Abstract
Objective
Although behavioral weight-loss interventions produce short-term weight loss, long-term maintenance remains elusive. This randomized trial examined whether learning a novel set of “stability skills” before losing weight improved long-term weight management. Stability skills were designed to optimize individuals’ current satisfaction with lifestyle and self-regulatory habits while requiring the minimum effort and attention necessary.
Methods
Overweight/obese women (N = 267) were randomly assigned to one of two 6-month interventions and assessed at baseline, 6, 12, and 18 months. Maintenance First women participated first in an 8-week stability skills maintenance module, then in a standard 20-week behavioral weight-loss program. Weight Loss First women participated first in a standard 20-week behavioral weight-loss program, then in a standard 8-week problem-solving skills maintenance module. There was no intervention staff contact during the 12-month follow-up (6–18 months).
Results
As designed, Maintenance First participants lost the same percent of initial weight during the 6-month intervention period as Weight Loss First participants (M = −8.6%, SD = 5.7 vs. M = −9.1%, SD = 6.9, t = −0.6, p = .52). However, Maintenance First participants regained significantly less weight during the 12-month follow-up (6–18 months) than Weight Loss First participants (M = 3.2 lbs, SD = 10.4 vs. M = 7.3 lbs, SD = 9.9, t = 3.3, p = .001, d = 0.4).
Conclusion
Learning stability skills before losing weight was successful for maintaining weight loss without intervention staff contact during follow-up. These results can inform the study design of future innovative interventions. Trial Registration: ClinicalTrials.gov-NCT00626457.
Keywords: Obesity, weight loss, weight maintenance, long-term, weight stability, randomized trial
Obesity is a precursor to significant health consequences such as heart disease and diabetes, yet modest weight losses of 5–10% of initial weight produce clinically significant improvements in cardiovascular disease risk factors (Diabetes Prevention Program Research Group, 2002; Stevens et al., 2001; Wing & Jeffery, 1995). Although behavioral weight-loss interventions effectively produce modest weight losses in the short term, defined as 5–10% of initial weight in six months (Wadden, Crerand, & Brock, 2005), long-term maintenance remains elusive. Overweight and obese individuals typically give up the lifestyle and self-regulatory changes they have made during a weight-loss program—such as healthy eating, physical activity, and vigilant record keeping—and regain 30–50% of the weight loss within one year after the program ends (Barte et al., 2010; Jeffery et al., 2000). Maintenance interventions based on a ‘continued care’ perspective rely on active intervention and staff contact over time to sustain skills learned during weight loss, such as record-keeping and problem-solving skills (Perri & Corsica, 2002; Svetkey et al., 2008; Wadden et al., 2005). Although often highly successful at improving weight-loss maintenance when intervention and staff contact are in place, these maintenance interventions may only delay rather than prevent weight regain (Perri & Corsica, 2002; Wadden et al., 2005). Discouragingly, individuals leaving interventions with active maintenance components typically regain weight at the same rate as individuals leaving weight-loss programs without such maintenance components, sometimes regaining weight even before maintenance contact ends (Perri & Corsica, 2002; Perri et al., 2001; Wadden et al., 2005).
Whereas these results could be interpreted as providing even stronger support for a continued care perspective, there may be additional skills that must be mastered for long-term weight maintenance. These skills may be independent from those needed for losing weight (King et al., 2002; Rothman, 2000; Sciamanna et al., 2011), but are not currently being incorporated into interventions. We hypothesized that individuals who want to successfully maintain a weight loss may need to proactively focus on weight “stability,” that is how to keep themselves at a stable and steady weight (Kiernan et al., 2005; Kiernan, Goldberg, & Durkin, 2003; Kiernan, Goldberg, Kirkpatrick, & Raymond, 2000). Consequently, they may need to learn a set of “stability skills” that optimizes satisfaction with the immediate day-to-day experience of engaging in lifestyle and self-regulatory habits. These skills include learning how to eat a healthy diet in appropriate portion sizes and be more physically active without feeling deprived or dissatisfied (relative deprivation theory; Kruglanski & Mayseless, 1990) and learning how to regulate or “fine-tune” the balance between their eating, activity, and weight with the minimum effort and attention necessary (limited resources theory; Baumeister, Bratslavsky, Muraven, & Tice, 1998). In addition, individuals may be more successful if they learn these stability skills before initiating weight loss, thus, capitalizing on initial motivation, providing a mastery experience for weight stability, and increasing perceived self-efficacy for maintaining a stable weight in the future (social cognitive theory; Bandura, 1986).
This randomized trial examined whether learning a novel set of stability skills before losing weight improved long-term weight management, i.e., during a 12-month follow-up period after intervention sessions and staff contact had ended. Overweight/obese women were randomly assigned to one of two 6-month interventions that differed in the content and order of the maintenance modules. In Maintenance First, women participated first in an 8-week weight stability skills maintenance module, then in a standard 20-week behavioral weight-loss program. In Weight Loss First, women participated first in a standard 20-week behavioral weight-loss program, then in an 8-week problem-solving skills maintenance module. Thus, Maintenance First (a multi-component approach) differed in two important ways from Weight Loss First (a standard care approach). The Maintenance First condition consisted of both novel content (learning a set of stability skills designed to optimize day-to-day satisfaction with lifestyle and self-regulatory habits) and a novel order (placing a maintenance module before a weight loss program). In this trial, we hypothesized that women randomized to Maintenance First would regain less weight over the 12-month follow-up than women randomized to Weight Loss First.
