Abstract
Background
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) studies have established multiyear mean hemoglobin A1c (HbA1c) as predictive of microvascular complications in persons with type 1 diabetes. However, multiyear mean HbA1c is not always available in the clinical setting. Skin advanced glycation end products (AGEs) are thought to partially reflect effects of hyperglycemia over time, and measurement of skin AGEs might be a surrogate for multiyear mean HbA1c. As certain AGEs fluoresce and skin fluorescence has been demonstrated to correlate with the concentration of skin AGEs, noninvasive measurement by skin intrinsic fluorescence (SIF) facilitates the exploration of the association of mean HbA1c and other clinical/technical factors with SIF using the detailed phenotypic database available in DCCT/EDIC.
Subjects and Methods
Of the subjects, 1,185 (53% male) had measurements of SIF during years 16/17 of EDIC with mean age and diabetes duration of 51.5 and 29.8 years, respectively. SIF measurements were obtained on the underside of the forearm near the elbow using a skin fluorescence spectrometer. Demographic data and health history were self-reported, and an annual standardized examination measured clinical status. Linear regression models were constructed to identify significant clinical and technical factors associated with SIF, and the final models only used factors that were significant.
Results
SIF ranged from 8.7 to 54.0 arbitrary units and was log-normally distributed. Log(SIF) correlated more with mean HbA1c as the time period increased. In multivariate analyses log(SIF) was significantly associated with mean HbA1c, age, estimated glomerular filtration rate <60 mL/min/m2, smoking status, skin tone, and clinic latitude <37° N.
Conclusions
SIF reflects age, mean HbA1c over time, smoking, and renal damage, which are known risk factors for diabetes complications.
Introduction
The Diabetes Control and Complications Trial1 (DCCT) and the Epidemiology of Diabetes Interventions and Complications2 (EDIC) study have extensively characterized a cohort of 1,441 subjects with type 1 diabetes mellitus (T1DM) over 28 years, resulting in a comprehensive phenotypic history of clinical risk factors, glycemic exposure, and development of micro- and macrovascular complications. The DCCT/EDIC studies have clearly demonstrated the causal role of glycemic exposure, as assessed by mean hemoglobin A1c (HbA1c), in development of diabetes-related microvascular complications. Skin advanced glycation end products (AGEs) are formed in part by exposure to glucose over time and have been shown to be independently associated with and predictive of the microvascular complications of diabetes.3,4 Certain skin AGEs fluoresce (e.g., pentosidine, crosslines), facilitating noninvasive measurement5–7 that could be used in clinical studies or settings.
Although the pathogenesis is poorly understood, hyperglycemia-associated tissue damage operates through formation and accumulation of AGEs.8,9 AGEs are the products of free radical oxidation between reducing sugars and amino groups in proteins. AGE formation is considered a process that is enhanced in diabetes by elevated glucose concentrations and oxidative stress.10,11 Studies have focused on AGE modification of skin collagen, which may reflect AGE-mediated tissue damage elsewhere in the body, including vessel wall stiffening, basement membrane thickening, and demyelination of nerve fibers.11–13
Skin AGE content accumulates with age, and this accumulation is accelerated in diabetes.14 In addition, studies have shown that AGE accumulation is higher with compromised kidney function because of reduced ability to clear AGEs from the body.15
Skin intrinsic fluorescence (SIF) provides an opportunity for noninvasive measurement5–7,16 and enables larger studies than possible with biopsies and chemical assays of skin AGEs. SIF may be affected by technical factors such as environmental sun exposure and skin tone because the melanin and hemoglobin in skin can absorb the light used to excite skin fluorescence more strongly than the emitted fluorescence, leading to distortion of SIF.5,6 The geographically diverse DCCT/EDIC study afforded the opportunity to examine the influence of these technical factors on SIF in concert with numerous clinical factors contained in the 28-year phenotypic history available in this cohort. The DCCT/EDIC study also facilitates examination of the association of SIF with time-weighted mean HbA1c to determine if SIF is an indicator of historic glycemic exposure that is often unavailable in normal clinical settings.
We therefore used the detailed phenotypic history available in the DCCT/EDIC study and measurements of SIF on 1,185 EDIC participants during years 16–17 to determine the association with SIF of glycemic exposure, other clinical factors, and technical factors that may impact the SIF measurement.
Subjects and Methods
Study population
The inclusion and exclusion criteria, treatment protocol, and baseline characteristics of the DCCT cohort have been previously described.17,18 In brief, 1,441 subjects with T1DM who were 13–39 years old were recruited between 1983 and 1989. The primary prevention cohort consisted of 726 subjects who, at study baseline, had no retinopathy, a urinary albumin excretion rate (AER) of <40 mg/24-h period, and diabetes duration of 1–5 years. The secondary intervention cohort consisted of 715 subjects who had very mild to moderate nonproliferative retinopathy, urinary AER ≤200 mg/24-h period, and diabetes duration 1–15 years. Individuals with hypertension (defined by systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg), a history of symptomatic ischemic heart disease, major electrocardiogram abnormalities, or severe hypercholesterolemia were excluded.
