Abstract
Multiple studies have shown that individuals with a reading disability (RD) demonstrate deficits in posterior left-hemispheric brain regions during reading-related tasks. These studies mainly focused on reading sub-skills, and it remains debated whether such dysfunction is apparent during more ecologically valid reading skills, such as reading fluency. In this fMRI study, reading fluency was systematically varied to characterize neural correlates of reading fluency in 30 children with (RD) and without (typical developing children, TYP) a RD. Sentences were presented at constrained, comfortable, and accelerated speeds, which were determined based on individual reading speed. Behaviorally, RD children displayed decreased performance in several reading-related tasks. Using fMRI, we demonstrated that both TYP and RD children display increased activation in several components of the reading network during fluent reading. When required to read at an accelerated speed, RD children exhibited less activation in the fusiform gyrus (FG) compared with the TYP children. A region of interest analysis substantiated differences in the FG and demonstrated a relationship to behavioral reading performance. These results suggest that the FG plays a key role in fluent reading and that it can be modulated by speed. These results and their implications for remediation strategies should be considered in educational practice.
Keywords: developmental dyslexia, fMRI, fusiform gyrus, reading disability, reading fluency
Introduction
Reading is a multicomponent process that requires the use of different neural systems to integrate phonology, orthography, syntax, and semantics with lower order perceptual, attentional, and motoric functions (Berninger et al. 2001; Kame'enui and Simmons 2001; Wolf and Katzir-Cohen 2001). Reading fluency in the English language often refers to the ability to read rapidly and accurately, with comprehension (NRP 2000; Lyon et al. 2003). Novice readers must learn that spoken words are composed of discrete sounds that can be mapped onto letters (Turkeltaub et al. 2003). With increasing experience, a connection develops between orthography and semantic units, which enables a child to decode printed words with increasing comprehension (Seidenberg 2005). Through automatization, greater accuracy and enhanced speed are achieved and, as a result, the act of reading itself requires less cognitive effort, which allows more cognitive resources for the task of comprehension (Norton and Wolf 2012). However, ∼5–17% of children in the USA have difficulty in learning to read despite adequate perceptual and general cognitive abilities, which can be defined as reading disability (RD; Lyon et al. 2003; Galaburda et al. 2006; Peterson and Pennington 2012). Although these disabilities can be (partly) remediated by interventions targeting reading sub-skills (e.g., phonemic awareness), many children still exhibit fluency and/or comprehension deficits after completing remediation (Lyon and Moats 1997) or may show reading fluency deficits, but accurate word identification (Thaler et al. 2004). The remaining fluency deficits are hard to remediate (Torgesen et al. 2001; Reynolds et al. 2003) and up to 30% of fourth graders exhibit reading fluency deficits (Perie et al. 2005; Lee et al. 2007). Nevertheless, the underlying mechanisms of fluency deficits have been less studied than other reading components and their neural correlates are almost unknown.
Most research studies investigating the neural correlates of typical and atypical reading development focus on lower level processes (e.g., phonological awareness) and single-word reading, both of which are easier to study and remediate. These studies have consistently shown that reading processes are integrated in distinct left-hemispheric posterior and anterior systems, which together form the neural reading network (Pugh et al. 2001; Turkeltaub et al. 2003; Schlaggar and McCandliss 2007). The posterior reading network includes fusiform gyrus (FG) and the occipitotemporal and parietotemporal junctions. Individuals with an RD tend to show hypoactivation within the posterior subsystem (e.g., Turkeltaub et al. 2003; Schlaggar and McCandliss 2007). The more anterior network includes mainly the inferior frontal gyrus and RD tends to hyperactivate this network (Temple 2002; Hoeft et al. 2006; Richlan et al. 2009).
In order to derive a more complete picture of the underlying neural processes of reading disabilities, increased research efforts simulating everyday reading fluency demands are needed. To our knowledge, no study has investigated neural differences in children with (RD) and without (typical developing children, TYP) reading disabilities when the opportunity to read fluently is manipulated. To date, only a few studies have investigated reading fluency in the brain, and these studies show inconsistent results. While some of these studies observed in a slow fixed presentation, speed decreased activation in reading-related brain areas in RD compared with age- or reading ability-matched peers in left temporoparietal (Meyler et al. 2007; Schulz et al. 2009) and occipitotemporal areas and increased activity in the left inferior frontal gyrus (Kronbichler et al. 2006). Other studies have revealed increased activity for RD compared with TYP in left temporoparietal areas, which were mainly driven by the incongruency effect (Rimrodt et al. 2009). Nevertheless, the focus of these studies has been mostly set on sentence comprehension, which is the product of fluent sentence reading, and neglected the differences in reading speeds that exist between RD and TYP. Importantly, if text is presented at a uniform rate during these experiments, slower readers (e.g., RD) are essentially completing a more difficult task than TYP. This introduces a systematic task-demand bias and could ultimately lead to false interpretations. In a small study by Karni et al. (2005), 8 typical and 8 reading-impaired Hebrew-speaking adults were compared at constrained and accelerated reading speeds based on the individually defined comfortable reading speed. However, the brain activations at different reading speeds were not directly statistically compared therefore an interpretation of the different word presentation rates is not possible. In addition, a comfortable reading speed was not used as a condition in the fMRI paradigm (Karni et al. 2005).
The present study aims to investigate “natural reading” at varying and individual-based reading speeds in RD and TYP children in order to investigate how the pattern of activation in core reading regions changes as the opportunity to read fluently is manipulated. Reading fluency is here defined as reading speed with accurate comprehension and does not include reading accuracy (e.g., while reading aloud). We previously employed a similar study design in typical adults (Benjamin and Gaab 2011) and demonstrated that brain regions engaged in reading respond selectively during fluent reading, and that these same regions (especially FG) increase their activity when fluent reading speed is accelerated (Benjamin and Gaab 2011). Based on the summarized evidence above, we aimed to first validate task-related reading network activations in TYP children and then compare activations between children with RD and TYP at varying reading speeds. Our main hypothesis was that children with RD activate posterior left-hemispheric brain regions (especially FG) to a lesser degree than TYP. Based on our previous results in adults and the literature on developmental dyslexia (showing hypoactivation in left-hemispheric occipitotemporal regions), we further hypothesized that, in TYP children, a faster reading rate would lead to increased activation within reading network components and that the group differences between RD and TYP would become more prominent as reading speed was accelerated.
Materials and Methods
Subjects
Fifteen typically reading children (TYP; mean age = 9.9 years, SD = 1.55, age range: 8.3–12.5, 7 females) and 15 children with a diagnosed RD (mean age = 10.0 years, SD = 1.42, age range: 8.5–12.5, 6 females) participated. Groups were matched according to gender, age (P = 0.48), and nonverbal IQ (Table 1). All children were native English speakers and right-handed. All children within the RD group were diagnosed by either a clinician or a reading specialist prior to their participation in the study. Our behavioral analysis revealed significantly lower performance in the RD compared with the typical children in all reading and reading-related tasks (as indicated in Table 1). The data for each subject are illustrated in the Supplementary Figure 1. All children classified as RD had at least one CTOPP and/or RAN score 2 standard deviations or more below the mean standardized score of 100 or scaled score of 10 for CTOPP, except 2 children who exhibited higher values (one child 76 and the other 83). All subjects completed the study with normal/corrected-to-normal vision, had no history of psychiatric or neurological diseases. This study was approved by the Institutional Review Board at Boston Children's Hospital.
Table 1.
Overview of the psychometric measures
| Psychometric measures | RD (mean ± SD) | TYP (mean ± SD) | P-values (two-tailed) |
|---|---|---|---|
| KBIT nonverbal | 104.17 ± 10.49 | 113.91 ± 12.22 | 0.062 |
| TOSWRF: reading fluency | 92.23 ± 13.45 | 107.60 ± 20.54 | 0.042* |
| WRMT-R passage comprehension | 95.58 ± 10.47 | 110.80 ± 6.53 | 0.001** |
| CTOPP: elision | 7.85 ± 4.36 | 11.36 ± 2.01 | 0.022* |
| CTOPP: nonword repetition | 8.25 ± 2.42 | 10.00 ± 1.26 | 0.044* |
| TOWRE sight word efficiency | 88.67 ± 14.91 | 116.09 ± 9.95 | 0.0004** |
| TOWRE decoding | 89.00 ± 11.81 | 107.18 ± 9.48 | 0.001** |
| RAN/RAS numbers | 91.17 ± 28.63 | 117.14 ± 10.12 | 0.035* |
| RAN/RAS letters | 81.67 ± 26.06 | 114.43 ± 8.18 | 0.005** |
Note: Standard scores: mean = 100 and SD = 15, except CTOPP, where the standard score mean = 10 with SD = 1.5 for children with a reading disability (RD) and typical developing children (TYP). Group mean scores, standard deviations (SD) and subsequent P-values (two-tailed) are reported. *P < 0.05 uncorrected, **P < 0.05 Bonferroni corrected for multiple comparisons. All other t-tests are nonsignificant at the threshold of P < 0.05.
