Abstract
Invasive opportunistic fungal infections of humans are common among those suffering from impaired immunity, and are difficult to treat resulting in high mortality. Amphotericin B (AmB) is one of the few antifungals available to treat such infections. The AmB resistance mechanisms reported so far mainly involve decrease in ergosterol content or alterations in cell wall. In contrast, depletion of sphingolipids sensitizes cells to AmB. Recently, overexpression of PMP3 gene, encoding plasma membrane proteolipid 3 protein, was shown to increase and its deletion to decrease, AmB resistance. Here we have explored the mechanistic basis of PMP3 effect on AmB resistance. It was found that ergosterol content and cell wall integrity are not related to modulation of AmB resistance by PMP3. A few prominent phenotypes of PMP3 delete strain, namely, defective actin polarity, impaired salt tolerance, and reduced rate of endocytosis are also not related to its AmB-sensitivity. However, PMP3 overexpression mediated increase in AmB resistance requires a functional sphingolipid pathway. Moreover, AmB sensitivity of strains deleted in PMP3 can be suppressed by the addition of phytosphingosine, a sphingolipid pathway intermediate, confirming the importance of this pathway in modulation of AmB resistance by PMP3.
Fungi cause superficial and invasive infections. Opportunistic invasive infections, though less prevalent, are of much greater concern because of high mortality (often over 50%) associated with them1. Many fungal species are responsible for these invasive infections, killing over one and half a million people every year, which is higher than that due to tuberculosis or malaria1. The treatment options for invasive infections are quite limited2. Amphotericin B (AmB) is a commonly used antifungal for over five decades. In spite of its toxicity, it is preferred for its broad-spectrum and fungicidal mode of action, particularly for treating invasive infections. Though echinocandins are also used for treating such infections, their use is limited in resource poor settings due to high cost. Moreover, Cryptococcus species do not respond to echinocandins and thus AmB alone (or in combination with flucytosine) is the mainstay to treat invasive infections caused by these species2,3.
AmB is currently considered to kill fungi by forming large, extramembranous fungicidal sterol sponge that depletes ergosterol from lipid bilayers4. Leakage of intracellular ions due to pore formation is thought to be a secondary effect of AmB5. Though AmB resistance is rare, it is seen in a significant percentage of pathogenic Candida species and filamentous fungi6,7. The AmB resistance mechanisms reported so far mainly involve reduction in ergosterol content or alterations in cell wall7,8,9,10,11. We have recently shown that sphingolipids also modulate AmB resistance12. A better understanding of AmB resistance/sensitivity mechanisms would facilitate developing therapeutic strategies to minimize evolution of AmB resistance, or to sensitize fungi to AmB such that lower AmB dose can be used to reduce toxicity.
While investigating apparent elevated AmB resistance of yeast cells in presence of farnesol (unpublished), we identified Saccharomyces cerevisiae PMP3 gene as conferring increased AmB resistance when present in a multicopy plasmid. Deletion of this gene rendered the cells hypersensitive to AmB. During the course of our studies, PMP3 gene's role in AmB resistance was also reported by Huang et al13, but the mechanism underlying this phenotype was not clear. PMP3 was first reported as a non-essential gene whose deletion results in plasma membrane hyperpolarization and salt sensitivity14. It encodes a 55 amino acid hydrophophic protein of plasma membrane. A homologous plant protein could complement salt sensitivity of a yeast strain deleted in PMP315. Here we have explored the mechanistic basis of PMP3 effect on AmB resistance. We show that certain prominent phenotypes of PMP3 delete strain, namely defects in salt tolerance, actin polarity and endocytosis, are not responsible for AmB-sensitivity of this strain. Instead, we demonstrate that modulation of AmB resistance by PMP3 is mediated through sphingolipid biosynthetic pathway.
Results and Discussion
PMP3 modulates AmB resistance
The S. cerevisiae PMP3 gene was isolated from a multicopy overexpression library (in plasmid pFL44L) as conferring higher resistance to AmB. A PMP3 clone with 165 bp ORF along with 1196 bp upstream and 275 bp downstream regions was used in further studies. To confirm the phenotype, PMP3 deletion and overexpression strains were compared with their parent strain for AmB resistance (Fig. 1a). While the delete strain was 8-fold more sensitive to AmB than the parent strain, the overexpression strain was about 4-fold more tolerant compared to the parent strain. During the course of this study, Huang et al13, while establishing a functional variomics tool for discovering drug-resistance genes and drug targets, also identified PMP3 as conferring AmB resistance when present at more than one copies. PMP3 (also known as SNA1) has three paralogs in S. cerevisiae, namely SNA2, SNA3 and SNA4, which encode proteins with 40%, 34% and 41% identity, respectively, to that of PMP316. Deletants of these genes were comparable to the parent strain in their susceptibility to AmB (results not shown), implying that these genes do not have any role in this phenotype.