Method
Participants and procedure
Participants were recruited from Northern California communities using multiple methods including targeted direct mail letters as well as newspaper and online advertisements (Brown et al., 2012). Participants were recruited in two waves (February-March 2008 and January-March 2009) with two cohorts per wave. Eligibility criteria included: being female, age ≥ 21 years, not pregnant or planning to be within the next two years, not planning to move out of the area within the next two years, body mass index (BMI) 27–40 kg/m2 (Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, 1998), free of heart disease, diabetes, and other chronic health conditions, able to participate in physical activity (Thomas, Reading, & Shephard, 1992), free of binge eating disorder or bulimic compensatory symptoms (Stice, Telch, & Rizvi, 2000), not currently on a special diet, not currently in another research study, reliable access to the Internet, and interest in attending sessions offered in English.
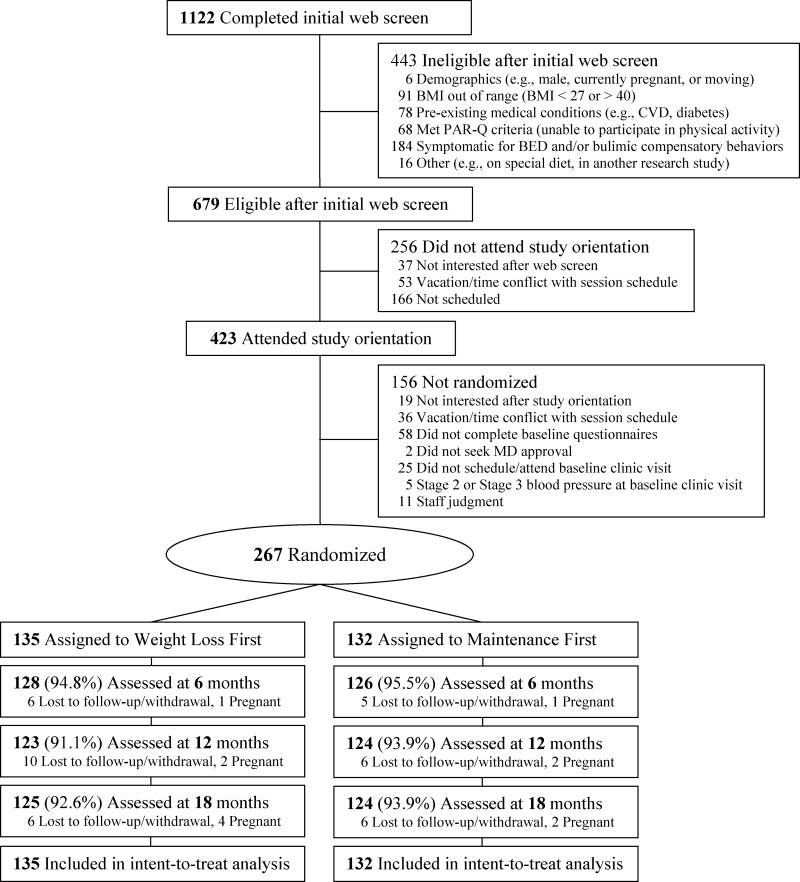
The participant flow diagram for recruitment, randomization and retention is presented in Figure 1 (Moher et al., 2010). Interested participants were directed to a study website to complete an online eligibility questionnaire (n = 1122). Eligible participants (n = 679) were invited to a group-based interactive orientation session (Goldberg & Kiernan, 2005). After attending the orientation session (n = 423), interested participants were asked to complete two online questionnaires and a baseline clinic assessment and were then randomized at the end of the baseline assessment to one of two 6-month interventions in a 1:1 allocation (N = 267).
Figure 1.
Participant flow of recruitment, randomization, and retention
BMI = body mass index; CVD = cardiovascular disease; PAR-Q = Physical Activity Readiness Questionnaire; BED = binge eating disorder
The randomization process was based on Efron randomization principles so that the sample sizes in each condition were nearly equal throughout recruitment to avoid possible biases at the end of recruitment (Efron, 1971). The allocation sequence was concealed prior to intervention assignment, and responsibility for the sequence generation, allocation concealment, and implementation steps was kept separate (Moher et al., 2010). The participants were actively involved in the process so that their commitment to whichever intervention they were randomly assigned to was enhanced (Langer, 1975). For the intervention assignment, participants were asked to draw a card out of a bag that contained six black cards and four red cards. Prior to each baseline visit, the project coordinator ran a statistical program based on Efron randomization principles that designated which intervention should be assigned to which card color. After each baseline visit, the coordinator recorded the intervention assignment that the participant drew from the bag; this also updated the database that the statistical program used for subsequent participants. The sample was stratified by racial/ethnic minority status (minority or White) within cohort for equal representation at the intervention sessions.
The 6-month interventions were followed by a 12-month follow-up period in which participants had no contact with intervention staff. Details about the two interventions are provided below. At the baseline, 6, 12, and 18 month clinic assessments, anthropometric data were assessed at the research center. Prior to each of the clinic assessments, participants completed online questionnaires at their convenience.
Trial retention did not differ at any of the assessments by intervention condition (ps > .38; Figure 1). Retention was excellent; 93.3% (n = 249) of the randomized participants were weighed at 18 months. All randomized participants (N = 267) were included in intent-to-treat statistical analyses. There were no serious adverse events related to the trial for either intervention condition. Participants were not financially compensated for their participation. The trial was approved by the Stanford University Institutional Review Board and all participants provided written informed consent.