Psychometric Measurements
In each child, we examined rapid automatized naming and rapid alternating stimulus tests (RAN/RAS Numbers and Letters) (Wolf and Denckla 2005), phonological processing [Comprehensive Test of Phonological Processing (CTOPP) Elision and Nonword Repetition] (Wagner et al. 1999); word-reading fluency [Test of Silent Word Reading Fluency (TOSWRF)] (Mather et al. 2004); single-word and -nonword reading [Test of Word Reading Efficiency (TOWRE), Sight Word Efficiency and Phonetic Decoding Efficiency (Torgesen et al. 1999)]: and reading comprehension [Woodcock Reading Mastery Test-Revised (WRMT-R), Passage Comprehension] (Woodcock 1987). RD and TYP children were compared with a between-groups test using t-tests for independent samples (statistical thresholds: P < 0.05 Bonferroni corrected for multiple comparisons).
Fluency Task
We used a modified version of the task and procedure as described in (Benjamin and Gaab 2011), who studied reading fluency in TYP adults. The task in the current study differed from (Benjamin and Gaab 2011) in the following respects: all sentences were 4 words long (in Benjamin and Gaab 2011: this was an average of 3, range 2–8); word characteristics were stringently controlled using the MRC; in the current task word speed in the constrained condition was fixed (Benjamin and Gaab 2011: this varied for each participant); in the current task, the accelerated condition was 65% of the comfortable reading speed condition (in Benjamin and Gaab 2011 this was 70%). For a detailed description of the task, please refer to (Benjamin and Gaab 2011). Here, a brief description is presented:
Determination of Sentence Reading Speed
The rate of comfortable speed was individually determined prior to entering the MR scanner by using a computer program to time the children as they read 3 paragraphs. Specifically, 3 passages from a standard reading inventory (Burns and Roe 2001) that are standard for children in first grade were used to ensure the text was comfortably below the expectations of children in the study. Further, all children were allowed as much time as necessary to read the passages (and were able to do so). By pressing a key on the laptop, the passage was presented on the screen. When they finished reading the passage, they needed to again press a key (this gave us the total reading time for the passage). After each passage, children answered standard questions to ensure comprehension. All children were able to answer the comprehension questions. Thus, each participant's average (comfortable) word reading speed was calculated. According to the “normal” comfortable speed, we determined each participant’s “accelerated” word reading speed (35% faster than the normal rate). All children also completed a constrained condition in which stimulus duration was fixed (1350 ms).
fMRI Task
In the MR scanner, each subject completed two 9.0-min runs of the fluency task. Each run consisted of 42 sentences. All sentences had 4 words and the number of letters was matched between conditions and runs. Sentences were constructed using the MRC database to allow word characteristics (the age of acquisition, word frequency, familiarity, concreteness, imageability, and number of phonemes and letters) to be controlled between conditions and runs (all P > 0.3). An overview for mean and standard deviation of all key characteristics can be found in the Supplementary Table 1. Identical to Benjamin and Gaab's study, we used a control condition, where word-like groups of the letter “n” were matched to sentence stimuli. Here, the participant's task was to identify the one oddball letter (either “f,” “p,” or “x”). The target letter was always displayed in 1 of the last 2 groups of the letter “n” of the sentence to ensure all letters were viewed. A similar control condition was also used by Kronbichler et al. (2006). Completion of the control task therefore involves basic visual attention/visual search processes and letter recognition, but does not rely heavily on more complex reading skills (orthographic processing; orthography-phonology mapping; semantic processing. Over both runs, 84 sentences (42 word sentences, 42 letter sentences) were presented at normal (14 word/letter sentences), constrained (14 word/letter sentences), or accelerated (14 word/letter sentences) speed.
The order of the 2 different conditions (fluent sentence reading, letter reading) and speed rates (constrained, comfortable, accelerated) was pseudorandomized across all subjects in an event-related design.
One sentence was presented per trial. The structure for all trials was identical (Supplementary Fig. 2). Before the sentences were presented an image cue (500 ms) indicated the following speed rate. This was followed by a blank screen (200 ms) and the words or control stimuli appearing from left to right at the determined speed until the complete sentence was displayed. Each word remained on the screen until all words were presented. Another blank screen followed (200 ms). Subsequently, the comprehension (fluent sentence reading) or text viewing (letter reading) was tested. For this purpose, the stimuli consisted of 3 cartoon images, 1 showing the key element of the sentence (fluent sentence reading) or 3 letters, one of which was the differing letter in the sentence (letter reading). The children had to choose the image that best represented the sentence (fluent sentence reading) or the letters that had differed from the others (letter reading), indicated by a button press within 3000 ms. In most trials, the correct picture did not just correspond to a single word (e.g., noun), so the child needed to read the whole sentence to discover the correct answer. The performance was measured by the percent correctly identified. The location of the correct response was pseudorandomized in each trial. Finally, a fixation cross was presented for a variable amount of time, being terminated by the following trial. In this rest period, the crosshair was presented and lasted up to 2 TRs.
Imaging Protocol and Analysis
The magnetic resonance imaging (MRI) scans were acquired on a SIEMENS 3.0T Trio MR whole-body scanner. All subjects underwent 2 fMRI runs, where 271 whole-brain images were acquired with a 32-slice functional echo-planar acquisition (interleaved ascending acquisition) using a repetition time (TR) of 2000 ms, and a field of view (FOV) 192 × 192 × 153 mm (full brain coverage). Further imaging parameters were voxel size = 3 × 3 × 4 mm; flip angle = 90°; and echo time (TE) = 30 ms. No parallel imaging was used. For the preprocessing and data analysis, FSL 4.1.9 (http://www.fmrib.ox.ac.uk/fsl) was used. The first 4 images were discarded to account for field effects. The preprocessing included motion correction (MC FLIRT), slice-timing correction, brain extraction (BET), spatial smoothing (4 mm FWHM kernel), high-pass filtering (50 s), and linear registration (12 degrees of freedom) to the MNI 152 T1 template (FLIRT). Because of the ages of participants, a careful procedure for artifact detection was applied. The art-imaging toolbox (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to reduce the residual errors that occur after realignment. Using an automated artifact detection software, artifactual time points were identified using a movement threshold of 2 mm and a rotation threshold of 0.02 mm and subsequent images with artifacts and voxelwise spikes were regressed out. The subjects were only included in the analysis if more than 80% of the images were artifact free. Two subjects were removed due to motion artifacts. Furthermore, the movement regressors for all images were used as regressors in the model. Whole-brain analysis was performed in 3 stages. A first-level model was conducted for each session. Data were prewhitened and regressors were modeled for the (1) speed cue: (2–4) constrained, comfortable and accelerated fluent sentence reading; (5–7) constrained, comfortable, and accelerated letter reading; (8–9) sentence and control comprehension stimuli; and (10) intertrial fixation. In addition, motion parameters were defined as confounding extraneous variables (EVs). We used an event-related design. The 4 words were entered as a single event with the total duration of all 4 words together. However, when directly modeling the first word of each trial, similar results could be obtained. It is important to mention that the event-related fMRI design allows the different duration of events at different reading speeds to be accommodated as sentences presented at slower speed will take longer than those at children's standard fluent reading speed. As the comfortable reading speed was faster in TYP than RD children, an unequal number of images was acquired in the 2 groups. However, the employed software package FSL accommodates for these differences in variance. For an extensive discussion about accommodation of variance, please refer to (Beckmann and Smith 2004; Smith et al. 2004). Another advantage of the employed software package FSL over other leading software packages for analyzing fMRI datasets is that low-level design matrices do not need to be identical to compare the subjects on a higher level analysis (Smith et al. 2004). As we deliberately matched the duration in task and control condition, the canonical HRF models the actual response for each subject identically. We additionally substantiated our results by comparing conditions with similar durations between the 2 groups (comfortable sentence reading in typicals vs. accelerated sentence reading in RD). The 2 runs of each subject were combined in a fixed-effects model. Subsequently, the data were entered onto a group random-effects analysis (FLAME 1). Contrasts were assessed between sentence, control, and rest regressors. Statistical inference was calculated using Z (Gaussianized t) statistic images, which were thresholded using a clusters determined threshold of Z = 2.3 and a corrected cluster significance threshold of P < 0.05 for the within-group contrast, as suggested by (Petersson et al. 1999). Because of the low signal-to-noise ratio and the generally high interindividual variance in pediatric datasets (Thomason et al. 2005), a threshold of P < 0.005 was used for the between-group comparison with a cluster extend threshold of k = 50 as previously employed by various studies (e.g., Raschle et al. 2012). This was done to reduce the probability of false positive findings.
