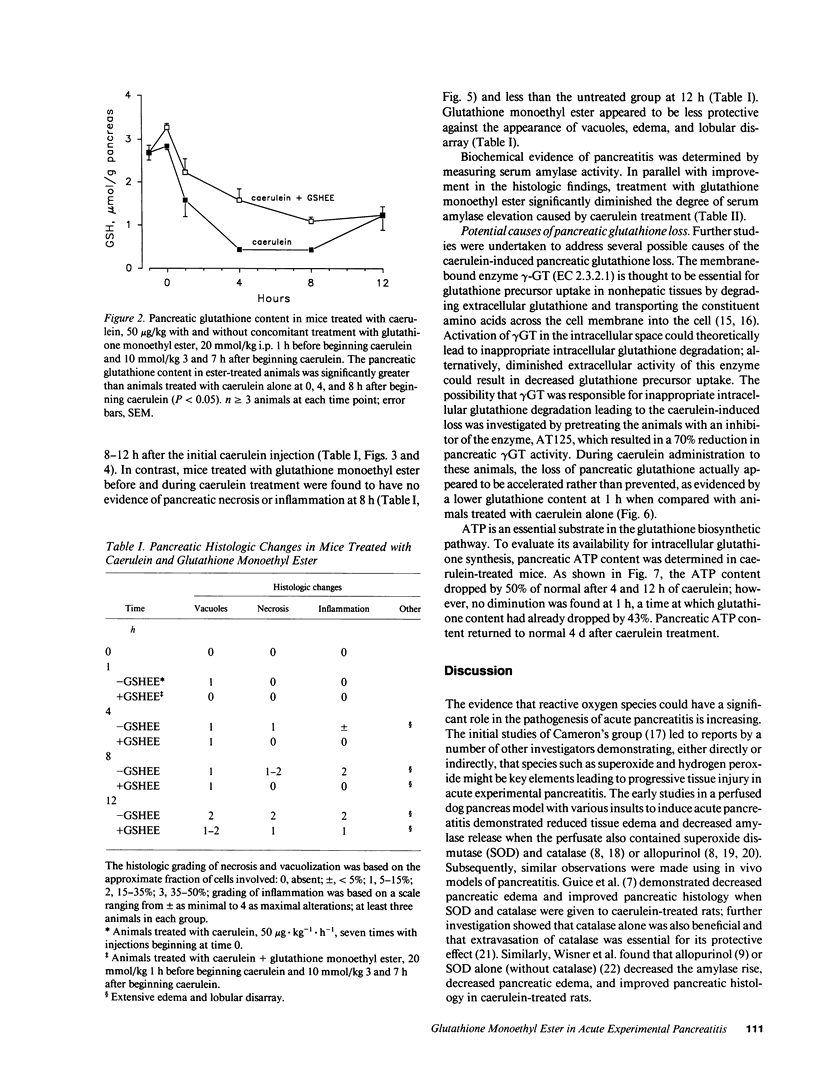
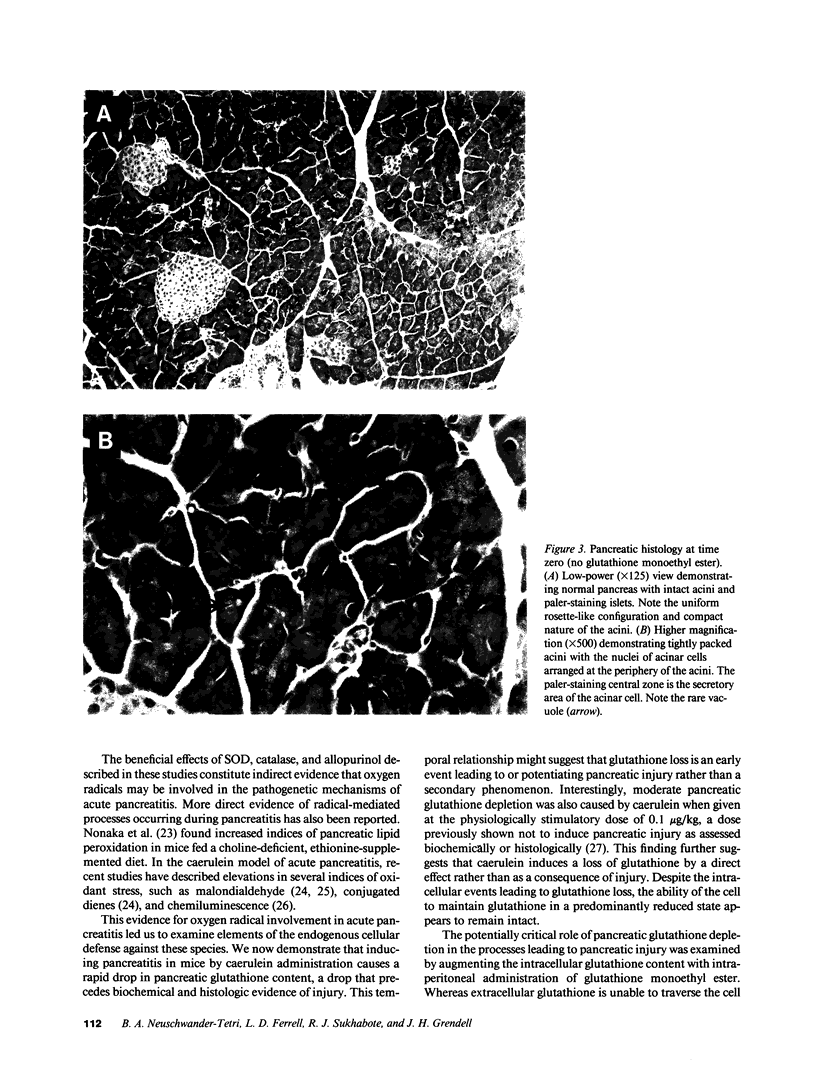
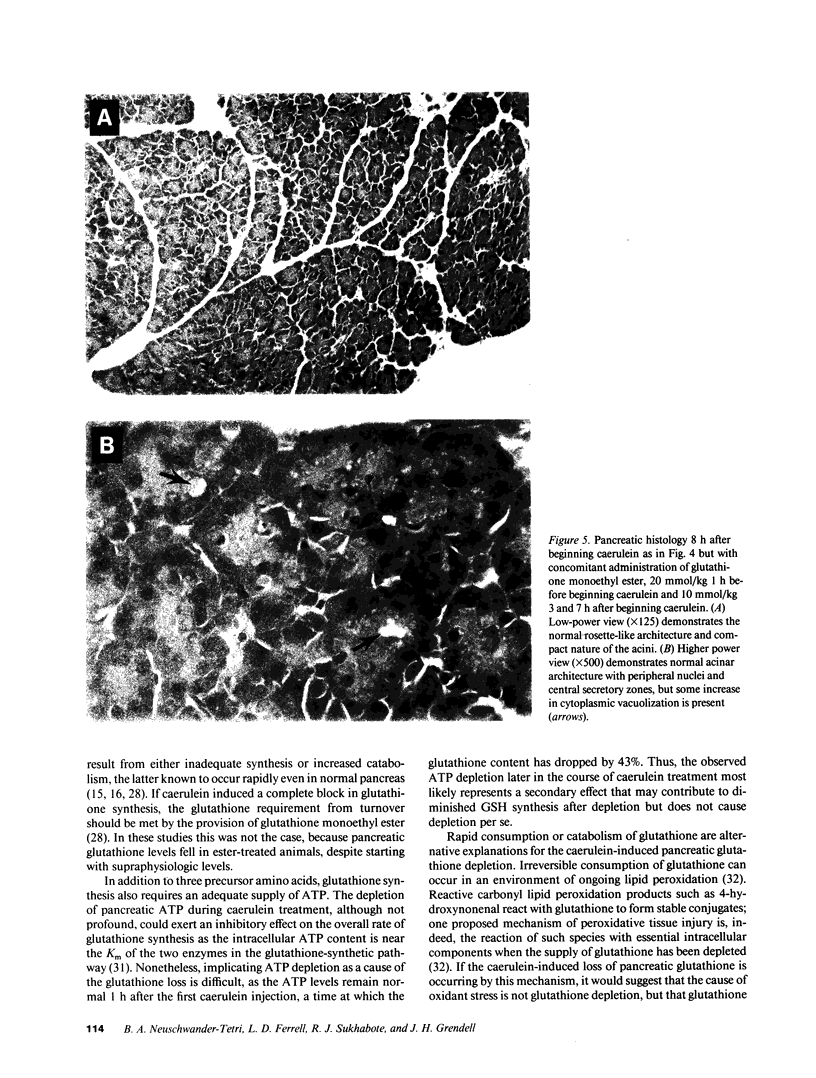
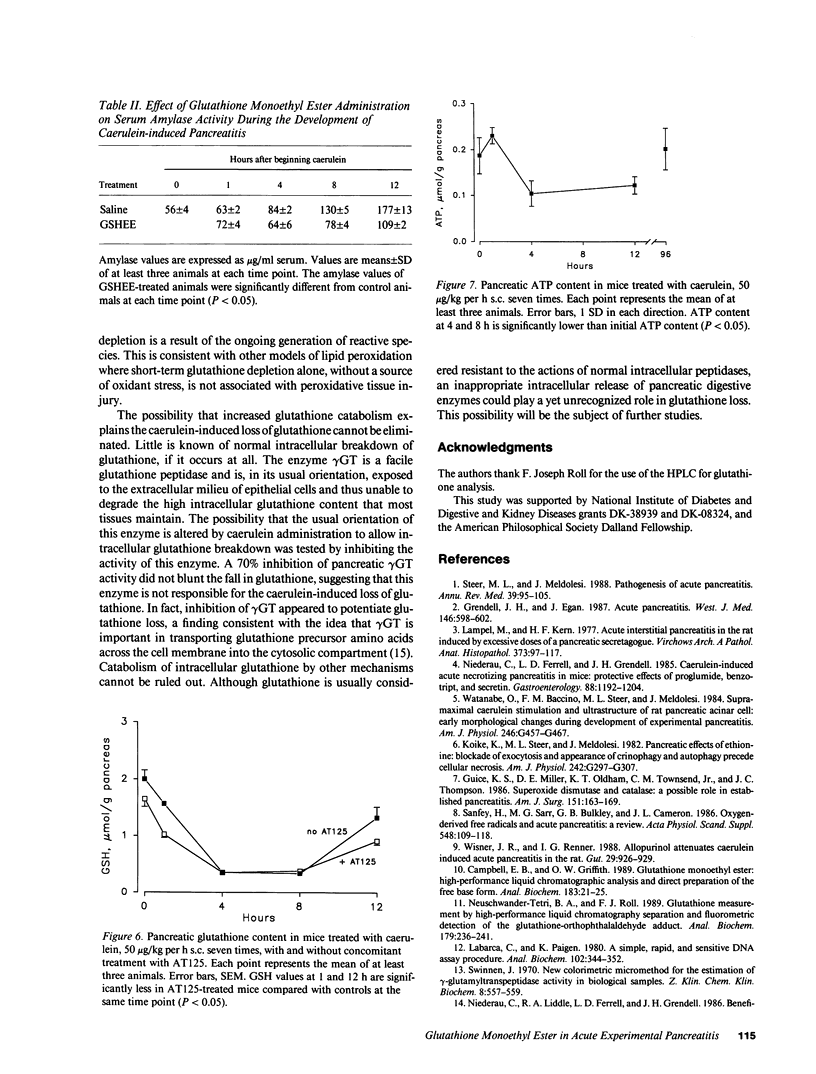
Studies in animal models suggest that oxygen radicals may be important in the pathogenesis of acute pancreatitis. Because glutathione is an essential component of the defense against radical-mediated cellular injury, we investigated whether pancreatic glutathione content is influenced by inducing acute pancreatitis and whether augmenting the intracellular supply of glutathione would alter the course of pancreatitis. Caerulein, a decapeptide cholecystokinin analogue, induces acute necrotizing pancreatitis in mice when given in high doses (50 micrograms/kg per h) over a period of 6 h. The pancreatic glutathione content (total, GSH + GSSG) in mice treated with high-dose caerulein fell to 17% of normal within 4 h of beginning caerulein and recovered toward normal after discontinuing caerulein treatment. Mice treated with glutathione monoethyl ester (20 mmol/kg 1 h before caerulein, 10 mmol/kg 3 and 7 h after starting caerulein) were found to have blunted depletion of pancreatic glutathione, diminished histologic evidence of pancreatitis (necrosis, inflammation, and vacuolization), and lower serum amylase values compared with mice treated with caerulein alone. These findings suggest that the profound depletion of pancreatic glutathione caused by hyperstimulation of the