Abstract
Savings is a fundamental property of learning. In motor adaptation, it refers to the improvement in learning observed when adaptation to a perturbation A (A1) is followed by re-adaptation to the same perturbation (A2). A common procedure to equate the initial level of error across sessions consists of restoring native sensorimotor coordinates by inserting null—unperturbed—trials (N) just before re-adaptation (washout). Here, we hypothesized that the washout is not innocuous but interferes with the expression of the new memory at recall. To assess this possibility, we measured savings following the A1NA2 protocol, where A was a 40° visual rotation. In Experiment 1, we increased the time window between N and A2 from 1 min to 24 h. This manipulation increased the amount of savings during middle to late phases of adaptation, suggesting that N interfered with the retrieval of A. In Experiment 2, we used repetitive TMS to evaluate if this interference was partly mediated by the sensorimotor cortex (SM). We conclude that the washout does not just restore the unperturbed sensorimotor coordinates, but inhibits the expression of the recently acquired visuomotor map through a mechanism involving SM. Our results resemble the phenomenon of extinction in classical conditioning.
Keywords: adaptation, anterograde interference, motor learning, null trials, sensorimotor cortex, transcranial magnetic stimulation
Introduction
Our body changes and so does our environment. The maintenance of accurate motor control despite this variable scenario is possible thanks to the establishment of new sensorimotor maps through adaptation learning. Motor adaptation has been studied extensively in the laboratory using experimental paradigms in which the visual or the proprioceptive feedback is altered during reaching. A typical experiment involves a session of familiarization during which subjects move to a visual target in native—unperturbed—sensorimotor coordinates (null trials), followed by an adaptation session to a visual or a force-field perturbation. Adaptation is characterized by an initial increment in motor error that subsides with practice, as the discrepancy between the predicted and the experienced error decreases, and the desired movement trajectory is attained (Shadmehr and Mussa-Ivaldi 1994). Retention of the acquired sensorimotor map over months, together with the improvement in learning upon subsequent encounters with the perturbation (savings), are consistent with the formation of long-term motor memories (Shadmehr and Brashers-Krug 1997; Della-Maggiore and McIntosh 2005). Neurophysiological and neuroimaging studies point to the primary motor cortex (M1) as a key cortical node of the motor network where sensorimotor maps may be stored (Li et al. 2001; Paz et al. 2003; Richardson et al. 2006; Landi et al. 2011).
In a typical savings protocol, adaptation to a perturbation A (A1) produces aftereffects that influence the initial level of error during re-adaptation to the same perturbation (A2). One experimental strategy to quantify savings independently from the level of retention of A has been to eliminate aftereffects from A1 by introducing null trials (N) immediately before A2 (e.g., Zarahn et al. 2008). This protocol (A1NA2) aims at restoring the native sensorimotor coordinates by bringing error down to baseline levels (Krakauer et al. 2005). Although introducing null trials before re-adaptation (washout) has proved successful at equating the initial level of error across training sessions, a couple of studies suggest it may hinder the occurrence of savings (Krakauer et al. 2005; Caithness et al. 2004).
Thus, it is possible that bringing behavior down to baseline levels does not just restore the native sensorimotor coordinates as previously thought but interferes with the expression of pre-existing memories at recall. Our hypothesis finds support in the classical conditioning literature. It has long been known that extinction does not destroy the original associative memory but temporally inhibits its expression returning spontaneously after a time interval (Rescorla 2004). In this study, we addressed this possibility through the A1NA2 protocol, where null trials are interposed between adaptation and re-adaptation to a 40° visual rotation. In Experiment 1, we examined if null trials interfered with the retrieval of the memory for the perturbation by manipulating the time elapsed between N and A2. To evaluate the possibility that interference may take place in the primary motor cortex and/or anatomically connected areas, we conducted Experiment 2 in which we used repetitive transcranial magnetic stimulation (rTMS) to prevent anterograde effects induced by the washout. We predicted that both experimental manipulations would release the memory for the new visuomotor map from interference thereby increasing the amount of savings.
Materials and Methods
Participants
Seventy-eight healthy right-handed subjects between 19 and 35 years old (26 males, mean ± SD: 25 ± 4) with no history of neurological or psychiatric disorders agreed to participate in the study. The experimental procedure was approved by the local Ethics Committee and carried out according to the Declaration of Helsinki.
Experimental Paradigm
Subjects were seated on a comfortable chair and performed a center-out-back task using a joystick operated with the thumb and index fingers of their right hand. The right elbow laid comfortably on the armrest whereas the wrist laid on a structure that fixated the joystick over a desktop. Subjects were told to maintain the same wrist posture across experimental sessions. Vision of the hand was occluded throughout the study. At the beginning of each trial, 8 potential targets (empty circles of 0.4 cm diameter) were displayed on a computer screen. Targets were located 2 cm from the start point and 45° from each other, in a concentric manner. Joystick position was represented on the screen with a gray cursor of the same size as the target. The gain of the joystick was set to 1.39 based on previous pilot tests (n = 6 subjects, data not shown) to avoid the occurrence of online corrections. Specifically, a displacement of 1.44 cm of the tip of the joystick moved the cursor on the screen by 2 cm. A trial began when 1 of the 8 potential targets was filled. There were 8 targets per cycle and 11 cycles per block. The order of target presentation was randomized within each cycle. Participants were instructed to make shooting movements through the targets starting as fast as possible from target onset.
There were 3 types of trials of which their occurrence varied depending on the session and the experiment. During null trials, participants performed shooting movements in the absence of a perturbation. During perturbed trials, a clockwise visual rotation of 40° was applied suddenly. Finally, during error-free trials visual feedback was manipulated to provide fake “straight” paths (angular deviation from target = 0 ± 10°; mean ± SD). The latter allowed estimating the internal state of the motor system while precluding subjects from perceiving their own errors and thus adjusting their aftereffects (Criscimagna-Hemminger and Shadmehr 2008). Feedback on aiming error was provided on each trial through the color of the cursor, which varied following a gradient between red (miss) and green (hit). Displayed feedback also took into consideration the time elapsed between target onset and the time when the target was reached (i.e., reaction time + movement time [RTMT]). Whenever RTMT was above 900 ms, the feedback provided in that trial was red even if it was a hit. In addition, hits with error <2.5° and RTMT < 900 ms were rewarded with a shooting sound. On average, movement time for correct trials was 125.5 ± 26.6 ms, consistent with the duration of an open-loop movement. The total score was displayed at the end of each block. The task was programmed using Matlab's Psychophysics Toolbox, Version 3 (Brainard 1997).