The 711 patients randomized to intensive treatment received either multiple daily insulin injections or continuous subcutaneous insulin infusion with external insulin pumps, along with frequent self-glucose monitoring. Conventional therapy patients (n=730) were treated with one or two daily insulin injections and daily urine or blood glucose testing. The intensive and conventional treatment groups maintained median HbA1c levels of 7.0% and 9.0%, respectively, during the 6.5-year mean DCCT follow-up. In 1994, 1,375 subjects (96% of the surviving cohort) agreed to participate in the EDIC study to examine DCCT treatment effects on longer-term complications of diabetes.2 With the initiation of the EDIC study, conventional treatment participants were offered instruction in intensive therapy. EDIC participants were evaluated annually.
For the SIF substudy, all living subjects who participated during year 16 or 17 were eligible for inclusion unless they met exclusion criteria: history of extreme photosensitivity (n=11), skin cancer (n=10), no informed consent (n=5), birthmarks, tattoos, skin rashes, or chemical hair removal (n=5), or missing the study participation window (n=73). As a result, 1,185 of 1,289 active EDIC participants were included (92%).
SIF measurement
Duplicate measurements of SIF were obtained from the skin on the underside of the left forearm using a SCOUT skin fluorescence spectrometer (VeraLight, Inc., Albuquerque, NM). SIF was excited with a light-emitting diode centered at 375 nm and was detected over the emission range of 435–655 nm. Skin reflectance was assessed with a white light-emitting diode over the 435–655 nm spectral region. The measured skin reflectance was used to compensate for absorbance due to melanin and hemoglobin as well as subject-specific light scattering using the intrinsic fluorescence correction5 formula expressed in Eq. 1:
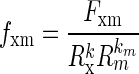 |
(1) |
where the measured fluorescence, Fxm, is divided by reflectance values from the excitation and white light-emitting diodes, Rx and Rm, respectively. The reflectance values are adjusted by the dimensionless exponents, kx and km. For these analyses, the 375-nm excited fluorescence was used with kx set to 0.6 and km set to 0.2. The resulting intrinsic fluorescence, fxm, was integrated over the 435–655 nm spectral region and multiplied by 1,000 to represent SIF, reported in arbitrary units (AU). These values of kx and km were previously determined to be relevant for the 375-nm excited fluorescence, which had the strongest association with diabetes-related complications in the Pittsburgh Epidemiology of Diabetes Complications cohort.19
Clinical outcomes
Demographic data and health history were self-reported. A physical examination measured clinical status. Laboratory measurements were performed at the DCCT/EDIC Central Biochemistry Laboratory as previously described.17 HbA1c was measured every 3 months during the DCCT and yearly during the EDIC study.2,17 Long-term stability of the HbA1c assay has been described.20 AER was measured annually during the DCCT and in alternate years during the EDIC study using a timed 4-h urine collection and expressed per 24-h period.2,17 Serum lipids were measured using enzymatic methods from fasting samples obtained yearly during the DCCT and in alternate years during the EDIC study. Serum creatinine was measured annually in the DCCT/EDIC. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine specific for age, sex, and race using the Chronic Kidney Disease–Epidemiology Collaboration equation.21 Time-weighted eGFR was computed by taking the mean of the eGFR values for each subject over the entire DCCT/EDIC time period up to the year the SIF measurement was performed.22 For all linear regression models a categorical variable of any eGFR <60 mL/min/m2 from DCCT enrollment to date of the SIF measurement was used because it was the renal variable most strongly correlated with SIF.
Clinic latitude was hypothesized to be a surrogate for potential differences in vitamin D levels due to sun exposure and was incorporated into the data analysis as a categorical variable, with EDIC clinics below latitude 37° N designated as southern clinics (n=7) and those above as northern clinics (n=21).23–26 Skin tone was considered a measure of the light reflected by the subject's skin. Skin tone was measured by the SCOUT device across the 435–655 nm spectral region and was calculated by summing light reflectance in that range. Smoking status was determined by subject self-report and categorized as “never smoked” (≤100 cigarettes in a subject's lifetime), “previous smoker” (quit ≥1 year ago), or “current smoker.” Subject age at the time of the SIF measurements was used for all analyses. Mean HbA1c for a given number of years was calculated by taking the mean of the HbA1c values of the given time period. Total mean HbA1c was calculated by summing (DCCT/EDIC eligibility HbA1c×duration of diabetes at study baseline), (DCCT mean HbA1c×years of follow-up in DCCT), and (EDIC mean HbA1c×years of follow-up in EDIC) and dividing by total duration of diabetes.
Statistical analyses
Demographic and clinical characteristics were compared using the Wilcoxon rank-sum test to evaluate treatment group differences for ordinal and numeric variables. The contingency χ2 test was used for categorical variables. The first SIF measurement per subject was used for all analyses except the intrasubject, same-day variation in SIF, which was assessed using the method of the Hoorn study.27 The correlations between log(SIF) and EDIC phenotype data were computed by linear regression, and variables that were significantly correlated with log(SIF) were used in multivariate analysis. Multivariate linear regression of log(SIF) on various glycemic exposure intervals was simultaneously adjusted for the following significant variables: age, any eGFR <60 mL/min/m2, smoking status, skin tone, and clinic latitude <37° N. In each linear regression model, the variance contribution of each term was estimated and reported as a squared semipartial correlation coefficient.