To investigate our hypotheses, we calculated the following contrasts:
Validation of task-related reading network activations in typical children: First, we intended to replicate results found with healthy adults (Benjamin and Gaab 2011). For this purpose, we analyzed each reading rate (constrained, comfortable, accelerated) separately versus the fixation cross (rest condition) for the sentence as well as the letter condition. This analysis was done for typical children (TYP) only.
Comparison between fluent sentence reading and letter reading tasks in children with and without a reading disability: In order to examine whether the brain regions activated during fluent sentence reading responded selectively to this condition or were also activated during lower order processes, we directly compared fluent sentence reading to letter reading. We computed the contrast (fluent sentence reading [all speeds] > letter reading [all speeds]), first for each group (RD and TYP) separately and then between the 2 groups.
Comfortable fluent sentence reading in typically developing children and children with a reading disability: In order to examine if there are neural differences in fluent sentence reading at the comfortable speed, we compared the groups on the contrast (fluent sentence reading [comfortable] > fixation cross [rest condition]).
The effect of accelerated reading speed in children with and without a reading disability: Differences between children with and without an RD are expected to be most prominent in the accelerated reading condition. Therefore, we first calculated the contrast (fluent sentence reading [accelerated] > fixation cross [rest condition]), for each group separately and subsequently compared the 2 groups. Moreover, we directly compared the groups as the opportunity to read fluently was varied (fluent sentences reading [accelerated] > fluent sentences reading [comfortable]) and (fluent sentences reading [accelerated] > fluent sentences reading [constrained]).
Region of Interest Analysis
Based on the results of the previous study (Benjamin and Gaab 2011) and our strong a priori hypotheses, a region of interest (ROI) analysis was performed for the bilateral FG. Instead of using arbitrary spheres as ROI, regions (one ROI per hemisphere) were extracted from the anatomical probability Harvard–Oxford atlas via featquery (http://www.FMRIb.ox.ac.uk/fsl/feat5/featquery.html), thus representing structure-based FG ROIs. The FG is subdivided into posterior (occipital FG) and anterior (temporal FG) divisions. Previous studies have shown that real words compared with letters and false fonts activate the more anterior FG, whereas letters activate only the posterior part of the FG (Vinckier et al. 2007; Brem et al. 2009). We first use the aggregate of those subdivision, because we were primarily interested in naturalistic sentence reading in RD compard with TYP. For the significant results, we refined our analysis to unveil whether the posterior or anterior part of the FG reflects group differences and is associated with behavioral measures. Each individual ROI was binarized. The mean contrasts of parameter estimates (COPEs) were then obtained from each ROI for fluent sentence and letter reading at each speeds (constrained, normal, accelerated). Fluent sentence reading was then compared with letter reading through 5 paired t-tests: fluent sentence reading (all speeds) > letter reading (all speeds); accelerated > comfortable fluent sentence reading; accelerated > constrained fluent sentence reading; accelerated > comfortable letter reading; accelerated > constrained letter reading. The statistical threshold was set to P < 0.05 and post hoc tests were corrected with a modified Bonferroni method: to optimally balance between Type-I and Type-II errors, we took the correlation between the dependent variables (values of the 5 ROI COPE contrasts) into account by using the Simple Interactive Statistical Analysis Bonferroni tool (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm). Using a Bonferroni correction that treats the variables as independent (proper Bonferroni: α/number of tests) would lead to a correction that is too stringent, as the dependent variables are not obtained from independent subgroups (Sankoh et al. 1997; Perneger 1998). Absolute volumes showed a mean correlation coefficient of r = 0.45, leading to an equivalent corrected α of 0.02 (number of tests = 6).
Furthermore, in order to analyze whether the fMRI results are related to behaviorally measured language/reading performance, correlation analyses were calculated between the COPEs of the contrasts (described above) and selected behavioral measures (RAN/RAS Letters; TOWRE Sight Word Efficiency; TOSWRF Reading Fluency and WRMT-R Passage Comprehension). The statistical threshold was set to P < 0.05 corrected with a modified Bonferroni method.
Results
Behavioral Data
Psychometric Measures
RD compared with TYP demonstrated decreased performance in several behavioral language and reading measures. All results are summarized in Table 1.
Determining Sentence Reading Speed
The rate of comfortable reading speed was individually determined beforehand (Materials and Methods section). RD showed a constrained comfortable reading speed compared with TYP (t = −3.30 P = 0.003) (Table 2).
Table 2.
Determined comfortable and accelerated (65% of comfortable) sentence reading speeds are presented for the children with a reading disability (RD) and typical developing children (TYP)
| Reading speed | RD (mean ± SD) (ms/word) | TYP (mean ± SD) (ms/word) | P-values RD versus TYP two-tailed |
|---|---|---|---|
| Comfortable | 686.93 ± 199.36 | 407.45 ± 213.17 | 0.003** |
| Accelerated | 449.49 ± 131.46 | 264.84 ± 138.56 | 0.003** |
Note: Group mean scores, standard deviations (SD), and subsequent P-values (two-tailed) are reported. **P < 0.05 Bonferroni corrected for multiple comparisons. For the constrained sentences, reading speed was set at a fixed duration of 1350 ms.
In-Scanner Performance
No significant groups difference between RD and TYP were observed for in-scanner comprehension accuracy in overall, constrained, comfortable, and accelerated sentences (Table 3).
Table 3.
No significant group differences were observed for in scanner sentence comprehension accuracy
| RD (% correct) | TYP (% correct) | P-values (two-tailed) | |
|---|---|---|---|
| Overall sentences accuracy | 77.74 | 82.25 | 0.619 |
| Constrained sentences accuracy | 78.53 | 83.33 | 0.629 |
| Comfortable sentences accuracy | 82.56 | 86.36 | 0.641 |
| Accelerated sentences accuracy | 71.8 | 77.27 | 0.564 |
Note: The % correct and the subsequent P-values (two-tailed) are stated for the children with a reading disability (RD) and typical developing children (TYP) for all conditions separately as well as combined.
Imaging Data
Validation of Task-Related Reading Network Activations in TYP
Each of the fluent reading contrasts (3 contrasts: constrained, comfortable, accelerated sentence reading > rest) revealed strong activation in left FG. Each sentence reading condition activated various components of the reading network, including right FG, supramarginal gyrus extending into superior temporal gyrus, and inferior frontal gyrus (Broca's area) (Table 4). The equivalent contrasts for letter reading compared with the rest condition activated similar regions including left inferior frontal gyrus (Broca's area) and left FG.
Table 4.
Results for the analysis of the typical developing children's (replication of the adult data) are presented
| Speed rate | Voxels | P-value corrected | Z-max | MNI coordinates (mm) |
Location (Z-max) | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Sentences | Constrained | 3852 | 1.56E−18 | 4.52 | 18 | −48 | −2 | Right lingual gyrus/fusiform gyrus |
| 338 | 0.0182 | 3.61 | 4 | 2 | 26 | anterior cingulate gyrus | ||
| 331 | 0.0207 | 3.12 | −62 | 12 | 18 | Left inferior frontal gyrus (Broca's area) | ||
| Comfortable | 1133 | 5.36E−07 | 4.55 | −26 | −72 | −8 | Left temporal occipital fusiform gyrus | |
| 568 | 0.000725 | 3.46 | 8 | −28 | −6 | Right thalamus | ||
| 417 | 0.0071 | 3.82 | 16 | −44 | −4 | Right lingual gyrus/fusiform gyrus | ||
| Accelerated | 2475 | 2.56E−15 | 3.99 | 20 | −44 | −14 | Right lingual gyrus/fusiform gyrus | |
| 1792 | 4.79E−12 | 4.7 | −26 | −72 | −8 | Left temporal occiptal fusiform gyrus | ||
| 481 | 0.0004 | 3.59 | −12 | 0 | 44 | Left juxtapositional lobule (SMA) | ||
| 311 | 0.0115 | 3.37 | −50 | −18 | 54 | Left sensorimotor area | ||
| 247 | 0.0453 | 3.29 | −48 | −44 | 8 | Left supramarginal gyrus/STG | ||
| Letters | Constrained | 2574 | 1.09E−13 | 4.14 | 18 | −48 | −2 | Right lingual gyrus |
| 1707 | 4.71E−10 | 3.72 | −20 | −52 | −2 | Left lingual gyrus/fusiform gyrus | ||
| 1043 | 7.75E−07 | 4.06 | 0 | 10 | 28 | anterior cingulate gyrus | ||
| 742 | 3.90E−05 | 3.73 | 36 | −36 | 40 | Right supramarginal gyrus | ||
| 577 | 4.10E−04 | 3.98 | −28 | −16 | 70 | Left premotor cortex | ||
| 380 | 9.38E−03 | 3.93 | −2 | −52 | −36 | Right cerebellum | ||
| Comfortable | 8925 | 1.42E−35 | 4.36 | −30 | −86 | −16 | Left temporal occipital fusiform gyrus | |
| 880 | 2.15E−06 | 3.57 | 26 | −56 | 44 | Right superior parietal lobe | ||
| 770 | 1.03E−05 | 3.73 | 32 | −66 | −26 | Right cerebellum | ||
| 477 | 9.80E−04 | 3.28 | −48 | 6 | 34 | Left inferior frontal gyrus (Broca's area) | ||
| 415 | 2.87E−03 | 3.37 | −14 | 12 | 2 | Left caudate | ||
| Accelerated | 13 535 | 0.00 | 4.41 | −38 | −36 | 48 | Left superior parietal lobe | |
| 1680 | 8.17E−11 | 3.87 | 40 | −34 | 44 | Right supramarginal gyrus | ||
| 699 | 2.40E−05 | 3.54 | −20 | −62 | −10 | Left lingual gyrus/fusiform gyrus | ||
Note: We computed the contrast of each separate speed rate (constrained, comfortable, accelerated) of the sentence and letter conditions versus the rest condition. Listed are the number of voxels of a significant cluster, corrected P-value, the maximum Z-value, the MNI coordinates of the local maximum, and the subsequent locations labeled via Harvard Oxford Cortical Atlas (Juelich for Broca Area).