Experimental Procedure
Experiment 1
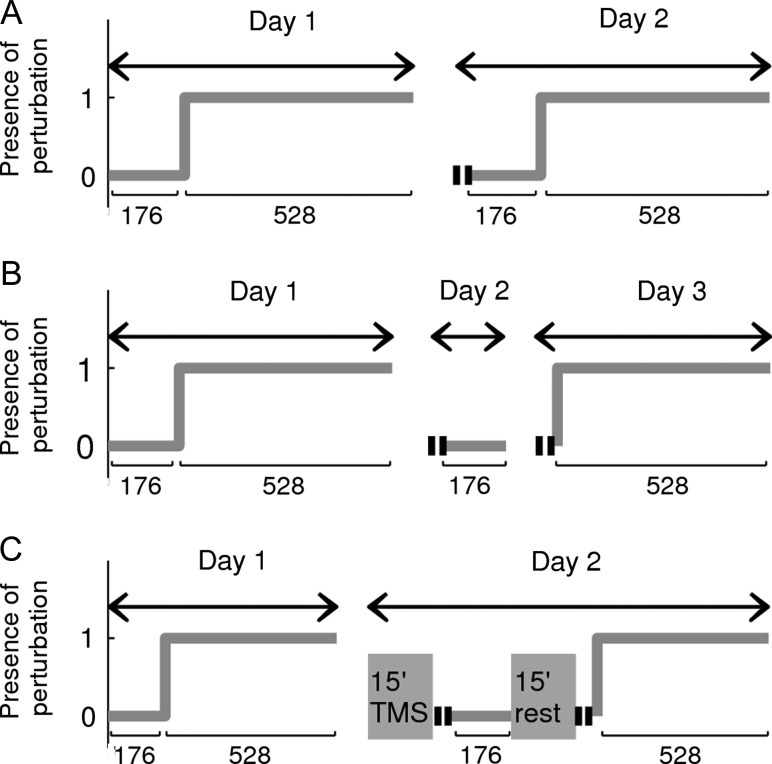
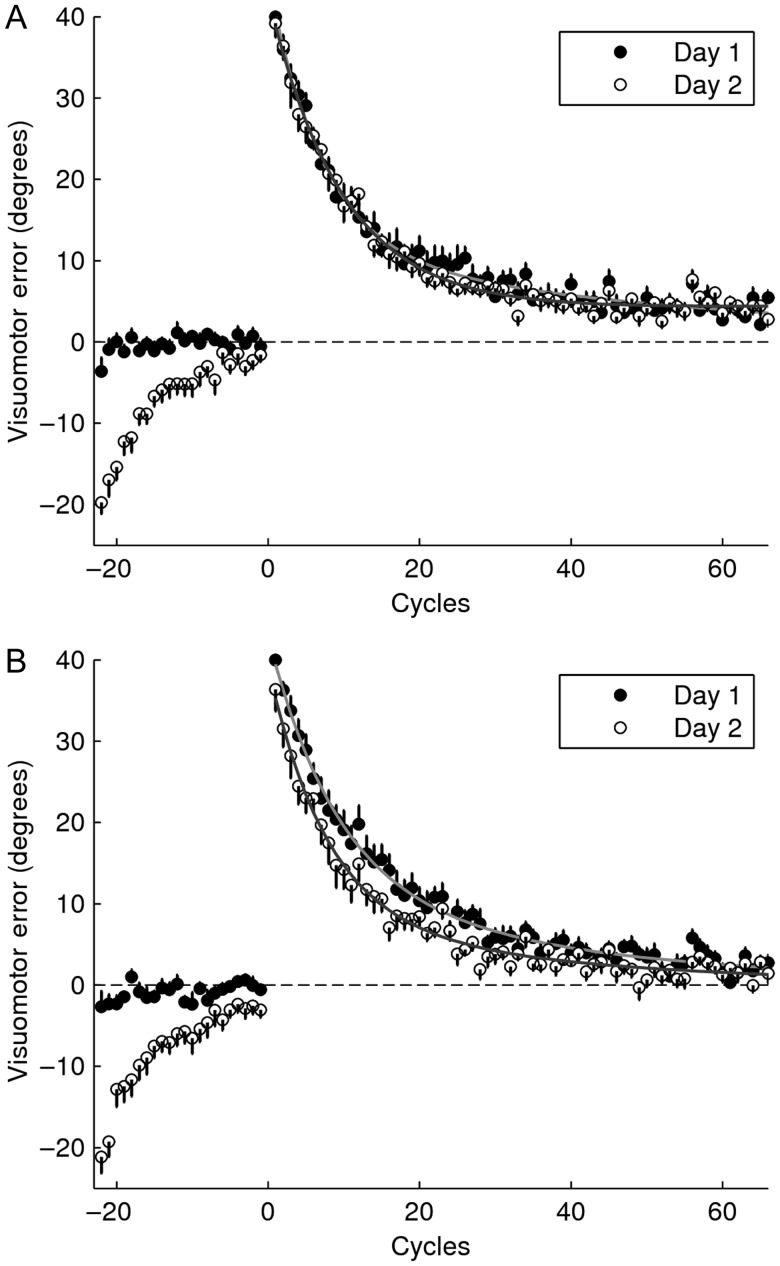
This experiment was designed to examine whether inserting null trials just before re-adaptation interferes with memory retrieval. Forty-eight subjects were randomly assigned to a control group (n = 24) or an experimental group (n = 24). The experimental design is shown in Figure 1. The control group (Fig. 1A) followed the typical experimental protocol used in the literature to assess the occurrence of savings (Krakauer et al. 2005; Zarahn et al. 2008), in which null trials (N) are inserted between adaptation and re-adaptation to a perturbation A. For practicality, we will refer to this group as A1NA2 throughout the manuscript. Specifically, subjects performed 2 blocks of null trials (baseline) followed by 6 blocks of perturbed trials (adaptation) on day 1. On day 2, 24 h later, they were exposed to 2 cycles of error-free trials followed by 2 blocks of null trials (washout) and 6 blocks of perturbed trials (re-adaptation). The experimental group (Fig. 1B) was exposed to the same training schedule with one difference: A 24-h interval was interposed between the end of the washout and re-adaptation. In addition, 2 cycles of error-free trials were introduced before re-adaptation on day 3 to assess the occurrence of spontaneous recovery (SR) (see below). For practicality, we will refer to the experimental group as A1NA2-24 h.
Figure 1.
Experimental design. Shown is the experimental design for Experiment 1 (A,B) and Experiment 2 (C). Each plot indicates the presence (1) or absence (0) of the perturbation as a function of the number of trials. (A) On day 1, control subjects of Experiment 1 (A1NA2) were exposed to 2 blocks of null trials (baseline) followed by 6 blocks of a 40° clockwise rotation. On day 2, they were exposed to 2 blocks of null trials (washout), followed by 6 blocks of the same clockwise rotation (re-adaptation). (B) Like control subjects, experimental subjects (A1NA2-24 h) were washed out on day 2 but re-adapted 24 h later, on day 3. (C) Sham and experimental subjects of Experiment 2 followed the same protocol as A1NA2, but before the washout were exposed to 15 min of rTMS over the sensorimotor cortex (A1NA2-M1) or the vertex (A1NA2-Sham). After the washout, there was a 15-min period to prevent the spread of rTMS effects into re-adaptation. Error-free trials are indicated by 2 parallel segments (||).
Experiment 2
This experiment was conducted to examine if the interference on memory retrieval induced by the washout was mediated by the primary motor cortex and/or anatomically connected areas. For this purpose, 30 subjects were recruited for this study (n = 16 experimental and n = 14 sham subjects). Repetitive TMS was applied over the region of the scalp covering the representation of the hand area of the left primary motor cortex for the experimental group and over the vertex for the sham group. Given that the effects of TMS can spread beyond M1 into sensory and premotor regions, we would refer to the stimulated region as the sensorimotor cortex (SM). Yet, for practicality, we will call the experimental group as A1NA2-M1 and the control group as A1NA2-Sham. Both groups followed the experimental protocol used in A1NA2 (Fig. 1C) with 2 differences: 1) rTMS was applied offline over the target region immediately before the washout and 2) after washout there was a 15-min interval to prevent the effects of rTMS from spreading into the re-adaptation session (Bolognini and Ro 2010). The total time elapsed between the end of rTMS and re-adaptation (preceded by 2 cycles of error-free trials) was ∼30 min.