Intervention Conditions
Participants were randomly assigned to one of two intervention conditions (Weight Loss First or Maintenance First). Each condition was comprised of a 6-month intervention followed by a 12-month follow-up period in which participants had no contact with intervention staff. Both 6-month interventions were identical in format: 90-minute group sessions were held weekly for 28 weeks in classrooms at the research center and were comprised of 15–18 participants. However, as designed, the two 6-month interventions differed by the content and order of their maintenance modules. In the Weight Loss First intervention, women participated first in a standard 20-week behavioral weight-loss program, then in an 8-week problem-solving skills maintenance module. In the Maintenance First intervention, women participated first in an 8-week stability skills maintenance module, then in a standard 20-week behavioral weight-loss program. Details of the weight-loss program and maintenance modules are described below. To capitalize on a challenging naturalistic environmental influence, we scheduled the 6-month interventions to end in October so that all women immediately had to navigate the traditional American holiday season (i.e., Halloween through New Year’s Eve) at the beginning of the 12-month follow-up period. Participants in both intervention conditions were given study-provided bathroom scales at their baseline clinic assessment to use at home for the entire 18-month trial.
Weight-loss program
Women in both intervention conditions participated in identical group-based 20-week behavioral weight-loss programs. The program was originally adapted by Perri and colleagues for a group format from the Diabetes Prevention Program (Diabetes Prevention Program Research Group, 2002) and has been shown to produce clinically significant weight loss (Perri et al., 2008). Participants were weighed before each session by intervention staff. The weekly sessions followed a three-part format. The group facilitator started with an opening “round robin” to review each woman’s behavioral goals and provide feedback on progress and problems. The facilitator then led an interactive discussion of printed session materials on key cognitive behavioral change techniques such as planning social support, stress management, relapse prevention (Abraham & Michie, 2008). The facilitator finished with a closing round robin to prompt intention formation and setting of specific behavioral goals for the coming week. Integral to this adapted behavioral weight-loss program was the group facilitator’s systematic use of the 5-step problem-solving model during weekly sessions to encourage participants to generate effective solutions to particular problems for one another (Perri et al., 2008; Perri et al., 2001; Perri, Nezu, & Viegener, 1992). The five steps included: (1) orientation (i.e., developing an appropriate coping perspective): “Problems are a normal part of managing your weight, but they can be dealt with effectively”; (2) definition (i.e., specifying the problem or barrier, and goal behaviors): “What is the particular problem facing you right now? What is your goal in this situation?”; (3) generation of alternatives (i.e., brainstorming potential solutions): “The greater the range of possible solutions you consider, the greater your chances of developing an effective solution”; (4) decision making (i.e., anticipating the probable outcomes of different options): “What are the likely short- and long-term consequences of each of your options?”; and (5) implementation and evaluation (i.e., trying out a plan and evaluating its effectiveness): “What solution plan are you going to try, and how will you know if it works?” Weekly behavioral goals for the weight loss program included: keeping diet and physical activity records 5–7 days per week; reaching personalized calorie targets designed to produce weight loss of ½-1 lb per week; reaching pedometer step goals designed to accrue 150 minutes per week of at least moderate intensity physical activity (Jakicic, Winters, Lang, & Wing, 1999), and carrying out one’s solution plans for particular problems. Participants were given pedometers to track physical activity. Participants were encouraged to weigh themselves daily at home using the study-provided scales.
Problem-solving maintenance module
Women in the Weight Loss First intervention condition participated in the 8-week problem-solving maintenance module after the 20-week weight-loss program. This maintenance module relied on a continued care approach by sustaining the behavioral goals and changes made in the weight-loss program (Perri et al., 2008). Participants were weighed before each session by intervention staff. The weekly sessions followed the same three-part format as the weight-loss program described above (round robin, discussion, round robin). Participants explicitly learned how to use the 5-step problem solving model as a skill to address barriers to weight maintenance on their own. For instance, participants completed formal written problem-solving worksheets using the 5-step model for three or more problems or barriers they expected to face during the 12-month follow-up period and discussed these with the group. Participants also took turns getting the group to use the 5-step problem-solving model to tackle problems for other participants in the group. Similar to “real world” weight management programs, participants could choose to lose weight or maintain their weight during the 8-week problem-solving maintenance module. Recommendations to lose weight included keeping diet and physical activity records at least 5–7 days per week and weighing daily whereas recommendations to maintain weight included keeping diet and physical activity records at least 3 days per week (with calorie and step goals adjusted for maintenance) and weighing daily.
At the end of the final session of the 6-month Weight Loss First intervention, participants reviewed behavioral recommendations for the upcoming 12-month follow-up period. Participants could choose to lose weight or maintain their weight. Consistent with the Weight Loss First intervention, recommendations to lose weight included keeping diet and physical activity records at least 5–7 days per week and weighing daily whereas recommendations to maintain weight included keeping diet and physical activity records at least 3 days per week and weighing daily.