Results
At the time of SIF measurement, the population had a mean age of 51.5 years and diabetes duration of 29.8 years (Table 1). The mean SIF values did not differ significantly by DCCT treatment cohort (P=0.82) or by gender (P=0.62). However, there was a significant difference in SIF between the primary prevention (22.2±4.8 AU) and secondary intervention (23.2±4.8 AU) cohorts of the DCCT (P<0.0001), which is explained by the younger age of the primary prevention cohort (51±7 vs. 52±7 years, P<0.01) and, at the start of DCCT, the shorter duration of diabetes (1–5 years vs. 1–15 years) and absence of renal compromise (AER <40 mg/24-h period).
Table 1.
Characteristics of the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications Skin Intrinsic Fluorescence (SIF) Population at the Time of SIF Assessment Overall and by Original DCCT Treatment Group
| Former treatment group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intensive | Conventional | P value | Overall | Univariate R2with log(SIF) | P value | Multivariate R2with log(SIF)a | P value | |
| Total number of patients | 612 | 573 | — | 1,185 | — | — | ||
| SIF (arbitrary units) | 22.6±4.7 | 22.7±4.9 | 0.8207 | 22.7±4.8 | — | — | ||
| Demographic characteristics | ||||||||
| Gender (male) | 317 (52) | 308 (54) | 0.5006 | 625 (53) | <0.001 | 0.8482 | 0.003 | 0.0237 |
| Age (years) | 51.9±6.9 | 51.0±6.9 | 0.0203 | 51.5±6.9 | 0.136 | <0.0001 | 0.110 | <0.0001 |
| Diabetes duration (years) | 30.0±4.9 | 29.5±4.9 | 0.0990 | 29.8±4.9 | 0.015 | <0.0001 | <0.001 | 0.2837 |
| Body mass index (kg/m2) | 29.4±9.3 | 28.3±4.9 | 0.0635 | 28.9±7.5 | <0.001 | 0.6615 | <0.001 | 0.2181 |
| Cohort assignment, primary | 298 (49) | 294 (51) | 0.3681 | 592 (50) | 0.014 | <0.0001 | <0.001 | 0.6387 |
| Skin tone, arbitrary units | 260.4±46.8 | 255.6±48.8 | 0.0513 | 258.1±47.8 | 0.015 | <0.0001 | 0.036 | <0.0001 |
| Clinic latitude >37° N | 444 (73) | 427 (75) | 0.4423 | 871 (74) | 0.017 | <0.0001 | 0.015 | <0.0001 |
| Smokingb | 0.7436 | 0.065 | <0.0001 | 0.048 | <0.0001 | |||
| Never smoker | 372 (61) | 352 (61) | 724 (61) | |||||
| Ever smoker | 154 (25) | 149 (26) | 303 (26) | |||||
| Current smoker | 86 (14) | 72 (13) | 158 (13) | |||||
| ESRD | 6 (1) | 8 (1) | 0.5080 | 14 (1) | 0.013 | <0.0001 | Not used | — |
| Time-weighted serum creatinine (mg/dL) | 0.8±0.2 | 0.8±0.2 | 0.4565 | 0.8±0.2 | 0.019 | <0.0001 | Not used | — |
| Time weighted AER (mg/24-h period) | 35.2±124.0 | 61.0±183.7 | <0.0001 | 47.7±156.1 | 0.019 | <0.0001 | Not used | — |
| Time-weighted log(AER) | 2.5±0.7 | 2.7±0.9 | 0.0002 | 2.6±0.8 | 0.026 | <0.0001 | Not used | — |
| Any eGFR <60 mL/min/m2 to date | 40 (7) | 44 (8) | 0.4436 | 84 (7) | 0.056 | <0.0001 | 0.017 | <0.0001 |
| Ever use of statins (yes vs. no) | 360 (59) | 329 (57) | 0.6238 | 689 (58) | 0.005 | 0.0130 | 0.001 | 0.1578 |
| Time-weighted HDLC (mg/dL) | 55.3±13.4 | 107.9±21.0 | 0.7300 | 55.3±12.8 | <0.001 | 0.9841 | Not used | — |
| Time-weighted LDLC (mg/dL) | 109.7±19.9 | 55.3±12.1 | 0.1789 | 108.9±20.5 | 0.013 | <0.0001 | Not used | — |
| Time-weighted triglycerides (mg/dL) | 86.6±42.2 | 83.1±42.1 | 0.0389 | 84.9±42.2 | 0.008 | 0.0018 | <0.001 | 0.3981 |
| Time-weighted BP (mm Hg) | ||||||||
| Diastolic | 74.4±5.2 | 74.1±5.1 | 0.3991 | 74.3±5.2 | <0.001 | 0.8704 | <0.001 | 0.3540 |
| Systolic | 118.8±8.1 | 118.5±8.4 | 0.6217 | 118.7±8.3 | 0.025 | <0.0001 | <0.001 | 0.5828 |
| Use of antihypertensive medications ever | 340 (56) | 337 (59) | 0.2575 | 677 (57) | 0.014 | <0.0001 | <0.001 | 0.1907 |
| Most recent HbA1c (%) | 7.9±1.2 | 7.9±1.2 | 0.3392 | 7.9±1.2 | 0.022 | <0.0001 | Not used | — |
| EDIC mean HbA1c (%) | ||||||||
| Last 5 years | 7.9±1.1 | 7.8±1.1 | 0.1637 | 7.9±1.1 | 0.035 | <0.0001 | Not used | — |
| Last 10 years | 7.9±1.1 | 7.8±1.1 | 0.1293 | 7.9±1.1 | 0.046 | <0.0001 | Not used | — |
| All years | 8.0±1.1 | 7.9±1.0 | 0.7549 | 8.0±1.0 | 0.063 | <0.0001 | Not used | — |
| EDIC+DCCT mean HbA1c (%) | 7.8±0.9 | 8.2±0.9 | <0.0001 | 8.0±1.0 | 0.058 | <0.0001 | Not used | — |
| EDIC+DCCT+baseline mean HbA1c (%)c | 8.0±0.9 | 8.4±0.9 | <0.0001 | 8.2±0.9 | 0.070 | <0.0001 | 0.043 | <0.0001 |
Data are n (%) or mean±SD values. P values are for treatment group differences comparing former intensive versus conventional therapy based on the contingency χ2 test for qualitative variables and the Wilcoxon rank-sum test for quantitative variables.
