Abstract
Because of their paternal antigens, the fetus and placenta may be considered an allograft in the maternal host. Understanding the mechanisms which prevent maternal immunological rejection of the fetus remains a fundamental unsolved problem in immunology. We have previously reported that macrophages are inhibited by maternal decidual stromal cells residing at the maternal-fetal interface. In view of the central role of macrophages in cell-mediated immunity, this inhibition may contribute to preventing maternal antifetal responses. We now report that it was the solid phase signals embedded in the extracellular matrix (ECM) made by decidual cells which are responsible for inhibiting macrophage-mediated lysis of TNF-alpha-resistant P815 mastocytoma cells. The latter macrophage function is acquired after stimulation by interferon gamma and endotoxin. All these macrophage functions were also inhibited by ECM isolated from the Engelberth-Holm-Swarme (EHS) tumor. This tumor ECM has a similar biochemical composition to decidual ECM. This ECM inhibited the effector, as opposed to the stimulator, phase of macrophage-mediated tumor lysis. Laminin, type IV collagen, and heparan sulfate proteoglycans, the major known components of decidual and EHS ECMs, did not inhibit the above macrophage functions. Altogether these data indicate that macrophages were inhibited by solid phase signals embedded in decidual and EHS ECMs. Whether the solid phase signals in these two ECMs are biochemically identical remains to be determined. To our knowledge, such signals are a novel pathway of inhibiting macrophage functions which may be important in understanding the maternal-fetal immunologic relationship, and the pathogenesis of perinatal infections. Furthermore, the ability of EHS tumor ECM to inhibit macrophage functions may indicate that some tumors may defend themselves against host macrophage responses using solid phase signals. This may be important in understanding some host-tumor relationships.
Full text
PDF

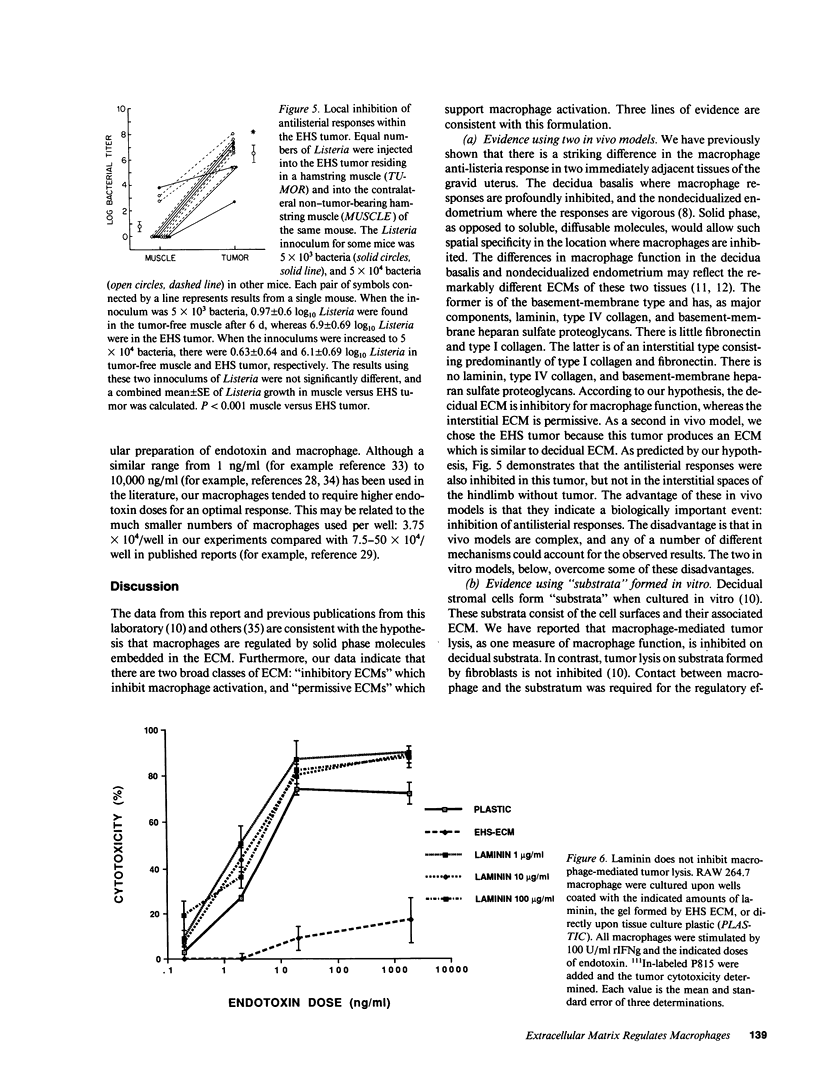
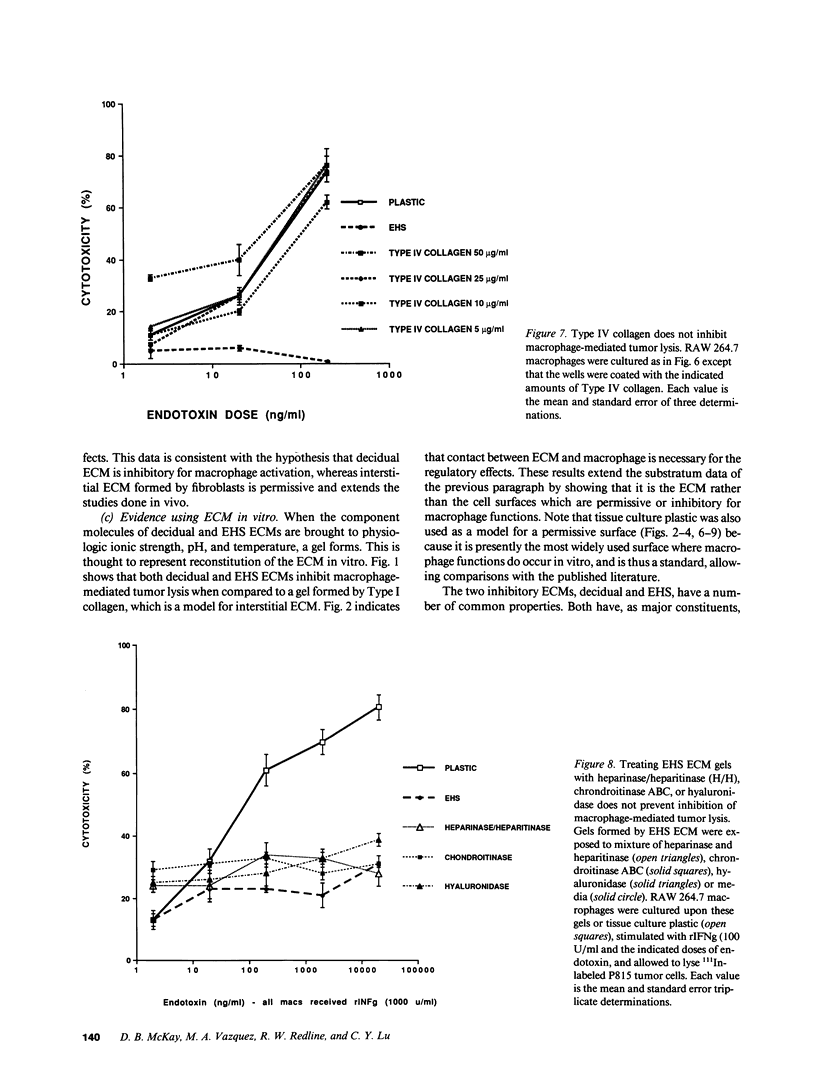
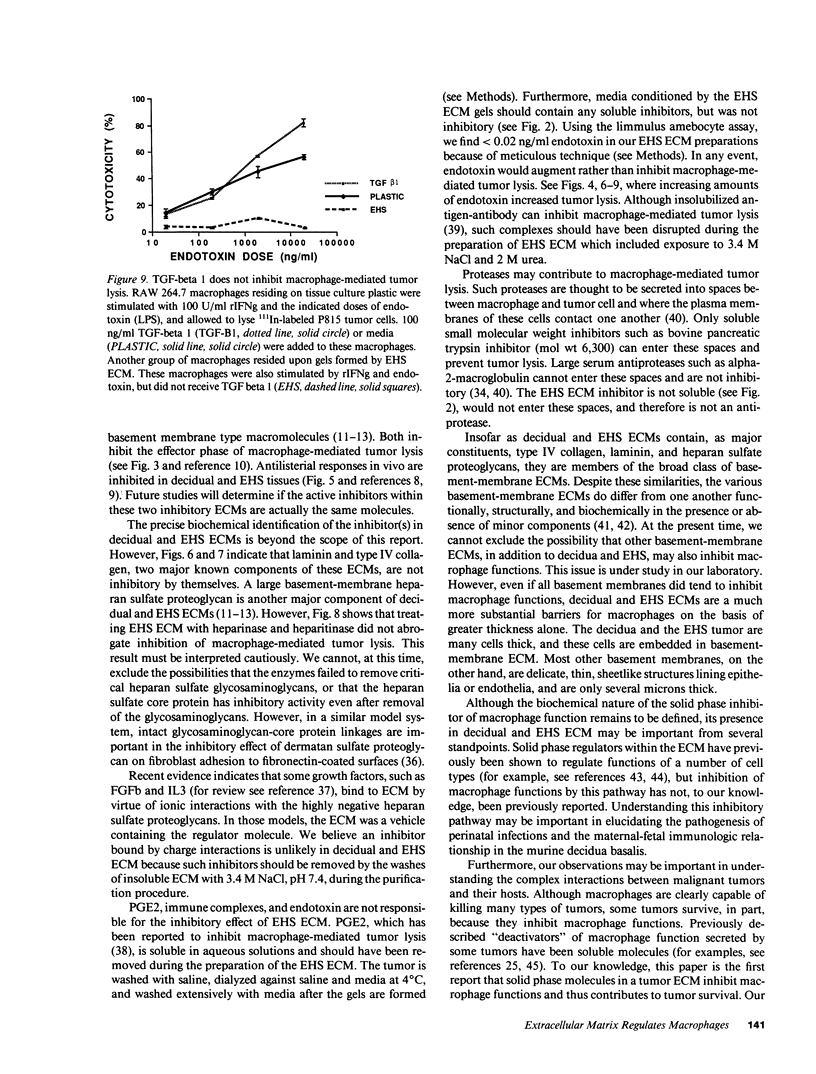