Transcranial Magnetic Stimulation
It is now well established that low-frequency rTMS applied during 15 min over the SM increases the motor threshold and depresses corticospinal excitability (Chen et al. 1997; Romero et al. 2002). This effect lasts ∼20 min and disappears 30 min poststimulation (Bolognini and Ro 2010). With the aim of releasing SM from the interference caused by the washout, 1 Hz rTMS was applied for 15 min over the representation of the first dorsal interosseous (FDI) of the right hand at an intensity of 90% of the resting motor threshold (rMT). This muscle, which is necessary to control the joystick with the index and thumb, has a large representation in the cortex and is easily recruited with TMS. This rTMS protocol has been previously used by Richardson and collaborators (2006) to interfere with motor memory consolidation during force-field adaptation. TMS pulses were delivered with an rTMS Magstim Rapid2 single PSU unit through a 70-mm figure-of-eight coil (Magstim, Whitland, UK) immediately before the washout. Subjects were seated on a reclining chair and rested their head on a neck rest, which restrained it from moving sideways. Head movements forward were unlikely since the subject was reclined. Earplugs were provided to protect their hearing. In A1NA2-Sham the coil was placed with the lateral edge perpendicular to the Vertex and the handle pointing backwards. In A1NA2-M1, the coil was positioned tangentially over the optimal scalp location of the left motor cortex, with the handle pointing backwards at 45° from the midline. To determine the motor threshold, superficial cup electrodes were placed following a belly-tendon mount over the FDI of the right hand. Electromyographical recordings were obtained using an AC amplifier (P5 series, Grass Instruments, USA) with a bandwidth between 10 and 1000 Hz. The signal was digitized at 5 kHz (National Instruments A/D card, Austin, TX, USA) and acquired in a PC using a script written in LabView (LabView 8.1, National Instruments). The optimal scalp position to stimulate the primary motor cortex was identified as that inducing the largest motor evoked potentials (MEPs) from the FDI. rMT was determined as the stimulation intensity that evoked 5 out of 10 MEPs ≥ 50 µV. Once over the hotspot, we fixed the coil in place using an articulated arm (Manfrotto, Venice, Italy), and marked the coil position over the scalp using a soft-tip pen. Before starting the experiment, we told participants to remain as still as possible throughout the 15 min of rTMS and until after a short test was conducted. This consisted in delivering 5 TMS pulses at an intensity of 120% rMT before and after rTMS to verify further coil position. MEPs can be elicited from nearby sites such as the premotor cortex. However, the intensity required to do so is much higher (e.g., Fridman et al. 2004). We reasoned that if the coil moved to neighboring areas, MEP amplitudes would drop significantly compared with prevalues (i.e., more than expected due to the manipulation of corticospinal excitability). Two participants were excluded following this criterion because they were unable to elicit 4 out of 5 MEPs of at least one-third of its prevalue amplitude (n = 2 from the Experimental group).
Data Analysis
Data analysis was carried out using custom scripts written in Matlab (Mathworks, MA, USA). Visuomotor error was computed for each trial as the angle defined by the line connecting the start position to the target and the line connecting the start position to the final cursor position. The level of error achieved during the second baseline block was subtracted from each subject. Subsequently, data for each learning session were normalized to the first cycle of A1 to reduce intersubject variability in performance. Subjects were considered outliers if the time course of the error achieved during adaptation or re-adaptation did not adjust to a double exponential function (coefficient of determination R2 < 0.1). This criterion was chosen to eliminate those subjects who used an explicit strategy to perform the task during initial practice, which would hinder the estimation of savings. Following this criterion, one subject was eliminated from Experiment 1 and one from Experiment 2.
SR, that is, the re-emergence of the memory for the perturbation after being washed out, was assessed based on error-free trials using the following equation:
| (1) |
where EF1 and EF2 indicate the average of the 2 cycles of error-free trials measured immediately before the washout and re-adaptation, respectively, and WO indicates the average of the last 2 cycles of the second washout block. SR was not computed for A1NA2 because only EF1 was measured. Due to technical difficulties, this group did not perform EF2.
Given that both experimental groups exhibited improvement in learning during initial and late stages of training, savings was computed based on 2 different methods. Savings during the initial phase of learning was computed based on the difference in the rate of learning across sessions. For this purpose, the visuomotor error for the first 11 cycles (Krakauer et al. 2005) of adaptation and re-adaptation was fit for each individual with a single exponential function, and the rate coefficients were subtracted (re-adaptation – adaptation). Differences larger than 0 indicated savings.
To quantify savings beyond the initial portion of training a different method based on the cumulative percent increment in learning from adaptation was used (e.g., Krakauer et al. 2005; Sing and Smith 2010). This method is more sensitive to measure additional improvement in learning later in training, as differences in slope become small or undetectable. We used the following equation modified from Sing and Smith (2010):
| (2) |
where S(n) represents the amount of savings accumulated from cycles 1 through n, and A1(i) and A2(i) represent the visuomotor error for the ith cycle of adaptation and re-adaptation, respectively. This metric allowed examining the progression of savings as a function of the number of cycles included (n = 1, 2, … , 66; S(1), S(2), … , S(66)). One limitation of this metric, is that it can be biased by differences in the initial level of error across sessions. Due to the experimental manipulation, the initial level of error was sometimes larger in A1 than in A2. This was the case for the first cycle of A1NA2-24 h. With the aim of assessing savings independently of SR (see Results), we corrected for this misalignment across sessions. Note that although the correction involved removing data, not adjusting for differences in the initial level of error would have led to an overestimation of savings for A1NA2-24 h, thereby biasing our effect of interest. Thus, before computing savings based on eq. 2, we removed the first cycle of A1 for that group and shifted the curve to the left so that the new first cycle of A1 matched the corresponding value of A2 (Sing and Smith 2010). Finally, to compensate for the difference in the total number of trials, the last cycle of A2 was excluded.
Statistics
Statistical differences were assessed at the 95% level of confidence (α = 0.05). Statistical differences in savings measured based on the initial rate of learning were assessed using t-tests. To assess group differences in savings measured using eq. 2, repeated-measures ANOVA was implemented with the Greenhouse–Geisser correction to adjust for autocorrelations. Additional statistical analyses to assess differences in the initial level of error or the final level of washout were carried out using ANOVA, one-sample, paired, or 2-sample t-tests as required.
Results
Experiment 1
Experiment 1 was designed to test whether inserting null trials just before re-adaptation interfered with memory retrieval. We predicted that interposing a 24-h interval between the washout and re-adaptation would weaken any interfering effects, thereby increasing the amount of savings. All subjects completed the experiment. Only one participant was not included in the analysis because he/she used an explicit strategy. Thus, results are reported on 23 control (A1NA2) and 24 experimental (A1NA2-24 h) subjects.