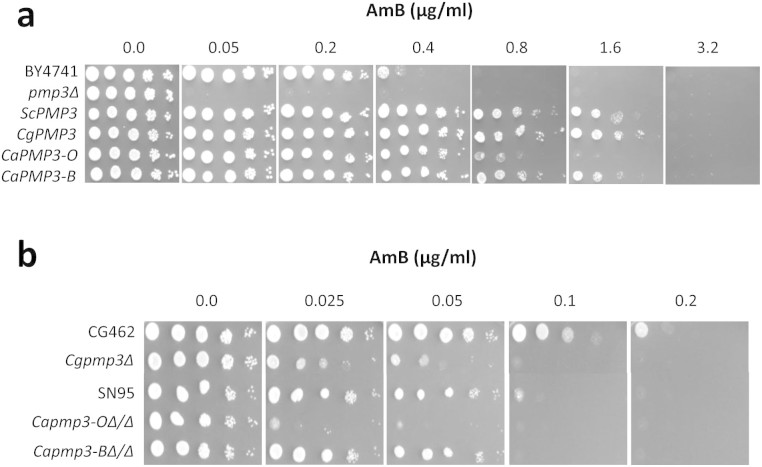
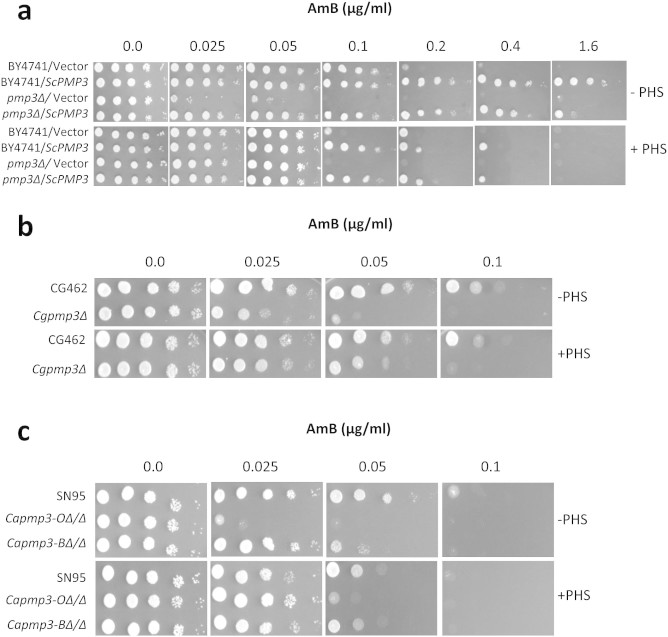
Figure 1. S. cerevisiae PMP3 and its homologs from C. albicans and C. glabrata modulate AmB resistance.
(a) Multicopy overexpression of S. cerevisiae PMP3 (ScPMP3) and its homologs from C. glabrata (CgPMP3) and C. albicans (CaPMP3-O: PMP3 ortholog, orf19.1655.3; CaPMP3-B: PMP3 best hit, orf19.2959.1) in pmp3Δ strain of S. cerevisiae enhance AmB resistance by about 4-fold with respect to wild-type strain (BY4741) and about 32-fold with respect to pmp3Δ strain. The relative growth of the strains on 0.1 μg/ml AmB (not shown) was comparable to that of respective strains on 0.2 μg/ml AmB. (b) AmB sensitivity of C. glabrata strain deleted in PMP3 ortholog (Cgpmp3Δ) and C. albicans strains deleted in both alleles of PMP3 ortholog (Capmp3-OΔ/Δ) and PMP3 best hit (Capmp3-BΔ/Δ), with respect to their respective parent strains CG462 and SN95. Five μl of 10-fold serial dilutions of cells were spotted starting from about 105 cells/spot, as described in Methods.
To test if PMP3 has a similar role in pathogenic yeasts, we searched for homologs in C. albicans and C. glabrata. C. albicans has two homologs, which encode proteins that show 51% and 45% identity at amino acid level to that of S. cerevisiae. The first one is referred to as CaPMP3 ortholog (orf19.1655.3) and the second one as CaPMP3 best hit (orf19.2959.1) in Candida Genome Database17. C. glabrata has a single ortholog CgPMP3 (CAGL0M08552g) encoding a protein with 76% identity to ScPmp3p. The open reading frames of these homologs were PCR amplified and used to replace the ORF in ScPMP3 clone, thereby placing these ORFs under the control of ScPMP3 promoter and terminator in pFL44L vector. These were tested for their ability to modulate AmB resistance after being transformed into pmp3Δ strain of S. cerevisiae. PMP3 ortholog from C. albicans was earlier shown to increase AmB resistance of S. cerevisiae13. In addition, we found CaPMP3 best hit and CgPMP3, besides complementing pmp3 mutation, provided resistance higher than that of wild-type strain (Fig. 1a). While the AmB resistance conferred by CgPMP3 and CaPMP3 best hit (CaPMP3-B) was similar to that of ScPMP3, i.e., 4-fold higher than that of wild-type strain, the CaPMP3 ortholog (CaPMP3-O) provided 2-fold higher resistance (Fig. 1a).
To study the role of CaPMP3 ortholog and CaPMP3 best hit in C. albicans, we deleted both alleles of these genes in strain SN95 and confirmed by diagnostic PCR (Fig. S1). The C. glabrata ortholog CgPMP3 (CAGL0M08552g) was also deleted and confirmed by diagnostic PCR (Fig. S2). The AmB susceptibility of these delete strains with respect to their parent strains was compared (Fig. 1b). While deletion of PMP3 orthologs in C. glabrata and C. albicans sensitized the cells to AmB by about 4-fold, deletion of CaPMP3 best hit did not have any effect. The AmB sensitivity of ortholog deletants in both these species provides strong evidence that PMP3 gene is important for modulation of AmB resistance in pathogenic fungi as well.
AmB resistance mediated by Pmp3p is not dependent on ergosterol or Hsp90 or cell wall integrity
As far as the mechanistic basis of PMP3 effect on AmB resistance is concerned, Huang et al13 showed that it is not related to its role in ion homeostasis. Absence or severe reduction in the amount of ergosterol in the fungal membranes and its replacement with certain other sterols results in AmB resistance in fungi7,10,11. To address this possibility total cellular content of ergosterol was estimated, as described18. The ergosterol content, as % wet weight of cells, of parent, delete and overexpression strains, was 0.021 ± 0.001, 0.023 ± 0.002 and 0.023 ± 0.001, respectively. Though these values are comparable, it is possible that the intracellular distribution of ergosterol might be affected. To check this, cells were stained with filipin, which is specific for sterols19, and observed (Fig. S3a). While wild-type and PMP3 overexpression strains showed intense fluorescent spots within cells, pmp3Δ strain lacked such spots. Thus, it is possible that more ergosterol is distributed in the plasma membrane of the delete strain, rendering it more accessible for AmB binding and killing. If this is true, then the delete strain should be more sensitive to other polyenes which also act by binding to ergosterol. However, the sensitivity pmp3Δ strain to the polyenes nystatin, natamycin and filipin was found to be comparable to that of wild-type and PMP3 overexpression strains (Fig. S3b), ruling out ergosterol distribution or content having any role in modulation of AmB resistance by PMP3. Huang et al13 have also ruled out the involvement of ergosterol in modulation of AmB resistance by PMP3, since this gene did not affect the resistance against other polyenes.