Comparison Between Fluent Sentence Reading and Letter Reading Tasks in RD and TYP
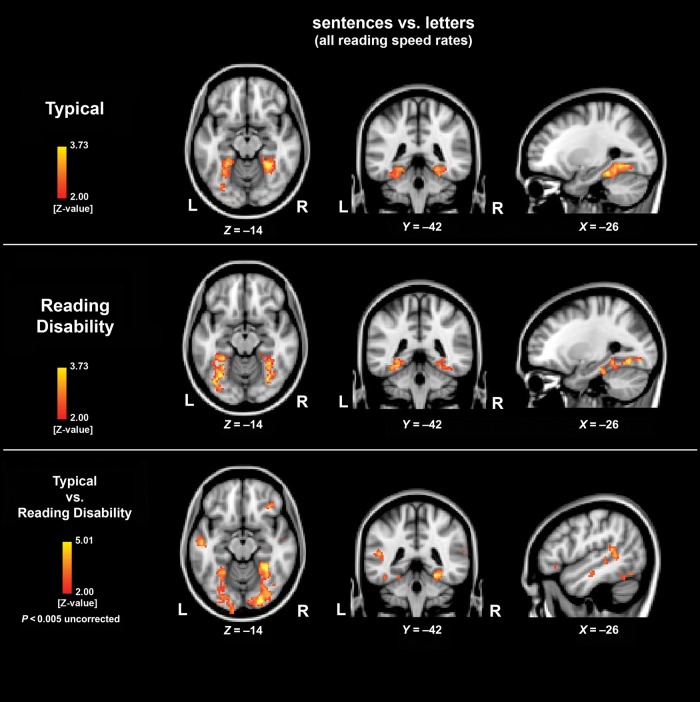
We computed the contrast (fluent sentence reading [all speeds] > letter reading [all speeds]), first for each group (RD and TYP) separately and then between the 2 groups. The within-group contrast revealed bilateral occipitotemporal and FG activation TYP and RD. TYP compared with RD showed increased brain activation in all reading network components, particularly in bilateral FG, left supramarginal gyrus, left superior temporal gyrus, and right inferior frontal regions (Fig. 1, Table 5). When regressing out the effects of nonverbal IQ, the group comparison exhibit equivalent results. The opposite contrast (RD > TYP) revealed no activation increases for this contrast.
Figure 1.
The contrasts (fluent sentence reading [all speeds] > letter reading [all speeds]) for the children with RD and typical developing children (TYP) are shown. Both the children with RD and TYP showed an increased BOLD response l in bilateral fusiform gyrus (FG) for the sentences versus letter fluent reading task. The level of significance was set at P < 0.05 cluster-corrected. The group comparison revealed significant differences in several regions of reading network at a threshold of P < 0.005 uncorrected. The exact values can be found in Table 5.
Table 5.
Displayed is the contrast (fluent sentence reading [all speeds] > letter reading [all speeds]) in children with (RD) and without (TYP) a reading disability and the direct comparisons (two-sample t-test) between typical developing children (TYP) and children with a reading disability (RD)
| Group | Voxels | P-value corrected | Z-max | MNI coordinates (mm) |
Location (Z-max) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| TYP | 637 | 0.000186 | 3.83 | −30 | −36 | −18 | Left temporal occipital fusiform gyrus |
| 417 | 0.00537 | 4.19 | 28 | −48 | −12 | Right temporal occipital fusiform gyrus/lingual gyrus | |
| RD | 847 | 0.0000106 | 4.09 | −30 | −62 | −10 | Left temporal occipital fusiform gyrus |
| 683 | 0.000097 | 4.07 | 30 | −68 | −14 | Right occipital fusiform gyrus | |
| TYP > RD (P < 0.005 uncorr) | 2726 | 7.52E-11 | 6.51 | 22 | −92 | −18 | Right occipital fusiform gyrus |
| 1512 | 0.000000017 | 5.64 | −34 | −72 | −20 | Left occipital fusiform gyrus | |
| 438 | 0.00000273 | 4.69 | −54 | −44 | 10 | Left supramarginal gyrus | |
| 266 | 0.00000236 | 4.72 | −52 | −14 | −12 | Left middle temporal gyrus | |
| 227 | 0.0000305 | 4.17 | 44 | 14 | 34 | Right inferior frontal gyrus (Broca's Area) | |
| 203 | 0.00000236 | 4.72 | −24 | −92 | 20 | Left lateral occipital cortex | |
Note: Listed are the number of voxels of a significant cluster, corrected P-value, the maximum Z-value, the MNI coordinates of the local maximum, and the subsequent locations labeled via Harvard Oxford Cortical Atlas (Juelich for Broca Area).
Comfortable Fluent Sentence Reading in RD and TYP
In the comfortable reading condition, each participant (RD/TYP) performed the task with his/her individually determined comfortable reading speed. Thus, finding group (RD vs. TYP) differences in this contrast (comfortable reading speed vs. rest) would suggest that previously reported differences in the activation patterns between RD and TYP are most likely not explainable by differences in task demands but rather suggest a fundamental difference in the reading network. This analysis revealed increased activation primarily within FG for TYP compared with RD during comfortable sentence reading > rest. Furthermore, increased activation was also observed in various other regions, such as the bilateral superior and inferior parietal lobe, including the supramarginal and angular gyrus (Supplementary Table 2). Moreover, RD children showed increased activation compared with TYP in left lateral occipital gyrus and the hippocampus.
In order to ensure that the observed group differences could not be explained by difference in the presentation rate and duration, which could potentially bias the data analysis (Price et al. 1996; Mechelli et al. 2000), we compared conditions with similar durations between the 2 groups, namely the accelerated reading condition in RD (average word reading speed = 449 ms) to the comfortable reading condition in TYP (average word reading speed = 407 ms). However, we used FSL to model the canonical HRF for each subject individually, which is able to take the accommodation effects into account (Beckmann and Smith 2004; Smith et al. 2004). For an extensive discussion on the individual task duration, see the Materials and methods section. We also observed increased activation within bilateral FG, validating the results reported above (Note: task demands may be different in this comparison).
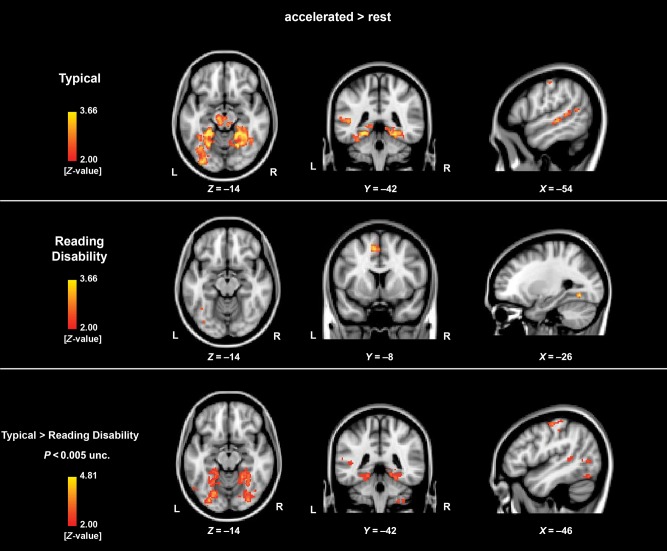
The Effect of Accelerated Reading Speed RD and TYP
We calculated the contrast accelerated fluent sentence reading > rest for each group separately and subsequently compared the 2 groups. TYP showed increased activation in FG (bilaterally), left supramarginal gyrus extending into the superior temporal gyrus, left supplementary motor (SMA) and sensorimotor areas during accelerated fluent sentence reading > rest. For the same contrast, RD children showed increased left lateralized FG activation and increased activation within the premotor area (Fig. 2, Supplementary Table 3). However, TYP compared with RD displayed increased activation within a variety of the reading network regions, including left FG, superior parietal cortex, supramarginal gyrus, and inferior frontal gyrus (Fig. 2, Supplementary Table 3). When regressing out the effects of nonverbal IQ, all group comparisons for the comfortable and accelerated conditions exhibit equivalent results. The opposite contrast (RD > TYP) revealed no activation increases for these contrasts.