Data are squared semipartial R2 correlations from a linear regression model regressing log(SIF) on all potential explanatory variables, resulting in a R2 of 0.345 for the full model. Variables that were redundant or weaker than other similar variables are marked as not used and were not included in the model. Specifically, Epidemiology of Diabetes Interventions and Complications (EDIC)+DCCT+baseline mean hemoglobin A1c (HbA1c) trumped all other mean HbA1c variables, triglycerides trumped high-density lipoprotein cholesterol (HDLC) and low-density lipoprotein cholesterol (LDLC), and any estimated glomerular filtration rate (eGFR) <60 mL/min/m2 trumped continuous eGFR, albumin excretion rate (AER), serum creatinine, and end-stage renal disease (ESRD).
Current smoker defined as currently smoking or smoking within the last year; ever smoker defined as smoking more than a year ago.
Total mean HbA1c is calculated by summing (DCCT/EDIC eligibility HbA1c×duration of diabetes at study baseline), (DCCT mean HbA1c×years of follow-up on DCCT), and (EDIC mean HbA1c×years of follow-up in EDIC) and dividing by total duration of diabetes.
In the 1,185 EDIC subjects with SIF measurement, the intraday Hoorn coefficient of variation was 4.2%, and the between-measurement correlation was 0.963 (R2=0.927), which explains 7.3% of the total variance in the SIF measurement. The range of SIF values in the EDIC cohort was 8.7–54.0 AU, with a mean value of 22.7±4.8 AU. The distribution had a log normal characteristic, so SIF was natural logarithm-transformed for further statistical analyses, resulting in a mean value of the log(SIF) of 3.10±0.21 AU.
The correlations (R2) of log(SIF) with increasing lengths of glycemic exposure as assessed by mean HbA1c are reported in Table 2. Each row represents a different interval of glycemic exposure, ranging from the most recent 5 years of EDIC to the subject's glycemic exposure from entry into DCCT to the most recent year of EDIC (29.8±4.9 years). For all time intervals, the intensive therapy group showed a lower correlation with current SIF than did the conventional therapy group. The correlation of log(SIF) with glycemic exposure increased with increasing length of glycemic exposure from an R2 of 0.035 for the most recent 5 years of EDIC to 0.063 for all of the EDIC study. As shown in Table 2, the trend of increasing correlation with time holds true for all time periods in the conventional therapy group, but in the intensive therapy group, the addition of the DCCT period to the EDIC period disrupts the trend, resulting in lower correlation.
Table 2.
Log(Skin Instrinsic Fluorescence) Correlation with Increasing Lengths of Glycemic Exposure as Assessed by Time-Weighted Mean Hemoglobin A1c, Stratified by Original Diabetes Control and Complications Trial Treatment Group
| R2for log(SIF) correlation with glycemic exposure | |||
|---|---|---|---|
| Former treatment group | |||
| Intensive | Conventional | Overall | |
| EDIC mean HbA1c | |||
| Last 5 years | 0.027 | 0.046 | 0.035 |
| Last 10 years | 0.036 | 0.059 | 0.046 |
| All years | 0.052 | 0.078 | 0.063 |
| EDIC+DCCT mean HbA1c | 0.041 | 0.085 | 0.058 |
| EDIC+DCCT+baseline mean HbA1ca | 0.054 | 0.094 | 0.070 |
Data are squared semipartial R2 correlations from five separate linear regression models regressing log(skin intrinsic fluorescence [SIF]) on various glycemic exposure intervals.
Total mean hemoglobin A1c (HbA1c) is calculated by summing (Diabetes Control and Complications Trial [DCCT]/Epidemiology of Diabetes Intervention and Control [EDIC] eligibility HbA1c×duration of diabetes at study baseline), (DCCT mean HbA1c×years of follow-up on DCCT), and (EDIC mean HbA1c×years of follow-up in EDIC) and dividing by total duration of diabetes.