A recent report suggested that AmB resistance of ergosterol biosynthetic pathway mutants is highly dependent on Hsp90 chaperone and these mutants are hypersensitive to Hsp90 inhibitors radicicol and geldanamycin as well as oxidative stress20. To check the Hsp90 dependence of AmB resistance conferred by PMP3, the sensitivity of this strain to radicicol and oxidative stress was checked along with erg6Δ strain as positive control (Table 1). The AmB resistance of erg6Δ strain and PMP3 overexpression strain was comparable. However, while erg6Δ strain was 8-fold and 4-fold, respectively, more sensitive to radicicol and oxidative stress, the sensitivity of PMP3 overexpression strain was comparable to wild-type, implying that Pmp3p is not dependant on Hsp90 for conferring AmB resistance. Cell wall alterations also can affect AmB resistance7. Compared to parent strain, PMP3 delete strain showed normal chitin deposition (Fig. S4a), as well as similar resistance to cell wall disrupting agents calcofluor white, sodium dodecyl sulphate and congo red (Fig. S4b), implying that AmB sensitivity of delete strain is not related to cell wall integrity.
Table 1. AmB resistance mediated by PMP3 is not dependant on HSP90.
| Strain | MIC | ||
|---|---|---|---|
| AmB, μg/ml | Radicicol, μM | TBH, mM | |
| BY4741/vector | 0.4 | 16 | 2 |
| pmp3Δ/vector | 0.05 | 8 | 2 |
| pmp3Δ/ScPMP3 | 1.6 | 16 | 2 |
| erg6Δ/vector | 1.6 | 2 | 0.5 |
MIC for AmB and radicicol was determined in SC-ura broth at 30°C. Sensitivity to oxidative stress was determined by dilution spotting on SC-ura agar medium with tert-butyl hydroperoxide (TBH) at 37°C. MIC is the concentration at which no growth was observed.
Actin polarity and endocytosis, though impaired in pmp3Δ strain, are not responsible for its AmB sensitivity
To gain further insight into PMP3 mechanism of action, we tried to predict its possible functions on the basis of biological roles of genes that interact with PMP3. The list of interacting genes was analyzed using DAVID Bioinformatics Resources21 for enrichment of gene ontology terms for biological processes. The top-two annotation clusters corresponded to endocytosis and actin cytoskeleton (Table 2). To check if impaired endocytosis would result in AmB sensitivity, we screened mutants of several genes having role in endocytosis for their AmB sensitivity. Deletants of RVS161 and RVS167 were about 4-fold more sensitive to AmB compared to the parent strain (Fig. S5). These strains, besides defects in endocytosis have several other phenotypes including salt sensitivity and altered actin cytoskeleton22,23,24,25. SUR7, encoding an eisosome protein involved in endocytosis, partially suppresses several of these phenotypes upon multicopy overexpression26,27,28. Thus, we exploited overexpression of SUR7 to understand if AmB sensitivity of pmp3Δ strain is a consequence of defects in actin cytoskeleton or endocytosis, or it is an independent phenotype.
Table 2. Functional Annotation Clustering of PMP3 interacting genes.
| Cluster 1 - Enrichment Score: 2.82 | ||
|---|---|---|
| Term | Count | P-Value |
| GO:0006897 ~ endocytosis | 12 | 2.07E-04 |
| GO:0010324 ~ membrane invagination | 13 | 1.32E-03 |
| GO:0016192 ~ vesicle-mediated transport | 23 | 4.18E-03 |
| GO:0016044 ~ membrane organization | 18 | 4.57E-03 |
| ACT1, AGE1, CMD1, CSR2, CYC2, ERG3, FEN1, GTS1, INP52, MGM1, MVB12, MYO5, PHO86, RIM8, RUD3, SHR3, SLA1, SSO2, SUR7, VAM10, VPS24, VPS27, VPS30, VPS8, VRP1, WHI2. | ||
| Cluster 2 - Enrichment Score: 2.40 | ||
|---|---|---|
| Term | Count | P-Value |
| GO:0006970 ~ response to osmotic stress | 14 | 2.38E-05 |
| GO:0048308 ~ organelle inheritance | 10 | 2.89E-04 |
| GO:0030036 ~ actin cytoskeleton organization | 11 | 1.88E-03 |
| GO:0008064 ~ regulation of actin polymerization or depolymerization | 4 | 2.39E-03 |
| GO:0032271 ~ regulation of protein polymerization | 4 | 2.39E-03 |
| GO:0030833 ~ regulation of actin filament polymerization | 4 | 2.39E-03 |
| GO:0030832 ~ regulation of actin filament length | 4 | 2.39E-03 |
| GO:0033043 ~ regulation of organelle organization | 10 | 2.63E-03 |
| GO:0043254 ~ regulation of protein complex assembly | 5 | 2.76E-03 |
| GO:0030029 ~ actin filament-based process | 11 | 2.85E-03 |
| GO:0032970 ~ regulation of actin filament-based process | 4 | 5.84E-03 |
| GO:0032956 ~ regulation of actin cytoskeleton organization | 4 | 5.84E-03 |
| GO:0031333 ~ negative regulation of protein complex assembly | 3 | 9.69E-03 |
| GO:0007010 ~ cytoskeleton organization | 15 | 1.13E-02 |
| GO:0044087 ~ regulation of cellular component biogenesis | 5 | 1.16E-02 |
| GO:0051493 ~ regulation of cytoskeleton organization | 5 | 3.28E-02 |
| GO:0032535 ~ regulation of cellular component size | 9 | 3.42E-02 |
| GO:0007015 ~ actin filament organization | 5 | 1.50E-01 |
| ABP1, ACT1, ARC15, ARC18, BIM1, CAP1, CAP2, CDC13, CMD1, DFG16, EMP70, ENA1, GLC7, GTS1, HSC82, HSP82, INP52, IST2, KTI12, MGM1, MVB12, MYO5, NIP100, NST1, PET122, SHE4, SKY1, SLA1, VMA9, VRP1, WHI2. | ||
List of PMP3 interacting genes were downloaded from SGD16 and analyzed with DAVID Functional Annotation Clustering tool (http://david.abcc.ncifcrf.gov/home.jsp) of DAVID Bioinformatics Resources v6.721 for enrichment of gene ontology terms using default parameters, but restricted to biological processes (GOTERM_BP_FAT category). Only the top-two annotation clusters with greater than 2-fold enrichment are listed, along with the genes grouped under each cluster.