Figure 2.
Displayed are the results for the single-group and between-group comparisons for the contrast (accelerated fluent sentence reading vs. rest). Increased activation was primarily observed in the occipitotemporal brain regions including the FG, the left supramarginal gyrus extending into the superior temporal gyrus in the typical developing children. The same contrast in children with RD showed minimal activation differences. The between-group comparison revealed an increased BOLD response primarily in the FG for the typical developing children compared with children with RD. The level of significance was set at P < 0.05 cluster-corrected. The exact values can be found in the associated Supplementary Table 3.
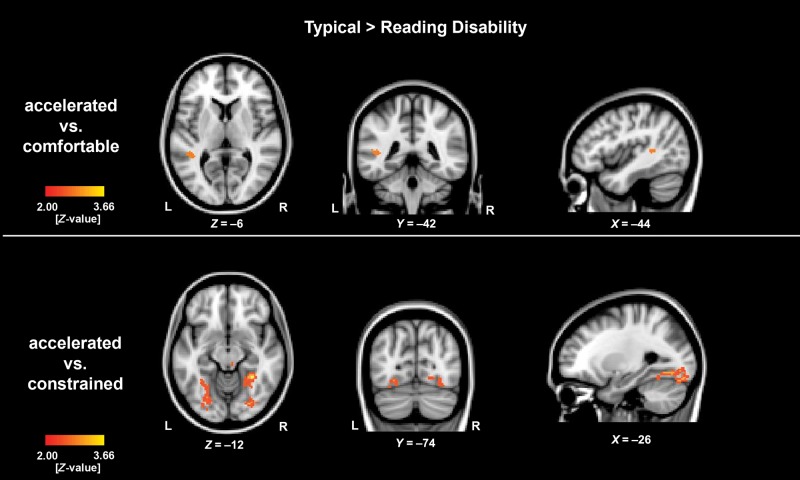
To specify if the effects are specific to the accelerated reading condition, we additionally computed the contrasts accelerated > constrained fluent sentence reading and accelerated > comfortable fluent sentence reading between the groups. The contrast accelerated > constrained fluent sentence reading revealed increased activation in several regions, including bilateral FG, for TYP compared with RD. The contrast accelerated > comfortable fluent sentence reading exhibited enhanced activation for TYP > RD within left superior temporal gyrus and left lateral occipital cortex extending to left FG (Fig. 3, Supplementary Table 3).
Figure 3.
Depicted are the between-group results for the analyses of the contrast of accelerated versus constrained and accelerated versus comfortable sentence reading speed. In the contrast accelerated versus comfortable reading speed rate, the typical developing children revealed an enhanced activation within the left superior temporal gyrus and left lateral occipital cortex. The contrast accelerated > constrained fluent sentence reading revealed an increased activation in several brain regions including bilateral FG for the typical developing children compared with the children with RD. The level of significance was set at P < 0.05 cluster-corrected. The exact values can be found in the associated Supplementary Table 3.
Region of Interest Analysis
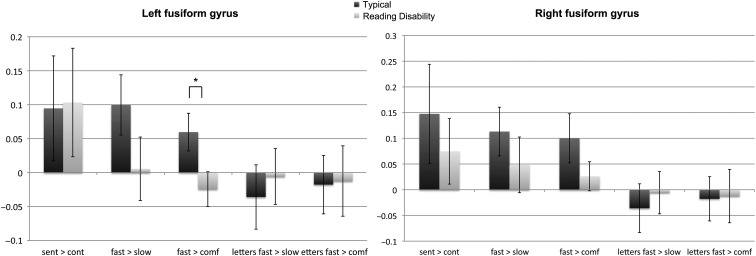
Significant group differences were observed for the contrast accelerated > comfortable fluent sentence reading, in which TYP demonstrated higher activation within the left FG ROI (P = 0.02, corrected) (Fig. 4). The group differences were driven by the left anterior part of the FG (P = 0.02), whereas the posterior part revealed no significant group differences. No significant differences could be found in the right hemisphere.
Figure 4.
Displayed are the results of the structural ROI analysis in bilateral FG. On the y-axis, the extracted COPE values for the children with and without a RD are presented. We only observed significant differences (P < 0.05 corrected for multiple comparisons) between TYP and RD in the left FG for the contrast accelerated (accelerated) > comfortable fluent sentences reading. There was a trend toward significance for the contrast accelerated > constrained fluent sentence reading. “Fast” represents the accelerated condition; “slow” is representative for constrained and “cont” for the letter control task.
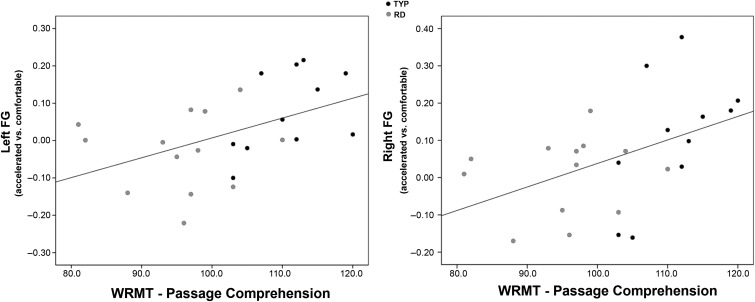
There was a significant positive correlation between ROI values for the contrast accelerated > comfortable fluent sentence reading and the WRMT-R Passage Comprehension in left FG (r = 0.477, P = 0.018, corrected) and right FG (r = 0.464, P = 0.022, corrected) (Fig. 5) The analysis of the subparts of FG revealed that the correlation was mainly driven by the anterior part of FG (left hemisphere: r = 0.552, P = 0.005; right hemisphere: r = 0.534, P = 0.005, corrected). There was no significant correlation between the FG and any of the other behavioral measures.
Figure 5.
The scatter plots for the significant correlation between the COPE values within the structural ROI for the FG in both hemispheres are presented. We observed a significant positive correlation between the contrast (accelerated > comfortable) in both the right and the left FG and the WRMT-R passage comprehension (P < 0.05 corrected for multiple comparisons). The black dots represent the TYP and the gray dots the RD children.
Discussion
The present study sought to delineate the neural basis of fluent sentence reading and the effects of different reading speed rates on reading fluency in RD and TYP children. We first demonstrated that RD compared with TYP displayed decreased performance in several reading-related psychometric tasks. Moreover, TYP revealed a significantly accelerated comfortable reading speed, which substantiates previous findings illustrating fluency impairments in RD (Norton and Wolf 2012; NRP 2000).
Validation of Task-related Reading Network Activations in TYP
Investigating the neural basis of fluent sentence reading in children, we first were able to replicate the results of our previous study, which examined healthy adults (Benjamin and Gaab 2011). For all fluent reading contrasts compared with a rest condition, TYP showed increased activation within reading network components, particularly within FG. These results demonstrate that the reading networks of typical children and adults are similar.
Comparison Between Fluent Sentence Reading and Letter Reading Task in RD and TYP
The comparison between the fluent sentence and letter reading tasks, averaged across all reading speeds in RD and TYP, revealed bilateral occipitotemporal and FG activation in both TYP and RD. In general, our results suggest that word stimuli compared with consonant strings modulate the FG preferentially. Interestingly, RD and TYP show a similar activation pattern for (fluent sentence reading [all speeds] > letter reading [all speeds]), which suggests that RD children are utilizing the same areas for fluent reading but to a lesser extent, which was confirmed by a group comparison. Such hypoactivation in the occipitotemporal area in RD has also been shown by several other studies investigating lower level reading processes (Schlaggar and McCandliss 2007; Richlan et al. 2009; van der Mark et al. 2009). Nevertheless, it is important to note that our control condition only involved 4 different letters altogether (2 in each given trial) and therefore does not entirely control for the visual (letter) complexity displayed in the fluent sentence task which could have affected activation within the FG for this contrast. However, a similar control task was utilized in (Kronbichler et al. 2006) whose results are in line with our findings. Further studies should address whether employing a control task that consists of letter strings with a variety of letters will elicit a similar response (when contrasted to the fluent sentence task) than here observed.