To determine the clinical factors associated with log(SIF), univariate and multivariate associations were examined for factors that could be influential, including time-weighted mean HbA1c, age, gender, body mass index, DCCT treatment group, DCCT cohort assignment, duration of diabetes, use of statins at any time, time-weighted mean high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides, time-weighted serum creatinine, time-weighted AER, any eGFR <60mL/min/1.73 m2, smoking status, time-weighted mean diastolic and systolic blood pressure, use of antihypertensive medications at any time, measure of skin tone, and EDIC clinic latitude >37° N. Univariate analysis found significant correlations with the above variables (Table 1) with the exceptions of gender, body mass index, high-density lipoprotein cholesterol, and diastolic blood pressure. In addition, we observed a loss of correlation of log(SIF) with time-weighted eGFR for the subset of those with an eGFR <60 mL/min/m2 (R2=0.0015, P=0.93, n=7), whereas time-weighted eGFR was significantly correlated with log(SIF) for those with an eGFR ≥60 mL/min/m2 (R2=0.0790, P<0.0001, n=1,178).
Through multivariate analysis (see Table 1), we found the following clinical/technical factors significantly associated with log(SIF): glycemic exposure, age, any eGFR <60 mL/min/m2, smoking status, skin tone, and clinic latitude. These variables were included in the multivariate models for log(SIF). Because there was not a significant interaction due to DCCT treatment group or primary versus secondary cohort, multivariate models were constructed using the entire SIF substudy cohort. Table 3 contains the multivariate model coefficients (β) and semipartial R2 for each significant independent variable in the model for log(SIF). Each column represents a different interval of glycemic exposure. The multivariate models for each interval of glycemic exposure explained 30.8–33.1% (total R2) of the variance and were not different from each other in their ability to predict log(SIF). Examining the model coefficients (β) for each time interval, the model coefficient for time-weighted mean HbA1c increased as the glycemic exposure interval increased, whereas the model coefficient for any eGFR <60 mL/min/m2 decreased as the glycemic exposure interval increased. The model coefficients for age, smoking status, skin tone, and clinic latitude were essentially constant across the various glycemic exposure intervals. Similarly, the semipartial R2 for time-weighted mean HbA1c increased with increasing glycemic exposure, decreased for any eGFR <60 mL/min/m2, and was essentially constant for age, smoking status, skin tone, and clinic latitude.
Table 3.
Association of Log(Skin Intrinsic Fluorescence) with Varying Glycemic Exposure Intervals
| β (R2) at glycemic exposure interval | |||||
|---|---|---|---|---|---|
| EDIC | |||||
| Last 5 years | Last 10 years | All years | EDIC+DCCT | EDIC+DCCT+baselinea | |
| Mean HbA1c (%) | 0.028 (0.023) | 0.032 (0.027) | 0.037 (0.033) | 0.043 (0.038) | 0.048 (0.045) |
| Age (years) | 0.011 (0.137) | 0.011 (0.137) | 0.011 (0.136) | 0.011 (0.146) | 0.012 (0.147) |
| Any eGFR <60 mL/min/m2 to date (yes vs. no) | 0.140 (0.030) | 0.133 (0.027) | 0.122 (0.022) | 0.113 (0.019) | 0.106 (0.016) |
| Smoking (current, former, never)b | 0.070 (0.058) | 0.069 (0.056) | 0.067 (0.053) | 0.067 (0.053) | 0.066 (0.052) |
| Skin tone (arbitrary units) | 0.0008 (0.034) | 0.0008 (0.034) | 0.0008 (0.034) | 0.0008 (0.036) | 0.0008 (0.034) |
| Clinic latitude >37o (south vs. north) | 0.055 (0.014) | 0.057 (0.015) | 0.056 (0.015) | 0.058 (0.015) | 0.057 (0.015) |
| Total R2 (all variables) | 0.308 | 0.314 | 0.320 | 0.325 | 0.331 |
Data are β coefficients and squared semipartial R2 correlations from five separate linear regression models regressing log(skin intrinsic fluorescence) on various glycemic exposure intervals. Each model was simultaneously adjusted for age, any estimated glomerular filtration rate (eGFR) <60 mL/min/m2, smoking status, skin tone, and clinic latitude.
Total mean hemoglobin A1c (HbA1c) is calculated by summing (Diabetes Control and Complications Trial [DCCT]/Epidemiology of Diabetes Intervention and Complications [EDIC] eligibility HbA1c×duration of diabetes at study baseline), (DCCT mean HbA1c×years of follow-up on DCCT), and (EDIC mean HbA1c×years of follow-up in EDIC) and dividing by total duration of diabetes.
Current smoker defined as currently smoking or smoking within the last year; ever smoker defined as smoking more than a year ago.