Stability skills maintenance module
Women in the Maintenance First intervention condition participated in the 8-week stability skills maintenance module before the 20-week behavioral weight-loss program. Importantly, to provide a mastery experience for weight stability per se, participants were asked not to lose weight during the initial 8 weeks, and if they lost a few pounds, were asked to gain the weight back. In addition, participants learned about distinguishing between weight loss and weight stability with regards to which skills to use and the nature of the experience. To optimize satisfaction with the immediate, day-to-day experience of maintaining a weight loss and thus increase intrinsic motivation for engaging in the lifestyle and self-regulatory behaviors themselves, Maintenance First participants engaged in experiential activities during weekly sessions and at home. These activities were designed to expose them to five stability skills and to provide opportunities for practice and mastery. Informed by social psychological theories (i.e., relative deprivation, limited resources, and social cognitive theory; Bandura, 1986; Baumeister et al., 1998; Kruglanski & Mayseless, 1990) and our formative research, the module’s five skills included: (1) learn about energy balance principles; (2) eat a healthy diet in appropriate portion sizes and be more physically active—without feeling deprived or dissatisfied; (3) weigh daily to monitor fluctuations in weight and interpret fluctuations using a personalized weight gain “alert”—without feeling badly or alarmed; (4) fine-tune lifestyle habits by making quick, small, and easy adjustments—without a lot of extra effort and attention; and (5) navigate inevitable disruptions—with confidence. Experiential activities were based on adult learning principles (Meyers & Jones, 1993), including hands-on activities designed to first change behaviors so that attitudes would follow, as well as small and large group discussions. Key experiential activities included: actively savoring favorite high-calorie foods in a mindful manner; weighing daily to collect “data” about one’s own weight fluctuations to eventually inform the choice of a personalized range (e.g., ~5 lbs or 2.3 kilograms); making quick, small, and easy lifestyle changes without keeping food records; and navigating a 1-week simulated disruption while remaining in their personalized range (i.e., eating five high-calorie meals in a week as if on vacation). The five stability skills and examples of experiential activities are listed in Table 1. During the 8-week stability skills maintenance module, participants were not weighed before each session by intervention staff. Instead, to foster autonomy and self-efficacy, participants were encouraged to weigh themselves daily at home using the study-provided scales as part of a set of experiential activities for “making peace with the scale” and using “fine-tuning habits” that promoted sustained non-effortful use of self-regulation skills.
Table 1.
Stability Skills First: Stability skills and experiential activities
| Stability skills | Experiential activities |
|---|---|
| 1. Be Savvy | Learn principles of energy balance, nutrition, and physical activity |
| 2. Enjoy Lifestyle Habits | Eat a healthy diet in appropriate portion sizes and be more physically active—without feeling deprived or dissatisfied |
|
|
| 3. Make Peace with the Scale | Weigh daily to monitor fluctuations in weight and interpret fluctuations using a personalized weight gain “alert”—without feeling badly about yourself or alarmed |
|
|
| 4. Fine-tune Lifestyle Habits | Make quick, small, and easy adjustments to remain at a steady and stable weight—without a lot of extra effort and attention |
|
|
| 5. Navigate Inevitable Disruptions | Navigate disruptions before, during, and after—with confidence |
|
At the end of the final session of the 6-month Maintenance First intervention (which was the final session of the 20-week behavioral weight-loss program for this intervention condition), participants reviewed behavioral recommendations for the upcoming 12-month follow-up period. Participants could choose to lose weight or maintain their weight. Recommendations to lose weight included keeping diet and physical activity records at least 5–7 days per week and weighing daily whereas recommendations to maintain weight included using the five stability skills (e.g., using a personalized range, fine-tuning lifestyle habits) and weighing daily.
Intervention fidelity and session attendance
Borrelli et al. (2005) have delineated a systematic intervention fidelity framework that considers procedures across multiple dimensions such as standardizing the number and format of intervention sessions (described above) and standardizing staff training and intervention delivery (Borrelli et al., 2005). In this trial, intervention staff included eight group facilitators (four facilitators per wave) who were registered dieticians or doctoral candidates in psychology. Staff training for facilitators was standardized via written manuals, case studies, and role play. To minimize any potential differential effect of facilitators by intervention condition, each facilitator was responsible for one group in each intervention condition. Intervention delivery was standardized via written manuals for facilitators and handouts for participants. In addition, throughout the 6-month interventions, facilitators’ adherence to intervention delivery was monitored through review of facilitators’ written summaries after each group session, weekly group and individual supervision, and direct observation.
To systematically assess adherence to intervention delivery and potential contamination, audio recordings were collected in the second wave for all sessions. Then, we a priori identified four sessions from the Weight Loss First intervention and four sessions from the Maintenance First intervention that had the highest potential for contamination by intervention condition (e.g., the last session of each intervention). A trained coder assessed the percentage of key intervention elements covered in each audio-recorded session using three checklists. The first checklist contained elements of the weight-loss program and, thus, were common to both intervention conditions (e.g., facilitator conducted the opening round robin). The remaining two checklists contained elements of the maintenance modules unique to each condition (e.g., the problem solving maintenance module encouraged participants to keep diet/physical activity records at least 3 days per week when trying to maintain weight; the stability skills maintenance module encouraged participants to consider the advantages of having a lower limit of one’s personalized range). In the audio-recorded sessions selected a priori as having highest potential for contamination, adherence to intervention delivery was excellent. For both intervention conditions, facilitators covered all weight-loss program elements (100.0%, range = 100.0%). In the Weight Loss First intervention sessions, facilitators covered almost all problem-solving maintenance module elements (95.0%, range = 80.0–100.0%) and none of the stability skills maintenance module elements (0.0%, range = 0.0%). In the Maintenance First sessions, facilitators covered almost all stability skills maintenance module elements (95.0%, range = 93.3–100.0%) and none of the problem-solving maintenance module elements (0.0%, range = 0.0%).