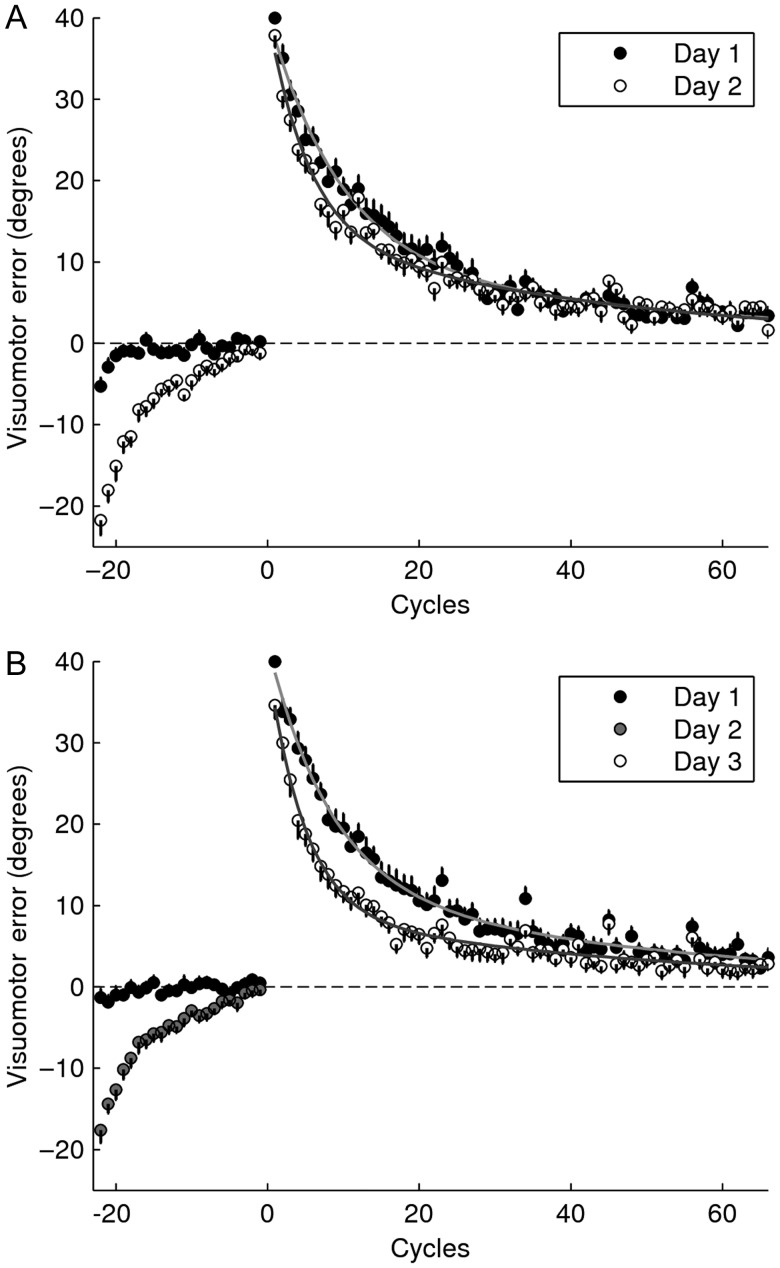
Figure 2 depicts the learning curves for both groups. As indicated by the initial portion of the plot, both groups approached baseline by the end of the second block of null trials (comparison of last 2 cycles of washout with last 2 cycles of baseline, A1NA2: P = 0.22; A1NA2-24h: P = 0.28, paired t-tests). Moreover, there was no difference in the level of washout achieved across groups (P = 0.72, two-sample t-test), suggesting that any group difference in the level of savings was attributable to the experimental manipulation.
Figure 2.
Learning curves for Experiment 1. Mean visuomotor error ± SE are shown as a function of cycle (one cycle = average of 8 trials) for the adaptation and re-adaptation sessions. (A) A1NA2, (B) A1NA2-24 h. The curves resulting from fitting the data with a double exponential function are displayed for the adaptation and re-adaptation sessions.
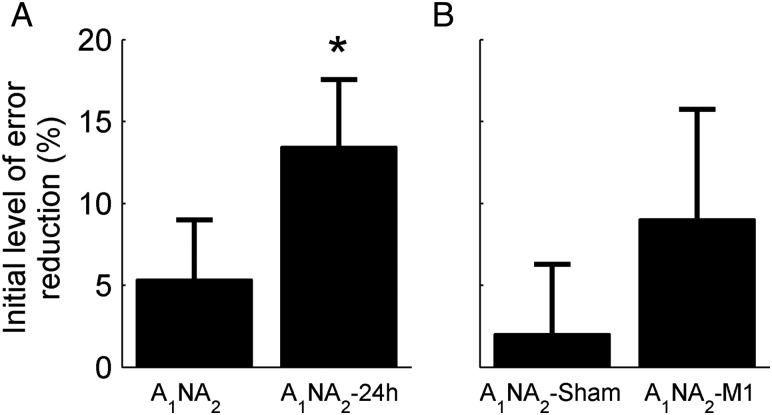
Savings, measured as the improvement in the initial rate of learning computed by fitting the first 11 cycles with a single exponential function, was evident in A1NA2-24 h (mean ± SE, slope(A2) – slope(A1) = 0.06 ± 0.01; P < 0.01; paired t-test) but marginally significant in A1NA2 (mean ± SE, slope(A2) – slope(A1) = 0.03 ± 0.01; P = 0.07; paired t-test. No statistical differences were found across groups (P = 0.12, two-sample t-test). Results are summarized in Table 1. Similar results were obtained based on the cumulative percent increment in learning across the first 11 cycles (eq. 2, n = 11). Savings was observed both for A1NA2 (S(11) = 12.54 ± 4.07%, mean ± SE; P < 0.01, one-sample t-test) and for A1NA2-24 h (S(11) = 22.12 ± 4.55%, mean ± SE; P < 0.01, one-sample t-test). No statistical differences were found across groups (P = 0.12, two-sample t-test). Given that A1NA2-24 h presented significant differences in the initial level of error across training sessions (Fig. 3A; P < 0.01, paired t-test for the first cycle) the first cycle of A1 was excluded from this analysis (paired t-test postcorrection: P = 0.74). The convergence of these 2 different methods suggests that, once corrected for differences in the initial level of error, the metric based on equation 2 can measure savings accurately and independently of SR.
Table 1.
Parameters of the single exponential fittings used to compute savings
| α1 (deg) | β1 (cycle−1) | α2 (deg) | β2 (cycle−1) | β2–β1 (cycle−1) | Within group comparison | |
|---|---|---|---|---|---|---|
| Experiment 1 | ||||||
| A1NA2 | 39.03 ± 0.48 | 0.10 ± 0.01 | 36.61 ± 1.36 | 0.12 ± 0.02 | 0.03 ± 0.01 | P = 0.07 |
| A1NA2-24h | 40.40 ± 0.67 | 0.10 ± 0.01 | 35.00 ± 2.05 | 0.16 ± 0.02 | 0.06 ± 0.01 | P < 0.01 |
| Between groups comparison | – | P = 0.87 | – | P = 0.20 | P = 0.12 | – |
| Experiment 2 | ||||||
| A1NA2-Sham | 39.99 ± 0.83 | 0.10 ± 0.01 | 40.32 ± 1.52 | 0.09 ± 0.01 | −0.01 ± 0.01 | P = 0.29 |
| A1NA2-M1 | 40.11 ± 0.74 | 0.09 ± 0.01 | 37.71 ± 1.65 | 0.12 ± 0.02 | 0.03 ± 0.01 | P = 0.05 |
| Between groups comparison | – | P = 0.76 | – | P = 0.20 | P = 0.03 | – |
Shown are the coefficients obtained from fitting the first 11 cycles of A1 and A2 with a single exponential function. Statistical comparisons within and between groups are reported. α1 and α2 refer to the Y intercept for A1 and A2, respectively. β1 and β2 refer to the slope for A1 and A2, respectively. β2–β1 refer to savings.