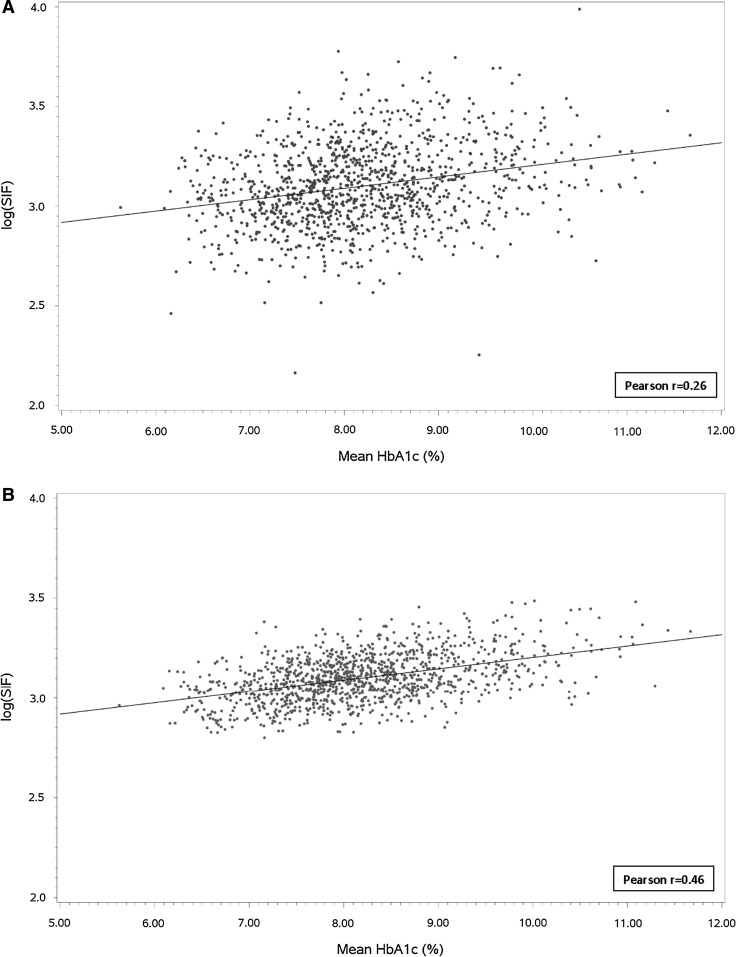
Finally, we compared the correlation of the unadjusted log(SIF) and the multivariate adjusted log(SIF) with glycemic exposure covering the longest time interval, from entry into DCCT to the most recent year of EDIC. As shown in Figure 1A, the Pearson correlation for the unadjusted log(SIF) with glycemic exposure over this interval (29.8±4.9 years) yielded an r=0.26, which though relatively weak was highly significant (P<0.0001). In Figure 1B, the adjusted log(SIF) (model based on total mean HbA1c, age, eGFR <60 mL/min/m2, smoking status, skin tone, and clinic latitude) had a higher Pearson correlation coefficient of r=0.46, which was both significant (P<0.0001) and more significantly correlated than the unadjusted log(SIF) with glycemic exposure.
FIG. 1.
(A) Unadjusted and (B) adjusted log(skin intrinsic fluorescence [SIF]) versus 25-year mean hemoglobin A1c (HbA1c). Adjustment was done simultaneously for age, any estimated glomerular filtration rate <60 mL/min/m2, smoking status, skin tone, and clinic latitude.
Discussion
We found that 33.1% of SIF was explained by the clinical and technical variables listed in Table 3 and another 7.3% by measurement-to-measurement variance. Of the former, age contributed nearly half (14.7%), smoking status 5.2%, skin tone 3.4%, “any eGFR” <60 mL/min/m2 1.6%, and clinic latitude 1.5%. As a measure of glycemic exposure, time-weighted mean HbA1c contributed 4.5%. These data demonstrate that, although there is an increasing correlation between SIF and longer periods of glycemic exposure, much of the SIF variance is not explained. Factors other than glycemic exposure that may influence SIF include age,28,29 individual variation in antioxidant defenses,10 renal damage,4,15,30,31 and smoking.32,33
The majority of SIF variance (60%) was not explained by study variables. Of the unknown contributing factors, individual variation in oxidative stress and in antioxidant defenses may be important. As shown in our own10 and other studies,5 among people without diabetes, the interindividual rate of accumulation of specific AGE products in skin can vary by a factor of 2. Also, in both people without diabetes and those with T1DM, we found similar interindividual variation in the rate of accumulation of oxidized methionine residues in skin collagen.10 Taken together, these findings suggest a hypothesis that, in the absence of diabetes, the large variation in the rate of AGE accumulation is possibly linked to individual specific oxidative stress. Unfortunately, there is no convenient measure for “oxidative stress,” so we were unable to assess its contribution to SIF.
We did find similarities with DCCT skin biopsy data collected in 1992. The article of Monnier et al.3 reports correlations with mean HbA1c and biopsy for the six different AGE assays after adjustment for age and duration of diabetes that range from 0.205 (R2=0.042) for acid-soluble collagen to 0.440 (R2=0.194) for furosine. The adjusted SIF measurement had a correlation of 0.46 (0.414–0.503) to 25-year mean HbA1c (Fig. 1B), which is of the same magnitude as the furosine correlation, although with the exception of age, the SIF adjustments were different than those used for furosine. It is interesting that SIF was correlated with the furosine assay (R=0.25) in the 175 subjects who had both the skin biopsy in 1992 and the SIF measurement 17–19 years later. Finally, although the 1992 biopsy data showed significant differences in AGE products between the intensive and conventional therapy groups, there was no difference in SIF between these groups, possibly due to study-wide adoption of intensive therapy and the passage of time.
Loss to follow-up during the EDIC study was higher in subjects who developed end-stage renal disease or who had a coronary artery calcification score of >200 Agatston units, potentially giving rise to a survivor bias, which may have eliminated differences in SIF between DCCT treatment groups for these outcomes (Table 1). However, compared with the few (n=104) active DCCT/EDIC participants without an SIF measure, those examined showed no differences in age (P=0.97) and duration of diabetes (P=0.64). Nonparticipants had a higher mean total (EDIC+DCCT+baseline) HbA1c (8.4±1.0% vs. 8.2±0.9%; P=0.02) (data not shown).