In both waves, facilitators were also evaluated by participants at the end of the 6-month interventions on five items (well-prepared, knowledgeable, organized, supportive, and attentive) using a 5-point Likert scale with response options labeled from not at all to extremely (1–5). Responses were summed and averaged so the mean score corresponded directly to response options. Scores for facilitators did not differ by intervention condition and participants rated all facilitators very to extremely highly (range = 4.2–4.9). Session attendance was recorded each week by group facilitators. Session attendance did not differ by intervention condition (p = .14). Participants attended 78.3% of 28 intervention sessions (M = 21.9, SD = 6.7).
Measures
The primary outcome was the number of pounds gained over the 12-month follow-up period. Anthropometric data were collected in duplicate using standardized protocols at the four clinic assessments (Lohman, Roche, & Martorell, 1988). Body weight was measured on a California state-certified standard beam balance scale with participants in light clothing and without shoes; height was measured using a wall stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Clinic assessment staff was blind to intervention condition (Moher et al., 2010). Demographic data were collected via the online questionnaires with validated single-item questions (Jeffery et al., 1984).
Statistical Analyses
For a two-sided test at α = 0.05, the planned sample size of 116 participants per intervention condition provided at least 80% power to detect an intervention standardized effect size of 0.4 as measured by Cohen’s d (Cohen, 1988), and is equivalent to a 5.2 lb difference (SD = 13.0). An approximately 5 lb difference (e.g., 2.3 kg or 2–3% loss of initial weight) in the amount of regain between intervention conditions is the magnitude of weight loss associated with small but clinically significant improvements in cardiovascular risk factors, such as blood pressure and insulin levels (Diabetes Prevention Program Research Group, 2002; Stevens et al., 2001; Wing & Jeffery, 1995).
Analyses followed intention-to-treat principles (Moher et al., 2010). As reported above, retention was excellent; 93.3% (n = 249) of the 267 randomized participants were weighed at 18 months at the research center. For descriptive statistics and t-tests, missing data were conservatively imputed using the baseline carried forward method (Perri et al., 2008). We used a spline multi-level model to estimate the effects of intervention condition on weight changes during the two time periods (the 6-month intervention period and the 12-month follow-up period) within a single parsimonious analysis. This model, which used maximum likelihood estimation, provided several advantages (Raudenbush & Bryk, 2002; Snijders & Bosker, 1999). First, it estimated the effect of intervention condition for the two time periods separately, thus accommodating the expected non-linear trajectory of weight change between the two time periods. Second, unlike traditional repeated measures analyses, this model retained participants with missing data, thus not deleting any cases and not relying on any imputation assumptions. The model was a two-level hierarchy in which Level-1 data (time invariant) consisted of weight and time point (baseline, 6, 12, and 18 months) and Level-2 data (time variant) consisted of intervention condition. The six fixed effects in the spline model consisted of the intercept (β00), intervention condition (β01, in which the Maintenance First condition was coded as the reference group to aid interpretation of the estimates), the 6-month intervention period (β10), the interaction between intervention condition and the 6-month intervention period (β11), the 12-month follow-up period (β20) estimated in two 6-month intervals, and the trial’s primary hypothesis of interest, the interaction between intervention condition and the 12-month follow-up period (β21).
Results
Demographic and initial weight characteristics
Demographic characteristics did not differ by intervention condition (see Table 2). In this sample (N = 267), most women were middle-aged, had a college degree, and were married or living with a partner. A third were from non-White racial/ethnic groups, reflecting the region’s racial/ethnic diversity (Brown et al., 2012). Most women had participated in a prior formal weight-loss program such as Weight Watchers. Most participants were obese, and initial weight did not differ by intervention condition. Weight change results are reported in pounds. For descriptive purposes, weight change results are also reported in kilograms and percentage change of initial weight in Table 2.
Table 2.
Demographic characteristics and weight change variables by intervention condition
| Variable | Weight Loss First
|
Maintenance First
|
p | ||
|---|---|---|---|---|---|
| M (%) | SD (n) | M (%) | SD (n) | ||
| N | 135 | 132 | |||
| Demographic characteristics | |||||
| Age, years | 48.0 | 10.6 | 48.8 | 11.0 | .54 |
| College degree, % | 68.9 | 93 | 65.2 | 86 | .52 |
| Race/Ethnicity1 | .52 | ||||
| White, % | 64.4 | 87 | 68.2 | 90 | |
| Latina/Hispanic, % | 11.1 | 15 | 9.9 | 13 | |
| Multiethnic (≥ 2 racial/ethnic groups), % | 10.4 | 14 | 9.9 | 13 | |
| Asian, % | 11.9 | 16 | 6.8 | 9 | |
| Black/African American, % | 0.7 | 1 | 5.3 | 7 | |
| Native Hawaiian/Pacific Islander, % | 1.5 | 2 | 0.0 | 0 | |
