Abstract
In 2014, numerous noteworthy papers focusing on adipose tissue physiology were published. Many of these articles showed the promise of adipose-tissue-targeted approaches for therapeutic intervention in obesity and type 2 diabetes mellitus. Here, we highlight advances in the development and maintenance of brown and/or beige adipocytes and the metabolic implications of infammation in adipose tissues.
Adipocytes that express UCP1, known as brown or beige adipocytes, can increase whole-body energy expenditure by dissipating excess energy through uncoupled mitochondrial respiration. With an increased appreciation that metabolically active brown adipose tissue (BAT) exists in adult humans,1 efforts have focused on adipose tissue as a key regulator of energy expenditure and homeostasis. We now know that adipose tissue displays enormous plasticity and is capable of changing its size, phenotype and metabolic functions. Several studies have advanced our understanding of key transcriptional mechanisms that manipulate these processes, including the ‘browning’ of white adipocytes.
Efforts to distinguish differences between brown adipocytes from that of the later-defined beige adipocytes (UCP1-expressing cells scattered between white adipocytes) have highlighted that brown and beige adipocytes represent two distinct cell types. Although both cell types share multiple biochemical and metabolic features, new evidence suggests that they have separate developmental origins. As such, the two cell types respond differently to various hormonal stimuli and differ in their gene expression profiles.2 As potential therapeutic targets for type 2 diabetes mellitus and obesity, there are vast clinical implications for understanding the origins and development of brown and beige adipocytes. In 2014, Long and colleagues elucidated key differences and similarities between these two cell types.3 By use of translating ribosome affinity purification technology, the authors isolated polysomes (clusters of ribosomes bound to mRNA) of UCP1-positive cells from mouse adipose tissue and used them to generate a comprehensive molecular description of brown and beige gene expression. The authors determined that both brown and beige cell types share a common thermogenic gene signature, regardless of location and cell type. However, classical brown, but not beige, adipocytes arise from a MYF5 and/or PAX7 skeletal-muscle lineage, which indicates that these two cell types have separate developmental origins (Figure 1). Mature beige, but not brown, adipocytes seem to have a gene expression pattern similar to that of smooth muscle cells and, thus, arise from a smooth-muscle-like origin.
Figure 1.
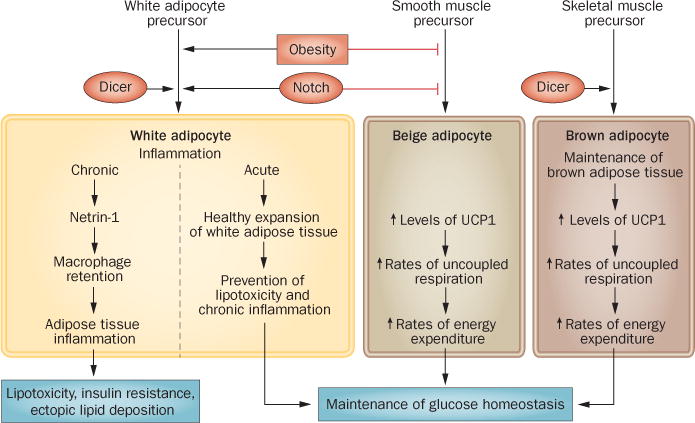
Adipose-tissue inflammation and adipocyte beigeing in the maintenance of glucose homeostasis.
First identified in Drosophila melanogaster, the evolutionarily conserved Notch signalling pathway regulates determination of cell fate and/or differentiation in various cell types. The Notch pathway has been associated with the pathophysiology of disease states caused by abnormal cellular differentiation (for exam ple, cancer and congenital heart disease). Notch signalling declines with age, which drives age-associated conditions, such as neurodegenerative disorders and impaired regeneration of aged skeletal muscle.4 Notch signalling has been implicated in the regulation of adipogenic differentiation. Although inhibition of Notch signalling was known to pro mote adipogenic differentiation in mouse and human primary cell cultures,5 in 2014 Bi et al. were the first to examine the role of Notch signalling in vivo in adipose tissue.6 Levels of activated Notch 1 inversely correlated with expression of UCP1 protein, indicating that Notch signalling might have a role in the expression of genes specific to brown adipose tissue.6 Adipocyte-specific Notch inactivation or pharmacological inhibition of Notch signalling increases the expression of UCP1, which leads to ‘browning’ of white adipose tissue (WAT). This ‘browning’ prevents high fat diet-induced obesity, increases energy expenditure, improves glucose tolerance and enhances insulin sensitivity. Conversely, adipocyte-specific Notch activation ‘whitens’ BAT and reduces glucose tolerance.6
Similar to Notch signalling, the aging process decreases the expression levels of the endo ribonuclease Dicer. Two years ago Mori et al. showed that caloric restriction prevents this age-related decline in levels of Dicer expression.7 In 2014, the same authors made the connection between levels of Dicer and BAT function and/or maintenance.8 Adipose-specific deletion of Dicer1 (encoding Dicer) led to ‘whitening’ and enlargement of inter-scapular brown fat, severe insulin resistance and lipodystrophy in white fat pads. Serum levels of adipo nectin were decreased in Dicer1−/− mice. Furthermore, cultured immortalized preadipo cytes isolated from Dicer1−/− mice expressed reduced levels of UCP1, had increased rates of coupled respiration and produced higher levels of leptin than preadipocytes isolated from wild-type mice.8
The chronic inflammation associated with pathological expansion of adipose tissue is well known in mice and humans. The proinflammatory molecules secreted by macro-phages and the adipocyte itself, which are enriched in expanding adipose tissue, contribute to both local and systemic inflammation and exacerbate the lipotoxicity and insulin resistance that is associated with obesity and type 2 diabetes mellitus. These findings indicate that factors involved in both regulating the recruitment of macrophages and the retention of macrophages in adipose tissue might be therapeutically relevant and could serve as targets for the prevention and treatment of obesity-associated local inflammation.