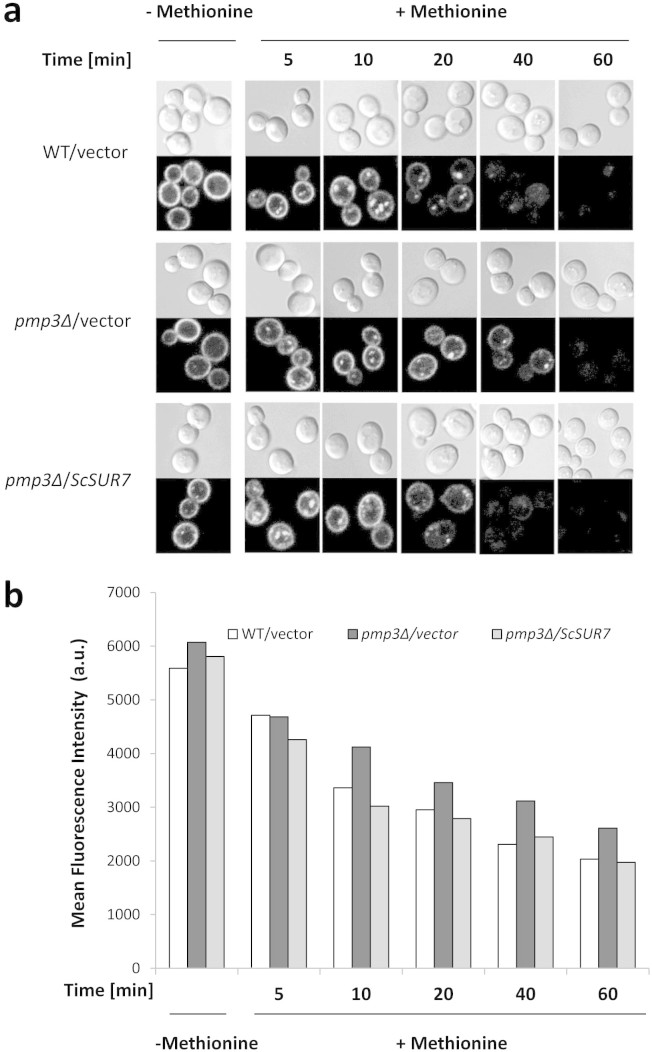
A large scale survey using GFP-Snc1-Suc2 reporter has indicated that endocytosis is decreased in pmp3Δ strain29. We monitored rate of endocytosis with a different reporter, namely methionine permease (Mup1) tagged with ecliptic pHluorin, which is a pH-sensitive green fluorescent protein variant that does not fluoresce after internalization to an acidic compartment like vacuole30,31. Mup1-pHluorin is internalized rapidly upon exposure to methionine. Wild-type cells showed substantial decrease in Mup1-pHluorin intensity within 20 min after adding methionine (Fig. 2a). However, in pmp3Δ strain 40 min was needed for a similar decrease, confirming that the rate of endocytosis is slowed down in this strain. SUR7 expressed from a multicopy plasmid restored the rate of endocytosis of pmp3Δ strain to normal level (Fig. 2a). Mup1-pHluorin fluorescence was also monitored by flow cytometry (Fig. 2b). Though background fluorescence was high for all the strains, the rate of decrease in fluorescence is indicative of rate of endocytosis. While it was slow in the pmp3Δ strain, it was restored to wild-type level upon SUR7 overexpression.
Figure 2. Slow rate of endocytosis of pmp3Δ strain is restored to normal level by overexpression of ScSUR7.
(a) Wild type strain 3818 (SEY6210-Mup1pHluorin) and pmp3Δ strain (3818 pmp3Δ::HIS3) transformed with either vector or ScSUR7, were grown without methionine to promote accumulation of Mup1-pHluorin in the plasma membrane. After addition of 20 μg/ml methionine, random fields of cells were imaged at different time intervals. All images were obtained at identical exposure conditions. (b) After addition of methionine, Mup1-pHluorin fluorescence was measured at indicated time intervals in a flow cytometer, as described in Methods. The values shown are average of two replicates from one representative experiment. Experiments were repeated thrice with comparable results.
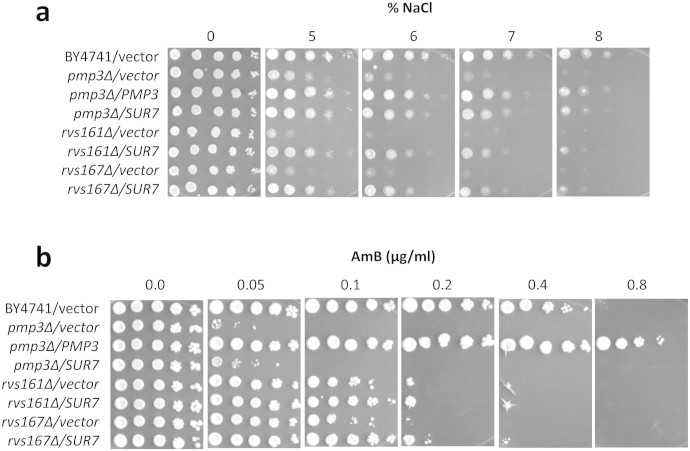
Actin cytoskeleton plays a central role in endocytosis25 and rvs161Δ and rvs167Δ strains impaired in endocytosis also have actin polarization defects23. Moreover, as PMP3 interacts with genes having role in actin cytoskeleton (Table 2), we visualized actin in PMP3 strains. The pmp3Δ strain showed pronounced defect in actin polarity, which is suppressed by overexpression of SUR7 (Fig. 3 and Fig. S6). SUR7 also suppressed the sensitivity of pmp3Δ, rvs161Δ and rvs167Δ strains to NaCl (Fig. 4a). However, it could not reverse the sensitivity of these strains to AmB (Fig. 4b), demonstrating that AmB sensitivity of these mutants is not mediated by defects in actin polarity, endocytosis or NaCl tolerance.