pancreas with caerulein is critically important in the pathogenesis of acute caerulein-induced pancreatitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acute pancreatitis. West J Med. 1987 May;146(5):598–602. [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Campbell E. B., Griffith O. W. Glutathione monoethyl ester: high-performance liquid chromatographic analysis and direct preparation of the free base form. Anal Biochem. 1989 Nov 15;183(1):21–25. doi: 10.1016/0003-2697(89)90165-6. [DOI] [PubMed] [Google Scholar]
- Dabrowski A., Chwiećko M. Oxygen radicals mediate depletion of pancreatic sulfhydryl compounds in rats with cerulein-induced acute pancreatitis. Digestion. 1990;47(1):15–19. doi: 10.1159/000200470. [DOI] [PubMed] [Google Scholar]
- Githens S. Glutathione metabolism in the pancreas compared with that in the liver, kidney, and small intestine. Int J Pancreatol. 1991 Feb;8(2):97–109. doi: 10.1007/BF02924424. [DOI] [PubMed] [Google Scholar]
- Gough D. B., Boyle B., Joyce W. P., Delaney C. P., McGeeney K. F., Gorey T. F., Fitzpatrick J. M. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br J Surg. 1990 Nov;77(11):1256–1259. doi: 10.1002/bjs.1800771119. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guice K. S., Miller D. E., Oldham K. T., Townsend C. M., Jr, Thompson J. C. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am J Surg. 1986 Jan;151(1):163–169. doi: 10.1016/0002-9610(86)90027-9. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Johnson K. J. Failure of antioxidant therapy (polyethylene glycol-conjugated catalase) in acute pancreatitis. Am J Surg. 1989 Jan;157(1):145–149. doi: 10.1016/0002-9610(89)90437-6. [DOI] [PubMed] [Google Scholar]
- Koike H., Steer M. L., Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982 Apr;242(4):G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri B. A., Roll F. J. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal Biochem. 1989 Jun;179(2):236–241. doi: 10.1016/0003-2697(89)90121-8. [DOI] [PubMed] [Google Scholar]
- Niederau C., Ferrell L. D., Grendell J. H. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985 May;88(5 Pt 1):1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- Niederau C., Liddle R. A., Ferrell L. D., Grendell J. H. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986 Oct;78(4):1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka A., Manabe T., Tamura K., Asano N., Imanishi K., Tobe T. Changes of xanthine oxidase, lipid peroxide and superoxide dismutase in mouse acute pancreatitis. Digestion. 1989;43(1-2):41–46. doi: 10.1159/000199859. [DOI] [PubMed] [Google Scholar]
- Poot M., Verkerk A., Koster J. F., Esterbauer H., Jongkind J. F. Influence of cumene hydroperoxide and 4-hydroxynonenal on the glutathione metabolism during in vitro ageing of human skin fibroblasts. Eur J Biochem. 