Comfortable Fluent Sentence Reading in RD and TYP
Comparing the 2 groups at an individually determined comfortable reading speed is important because most of the previous studies investigating reading skills have not controlled for differences in task demands between age-matched TYP and RD. An alternative approach to control for different task demands between RD and TYP has been previously employed (Brown et al. 2005; Hoeft et al. 2006; Schlaggar et al. 2002), and matches groups according to performance, but allows group differences in age. Such an approach was also used in a combined fMRI and ERP study by Schulz et al. (2009) investigating sentence reading. They observed decreased activation in children with dyslexia and in a control group at a similar reading level in the inferior parietal cortex in response to sentence reading compared with age-matched controls as well as a decreased AND word presentation, which amplitude 400 ms after word presentation which suggests a developmental and dyslexia-specific word processing deficit. In studies with constant presentation speed, TYP children may be forced to read at a more constrained level than their comfortable level while RD may be forced to read at a more accelerated level than their comfortable levels. For the contrast comfortable reading speed versus rest, we observed increased activation within FG for TYP compared with RD. Furthermore, increased activation was also observed in other reading network components for TYP compared with RD, including right middle frontal, left middle/superior temporal, and bilateral supramarginal and angular gyrus. These temporoparietal and frontal regions are most likely not engaged by orthographic processes but may reflect other lower and higher order processes, such as phonological processing, lexical-semantic processes, and retrieval operations (Price et al. 1997; Bookheimer 2002; Cao et al. 2006; Vigneau et al. 2006; Booth et al. 2007) and therefore suggest atypical reading development in RD in several reading network components.
Interestingly, the increased activation within FG for TYP compared with RD could also be observed when comparing the accelerated reading condition in RD to a similar word reading speed (comfortable) in TYP. This suggests that our results cannot be explained by the objective difference in reading speed but rather point to a fundamental difference within the posterior reading network in RD, which is observable regardless of task demands.
The Effect of Accelerated Reading Speed in RD and TYP
TYP but not RD showed increased activation in multiple reading network components when reading speed was increased, especially within FG. This is in line with the study of Kronbichler et al. (2006) demonstrating reduced left FG activation in adolescents with RD compared with TYP during a similar paradigm with a fixed word presentation rate and a very similar control task. In addition, Karni et al. (2005) studied sentence reading in adults with and without dyslexia, and found increased left FG activation in typical adults in an accelerated reading task. They further describe less activation in accelerated as compared with constrained sentence reading in typical adults, although different reading speeds were not directly statistically compared.
Our anatomically defined ROI analysis highlighted significant group differences in left FG, particularly when comparing accelerated versus constrained sentences, and further demonstrated a parametric relationship between FG and behavioral reading skills. In the following paragraphs, we will discuss the role of the FG within the reading network and incorporate our findings into current literature.
The Role of the FG During Fluent Reading
It has been suggested that the FG is engaged in the processing of word form-related stimuli, particularly in the (mid-)part of the FG, also called the visual word form area (VWFA) (McCandliss et al. 2003; Kronbichler et al. 2004; Vinckier et al. 2007). Furthermore, Dehaene et al. (2010) demonstrated that literacy enhances the brain responses in the VWFA and that the VWFA area is significantly more sensitive to words than other visual stimuli, such as checkerboards and faces. Several studies argue that the FG is a processing center for category-specific perception (Martin et al. 1996), expert perception (Wong et al. 2009) or logical rule identification (Tachibana et al. 2009). Although a recent debate centers on the contribution of this area to nonreading tasks, such as facial recognition and the perception of other well-known objects, one can conclude from the majority of studies that the FG is more generally involved in expert perception (Price and Devlin 2003; Rogers et al. 2005; Wong et al. 2009). Because printed word recognition requires the perception of a visual stimulus, the FG involvement in visual word processing aligns with this concept as this is an area of perceptive expertise specific to humans. Furthermore, research on the engagement of the FG in visual word recognition has suggested a role in the bottom-up encoding of orthographic characteristics of consecutive letter chains, with a positive correlation between activation in this region and the statistical properties by which letters are organized into word forms within a given writing context (Dehaene et al. 2004; Binder et al. 2006). Recent studies also demonstrate a posterior-to anterior gradient within the FG with increasing specialization to real words in the anterior FG (Vinckier et al. 2007; Brem et al. 2009; Yeatman et al. 2012). Our ROI results suggest that RD fail to recruit the anterior part of the FG, which is also related to behavioral performance of sentence comprehension. Several research studies have shown that individuals with an RD demonstrate a hypoactivation in the FG during reading and reading-related tasks (Kronbichler et al. 2004, 2008; Rykhlevskaia et al. 2009; Raschle et al. 2011, 2012; Peterson and Pennington 2012). Cohen et al. (2002) first interpreted this hypoactivation as a deficit in accelerated parallel processing of letter strings. Our results, which demonstrate that the FG (especially within the left hemisphere) is preferentially engaged in the processing of sentences compared with letter reading, align with the previous findings but do not suggest a general deficit for the parallel processing of letter strings as suggested by Cohen et al. Other research groups have hypothesized that the hypoactivation within the FG reflects a general impairment in linking visual or other sensory information to higher order representations (Price and Devlin 2003; Devlin et al. 2006) or an impairment in amassing or using orthographic word recognition (Richlan et al. 2009; van der Mark et al. 2009). Moreover, several studies suggested that the FG operates as a “gateway” through which critical invariant information extracted from visual forms makes contact with linguistic representations (Carr and Posner 1995; Schlaggar and McCandliss 2007). We observed a significant relationship between activation within the FG during fluent sentence reading (accelerated > comfortable) and the Passage Comprehension subtest of the WRMT-R, which is strongly linked to reading fluency (Rimrodt et al. 2009). Readers must attain a level of reading fluency in order to dedicate cognitive capacity to the cognitive process of comprehending the read passage (Breznitz 2005; Norton and Wolf 2012). This correlation suggests that our results align with this general idea that children with an RD are equipped with a dysfunctional “gateway” which may lead to a dysfunctional connection when relating visual information to higher order linguistic and language representations. The detected correlation (Fig. 5) indicates that the engagement of the FG may be an important prerequisite for comprehension. This does not imply that comprehension takes place in the FG but rather suggests a strong relationship/connectivity between brain areas involved in reading fluency and reading comprehension and may suggest less efficient connections between the FG and brain areas involved in reading comprehension in children with reading fluency and accompanying reading comprehension deficits. However, several studies were particularly critical of the WRMT-R Passage Comprehension subtest and especially criticize its cloze procedure [see review in De Rose (1999)] which may not be the most effective way of measuring across-sentence comprehension (Shanahan et al. 1982; Leys et al. 1983). Nevertheless, decent correlations between the WRMT-R passage comprehension subtest and other comprehension measures without the cloze procedure have been reported (Harlaar et al. 2010). Future studies are needed to further examine this relationship, and it would be particularly interesting to examine the relationship with a comprehension measure that does not employ the cloze procedure [e.g., PIAT-R (Markwardt 1989)].
Interestingly, we did not observe a correlation between activation within the FG and the reading sub-skill rapid automatized naming (RAN). This is somewhat surprising since several research studies have linked RAN to reading fluency (Rimrodt et al. 2009; Norton and Wolf 2012). However, several studies have shown that the relationship between RAN and reading is much stronger in poor readers than it is in typical readers (Meyer et al. 1998; Scarborough 1998; Rimrodt et al. 2009; Frijters et al. 2011) and our regression analysis included typical as well as reading-disabled children. A follow-up analysis with only the reading-disabled children also did not display a positive correlation between RAN and the engagement of the FG, which could be due to the fact that the sample size for this subanalysis was relatively small and may therefore not yield statistical significance. However, the aforementioned studies strongly accentuated the comprehension portion of reading fluency, whereas in our study, comprehension was held relatively constant in all conditions and more focus was placed on the speed factor. We also did not examine the neural correlates of reading accuracy (e.g., while reading aloud) in our study but this component is often sought to be part of reading fluency as a whole. An alternative explanation for the lack of the correlation between activation in FG and RAN could be that the FG is not one of the key regions involved during the rapid automatized naming task, which is supported by the fact that previous studies have shown more frontal regions being engaged during RAN (Misra et al. 2004; Norton and Wolf 2012).
Implications for Intervention Strategies Targeting Fluency Deficits
The present study has implications for intervention programs designed to specifically target reading fluency deficits. Although there are many different remediation programs for RD children, many research studies evaluating intervention strategies have focused on programs that aim to remediate lower level processes. Fluency deficits are often not remediated by interventions targeting these skills (Lyon and Moats 1997). Several intervention studies come to the conclusion that remediating reading in general is extremely time-consuming and that improvements are mainly shown for word-reading accuracy with no accompanying enhancement of fluency (Torgesen et al. 2001; Wolf and Katzir-Cohen 2001; Reynolds et al. 2003; Katzir et al. 2006; Morris et al. 2010; Hofstadter-Duke and Daly 2011). It has been suggested that intervention strategies that focus on repeated reading of the same text with various speeds have been successful and also show generalizable increases for speed and decoding accuracy (Young et al. 1996; Meyer and Felton 1999; Stahl and Heuback 2005; Norton and Wolf 2012). It needs to be determined whether these intervention strategies specifically alter the engagement of the FG during the intervention process and/or lead to changes in the overall reading network and its connections.