Figure 3. Actin polarization defect of pmp3Δ strain is suppressed by multicopy SUR7 overexpression.
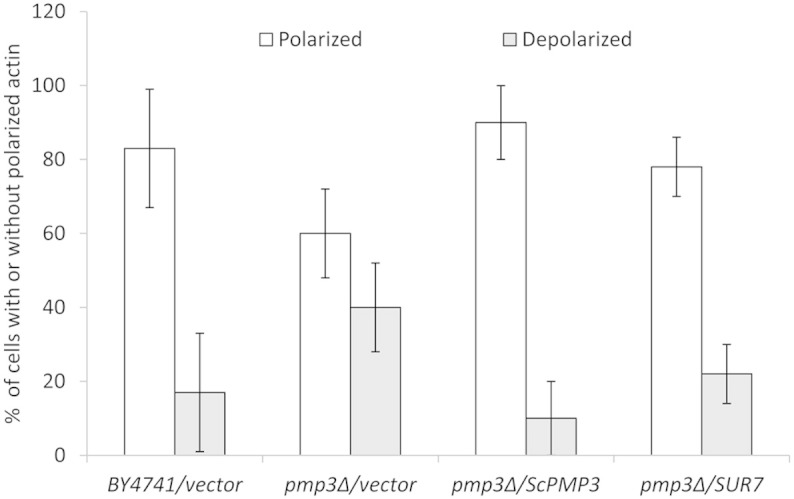
Cells were grown to log phase and actin was visualized by rhodamine phalloidin staining. About 200 cells with small buds were scored according to their polarization state. Cells with actin patches concentrated in the small bud, with fewer than four patches in the mother cell, were classified as polarized cells. Other cells with more actin patches in the mother cell than in the small bud were classified as depolarized cells. Representative images are shown in Figure S6. Mean values of two independent experiments are given. The error bars indicate the range.
Figure 4.
SUR7 overexpression can suppress salt sensitivity (a), but not AmB sensitivity (b) of strains deleted in PMP3, RVS161 or RVS167. Wild-type (BY4741) and PMP3 overexpression strains are included as controls.
Sphingolipid biosynthetic pathway is essential for PMP3 mediated increase in AmB resistance
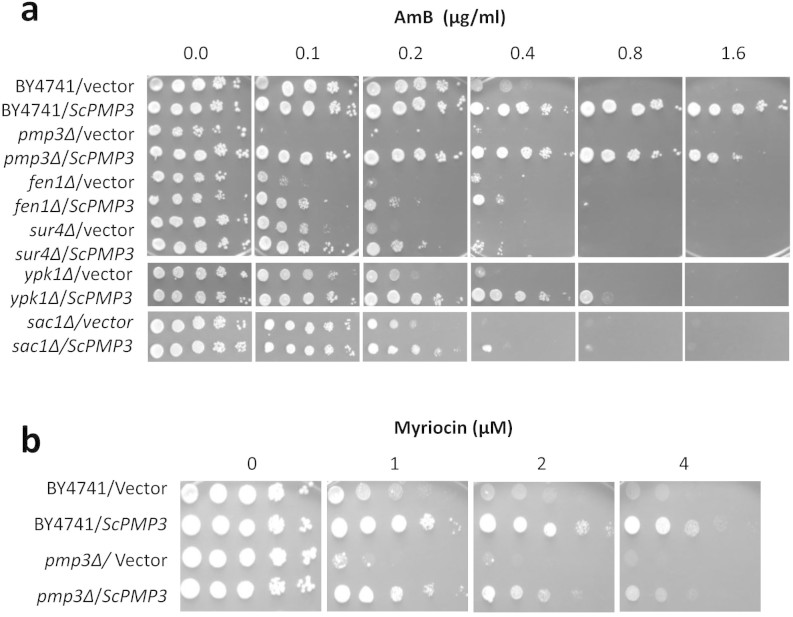
We had recently shown that sphingolipid biosynthetic pathway genes FEN1 (ELO2) and SUR4 (ELO3) modulate AmB resistance12. While inhibition of sphingolipid biosynthesis with myriocin sensitized cells to AmB, addition of phytosphingosine, a sphingolipid pathway intermediate, reversed this phenotype12. To check the importance of this pathway for PMP3 mediated increase in AmB resistance, multicopy ScPMP3 was transformed into a few sphingolipid pathway mutants and the resistance was checked (Fig. 5a). In the wild-type parent strain (BY4741) ScPMP3 could increase AmB resistance at least by 4-fold. However, it increased AmB resistance by 2-fold or less in mutants of sphingolipid biosynthetic genes FEN1 and SUR4, and regulatory genes YPK132,33 and SAC134. If PMP3 overexpression effect is independent of sphingolipid pathway, then fold-increase in AmB resistance by PMP3 in these mutants should have been comparable to that of the parent strain. Only 2-fold or less increase in resistance shows that PMP3 is dependent on this pathway for enhancing AmB resistance. Even this increase appears to be due to genetic redundancy. FEN1 and SUR4 are involved in fatty acid elongation and can partly compensate for each other's loss, since double deletion is lethal35. YPK1 and YPK2 are synthetic lethal36 and arose from the whole genome duplication37. Sac1p is a phosphatidylinositol phosphate phosphatase, and its catalytic domain (Sac1-like domain) is seen among several phosphatases with partially overlapping function38. Sac1p is known to modulate sphingolipid metabolism34,39. Physical interaction of Pmp3p and Sac1p has also been reported in a large-scale study40. Thus it appears likely that Pmp3p modulates sphingolipid biosynthesis and AmB resistance by interacting with Sac1p. Dependence of Pmp3p on Sac1p provides possible link between Pmp3p and sphingolipid pathway.