Figure 3.
Initial level of error reduction. Shown is the mean ± SE percent reduction in the initial visuomotor error (first cycle) across learning sessions for Experiment 1 (A) and Experiment 2 (B). Only the experimental group of Experiment 1 (A1NA2-24 h) showed a significant increment in the initial level of error reduction as indicated by an asterisk (*P < 0.01).
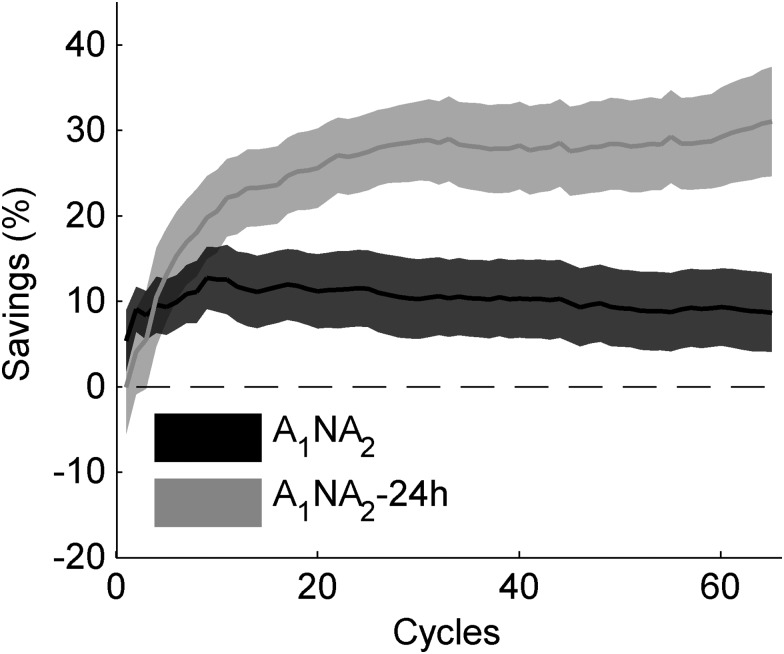
Given that the improvement in learning observed for A1NA2-24 h appeared to endure beyond the initial phase of adaptation (see Fig. 2B), we examined the progression of savings as a function of the number of cycles (n) included in the analysis (eq. 2: S(1), S(2), … , S(65)). As depicted by Figure 4, savings for A1NA2 increased during the early portion of learning (up to cycle 11) but decreased thereafter, whereas savings for A1NA2-24 h increased throughout the whole learning session (group-by-cycle interaction P < 0.01, repeated-measures ANOVA with Greenhouse–Geisser correction). In fact, the amount of savings yielded by all 65 cycles was much stronger for the experimental than for the control group (mean ± SE: S(65) for A1NA2-24 h = 34.94 ± 6.09%, P < 0.01, one-sample t-test; S(65) for A1NA2 = 8.88 ± 4.62%, P = 0.07, one-sample t-test; A1NA2-24 h vs. A1NA2: P < 0.01, two-sample t-test).
Figure 4.
Progression of savings as a function of cycle for Experiment 1. (A) Savings (S) was computed as the cumulative percent increment in learning from adaptation, according to equation 2. Shown are the mean ± SE of S for each cycle of the experimental (A1NA2-24 h) and the control (A1NA2) group. Note that A1NA2-24 h starts from zero because the first cycle was removed by the correction.
Finally, we assessed whether the experimental manipulation led to SR of the memory for the perturbation. We hypothesized that, despite the memory decline associated with interposing a 24-h interval in A1NA2-24 h (see Krakauer et al. 2005), release from interference would result in SR. SR (eq. 1) was 24.89 ± 11.62% (mean ± SE), which was significantly different from 0 (P < 0.01, one-sample t-test). This result was consistent with the large difference in the initial level of error observed for this group (see Figs 2A and 3A).
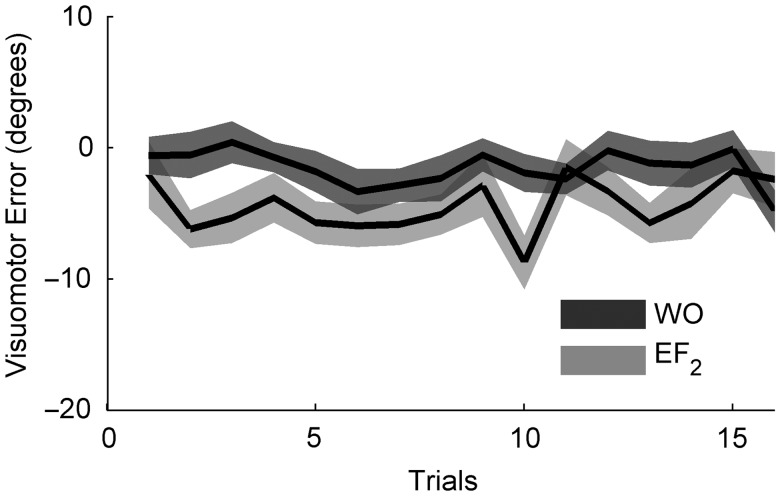
SR could not be assessed for A1NA2 given that only one measure of error-free trials was recorded before the washout (EF1). Due to the close proximity between the end of the washout and EF2 (1 min) we expected little or no SR for the control group (see eq. 1). However, the lack of these data for the control group opens the possibility that increased savings in A1NA2-24 h could have resulted from the reactivation of the memory for A during EF2. To rule out this option, we examined the 16 EF2 trials of A1NA2-24 h for possible signs of memory reactivation. Figure 5 shows the corresponding time course of visuomotor error. Neither the results of t-test comparing first vs. last trial of EF2 (P = 0.99) nor the linear fitting of all 16 trials of EF2 (slope (mean ± SE) = −0.002 ± 0.002; P = 0.34), showed evidence of a linear reactivation of the memory for A during error-free trials. Moreover, no obvious evidence of sustained nonlinear changes indicative of memory reactivation was detectable at the eye level (for an example of memory reactivation, see Fig. 6A of Pekny et al. 2011). Furthermore, comparison of the 16 trials of EF2 to the last 16 trials of the washout (see Fig. 5) suggests that SR in A1NA2-24 h took place sometime during the 24-h interval elapsed between the 2 events (paired t-test comparing all 16 cycles of the washout vs. all 16 cycles of EF2: P < 0.01). Therefore, despite lacking the corresponding data for A1NA2, this analysis rules out the possibility that savings in A1NA2-24 h could be explained by memory reactivation during EF2.
Figure 5.
Time course of EF2 and the end of the washout for A1NA2-24 h. Shown is the time course of the mean ± SE of the visuomotor error for the last 16 trials of the washout (WO) and the 16 trials of the second set of error-free trials (EF2).