Integrating evidence from neuroscientific studies that examine reading fluency deficits in the brain may help to develop and/or evaluate new and existing remediation strategies. To date, few neuroimaging studies have evaluated reading fluency and its remediation. For example, Shaywitz et al. (2004) demonstrated that a phonologically mediated reading intervention in RD children led to significant gains in reading fluency and demonstrated increased activation in left-hemispheric regions. Our results suggest that training for specific functions that are processed by the FG might be beneficial for reading fluency. Furthermore, TYP compared with RD exhibited stronger activation not exclusively in the FG area but also in the whole reading network (Figs 1 and 2). This suggests that interventions to improve reading fluency skills for RD might be most successful if they focused not only on repetition of sentence reading to increase the speed of reading, but also on all aspects of reading. This is in line with Morris et al. (2010), who showed in their randomized treatment-control study that the best remediation outcome was obtained through a multicomponent intervention (Wolf and Katzir-Cohen 2001; Norton and Wolf 2012).
Alternatively, a new remediation approach could focus on improving the buffer and storage system for word chains. A recent fMRI study investigated how spoken or written sentences presented at different speed rates influenced brain activation in healthy adults (Vagharchakian et al. 2012). The study indicates that visual and frontal brain regions are more engaged when written sentences are presented at constrained compared with accelerated rates. The authors explain the increased activation as a result of the greater need to buffer and store the chains of single words to assure successful retrieval and maintain their appropriate order (Vagharchakian et al. 2012). Children with an RD may have deficits with the buffer and storage system. Subsequently, they may read at a more constrained level than their peers and need more cognitive resources for successful retrieval and maintenance of the appropriate order. This in turn could lead to reading fluency deficits and deficient text comprehension and/or retention. Our results demonstrated that children with an RD display a reduced comfortable reading speed, which might be the results of a deficient ventral system, which is then associated with reading comprehension as we have shown in the ROI analysis. This may suggest that future intervention studies should aim to enhance this buffer system in order to improve fluent reading and that this may be most successful if the ventral components of the reading networks, especially the FG, are enhanced. Furthermore, focusing on automatization of the reading process could improve this buffer and storage system and consequently improve reading fluency and comprehension. There is a need to further investigate the functionality of the fusiform gyri within the reading network especially during reading fluency and its relation to reading comprehension and to connect these findings to the past and future literature on evaluating reading remediation programs.
Future studies should focus on the functional and structural connections between the FG and other components of the reading network in order to uncover the underlying mechanisms of deficits in reading fluency and comprehension. In addition, more studies evaluating reading fluency programs through brain imaging should investigate which areas of the reading network benefit from which remediation programs.
The present study is the first to differentiate TYP and RD children during “natural reading” at varying and individual-based reading speeds. We demonstrated that RD compared with TYP has lower performance scores on several reading measures and that their comfortable reading speed is constrained. Our fMRI results suggest that both TYP and RD show activation within several reading network components during sentence reading, similar to what has been reported in adults. Specifically, during reading at comfortable speeds RD children are utilizing the same areas for fluent reading as TYP but to a lesser extent as shown by the direct group comparison. Furthermore, when required to read sentences faster than their comfortable reading speed, decreases in activation within FG were observed for RD compared with TYP (Fig. 4).
This suggests that FG plays a key role in sentence reading and further supports the notion that functional deficits in FG could be an underlying mechanism of reading fluency deficits in RD. Given that reading fluency is one of the key deficits in RD, and that remediation programs often fail to remediate deficits in fluent reading, these results and their implications should be carefully considered in clinical and educational practice.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by National Institutes of Health (grant number: 5RO1HD065762–01/02).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Beckmann CF, Smith SM. 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 23:137–152. 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- Benjamin CF, Gaab N. 2011. What's the story? The tale of reading fluency told at speed. Hum Brain Mapp. 33:2572–2585. 10.1002/hbm.21384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger VW, Abbott RD, Billingsley R, Nagy W. 2001. Processes underlying timing and fluency of reading. In: Wolf M, editor. Dyslexia, fluency, and the brain. Baltimore: York Press. [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. 2006. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 33:739–748. 10.1016/j.neuroimage.2006.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. 2002. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 25:151–188. 10.1146/annurev.neuro.25.112701.142946 [DOI] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. 2007. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 45:775–783. 10.1016/j.neuropsychologia.2006.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. 2009. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Hum Brain Mapp. 30:1833–1844. 10.1002/hbm.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznitz Z. 2005. Brain activity during performance of naming task: comparison between dyslexic and regular readers. Sci Stud Read. 9:17–42. 10.1207/s1532799xssr0901_3 [DOI] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. 2005. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 15:275–290. 10.1093/cercor/bhh129 [DOI] [PubMed] [Google Scholar]
- Burns P, Roe B. 2001. Informal reading inventory: preprimer to twelfth grade. Boston: Houghton Mifflin. [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. 2006. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 47:1041–1050. 10.1111/j.1469-7610.2006.01684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr TH, Posner MI. 1995. The impact of learning to read on the functional anatomy of language processing. In: de Gelder B, Morais J, editors. Language and literacy: comparative approaches. Cambridge: (MA: ): MIT Press; p. 267–294. [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. 2002. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 125:1054–1069. 10.1093/brain/awf094 [DOI] [PubMed] [Google Scholar]
- De Rose M. 1999. A review of the Woodcock reading mastery test-revised (WRMT-R). TESL Can J. 16:86–93. [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. 2004. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol Sci. 15:307–313. 10.1111/j.0956-7976.2004.00674.x [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. 2010. How learning to read changes the cortical networks for vision and language. Science. 330:1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. 2006. The role of the posterior fusiform gyrus in reading. J Cogn Neurosci. 18:911–922. 10.1162/jocn.2006.18.6.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijters JC, Lovett MW, Steinbach KA, Wolf M, Sevcik RA, Morris RD. 2011. Neurocognitive predictors of reading outcomes for children with reading disabilities. J Learn Disabil. 44:150–166. 10.1177/0022219410391185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. 2006. From genes to behavior in developmental dyslexia. Nat Neurosci. 9:1213–1217. 10.1038/nn1772 [DOI] [PubMed] [Google Scholar]
- Harlaar N, Cutting L, Deater-Deckard K, Dethorne LS, Justice LM, Schatschneider C, Thompson LA, Petrill SA. 2010. Predicting individual differences in reading comprehension: a twin study. Ann Dyslexia. 60:265–288. 10.1007/s11881-010-0044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. 2006. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 26:10700–10708. 10.1523/JNEUROSCI.4931-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstadter-Duke KL, Daly EJ., 3rd 2011. Improving oral reading fluency with a peer-mediated intervention. J Appl Behav Anal. 44:641–646. Available from: URL http://www.FMRIb.ox.ac.uk/fsl/feat5/featquery.html In. 10.1901/jaba.2011.44-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kame'enui EJ, Simmons DC. 2001. Introduction to this special issue: the DNA of reading fluency. Sci Stud Read. 5:203–210. 10.1207/S1532799XSSR0503_1 [DOI] [Google Scholar]
- Karni A, Morocz IA, Bitan T, Shaul S, Kushnir T, Breznitz Z. 2005. An fMRI study of the differential effects of word presentation rates (reading acceleration on dyslexic readers’ brain activity patterns). J Neurolinguistics. 18:197–219. 10.1016/j.jneuroling.2004.11.002 [DOI] [Google Scholar]
- Katzir T, Kim Y, Wolf M, O'Brien B, Kennedy B, Lovett M, Morris R. 2006. Reading fluency: the whole is more than the parts. Ann Dyslexia. 56:51–82. 10.1007/s11881-006-0003-5 [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. 2006. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 44:1822–1832. 10.1016/j.neuropsychologia.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. 2004. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 21:946–953. 10.1016/j.neuroimage.2003.10.021 [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. 2008. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 29:613–625. 10.1002/hbm.20425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Grigg W, Donahue P. 2007. National Assessment of Educational Progress (NAEP): the nation's report card, reading 2007. Washington: (DC: ): Institute of Education Sciences, U.S. Department of Education. [Google Scholar]
- Leys M, Fielding L, Herman P. 1983. Does cloze measure intersentence comprehension? A modified replication of Shanahan, Kamil, and Tobin. In: Niles JA, Harris AL, editors. Searches for meaning in reading/language processing and instruction. Rochester: (NY: ): National Reading Conference; p. 111–114. [Google Scholar]
- Lyon GR, Moats LC. 1997. Critical conceptual and methodological considerations in reading intervention research. J Learn Disabil. 30:578–588. 10.1177/002221949703000601 [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA. 2003. Defining dyslexia, comorbidity, teachers knowledge of language and reading. A definition of dyslexia. Ann Dyslexia. 53:1–14. 10.1007/s11881-003-0001-9 [DOI] [Google Scholar]
- Markwardt FC. 1989. Peabody individual achievement test-revised: PIAT-R. Circle Pines: (MN: ): American Guidance Service. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. 1996. Neural correlates of category-specific knowledge. Nature. 379:649–652. 10.1038/379649a0 [DOI] [PubMed] [Google Scholar]
- Mather N, Hammill DD, Allen EA, Roberts R. 2004. Test of silent word reading. Austin, TX: PRO-ED. [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. 2003. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 7:293–299. 10.1016/S1364-6613(03)00134-7 [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Price CJ. 2000. The effects of presentation rate during word and pseudoword reading: a comparison of PET and fMRI. J Cogn Neurosci. 12(Suppl 2):145–156. 10.1162/089892900564000 [DOI] [PubMed] [Google Scholar]
- Meyer AS, Felton RH. 1999. Repeated reading to enhance fluency: old approaches and new directions. Ann Dyslexia. 1:283–306. [Google Scholar]
- Meyer MS, Wood FB, Hart LA, Felton RH. 1998. Selective predictive value of rapid automatized naming in poor readers. J Learn Disabil. 31:106–117. 10.1177/002221949803100201 [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JD, Just MA. 2007. Brain activation during sentence comprehension among good and poor readers. Cereb Cortex. 17:2780–2787. 10.1093/cercor/bhm006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzir T, Wolf M, Poldrack RA. 2004. Neural systems for rapid automatized naming in skilled readers: unraveling the RAN-reading relationship. Sci Stud Read. 8:241–256. 10.1207/s1532799xssr0803_4 [DOI] [Google Scholar]
- Morris RD, Lovett MW, Wolf M, Sevcik RA, Steinbach KA, Frijters JC, Shapiro MB. 2010. Multiple-component remediation for developmental reading disabilities: IQ, socioeconomic status, and race as factors in remedial outcome. J Learn Disabil. 45(2):99–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton ES, Wolf M. 2012. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 63:427–452. 10.1146/annurev-psych-120710-100431 [DOI] [PubMed] [Google Scholar]
- NRP. 2000. Fluency. Report of the National Reading Panel Available from: URL http://www.nationalreadingpanel.org/.