Figure 5. PMP3 modulates AmB resistance through sphingolipid biosynthetic pathway.
(a) Sphingolipid biosynthetic pathway genes FEN1 and SUR4 and regulatory genes YPK1 and SAC1 are important for PMP3 mediated increase in AmB resistance. Wild-type (BY4741) and pmp3Δ strains overexpressing ScPMP3 serve as positive controls. (b) PMP3 modulates tolerance to myriocin, a sphingolipid biosynthetic pathway inhibitor. While strains overexpressing PMP3 are about 4-fold more tolerant, the strain deleted in PMP3 is about 2-fold more sensitive to myriocin, compared to the wild-type strain BY4741.
Myriocin inhibits the first committed step of sphingolipid biosynthesis catalyzed by serine palmitoyltransferase33. Sphingolipid pathway regulatory genes YPK132,33 and SAC134 modulate myriocin resistance. To test if PMP3 also regulates sphingolipid pathway, we checked myriocin resistance of deletion and overexpression strains. While deletion of PMP3 decreased myriocin resistance by 2-fold, its overexpression increased myriocin resistance by 4-fold, both with respect to parent strain (Fig. 5b), indicating that PMP3 is possibly involved in regulation of this pathway in S. cerevisiae. We also checked the myriocin sensitivity of C. glabrata strain deleted in PMP3 ortholog, and C. albicans strains deleted in PMP3 ortholog or best hit. However, the sensitivity of these strains was found to be comparable to that of their respective parent strains (Fig. S7). Another approach used to establish the role or dependence of genes on sphingolipid pathway is by supplementing with phytosphingosine (PHS), a sphingolipid pathway intermediate33,41. Addition of PHS increased the AmB resistance of pmp3Δ strain of S. cerevisiae to wild type level. It also decreased the AmB resistance of PMP3 overexpression strain to nearly wild type level (Fig. 6a), perhaps by its known antifungal activity at high concentration42. PHS also suppressed AmB sensitivity of C. glabrata and C. albicans strains deleted in PMP3 orthologs (Figs. 6b and 6c). These results further establish that PMP3 modulates AmB resistance through sphingolipid pathway in S. cerevisiae as well as in pathogenic Candida species.
Figure 6. Phytosphingosine (PHS), a sphingolipid pathway intermediate, modulates AmB resistance.
(a) Growth of wild-type (BY4741), PMP3 deletion and overexpression strains of S. cerevisiae on indicated concentrations of AmB alone or in combination with 5 μM phytosphingosine (PHS). Relative growth of strains at 0.8 μg/ml AmB (not shown) was comparable to their growth at 1.6 μg/ml. (b) Growth of wild-type (CG462) and PMP3 delete (Cgpmp3Δ) strains of C. glabrata on indicated concentrations of AmB alone or in combination with 5 μM PHS. (c) Growth of C. albicans strains deleted in both alleles of PMP3 ortholog (Capmp3-OΔ/Δ) or PMP3 best hit (Capmp3-BΔ/Δ), with respect to their parent SN95 on indicated concentrations of AmB alone or in combination with 10 μM PHS.
Sphingolipid bases and complex sphingolipids have multiple roles in cells, both as structural components and as signalling molecules43,44. Mutants of sphingolipid pathway show pleiotropic phenotypes44, of which those affected in actin cytoskeleton45, endocytosis46 and AmB resistance12 are pertinent here. Since actin is critical for endocytosis25, defective endocytosis could be a consequence of impaired actin polarity. Thus, impaired actin cytoskeleton and slow rate of endocytosis of pmp3Δ strain are consistent with the regulatory role played by PMP3 in sphingolipid pathway.
In conclusion, we have shown that a few striking phenotypes of PMP3 mutant, such as impaired actin polarity, endocytosis and salt tolerance are not related to its AmB-sensitivity. Rather, we show that modulation of AmB resistance by PMP3 is dependent on sphingolipid biosynthetic pathway, since AmB sensitivity of PMP3 deletants is suppressed by phytosphingosine, a sphingolipid pathway intermediate. Moreover, enhanced AmB resistance conferred by overexpression of PMP3 is dependent on functional sphingolipid biosynthetic and regulatory genes. Efforts are underway to elucidate the precise mechanism underlying PMP3 effect or dependence on sphingolipid pathway for modulating AmB resistance.
Methods
Fine chemicals and yeast synthetic drop-out medium supplements without uracil were procured from Sigma. All other media components were obtained from BD (Difco). Oligonucleotides were custom synthesised from Sigma-Genosys, India. Restriction enzymes, DNA polymerases and other DNA modifying enzymes were obtained from New England Biolabs, and DNA purification kits were obtained from Qiagen.
Strains, media and growth conditions
S. cerevisiae and Candida strains and plasmids used in this study are listed in Table S1 and S2. The Escherichia coli strain DH5α was used as a cloning host. YPD and Synthetic complete (SC) media were prepared and used as described12. Uracil supplement is omitted in SC medium to provide SC-ura medium. Yeast transformations were carried out using the modified lithium acetate method47. Stock solutions of AmB (2 mg/ml), myriocin (5 mM), phytosphingosine (15 mM) and radicicol (5 mM) were prepared in DMSO. Stock solutions of nourseothricin (200 mg/ml) and tert-butyl hydroperoxide (500 mM) were made in water.