| Married/living with partner, % | 74.1 | 100 | 63.6 | 84 | .07 |
| Prior formal weight loss program, % | 65.9 | 89 | 69.7 | 92 | .51 |
| Baseline weight | |||||
| Weight, lb | 189.3 | 27.9 | 188.5 | 26.7 | .83 |
| Weight, kg | 85.8 | 12.7 | 85.5 | 12.1 | |
| BMI, kg/m2 | 32.1 | 3.5 | 32.1 | 3.4 | |
| BMI ≥ 30 (Obese), % | 62.2 | 84 | 66.7 | 88 | |
| Weight changes over 6-month intervention | |||||
| Weight loss, 0–6 months, lb | −17.1 | 13.4 | −16.1 | 10.9 | .52 |
| Weight loss, 0–6 months, kg | −7.7 | 6.1 | −7.3 | 5.0 | |
| % loss of initial weight, 0–6 months, mean | −9.1 | 6.9 | −8.6 | 5.7 | |
| Weight changes over 12-month follow-up period | |||||
| Weight gain, 6–18 months, lb | 7.3 | 9.9 | 3.2 | 10.4 | .001 |
| Weight gain, 6–18 months, kg | 3.3 | 4.5 | 1.4 | 4.7 | |
| % gain of initial weight, 6–18 months, mean | 4.4 | 6.0 | 2.1 | 6.1 | |
| Weight gain, 6–12 months, lb | 4.0 | 7.4 | 0.6 | 7.3 | .0002 |
| Weight gain, 6–12 months, kg | 1.8 | 3.4 | 0.3 | 3.3 | |
| % gain of initial weight, 6–12 months, mean | 2.4 | 4.3 | 0.5 | 4.4 | |
| Weight gain, 12–18 months, lb | 3.3 | 8.0 | 2.5 | 7.0 | .38 |
| Weight gain, 12–18 months, kg | 1.5 | 3.6 | 1.1 | 3.2 | |
| % gain of initial weight, 12–18 months, mean | 2.0 | 4.6 | 1.6 | 4.0 | |
| ‘Model’ pattern over 0–18 months | |||||
| Lost ≥ 5% from 0–6 months, % | 71.1 | 96 | 73.5 | 97 | |
| Lost ≥ 5% from 0–6 months and gained ≤ 5 lbs at any assessment between 6–18 months (including 6–12, 12–18, and 6–18 months), % |
17.8 | 24 | 33.3 | 44 | .004 |
Percentages for Maintenance First add up to 100.1 due to rounding. Chi-square test conducted for Race/Ethnicity by intervention condition using a dichotomous race/ethnicity variable (White versus non-White) given some cells had expected counts less than 5 (e.g., Native Hawaiian).
Changes over the 6-month intervention
As designed, Maintenance First participants were willing to wait before losing weight. Maintenance First participants kept their weight stable as measured at 9 weeks (i.e., the first session of the weight-loss program for participants in this condition) whereas Weight Loss First participants lost weight as measured at 9 weeks at an amount consistent with the ½-1 lb recommended rate per week (M = −0.3 lbs, SD = 3.1 vs. M = −7.0 lbs, SD = 5.4, respectively, t = −12.5, p < .0001, d = 1.6). Over 90% (90.9%, n = 120) of Maintenance First participants kept their weight within 5 lbs or less of their baseline weight; only 7.6% (n = 10) lost > 5 pounds and 1.5% (n = 2) gained > 5 lbs (inter-quartile range −2.1 to 1.4 lbs).
As designed, Maintenance First participants lost the same amount of weight by the end of the 6-month intervention period as Weight Loss First participants (M = −16.1 lb, SD = 10.9 vs. M = −17.1 lbs, SD = 13.4, respectively, t = −0.6, p = .52).
Changes over the 12-month follow-up period
Maintenance First participants regained significantly less weight during the 12-month follow-up period (6–18 months) than Weight Loss First participants (M = 3.2 lbs, SD = 10.4 vs. M = 7.3 lbs, SD = 9.9, t = 3.3, p = .001, d = 0.4). Moreover, almost twice as many Maintenance First participants displayed a “model” pattern of weight change than Weight Loss First participants (33.3% vs. 17.8%, p = .004, odds ratio = 2.3, 95% confidence interval 1.3–4.1), defined as losing ≥ 5% of initial weight at 6 months and gaining ≤ 5 pounds at any time point from 6 to 18 months including 6–12, 12–18, and 6–18 months. This definition conservatively excludes participants who may have gained > 5 lbs at 12 months but were able to lose it by 18 months.
The spline multi-level model, which parsimoniously estimated the effects of intervention condition for the two time periods of weight change (the 6-month intervention period and 12-month follow-up period), revealed results virtually identical to the t-test analyses. There was no difference in the estimated baseline weight by intervention condition; Maintenance First participants were 188.5 lbs (β00 = 188.5, SE = 2.4, t = 79.3, p < .0001, 95% confidence interval 183.9 to 193.2) and Weight Loss First participants were less than a pound heavier (β01 = 0.7, SE = 3.3, t = 0.2, p = .83, 95% confidence interval −5.8 to 7.3). There was no difference in the estimated weight loss at the end of the 6-month intervention period by intervention condition; Maintenance First participants lost an estimated 17.1 lbs at the end of the 6-month intervention (β10 = −17.1, SE = 1.1, t = −15.2, p < .0001, 95% confidence interval −19.3 to −14.9) whereas Weight Loss First participants only lost an additional −0.5 lbs (β11 = −0.5, SE = 1.6, t = −0.3, p = .74, 95% confidence interval −3.6 to 2.6). However, for the trial’s primary hypothesis of interest, there was a difference in estimated weight gain during the 12-month follow-up period by intervention condition; Maintenance First participants gained 1.5 lbs per six-month interval (β20 = 1.5, SE = 0.4, t = 3.5, p = .0005, 95% confidence interval 0.7 to 2.4) whereas Weight Loss First participants gained an additional 2.0 pounds per six-month interval (β21 = 2.0, SE = 0.6, t = 3.3, p = .001, 95% confidence interval 0.8 to 3.2). Thus, Maintenance First participants gained an estimated total of 3.0 lbs over the 12-month follow-up period whereas Weight Loss First participants gained an estimated total of 7.0 lbs. Adjusting for baseline weight or demographic characteristics did not substantively alter the magnitude or statistical significance of the estimated effects.