This year, Ramkhelawon and colleagues made advances in our understanding of macrophage migration in adipose tissue in their investigation of Netrin-1, a secreted laminin-related neuroimmune molecule that regulates cell migration.9 The authors demonstrated that high fat-diet-induced obesity increases expression levels of Netrin-1 in adipose tissue, which, in turn, reduces the migratory capacity of macrophages and leads to an increased accumulation of macrophages in adipose tissue. Haematopoietic deletion of Ntn1 (encoding Netrin-1) led to increased migration of macrophages out of the adipose tissue, suggesting that in the obese state Netrin-1 functions downstream of monocyte chemoattractant signals and promotes local macrophage retention in WAT. Retention of macrophages might limit insulin sensitivity, as Ntn1−/− mice are protected from diet-induced adipose-tissue inflammation and insulin resistance. Thus, pharmacological inhibition of adipose-tissue macrophage retention leads to a reduction in the complication s of obesity.9
As outlined in Ramkhelawon et al., chronic adipose-tissue inflammation exerts deleterious systemic effects.9 However, in 2014, Wernstedt Asterholm et al. provided evidence that an acute, local inflammatory response in adipose tissue might be required for benign ‘healthy’ expansion of WAT,10 suggesting that inflammation inherent in the expansion of adipose tissue is a protective force, as long as it does not become a chronic phenomenon. The ‘healthy’ expansion of adipose tissue is a bene ficial, adaptive response to over-nutrition that prevents obesity-associated insulin resistance, hepatic steatosis and systemic inflammation. In three mouse models with adipose-tissue-specific reductions in pro inflammatory signalling, acute inflammation was required for full functionality of adipose tissue. When challenged with a high fat diet, mice that were unable to mount a pro inflammatory response had reduced glucose tolerance, decreased circulating levels of adipo nectin, WAT depots of reduced size, along with increased macrophage infiltration and increased hepatic steatosis. An acute and local inflammatory response within adipose tissue is thus crucial for the healthy expansion of this tissue and for prevention of lipo toxicity and ectopic lipid deposition that occurs in response to overnutrition.
Our understanding of the biology of adipose tissue has come a long way. Contrary to previously held beliefs that adipose tissue is a simple benign storage depot for lipids, we now appreciate the pivotal role of this tissue in maintaining systemic metabolic homeostasis and it’s capability for expansion, which protects peripheral tissues from lipotoxic damage. Adipose tissue serves as an endocrine organ that is capable of crosstalk with peripheral organs via adipokine production and secretion, and is a regulator of energy expenditure. The 2014 studies highlighted here expand our understanding even further and provide a more nuanced view of what is protective or harmful to the tissue, how precursors are differentially activated under distinct physiological conditions to transform a white adipocyte into one resembling a brown adipo cyte (and vice versa), as well as the origins of brown versus beige adipocytes. These newly discovered, distinctive pathways yield fur ther insights into the physiology of adipose tis sue and lead to a better understanding of the pathophysiological complications that are common to increased adiposity.
Key advances.
-
▪
A distinguishing feature between beige and brown adipocytes is the smooth muscle versus skeletal-muscle origin of these two cell types3
-
▪
Distinct signalling pathways, such as the Notch pathway, influence the commitment of these cells towards differentiation6
-
▪
The endoribonuclease Dicer regulates thermogenic gene expression, highlighting the importance of microRNA processing in the determination of white and brown adipose tissue gene expression8
-
▪
A unique set of molecules, including Netrin-1, is responsible for the retention of proinflammatory cells in adipose tissue9
-
▪
A more nuanced view of the role of adipose tissue inflammation is emerging with a need for transient activation of proinflammatory cascades for remodelling versus chronic elevation of proinflammatory pathways in adipose tissue10
Acknowledgments
The authors acknowledge support from the NIH (Grants R01-DK55758, R01-DK099110 and P01-DK088761 [P.E.S.], T32-DK007307 [J.H.S.]).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 2.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 3.Long JZ, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 5.Osathanon T, Subbalekha K, Sastravaha P, Pavasant P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol Int. 2012;36:1161–1170. doi: 10.1042/CBI20120288. [DOI] [PubMed] [Google Scholar]
- 6.Bi P, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori MA, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori MA, et al. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014;124:3339–3351. doi: 10.1172/JCI73468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramkhelawon B, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20:377–384. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wernstedt Asterholm I, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