Growth assays by dilution spotting
For dilution spotting assays, the strains/transformants were grown overnight in SC or SC-ura medium, reinoculated in fresh medium to an A600 of 0.1 and grown for 6 h. The exponential phase cells were harvested, washed and resuspended in sterile water to an A600 of 1.0 (~2 × 107 cells/ml). Ten-fold serial dilutions were made in water and 5 μl of each dilution was spotted on SC or SC-ura plates with desired concentration of compounds, as mentioned in Figures. DMSO alone was included in control plates, corresponding to its concentration in experimental plates, where appropriate. Plates were incubated for 2 days at 30°C before taking photographs. These experiments were repeated at least three times with comparable results.
Cloning methods
The ORFs of putative homologs of ScPMP3 in C. albicans [CaPMP3-ortholog (orf19.1655.3), CaPMP3-Best hit (orf19.2959.1)] and C. glabrata (CAGL0M08552g) were PCR amplified from the genomic DNA of C. albicans and C. glabrata with specific primers sets (Table S3). The PCR products were then used to replace the ScPMP3 ORF in a ScPMP3 clone in multicopy vector pFL44L, using Circular Polymerase Extension Cloning (CPEC) method48,49, thereby retaining the ScPMP3 promoter and terminator regions for all PMP3 orthologs as well. For cloning ScSUR7 gene, the SUR7 ORF of S. cerevisiae along with its promoter and terminator (+568 to −326 bp) was amplified from strain BY4741 with forward primer ScSUR7-OCS1 and reverse primer ScSUR7-OCA1 (Table S3) and cloned in pFL44L by CPEC method48,49.
Construction of C. albicans strains deleted in CaPMP3-ortholog and CaPMP3-best hit
Both alleles of CaPMP3-ortholog (orf19.1655.3) and CaPMP3-Best hit (orf19.2959.1) were deleted in C. albicans, using HAH2 cassette and gene-specific primers, as described12, and confirmed by diagnostic PCR with appropriate primers (Table S3).
Construction of C. glabrata strain deleted in CgPMP3
PMP3 ortholog in C. glabrata (CAGL0M08552g) was deleted using a selection cassette conferring nourseothricin resistance containing CaNAT1 gene with codon usage adapted for Candida species50. A 508 bp region upstream of, and 472 bp region downstream of CgPMP3 ORF were PCR amplified from wild type genomic DNA using primers for upstream (CgPMP3-US1 and CgPMP3-UA1) and downstream regions (CgPMP3-DS1 and CgPMP3-DA1). The upstream flanking region was fused with the 5′ region of CaNAT1 cassette using amplified upstream region and plasmid (pCR2.1-NAT51) with CaNAT1 as templates and primers CgPMP3-US1 and CaNAT1-US-R1 to generate upstream split marker. Similarly, the downstream flanking region was fused to 3′ region of CaNAT1 cassette with amplified downstream region and pCR2.1-NAT as templates and primers CaNAT1-DS-F1 and CgPMP3-DA1 to generate downstream split marker. These fusion products, which share 401 bp homology between them in the cassette, were mixed together, transformed52 into C. glabrata wild type strain CG462, and plated on YPD plate. After incubation at 30°C for 24 h, cells were replica-plated onto YPD plate with 200 μg/ml nourseothricin and further incubated for 24 h. Nourseothricin resistant colonies were purified and checked for gene deletion by diagnostic PCR using cassette specific primers and primers outside the flanking region of homology (Table S3).
Fluorescence microscopy
Mup1-pHluorin internalization assay was performed as reported31,53. Mup1- pHluorin localization was visualized using a Nikon A1R confocal microscope using FITC optics and 100X oil immersion objective. Images were analysed using NIS Elements software. Visualization of actin by rhodamine phalloidin staining was carried out as described54. Calcofluor staining of cell wall was done as described55. The subcellular localization of sterols was monitored by staining with filipin as described19 with slight modification. Exponentially growing cells (0.5 OD cells/ml) were fixed with 3.7% paraformaldehyde for 10 min at 30°C, washed with phosphate-buffered saline (PBS) and incubated with 5 μg/ml of filipin (Sigma F9765) in the dark at 30°C for 5 min. The stained cells were directly observed under a confocal laser scanning microscope (Nikon A1R) using 405 nm laser and images were analysed using NIS element software.
Flow cytometry
Log-phase cells were grown in SC medium without uracil and methionine for 6 hours, and then methionine was added to 20 μg/ml final concentration. At different time intervals cells were collected by centrifugation, washed and resuspended in PBS. Mup1-pHluorin fluorescence was measured with BD Accuri™ C6 flow cytometer in FL1 channel. Excitation and emission wavelengths were 488 nm and 530 nm, respectively. For each sample 104 cells were analysed. Three independent experiments were done with two replicates each time.
Author Contributions
K.G. designed the project and provided overall guidance. V.K.B. and S.S. carried out the experiments and collected data. V.K.B. and K.G. drafted and finalized the manuscript. A.K.M. provided technical inputs and guidance for confocal microscopy. S.S., M.A. and A.K.M. provided critical input during group meetings and on the manuscript. All authors reviewed the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Beverly Wendland and Suzanne Noble for S. cerevisiae and C. albicans strains, and Rupinder Kaur for C. glabrata strain and pCR2.1-NAT plasmid. Vinay K. Bari, Sushma Sharma and Md. Alfatah acknowledge the Council of Scientific and Industrial Research, New Delhi, for fellowships. This work was supported by a CSIR project “Understanding the molecular mechanism of diseases of national priority: Developing novel approaches for effective management” (SIP10), and a Supra Institutional Project on Infectious Diseases (BSC0210).