Experiment 2
Experiment 2 was designed to assess if the interfering effect of the washout was mediated by the primary motor cortex and/or anatomically connected structures. This hypothesis was based on previous rTMS evidence pointing to SM as a key region mediating anterograde interference during adaptation to opposite force-field perturbations (Cothros et al. 2006). We predicted that the application of rTMS would release the memory of A from the interference induced by the washout.
Two out of 30 subjects were not included in the analysis because the verification procedure indicated they had moved during rTMS. In addition, one participant from A1NA2-Sham was discarded because he/she used explicit strategies to learn the task. Thus, results are reported on 13 sham and 14 experimental subjects. As indicated by Figure 6, both groups approached baseline by the end of the second block of null trials (comparison of last 2 cycles of washout vs. last 2 cycles of baseline, A1NA2-M1: P = 0.07; A1NA2-Sham: P = 0.12). Given that A1NA2-M1 approached significance, we contrasted the final level of washout across groups. No significant differences were found (P = 0.86, two-sample t-test), suggesting that any group difference in the level of savings was attributable to the experimental manipulation. The lack of a TMS effect over the final level of the washout is consistent with previous reports indicating no effect of the procedure on motor performance (Muellbacher et al. 2002; Richardson et al. 2006).
Figure 6.
Learning curves for Experiment 2. Mean visuomotor error ± SE are shown as a function of cycle (1 cycle = average of 8 trials) for the adaptation and re-adaptation sessions. (A) A1NA2-sham, (B) A1NA2-M1. The curves resulting from fitting the data with a double exponential function are displayed for the adaptation and re-adaptation sessions.
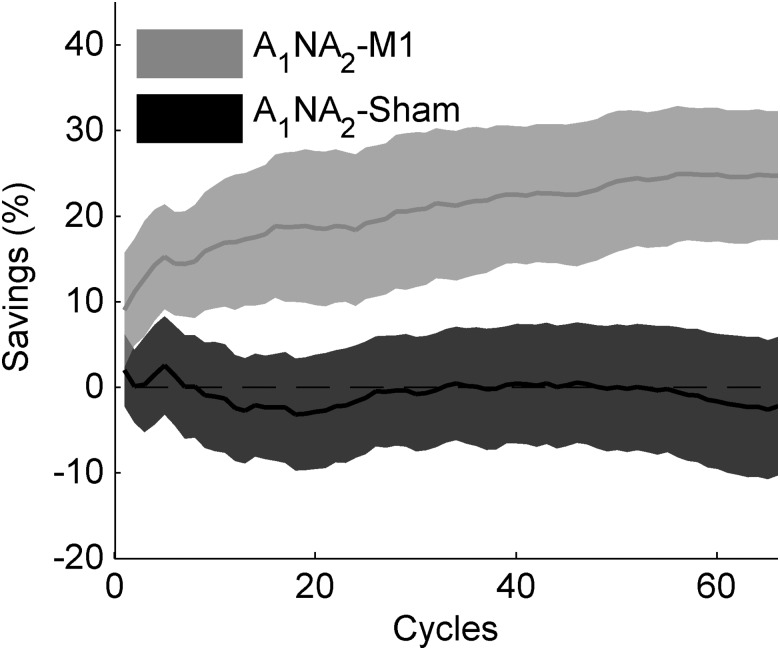
Figure 6 depicts the learning curves for both groups. Savings measured as the improvement in the initial rate of learning computed by fitting the first 11 cycles with a single exponential function, was present in A1NA2-M1 (mean ± SE, slope(A2) – slope(A1) = 0.025 ± 0.012; P = 0.05; paired t-test) but not in A1NA2-Sham (mean ± SE, slope(A2) – slope(A1) = −0.009 ± 0.008; P = 0.29; paired t-test). This difference was statistically significant across groups (A1NA2-M1 vs. A1NA2-Sham: P = 0.03, two-sample t-test). Results are summarized in Table 1. Similarly, savings measured as the cumulative percent increment in learning during the first 11 cycles (n = 11 in eq. 2) was observed for A1NA2-M1 (S(11) = 16.92 ± 7.48%, mean ± SE; P = 0.04, one-sample t-test) but not for A1NA2-Sham (S(11) = −1.3 ± 6.34%, mean ± SE; P = 0.84, one-sample t-test). Statistical comparison across groups was marginally significance (P = 0.08, two-sample t-test). Correction for differences in the initial level of error was not applied for this Experiment (see Fig. 3B), as statistical assessment yielded no significant differences across sessions for either group (A1NA2-M1, P = 0.398; A1NA2-Sham, P = 0.557, paired t-test).
Examination of the progression of savings as a function of the number of cycles included in the analysis (Fig. 7) indicated that learning improved steadily for A1NA2-M1 throughout the whole training session, whereas A1NA2-Sham showed no evidence of savings at any time (group-by-cycle interaction P < 0.05, repeated-measures ANOVA with Greenhauser–Geisser correction). In fact, the amount of savings yielded by all 66 cycles was stronger for the experimental than for the sham group (mean ± SE: S(66) for A1NA2-M1 = 24.73 ± 7.52%, P < 0.01, one-sample t-test; S(66) for A1NA2-Sham = −2.19 ± 8.09%, P = 0.79, one-sample t-test; A1NA2-M1 vs. A1NA2-Sham, P = 0.02, two-sample t-test).
Figure 7.
Progression of Savings as a function of cycle for Experiment 2. (A) Savings (S) was computed as the cumulative percent increment in learning from adaptation, according to equation 2. Shown are the mean ± SE of S for each cycle of the experimental (A1NA2-M1) and the control (A1NA2-Sham) group.
Statistical assessment of SR for Experiment 2 yielded no significant differences in either group (mean SR ± SE: A1NA2-M1 = 1.72 ± 5.86%, P = 0.77, one-sample t-test; A1NA2-Sham = −4.81 ± 6.12%, P = 0.45, one-sample t-test). These results are in agreement with the similarity in the initial level of error observed across groups (see Fig. 3B).
Discussion
Savings refers to the ability of previous learning to improve future learning (Sing and Smith 2010). A common procedure to measure savings in motor adaptation consists of inserting null trials immediately before re-adaptation to bring motor error down to baseline levels. Here, we examined the possibility that null trials interfere with the retrieval of the memory for a new visuomotor map, thereby reducing the level of savings. For this purpose, we measured savings using the A1NA2 protocol in which null trials are interposed between adaptation and re-adaptation. In Experiment 1, we manipulated the time window elapsed between the washout and re-adaptation from 1 min to 24 h to allow the decay of anterograde effects. Separating these events by 24 h increased the amount of savings significantly during middle to late phases of adaptation. In Experiment 2, we examined whether this interference took place at least partly in the primary motor cortex, a region implicated in the storage of motor memories for this type of learning. The application of rTMS before the washout led to an increase in savings suggesting release from interference. Our results demonstrate for the first time, that inserting null trials before re-adaptation does not just restore the unperturbed sensorimotor coordinates but inhibits the expression of the recently acquired visuomotor map through a mechanism involving the primary motor cortex.