In subjects without diabetes, chronological age correlates more strongly with SIF (R2=0.261)28,29 than in the current study (R2=0.147). We hypothesize that the higher and more variable glycemic exposure in subjects with T1DM as well as increased likelihood of compromised renal function contributed to the weakened correlation of chronological age with SIF in the EDIC SCOUT substudy cohort. Several studies have shown that renal failure leads to increased levels of skin AGEs independent of glycemic control, and the combination of diabetes plus renal failure can lead to high levels of skin AGEs and SIF.15,30,31
We hypothesized the variance explained by skin tone was due to imperfections in the intrinsic correction of the measured fluorescence. To test this hypothesis, we changed the intrinsic correction coefficient, kx, from a value of 0.6 to 0.8 to compute new SIF values and then rebuilt the multivariate models. For the new SIF variable, the model R2 increased from 0.33 to 0.46, and the variance explained by skin tone increased from 0.034 to 0.161, whereas the variance explained by the other model variables decreased. This analysis confirmed that the primary function of the skin tone variable was to compensate for imperfections in the intrinsic correction.
The clinic latitude variable was used to denote subjects who attended EDIC clinics above 37° N latitude. As shown in the multivariate analysis, clinic latitude did contribute to a small but statistically significant portion of the variance in SIF. However, we cannot definitively say whether this was due to differences in vitamin D, sun exposure, genetics, or lifestyle.
Approximately 13% of the study cohort were current smokers, and another 26% were former smokers. As smoking is known to enhance AGE formation and is a source of oxidative stress,28 the finding that smoking is a contributor to the variance in SIF is consistent with the literature.
Imperfections of SIF that could contribute to the unexplained variance include the superposition of epidermal fluorescence from NADH and FAD28 on dermal AGE fluorescence and interday, within-subject measurement variance. As NADH and FAD are not as stable as dermal AGEs and their levels fluctuate in response to changes in metabolism and oxidative stress,34 their superposition on the dermal AGE signal could reduce the reflection of glycemic exposure in SIF because the SIF algorithm only takes into account the intensity of the fluorescence and not the spectral shapes.
Finally, we tested to see if instrument bias was a source of error by adding clinic number to the multivariate model because there was a one-to-one mapping between instruments and clinics. This test showed that instrument bias was not a significant contributor to the overall variance in SIF.
Strengths of this study include the excellent characterization of 92% of all living EDIC subjects. Potential weaknesses include the absence of measures of smoking by pack-years and the absence of measures of long-term oxidative stress. In addition, there are gaps in the HbA1c history due to annual measurement of HbA1c during the EDIC study instead of quarterly during the DCCT. Finally, use of clinic latitude as an indicator of vitamin D levels or sun exposure is an approximation.
Conclusions
In the DCCT/EDIC cohort, clinical factors significantly associated with SIF include age, smoking status, time-weighted mean HbA1c, skin tone, clinic latitude >37° N, and any eGFR <60 mL/min/m2. The unadjusted SIF is weakly but significantly correlated (R=0.26) with glycemic exposure, and the strength of the correlation increases with increasing length of glycemic exposure with the exception that the HbA1c during the DCCT did not contribute to current SIF values for the intensive treatment group. When SIF is predicted by a multivariate model that uses the aforementioned clinical factors, the correlation with glycemic exposure becomes modest (R=0.46).
These findings suggest that SIF may be associated with diabetes-related complications because it reflects age, long-term glycemic exposure, smoking, and chronic kidney disease, which are known correlates and risk factors.
Contributor Information
Collaborators: the DCCT/EDIC Research Group
Acknowledgments
The authors would like to thank all members of the DCCT/EDIC Research Group.22 The authors would also like to thank the contributors of free or discounted supplies and/or equipment: Abbott, Animas, Aventis, BD, Bayer, Can-AM, Eli Lilly, Lifescan, Medtronic Minimed, Omron, and Roche. In addition, the authors would like to acknowledge support from contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and VeraLight and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. P.A.C. and J.M. researched the data, wrote significant portions of the manuscript, reviewed/edited the manuscript, and contributed to the discussion. B.H.B. researched the data, wrote a portion of the manuscript, reviewed/edited the manuscript, and contributed to the discussion. T.O., T.J.L., C.C., and R.A.G.-K. each wrote a portion of the manuscript, reviewed/edited the manuscript, and contributed to the discussion. J.W. and K.A. researched the data. A.B. reviewed/edited the manuscript and contributed to the discussion. S.V. reviewed/edited the manuscript.