References
- Brown G. D. et al. Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113 (2012). [DOI] [PubMed] [Google Scholar]
- Roemer T. & Krysan D. J. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4, a019703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. N. et al. Combination Antifungal Therapy for Cryptococcal Meningitis. New England Journal of Medicine 368, 1291–1302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10, 400–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. C. et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109, 2234–2239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother 49 Suppl 1, 7–10 (2002). [DOI] [PubMed] [Google Scholar]
- Shaughnessy E. M. O., Lyman C. A. & Walsh T. J. in Antimicrobial Drug Resistance (ed Mayers D. L., ed. ) Ch. 25, 295–305 (Humana Press, 2009). [Google Scholar]
- Bahmed K., Bonaly R. & Coulon J. Relation between cell wall chitin content and susceptibility to amphotericin B in Kluyveromyces, Candida and Schizosaccharomyces species. Res Microbiol 154, 215–222 (2003). [DOI] [PubMed] [Google Scholar]
- Seo K., Akiyoshi H. & Ohnishi Y. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol Immunol 43, 1017–1025 (1999). [DOI] [PubMed] [Google Scholar]
- Hull C. M. et al. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob Agents Chemother 56, 6417–6421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. Y., Hull C. M. & Heitman J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47, 2717–2724 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. et al. Sphingolipid biosynthetic pathway genes FEN1 and SUR4 modulate amphotericin B resistance. Antimicrob Agents Chemother 58, 2409–2414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. et al. A functional variomics tool for discovering drug-resistance genes and drug targets. Cell Rep 3, 577–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre C. & Goffeau A. Membrane hyperpolarization and salt sensitivity induced by deletion of PMP3, a highly conserved small protein of yeast plasma membrane. EMBO J 19, 2515–2524 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander M. et al. The low-temperature- and salt-induced RCI2A gene of Arabidopsis complements the sodium sensitivity caused by a deletion of the homologous yeast gene SNA1. Plant Mol Biol 45, 341–352 (2001). [DOI] [PubMed] [Google Scholar]
- Cherry J. M. et al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res 40, D700–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis D. O. et al. The Candida genome database incorporates multiple Candida species: multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata. Nucleic Acids Res 40, D667–674 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthington-Skaggs B. A., Jradi H., Desai T. & Morrison C. J. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37, 3332–3337 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh C. T. & Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci 117, 2983–2996 (2004). [DOI] [PubMed] [Google Scholar]
- Vincent B. M., Lancaster A. K., Scherz-Shouval R., Whitesell L. & Lindquist S. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11, e1001692 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B. T. & Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- Crouzet M., Urdaci M., Dulau L. & Aigle M. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast 7, 727–743 (1991). [DOI] [PubMed] [Google Scholar]
- Munn A. L., Stevenson B. J., Geli M. I. & Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. .Mol Biol Cell 6, 1721–1742 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. Y. et al. Dissecting BAR domain function in the yeast Amphiphysins Rvs161 and Rvs167 during endocytosis. Mol Biol Cell 21, 3054–3069 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren O. L., Galletta B. J. & Cooper J. A. Roles for actin assembly in endocytosis. Annu Rev Biochem 81, 661–686 (2012). [DOI] [PubMed] [Google Scholar]
- Sivadon P., Peypouquet M. F., Doignon F., Aigle M. & Crouzet M. Cloning of the multicopy suppressor gene SUR7: evidence for a functional relationship between the yeast actin-binding protein Rvs167 and a putative membranous protein. Yeast 13, 747–761 (1997). [DOI] [PubMed] [Google Scholar]
- Walther T. C. et al. Eisosomes mark static sites of endocytosis. Nature 439, 998–1003 (2006). [DOI] [PubMed] [Google Scholar]
- Young M. E. et al. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol Cell Biol 22, 927–934 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston H. E. et al. Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol 185, 1097–1110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G., De Angelis D. A. & Rothman J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998). [DOI] [PubMed] [Google Scholar]
- Prosser D. C., Drivas T. G., Maldonado-Baez L. & Wendland B. Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J Cell Biol 195, 657–671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants F. M., Torrance P. D. & Thorner J. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology 150, 3289–3304 (2004). [DOI] [PubMed] [Google Scholar]
- Sun Y. et al. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol 20, 4411–4419 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow D. K. et al. Orm family proteins mediate sphingolipid homeostasis. Nature 463, 1048–1053 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. S., Toke D. A., Mandala S. & Martin C. E. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem 272, 17376–17384 (1997). [DOI] [PubMed] [Google Scholar]
- Chen P., Lee K. S. & Levin D. E. A pair of putative protein kinase genes (YPK1 and YPK2) is required for cell growth in Saccharomyces cerevisiae. Mol Gen Genet 236, 443–447 (1993). [DOI] [PubMed] [Google Scholar]
- Byrne K. P. & Wolfe K. H. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res 15, 1456–1461 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl T. & Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 1771, 353–404 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T. C. Keeping sphingolipid levels nORMal. Proc Natl Acad Sci U S A 107, 5701–5702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P. et al. Large-scale identification of yeast integral membrane protein interactions. Proc Natl Acad Sci U S A 102, 12123–12128 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag A., Fadri M., Jung S. Y., Qin J. & Kunz J. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol 27, 633–650 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman E. C. et al. Phytosphingosine kills Candida albicans by disrupting its cell membrane. Biol Chem 391, 65–71 (2010). [DOI] [PubMed] [Google Scholar]
- Dickson R. C. Roles for sphingolipids in Saccharomyces cerevisiae. Adv Exp Med Biol 688, 217–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefusco D. J., Matmati N. & Hannun Y. A. The yeast sphingolipid signaling landscape. Chem Phys Lipids 177, 26–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B. et al. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J 19, 2824–2833 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant S., Lombardi R., Schmelzle T., Hall M. N. & Riezman H. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J 20, 6783–6792 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D. & Schiestl R. H. Large-scale high-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2, 38–41 (2007). [DOI] [PubMed] [Google Scholar]
- Bryksin A. V. & Matsumura I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques 48, 463–465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J. & Tian J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS One 4, e6441 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Guo W. & Kohler J. R. CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun 73, 1239–1242 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B., Bouchier C., Fairhead C., Craig N. L. & Cormack B. P. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mob DNA 3, 3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Castano I. & Cormack B. P. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother 48, 1600–1613 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser D. C., Whitworth K. & Wendland B. Quantitative analysis of endocytosis with cytoplasmic pHluorin chimeras. Traffic 11, 1141–1150 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E. & Pringle J. R. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol 194, 729–731 (1991). [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol 194, 732–735 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information