Discussion
This randomized trial demonstrated that learning a novel set of stability skills before losing weight improved long-term weight management. Maintenance First participants (who participated first in an 8-week stability skills maintenance module and then in a standard 20-week behavioral weight-loss program) regained half as much weight over a 12-month follow-up than Weight Loss First participants (who participated first in a standard 20-week behavioral weight-loss program and then in a standard 8-week problem-solving skills maintenance module). These results are striking given there was no intervention staff contact during follow-up.
Maintenance First participants regained only 3.2 lbs or 20% of the weight they lost during the 12-month follow-up. In contrast, Weight Loss First participants regained 7.3 lbs or 43% of the weight they lost, a percentage consistent with prior trials without maintenance components where 30–50% of weight loss was regained one year after treatment (Jeffery et al., 2000). The small amount of regain for Maintenance First participants during the 12-month follow-up in which there was no intervention staff contact compares favorably to results from a highly successful randomized trial that examined the cost effectiveness of different types of staff contact during a 1-year maintenance intervention (Perri et al., 2008). Participants in that trial who received either biweekly face-to-face group sessions or biweekly individual telephone calls regained 2.6 lbs over the year of intervention staff contact whereas participants in an education newsletter control group regained 8.1 lbs (Perri et al., 2008). Taken together, Maintenance First participants in the current trial regained 4.1 fewer pounds (or 2.2% of initial weight) than Weight Loss First participants, a magnitude that corresponds with small but clinically significant improvements in cardiovascular risk factors, such as blood pressure and insulin levels (Diabetes Prevention Program Research Group, 2002; Stevens et al., 2001; Wing & Jeffery, 1995).
Although one could conjecture that regain for Maintenance First participants may only have been delayed rather than prevented (Perri & Corsica, 2002; Spring et al., 2004; Wadden et al., 2005), suggestive evidence in the current trial argues against this interpretation. Maintenance First participants gained less than a pound during the first six months of the 12-month follow-up, thus successfully navigating the challenging American holiday period without any contact from intervention staff whereas Weight Loss First participants gained 4 pounds. In addition, Maintenance First participants only gained a total of 3.2 lbs over the entire 12-month follow-up period which still fell within the typical personalized range recommended during the stability skills maintenance module (e.g., ~5 lbs or 2.3 kilograms). Finally, Maintenance First participants were almost twice as likely to display a model pattern of weight change over the entire 18-month trial as Weight Loss First participants (i.e., losing ≥ 5% from baseline to 6 months and gained ≤ 5 lbs at any assessment between 6–18 months including 6–12, 12–18, and 6–18 months). Such suggestive findings notwithstanding, a randomized trial with a longer follow-up is needed to definitively answer whether regain was only delayed or indeed prevented for Maintenance First participants.
The study design of this first-generation trial did not provide the opportunity to independently examine the effect of the order of the stability skills maintenance module and the effect of the content of the module, the stability skills themselves. Thus, although the Maintenance First approach was efficacious, we do not know whether one component was responsible or both. As is often the case in applied research, incorporating multiple components into an intervention and comparing the intervention to standard care constitutes the first stage of inquiry (Mohr et al., 2009). After demonstrating that a multi-component intervention is effective, researchers should then turn to testing which particular components are responsible for beneficial outcomes (Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Kazdin, 1992). Future research can explicitly examine whether the two components of Maintenance First may have independent effects including possible additive or multiplicative effects.
Placing the order of the stability skills maintenance module before the weight loss program was informed by social cognitive theory and designed to capitalize on initial motivation, provide a mastery experience for weight stability, and increase perceived self-efficacy for maintaining a stable weight in the future. However, alternative mechanisms may exist. Given Maintenance First participants had to wait to lose weight (and if they did lose weight, were asked to gain it back), such a context could have fostered a learning environment without emotional distractions which has been shown in social psychological laboratory research to increase learning and memory (Bower, 1992); this context could have served as an experimental intervention that addressed an inability to delay gratification shown to be a risk factor for weight gain in children (Epstein, Salvy, Carr, Dearing, & Bickel, 2010); or this context could have provided exposure and fostered acceptance prior to initiating subsequent behavior changes, deemed to be important constructs in recent psychotherapy frameworks (Pull, 2008). More broadly, providing time for other kinds of preparatory modules prior to behavioral interventions is an innovative and potentially fruitful avenue for future research, and complements other recent creative manipulations of intervention timing including examining the effect of sequential versus simultaneous interventions for changes in multiple lifestyle behaviors (King et al., 2009; Spring et al., 2004) or providing explicit 4-week breaks from weight loss to counter boredom and habituation (Jeffery et al., 2009).
The stability skills maintenance module was comprised of a complementary set of skills to foster weight stability and was presented as distinct from weight loss. The stability skills were designed to explicitly optimize satisfaction with the immediate day-to-day experience of engaging in lifestyle and self-regulatory behaviors, thus promoting sustained use of these behaviors over time and avoiding an “on/off” approach. Some stability skills in the maintenance module ran counter to more established behavioral recommendations. Participants experienced how to self-regulate the balance among food, activity, and weight without keeping daily food records. Instead, along with the combination of daily weighing and a personalized range, they were encouraged to approach the process with relaxed rather than typically recommended vigilant awareness (Baker & Kirschenbaum, 1998). Participants learned to view the lower limit of their personalized range as an indication of when they could indulge a bit or exercise a little less. Participants practiced navigating a 1-week simulated disruption by going on “vacation” and eating five high-fat/calorie meals while using fine-tuning habits to remain within their personalized range. This included strategically losing a few pounds before the simulated vacation to have a little room to gain during the disruption. Aspects of other stability skills in the maintenance module complement recent strategies tested in obesity prevention and treatment trials including daily weighing (Wing, Tate, Gorin, Raynor, & Fava, 2006), mindful eating (Kristeller & Wolever, 2010), eating in moderation (Sbrocco, Nedegaard, Stone, & Lewis, 1999), cumulative effects of making small changes (Jeffery & French, 1999; Lutes et al., 2008), and non-food related reinforcements (West et al., 2011). Taken together, these recent efforts to design and test innovative intervention strategies are vitally needed and promising for the field.