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Altman D. J., Schneider S. L., Thompson D. A., Cheng H. L., Tomasi T. B. A transforming growth factor beta 2 (TGF-beta 2)-like immunosuppressive factor in amniotic fluid and localization of TGF-beta 2 mRNA in the pregnant uterus. J Exp Med. 1990 Nov 1;172(5):1391–1401. doi: 10.1084/jem.172.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Role of protein kinase C and intracellular calcium mobilization in the induction of macrophage tumoricidal activity by interferon-gamma. J Immunol. 1986 Oct 1;137(7):2373–2379. [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. A., Falbo M., Rowley R. B., Banwatt D., Stedronska-Clark J. Active suppression of host-vs graft reaction in pregnant mice. IX. Soluble suppressor activity obtained from allopregnant mouse decidua that blocks the cytolytic effector response to IL-2 is related to transforming growth factor-beta. J Immunol. 1988 Dec 1;141(11):3833–3840. [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Vesalius and Hunter were right: decidua is a membrane! Lab Invest. 1985 Dec;53(6):597–598. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Esparza I., Green R., Schreiber R. D. Inhibition of macrophage tumoricidal activity by immune complexes and altered erythrocytes. J Immunol. 1983 Nov;131(5):2117–2121. [PubMed] [Google Scholar]
- Finn C. A. The biology of decidual cells. Adv Reprod Physiol. 1971;5:1–26. [PubMed] [Google Scholar]
- Gorecka-Tisera A. M., Snowdowne K. W., Borle A. B. Implications of a rise in cytosolic free calcium in the activation of RAW-264 macrophages for tumor cell killing. Cell Immunol. 1986 Jul;100(2):411–421. doi: 10.1016/0008-8749(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harrell R. A., Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Suppression of the respiratory burst of human monocytes by a synthetic peptide homologous to envelope proteins of human and animal retroviruses. J Immunol. 1986 May 15;136(10):3517–3520. [PubMed] [Google Scholar]
- Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988 Mar 1;140(5):1690–1694. [PubMed] [Google Scholar]
- Hunziker R. D., Wegmann T. G. Placental immunoregulation. Crit Rev Immunol. 1986;6(3):245–285. [PubMed] [Google Scholar]
- Inaba K., Kitaura M., Kato T., Watanabe Y., Kawade Y., Muramatsu S. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J Exp Med. 1986 Apr 1;163(4):1030–1035. doi: 10.1084/jem.163.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T. G. Intrauterine infusion of prostaglandins and decidualization in rats with uteri differentially sensitized for the decidual cell reaction. Biol Reprod. 