The insertion of null trials before re-adaptation has also been implemented to eliminate unwanted anterograde effects in protocols where retrograde interference is used to unveil the time course of memory consolidation (Shadmehr and Brashers-Krug 1997; Krakauer et al. 2005; Zarahn et al. 2008). Specifically, adaptation to a perturbation A (A1) followed by adaptation to the opposite perturbation B and subsequent re-adaptation to A (A2) often results in retrograde interference from B to A1, leading to the reduction of savings (Brashers-Krug et al. 1996; Krakauer et al. 2005). Yet, B often interferes with the learning of A2 leading to an increment in the initial error and/or reduced savings. Even though anterograde effects tend to decay with the passage of time, in visuomotor adaptation, this phenomenon may persist even after a 24-h interval, thereby masking any retrograde effects of interest (Caithness et al. 2004; Krakauer et al. 2005). Our results suggest that, like counter perturbations, null trials can also interfere with future learning. In contrast with previous studies, we found that 24 h were enough to achieve, at least in part, release from interference. This may be due to the fact that only 2 blocks of null trials were inserted before re-adaptation (the necessary amount to washout). Thus, it is likely that using as many blocks of null and perturbed trials (6 blocks) would have led to even stronger and probably, longer-lasting interference.
One may wonder if our results can be explained by a retrograde effect of null trials over the consolidation of the memory for the perturbation. We have not directly addressed this possibility here. Yet, the available evidence suggests that consolidation for proprioceptive and visual perturbations appears to be completed within 24 h (Shadmehr and Brashers-Krug 1997; Krakauer et al. 2005), the time interval that separated adaptation from the washout. If, notwithstanding, a residual retrograde effect had taken place, it would have affected equally experimental and control groups. Despite this possibility, the fact that savings increased significantly for both experimental groups points to an anterograde effect underlying our findings.
Our results find echo in the classical conditioning literature. Associations formed by pairing a conditioning stimulus (CS) to an unconditioned stimulus (US) can be extinguished after several presentations of the CS in the absence of the US. Today, it is known that despite the drastic reduction in memory retention evident at the behavioral level, extinction does not destroy the original memory but temporally inhibits its expression returning spontaneously after a time interval (Rescorla 2004). The fact that extinction depends on protein synthesis supports the hypothesis that, during this period, synapses are not reset to the initial state but instead new memories are formed (Bouton 2002). The enhancement of savings reported in Experiment 1 together with the occurrence of SR, a requirement for extinction in classical conditioning, suggests the possibility that a similar mechanism may take place in adaptation learning. Like extinction, it is possible that during the washout the association between unperturbed sensorimotor coordinates and the experimental context is strengthened. This association may go unnoticed as usually occurs, unless blocks of perturbed and null trials are juxtaposed in time. If, as implemented here, the washout precedes re-adaptation, then the strengthened association would interfere anterogradely with the retrieval of the memory for the new visuomotor map. Such mechanism could also mediate retrograde interference if the washout follows adaptation. Recent evidence suggests that null trials may indeed interfere retrogradely with the consolidation of the visuomotor map (Hinder et al. 2007).
Our proposal is consistent with the coexistence of multiple memories in the brain, a hypothesis becoming popular in the field of motor adaptation (Krakauer 2009; Pekny et al. 2011; Ogawa and Imamizu 2013; Vaswani and Shadmehr 2013). It has been proposed that anterograde effects of one perturbation on another reflect the competition of their coexisting memories for retrieval (Krakauer 2009). Our findings suggest that contextual associations in unperturbed coordinates may behave similarly to contextual associations in perturbed coordinates. We propose that the interference reported here occurs because, after washout, the memory for the perturbation learned 24 h earlier is no longer associated with the experimental context and therefore cannot be retrieved. The fact that the original memory emerges after an interval of 24 h in the form of SR and increased savings (A1NA2-24 h) suggests that the unperturbed mapping overrides—not overwrites—the memory for the perturbed mapping. This finding is consistent with the coexistence of both representations. In light of our results, the success of null trials at “removing” anterograde interference between counter perturbations (Krakauer et al. 2005) may not stem solely from weakening the association between the interfering perturbation and the experimental context as previously suggested (Krakauer 2009) but also from strengthening the association between the experimental context and native coordinates. The observation that the amount of savings produced in the protocol A1A2 (without a washout) is reduced when null trials precede re-adaptation in the protocol A1NA2 (Krakauer et al. 2005; Krakauer 2009) is consistent with this hypothesis.
What is the neural basis of the anterograde effect reported in Experiment 1? This question was addressed in Experiment 2 using rTMS. The rTMS protocol chosen for our study has been implemented to interfere with the consolidation of force-field adaptation when administered before learning (Richardson et al. 2006). Here, we applied this protocol before the washout to prevent the reinforcement of the association between the experimental context and unperturbed sensorimotor coordinates and thus, release the memory for the perturbation from interference. A previous study has shown that the SM partly mediates anterograde interference during adaptation to opposite force fields (Cothros et al. 2006). The results from Experiment 2 are in agreement with this finding. Remarkably, interference was observed from the start of the session in A1NA2-Sham. It is worth noticing that a 15-min interval was introduced after the washout to avoid the spread of rTMS effects into re-adaptation. Thus, a complete interference could be explained by further stabilization of the association between the experimental context and the unperturbed sensorimotor coordinates during this time period. This is in line with a large body of evidence suggesting that neural events leading to motor memory consolidation begin with practice but continue after training (Albert et al. 2009; see Dayan and Cohen 2011 for a recent review in motor skill learning). Interestingly, a force-field adaptation study carried out by Criscimagna-Hemminger and Shadmehr (2008) indicates that 10 min are sufficient to convert the memory acquired during a similar number of trials (n = 20), from a fragile into a more stable state. The application of rTMS over SM likely prevented the strengthening of the association during the washout thereby increasing the amount of savings throughout the whole learning period.
It is worth noticing that SR was not observed in A1NA2-M1 despite a significant increment in the level of savings compared with A1NA2-Sham. As previously reported the initial level of error, which relates to the measure recorded in error-free trials, is not necessarily linked to the ability of improving subsequent learning (Sing et al. 2009). In fact, the initial level of error appears to reflect the performance or the retention associated with the last learned sensorimotor map (see Sing and Smith 2010). Thus, it is possible that applying an rTMS protocol known to decrease corticospinal excitability, allowed accessing the memory previously masked by the washout resulting in enhanced learning, but had no effect on the level of performance attained toward the end of the washout. Taken together, our results suggest that the representation strengthened during the washout interfered with the retrieval of the memory for the perturbation at the level of the SM and/or anatomically connected structures.
Increasing experimental evidence now links SM to the formation (Li et al. 2001; Hadipour-Niktarash et al. 2007; Orban de Xivry et al. 2010) and the persistence (Landi et al. 2011) of memories acquired during adaptation. Our findings together with Cothro's et al. (2006) and those from a recent fMRI study by Ogawa and Imamizu (2013) suggest further that SM may be the node where multiple memories for different sensorimotor maps coexist. Recent evidence showing that rTMS can block the modification of a reactivated memory trace for a motor sequence suggests that SM may also support multiple memory traces in motor skill learning (Censor et al. 2010). Most importantly, the fact that, in our study, TMS increased savings throughout the whole session, and not just during its initial phase, strengthens the possibility that the motor cortex may be part of a slow process involved in motor memory consolidation (Richardson et al. 2006; Smith et al. 2006; Joiner and Smith 2008; Orban de Xivry et al. 2010; Landi et al. 2011; Censor et al. 2012).