1987 Jan 15;162(2):287–291. doi: 10.1111/j.1432-1033.1987.tb10598.x. [DOI] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–639. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Sarr M. G., Bulkley G. B., Cameron J. L. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109–118. [PubMed] [Google Scholar]
- Sarr M. G., Bulkley G. B., Cameron J. L. Temporal efficacy of allopurinol during the induction of pancreatitis in the ex vivo perfused canine pancreas. Surgery. 1987 Mar;101(3):342–346. [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Gaspar M., Stinner A., Younes M., Melzner I., Bültmann B., Beger H. G. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990 Oct;31(10):1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J. Pathogenesis of acute pancreatitis. Annu Rev Med. 1988;39:95–105. doi: 10.1146/annurev.me.39.020188.000523. [DOI] [PubMed] [Google Scholar]
- Swinnen J. New colorimetric micromethod for the estimation of gamma-glutamyl transpeptidase activity in biological samples. Z Klin Chem Klin Biochem. 1970 Nov;8(6):557–563. doi: 10.1515/cclm.1970.8.6.557. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Wisner J. R., Renner I. G. Allopurinol attenuates caerulein induced acute pancreatitis in the rat. Gut. 1988 Jul;29(7):926–929. doi: 10.1136/gut.29.7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner J., Green D., Ferrell L., Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988 Nov;29(11):1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]