1986 Mar;34(2):327–335. doi: 10.1095/biolreprod34.2.327. [DOI] [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Brenchley P. E., Anderson J. C., Dickinson M. A., Horigan E. A., Hassell J. R. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988 Mar;106(3):963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Martin G. R. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983 Oct 11;22(21):4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Lewandowska K., Choi H. U., Rosenberg L. C., Zardi L., Culp L. A. Fibronectin-mediated adhesion of fibroblasts: inhibition by dermatan sulfate proteoglycan and evidence for a cryptic glycosaminoglycan-binding domain. J Cell Biol. 1987 Sep;105(3):1443–1454. doi: 10.1083/jcb.105.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay D. R., Ettensohn C. A. Cell adhesion in morphogenesis. Annu Rev Cell Biol. 1987;3:319–345. doi: 10.1146/annurev.cb.03.110187.001535. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989 Jul;61(1):27–36. [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988 Jun 1;140(11):3947–3955. [PubMed] [Google Scholar]
- Redline R. W., McKay D. B., Vazquez M. A., Papaioannou V. E., Lu C. Y. Macrophage functions are regulated by the substratum of murine decidual stromal cells. J Clin Invest. 1990 Jun;85(6):1951–1958. doi: 10.1172/JCI114658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Shea C. M., Papaioannou V. E., Lu C. Y. Defective anti-listerial responses in deciduoma of pseudopregnant mice. Am J Pathol. 1988 Dec;133(3):485–497. [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Pace J. L. Comparison of responses to activating agents by mouse peritoneal macrophages and cells of the macrophage line RAW 264. J Reticuloendothel Soc. 1980 Jun;27(6):607–619. [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Crossin K. L., Hoffman S., Edelman G. M. Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7977–7981. doi: 10.1073/pnas.84.22.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Macrophage deactivation. Altered kinetic properties of superoxide-producing enzyme after exposure to tumor cell-conditioned medium. J Exp Med. 1986 Oct 1;164(4):1319–1331. doi: 10.1084/jem.164.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Damjanov A., Weiss J., Liotta L. A., Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32(1):49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]