Despite its strengths, there were certain limitations to our study that need to be addressed. First, due to technical difficulties, we were unable to acquire the second set of error-free trials (EF2) for A1NA2. This probably had little impact on the level of SR of this group since the washout and EF2 were too close in time, what would have led to little or no SR (see eq. 1). Yet, there is evidence suggesting that, under particular circumstances, memories can be reactivated during error-free trials (Pekny et al. 2011). Thus, one may wonder if the larger level of savings observed in Experiment 1 for A1NA2-24 h was due to the reactivation of the motor memory for the perturbation during EF2, which was absent in A1NA2. Two findings are against this possibility: 1) the lack of linear and nonlinear trends during EF2 and 2) the absence of SR or savings observed in A1NA2-Sham, which experienced both EF1 and EF2. Furthermore, the data depicted in Figure 5, suggests that SR in A1NA2-24 h took place sometime during the 24 h interval and not during EF2. Second, one may question whether the larger level of savings measured in A1NA2-24 h could be explained by further consolidation of the memory of A during the additional 24 h inserted after the washout. Recent data from our laboratory, using the same experimental paradigm (Caffaro et al. submitted for publication) indicates that memories formed during visuomotor adaptation decay to a 35% after 72 h. This is in line with a reaching study showing that re-adaptation to a visual rotation is faster when tested 24 h (1 night of sleep) than when tested 48 h (2 nights of sleep) after adaptation (see Krakauer et al. 2005, Experiment 4). Thus, had there been no interfering effect of the washout, we would have expected the level of savings in A1NA2-24 h to be even smaller than in A1NA2. The fact that the opposite pattern was found is rather consistent with release from interference of the memory for A. The presence of SR in A1NA2-24 h strengthens our interpretation further, since this phenomenon occurs following an adaptation–extinction training episode (Smith et al. 2006).
In sum, we have shown that, when preceding re-adaptation, null trials can interfere with future learning through a mechanism that takes place, at least in part, in the SM. Our results indicate that the washout not only restores the unperturbed sensorimotor coordinates but interferes with the retrieval of the recently acquired visuomotor map. These findings are reminiscent of the phenomenon of extinction in classical conditioning. We recommend that A1A2 protocols along with an unbiased measure of savings be chosen over A1NA2 protocols to avoid unwanted anterograde effects.
Funding
This work was supported by the Fogarty International Research Collaboration Award of the National Institutes of Health with Dr ST Grafton (R03 TW007815-01A1), the National Agency for the Promotion of Science and Technology of Argentina (PICT-0063), and the University of Buenos Aires (UBACyT-090100157).
Notes
We thank Arturo Romano for his intellectual contribution to this work. Conflict of Interest: None declared.
References
- Albert NB, Robertson EM, Miall RC. 2009. The resting human brain and motor learning. Curr Biol. 19:1023–1027. 10.1016/j.cub.2009.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Ro T. 2010. Transcranial magnetic stimulation: disrupting neural activity to alter and assess brain function. J Neurosci. 30:9647–9650. 10.1523/JNEUROSCI.1990-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. 2002. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 52:976–986. 10.1016/S0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- Brainard DH. 1997. The Psychophysics Toolbox. Spat Vis. 10:433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. 1996. Consolidation in human motor memory. Nature. 382:252–255. 10.1038/382252a0 [DOI] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. 2004. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci. 24:8662–8671. 10.1523/JNEUROSCI.2214-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. 2010. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol. 20:1545–1549. 10.1016/j.cub.2010.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Sagi D, Cohen LG. 2012. Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci. 13(9):658–664. 10.1038/nrn3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. 1997. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 48:1398–1403. 10.1212/WNL.48.5.1398 [DOI] [PubMed] [Google Scholar]
- Cothros N, Kohler S, Dickie EW, Mirsattari SM, Gribble PL. 2006. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci. 18:2167–2176. 10.1162/jocn.2006.18.12.2167 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Shadmehr R. 2008. Consolidation patterns of human motor memory. J Neurosci. 28:9610–9618. 10.1523/JNEUROSCI.3071-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. 2011. Neuroplasticity subserving motor skill learning. Neuron. 72(3):443–454. 10.1016/j.neuron.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, McIntosh AR. 2005. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. J Neurophysiol. 93:2254–2262. 10.1152/jn.00984.2004 [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. 2004. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 127(Pt 4):747–758. 10.1152/jn.00984.2004 [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. 2007. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 27:13413–13419. 10.1523/JNEUROSCI.2570-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Walk L, Woolley DG, Riek S, Carson RG. 2007. The interference effects of non-rotated versus counter-rotated trials in visuomotor adaptation. Exp Brain Res. 180:629–640. 10.1007/s00221-007-0888-1 [DOI] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. 2008. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol. 100:2948–2955. 10.1152/jn.90706.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. 2009. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 629:405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. 2005. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 25:473–478. 10.1523/JNEUROSCI.4218-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi SM, Baguear F, Della-Maggiore V. 2011. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J Neurosci. 31:11808–11813. 10.1523/JNEUROSCI.2253-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. 2001. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 30:593–607. 10.1016/S0896-6273(01)00301-4 [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. 2002. Early consolidation in human primary motor cortex. Nature. 23:23. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Imamizu H. 2013. Human sensorimotor cortex represents conflicting visuomotor mappings. J Neurosci. 33:6412–6422. 10.1523/JNEUROSCI.4661-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Criscimagna-Hemminger SE, Shadmehr R. 2010. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex. 21(7):1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Boraud T, Natan C, Bergman H, Vaadia E. 2003. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci. 6:882–890. 10.1038/nn1097 [DOI] [PubMed] [Google Scholar]
- Pekny SE, Criscimagna-Hemminger SE, Shadmehr R. 2011. Protection and expression of human motor memories. J Neurosci. 31:13829–13839. 10.1523/JNEUROSCI.1704-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. 2004. Spontaneous recovery varies inversely with the training-extinction interval. Learn Behav. 32:401–408. 10.3758/BF03196037 [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. 2006. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 26:12466–12470. 10.1523/JNEUROSCI.1139-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. 2002. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 113:101–107. 10.1016/S1388-2457(01)00693-9 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. 1997. Functional stages in the formation of human long-term motor memory. J Neurosci. 17:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. 1994. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 14:3208–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing G, Joiner WM, Nanayakkara T, Brayanov JB, Smith MA. 2009. Primitives for motor adaptation reflect correlated neural tuning to position and velocity. Neuron. 64:575–589. 10.1016/j.neuron.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Sing GC, Smith MA. 2010. Reduction in learning rates associated with anterograde interference results from interactions between different timescales in motor adaptation. PLoS Comput Biol. 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. 2006. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 4:e179 10.1371/journal.pbio.0040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaswani PA, Shadmehr R. 2013. Decay of motor memories in the absence of error. J Neurosci. 33:7700–7709. 10.1523/JNEUROSCI.0124-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Weston GD, Liang J, Mazzoni P, Krakauer JW. 2008. Explaining savings for visuomotor adaptation: linear time-invariant state-space models are not sufficient. J Neurophysiol. 100:2537–2548. 10.1152/jn.90529.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]