- Perie M, Grigg W, Donahue P. 2005. National Assessment of Educational Progress: the nation's report card, reading. In: Sci. USDEIE, editors. Washington: (DC: ): U.S. Government Printing Office. [Google Scholar]
- Perneger TV. 1998. What's wrong with Bonferroni adjustments. BMJ. 316:1236–1238. 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson KM, Nichols TE, Poline JB, Holmes A. 1999. Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Phil Trans R Soc Lond B. 354:1261–1281. 10.1098/rstb.1999.0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. 2012. Developmental dyslexia. Lancet. 379:1997–2007. 10.1016/S0140-6736(12)60198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. 2003. The myth of the visual word form area. Neuroimage. 19:473–481. 10.1016/S1053-8119(03)00084-3 [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Frackowiak RS. 1996. The effect of varying stimulus rate and duration on brain activity during reading. Neuroimage. 3:40–52. 10.1006/nimg.1996.0005 [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. 1997. Segregating semantic from phonological processes during reading. J Cogn Neurosci. 9:727–733. 10.1162/jocn.1997.9.6.727 [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. 2001. Neurobiological studies of reading and reading disability. J Commun Disord. 34:479–492. 10.1016/S0021-9924(01)00060-0 [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. 2011. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 57:742–749. 10.1016/j.neuroimage.2010.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. 2012. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA. 109:2156–2161. 10.1073/pnas.1107721109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D, Nicolson RI, Hambly H. 2003. Evaluation of an exercise-based treatment for children with reading difficulties. Dyslexia. 9:48–71. 10.1002/dys.235 [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2009. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 30:3299–3308. 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. 2009. Functional MRI of sentence comprehension in children with dyslexia: beyond word recognition. Cereb Cortex. 19:402–413. 10.1093/cercor/bhn092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Mechelli A, Patterson K, Price C. 2005. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 17:434–445. 10.1162/0898929053279531 [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Uddin LQ, Kondos L, Menon V. 2009. Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front Hum Neurosci. 3:51 10.3389/neuro.09.051.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. 1997. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 16:2529–2542. [DOI] [PubMed] [Google Scholar]
- Scarborough H. 1998. Predicting the future achievement of second graders with reading disabilities: contributions of phenemic awareness, verbal memory, rapid naming and IQ. Ann Dyslexia. 48:115–136. 10.1007/s11881-998-0006-5 [DOI] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. 2002. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 296:1476–1479. 10.1126/science.1069464 [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. 2007. Development of neural systems for reading. Annu Rev Neurosci. 30:475–503. 10.1146/annurev.neuro.28.061604.135645 [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. 2009. Reading for meaning in dyslexic and young children: distinct neural pathways but common endpoints. Neuropsychologia. 47:2544–2557. 10.1016/j.neuropsychologia.2009.04.028 [DOI] [PubMed] [Google Scholar]
- Seidenberg MS. 2005. Connectionist models of word reading. Curr Dir Psychol Sci. 14:238–243. [Google Scholar]
- Shanahan T, Kamil ML, Tobin AW. 1982. Cloze as a measure of intersentential comprehension. Read Res Q. 17:229–255. 10.2307/747485 [DOI] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, et al. 2004. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 55:926–933. 10.1016/j.biopsych.2003.12.019 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stahl S, Heuback K. 2005. Fluency-oriented reading instruction. J Literacy Res. 37:25–60. [Google Scholar]
- Tachibana K, Suzuki K, Mori E, Miura N, Kawashima R, Horie K, Sato S, Tanji J, Mushiake H. 2009. Neural activity in the human brain signals logical rule identification. J Neurophysiol. 102:1526–1537. 10.1152/jn.90659.2008 [DOI] [PubMed] [Google Scholar]
- Temple E. 2002. Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol. 12:178–183. 10.1016/S0959-4388(02)00303-3 [DOI] [PubMed] [Google Scholar]
- Thaler V, Ebner EM, Wimmer H, Landerl K. 2004. Training reading fluency in dysfluent readers with high reading accuracy: word specific effects but low transfer to untrained words. Ann Dyslexia. 54:89–113. 10.1007/s11881-004-0005-0 [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JD, Glover GH. 2005. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage. 25:824–837. 10.1016/j.neuroimage.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Alexander AW, Wagner RK, Rashotte CA, Voeller KK, Conway T. 2001. Intensive remedial instruction for children with severe reading disabilities: immediate and long-term outcomes from two instructional approaches. J Learn Disabil. 34:33–58, 78. 10.1177/002221940103400104 [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. 1999. Test of word reading efficiency (TOWRE). Austin: (TX: ): PRO-ED. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. 2003. Development of neural mechanisms for reading. Nat Neurosci. 6:767–773. 10.1038/nn1065 [DOI] [PubMed] [Google Scholar]
- Vagharchakian L, Dehaene-Lambertz G, Pallier C, Dehaene S. 2012. A temporal bottleneck in the language comprehension network. J Neurosci. 32:9089–9102. 10.1523/JNEUROSCI.5685-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmuller J, Kronbichler M, Loenneker T, Klaver P, Martin E, et al. 2009. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 47:1940–1949. 10.1016/j.neuroimage.2009.05.021 [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. 2006. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 30:1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. 2007. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 55:143–156. 10.1016/j.neuron.2007.05.031 [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. 1999. Comprehensive test of phonological processing. Austin: (TX: ): PRO-ED. [Google Scholar]
- Wolf M, Denckla MB. 2005. RAN/RAS: rapid automatized naming and rapid alternating. Austin: (TX: ): PRO-ED, Inc. [Google Scholar]
- Wolf M, Katzir-Cohen T. 2001. Reading fluency and its intervention. Sci Stud Read. 5:211–239. 10.1207/S1532799XSSR0503_2 [DOI] [Google Scholar]
- Wong ACN, Palmeri TJ, Rogers BP, Gore JC, Gauthier I. 2009. Beyond shape: how you learn about objects affects how they are represented in visual cortex. PLoS One. 4:e8405 10.1371/journal.pone.0008405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. 1987. Woodcock reading mastery tests—revised/normative update. Bloomingtion: (MN: ): Pearson Assessment. [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. 2012. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 125:146–155. 10.1016/j.bandl.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Bowers P, MacKinnon G. 1996. Effects of prosodic modeling and repeated reading on poor readers' fluency and comprehension. Appl Psycholinguist. 17:59–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.