Author Disclosure Statement
P.A.C., B.H.B., C.C., R.A.G.-K., K.A., and S.V. have no relevant conflicts of interest to disclose. J.M. and J.W. are employees and stock option holders of VeraLight, Inc. A.B. is an independent insulin pump trainer who receives payment from Medtronic, Animas, Spirit, and Omnipod pumps. T.O. has received grant support from VeraLight, Inc. and serves as a consultant to Gilead and Aegerion. T.J.L. has received grant support from VeraLight, Inc. in the past.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnier VM. Bautista O. Kenny D. Sell DR. Fogarty J. Dahms W. Cleary PA. Lachin J. Genuth S. Skin collagen glycation, glycoxidation, crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genuth S. Sun W. Cleary P. Sell DR. Dahms W. Malone J. Sivitz W. Monnier VM DCCT Skin Collagen Ancillary Study Group. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull E. Ediger M. Unione A. Deemer E. Stroman M. Baynes J. Noninvasive, optical detection of diabetes: model studies with porcine skin. Optics Express. 2004;12:4496–4510. doi: 10.1364/opex.12.004496. [DOI] [PubMed] [Google Scholar]
- 6.Meerwaldt R. Graaf R. Oomen PH. Links TP. Jagger JJ. Alderson NL. Thorpe SR. Baynes JW. Gans RO. Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 7.Beisswenger PJ. Howell S. Mackenzie T. Corstjens H. Muizzuddin N. Matsui MS. Two fluorescent wavelengths, 440ex/520em nm and 370ex/440em nm, reflect advanced glycation and oxidation end products in human skin without diabetes. Diabetes Technol Ther. 2012;14:285–292. doi: 10.1089/dia.2011.0108. [DOI] [PubMed] [Google Scholar]
- 8.Thorpe S. Lyons T. Baynes J. Glycation and glycoxidation in diabetic vascular disease. In: Keaney JF, editor. Oxidative Stress and Vascular Disease. Norwell, MA: Kluwer; 2000. pp. 259–285. [Google Scholar]
- 9.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y. Thorpe SR. Jenkins AJ. Shaw JN. Sochaski MA. McGee D. Aston CE. Orchard TJ. Silvers N. Peng YG. McKnight JA. Baynes JW. Lyons TJ DCCT/EDIC Research Group. Advanced glycation end-products and methionine sulphoxide in skin collagen of patients with type 1 diabetes. Diabetologia. 2006;49:2488–2498. doi: 10.1007/s00125-006-0355-8. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Conway BN. Aroda VR. Maynard JD. Matter N. Fernandez S. Ratner RE. Orchard TJ. Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care. 2011;34:1000–1005. doi: 10.2337/dc10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sell DR. Monnier VM. Molecular basis of arterial stiffening: role of glycation—a mini-review. Gerontology. 2012;58:227–237. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 14.Dyer DG. Dunn JA. Thorpe SR. Bailie KE. Lyons TJ. McCance DR. Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meerwaldt R. Hartog JW. Graaf R. Huisman RJ. Links TP. den Hollander NC. Thorpe SR. Baynes JW. Navis G. Gans RO. Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3687–3693. doi: 10.1681/ASN.2005020144. [DOI] [PubMed] [Google Scholar]
- 16.Monnier VM. Vishwanath V. Frank KE. Elmets CA. Dauchot P. Kohn RR. Relation between complications of type 1 diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications Trial (DCCT) Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 18.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway B. Edmundowicz D. Matter N. Maynard J. Orchard T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther. 2010;12:339–345. doi: 10.1089/dia.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffes M. Cleary P. Goldstein D. Little R. Wiedmeyer HM. Rohlfing C. England J. Bucksa J. Nowicki M. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51:753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS. Stevens LA. Schmid CH. Zhang YL. Castro AF3. Feldman HI. Kusek JW. Eggers P. Van Lente F. Greene T. Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DCCT/EDIC Research Group. de Boer IH. Sun W. Cleary PA. Lachin JM. Molitch ME. Steffes MW. Zinman B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablonski KL. Chonchol M. Pierce GL. Walker AE. Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarcin O. Yavuz DG. Ozben B. Telli A. Ogunc AV. Yuksel M. Toprak A. Yazici D. Sancak S. Deyneli O. Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 25.Yiu YF. Chan YH. Yiu KH. Siu CW. Li SW. Wong LY. Lee SW. Tam S. Wong EW. Cheung BM. Tse HF. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:E830–E835. doi: 10.1210/jc.2010-2212. [DOI] [PubMed] [Google Scholar]
- 26.Danescu LG. Levy S. Levy L. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–17. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 27.Mooy JM. Grootenhuis PA. de Vries H. Kostense PJ. Popp-Snijders C. Bouter LM. Heine RJ. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn study. Diabetologia. 1996;39:298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 28.Na R. Stender IM. Henriksen M. Wulf HC. Autofluorescence of human skin is age-related after correction for skin pigmentation and redness. J Invest Dermatol. 2001;116:536–540. doi: 10.1046/j.1523-1747.2001.01285.x. [DOI] [PubMed] [Google Scholar]
- 29.Maynard J. Matter N. Non-invasive skin fluorescence age trends in subjects with normal health are gender dependent [abstract] Diabetologia. 2011;54(Suppl 1):S147. [Google Scholar]
- 30.Gerrits EG. Lutgers HL. Kleefstra N. Graaf R. Groenier KH. Smit AJ. Gans RO. Bilo HJ. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 31.Chabroux S. Canoui-Poitrine F. Reffet S. Mills-Joncour G. Morelon E. Colin C. Thivolet C. Advanced glycation end products assessed by skin autofluorescence in type 1 diabetics are associated with nephropathy, but not retinopathy. Diabetes Metab. 2010;36:152–157. doi: 10.1016/j.diabet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Jee SH. Foong AW. Hur NW. Samet JW. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care. 2010;33:2567–2572. doi: 10.2337/dc10-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickerson TJ. Janda KD. A previously undescribed chemical link between smoking and metabolic disease. Proc Natl Acad Sci U S A. 2002;99:15084–15088. doi: 10.1073/pnas.222561699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heikal AA. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark Med. 2010;4:241–263. doi: 10.2217/bmm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]