In this trial, over a third of the Maintenance First participants lost ≥ 5% of their initial weight and regained ≤ 5 pounds at any assessment point from 6 to 18 months without intervention staff contact over the 12-month follow-up. Based on this finding, one could interpret that continued intervention staff contact is not needed. However, it would be more productive to determine how to double or triple the percentage of individuals able to achieve a model pattern, amplifying clinical significance. Future research could examine how to tailor optimal maintenance combinations for individuals on their own or for individuals needing extended support. Future research should also examine cost-effectiveness.
One study limitation was that two-thirds of the sample was comprised of healthy, middle-aged, educated, White women. Future research needs to examine whether the stability skills first approach resonates with younger women and men of all ages, as well as individuals who are less educated, from different ethnic/racial backgrounds, heavier, have co-morbid conditions, attempt weight loss on their own, or experience binge eating disorder symptoms. For this community study sample, women with binge eating disorder and/or bulimic compensatory symptoms were excluded. Women with these symptoms typically comprise 10%–30% of women in standard behavioral weight-loss programs (Gorin et al., 2008) but comprise only 3.5% of adult-aged women in population-based samples (Hudson, Hiripi, Pope Jr., & Kessler, 2007), and are less likely to experience long-term remission of binge eating in behavioral weight-loss programs than women in specialty treatment programs for binge eating disorder such as interpersonal psychotherapy (Wilson, Wilfrey, Agras, & Bryson, 2010). Despite study limitations, this trial had multiple strengths including an experimental design; matching for number and length of sessions in the two 6-month interventions; use of social psychological theory to inform the skills and experiential activities in the stability skills first maintenance module; and thorough trial implementation as evident by the excellent attendance and long-term retention at clinic assessments that did not differ by intervention condition.
In summary, learning stability skills first, a multi-component approach, appeared successful for maintaining weight loss in the absence of intervention staff contact during a 12-month follow-up period. These results can inform the study design and development of future innovative interventions aimed at the long-term regulation of lifestyle and self-regulatory behaviors essential for the treatment of obesity.
Acknowledgments
The research was supported by Public Health Service Grant R01 CA112594 to Michaela Kiernan from the National Institutes of Health. The funding source did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We gratefully thank the trial participants and research staff, and also thank Dr. Joan Fair for her insightful expertise.
Footnotes
Requests for reprints should be sent to the corresponding author.
The authors have no potential conflicts of interest and no financial disclosures.
References
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology. 2008;27(3):379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- Baker RC, Kirschenbaum DS. Weight control during the holidays: Highly consistent self-monitoring as a potentially useful coping mechanism. Health Psychology. 1998;17(4):367–370. doi: 10.1037/0278-6133.17.4.367. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: Social cognitive theory. Englewood Cliffs, New Jersey: Prentice Hall, Inc; 1986. [Google Scholar]
- Barte JCM, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obesity Reviews. 2010;11(12):899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74(5):1252–1265. doi: 10.1037/0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology. 2005;73(5):852–860. doi: 10.1037/0022-006X.73.5.852. [DOI] [PubMed] [Google Scholar]
- Bower GH. How might emotions affect learning? In: Christianson S, editor. The Handbook of Emotion and Memory: Research and Theory. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Brown SD, Lee K, Schoffman DE, King AC, Crawley LM, Kiernan M. Using direct mail to enhance minority recruitment to clinical trials: Experimental findings and practical recommendations. Contemporary Clinical Trials. 2012;33(4):620–623. doi: 10.1016/j.cct.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. American Psychologist. 1986;41(7):765–782. doi: 10.1037/0003-066X.41.7.765. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58(3):403–417. doi: 10.1093/biomet/58.3.403. [DOI] [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & Behavior. 2010;100(5):438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on the Identification Evaluation and Treatment of Overweight and Obesity in Adults. Executive Summary of the Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Archives of Internal Medicine. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Education and Research. 2005;20(4):439–447. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- Gorin AA, Niemeier HM, Hogan P, Coday M, Davis C, DiLillo VG, et al. Binge eating and weight loss outcomes in overweight and obese individuals with type 2 diabetes: Results from the Look AHEAD trial. Archives of General Psychiatry. 2008;65(12):1447–1455. doi: 10.1001/archpsyc.65.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women. Journal of the American Medical Association. 1999;282(16):1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL. Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Preventive Medicine. 1984;13(2):155–168. doi: 10.1016/0091-7435(84)90048-3. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR. Long-term maintenance of weight loss: Current status. Health Psychology. 2000;19(1 Suppl):5–16. doi: 10.1037//0278-6133.19.1. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, French SA. Preventing weight gain in adults: The Pound of Prevention study. American Journal of Public Health. 1999;89(5):747–751. doi: 10.2105/ajph.89.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Levy RL, Langer SL, Welsh EM, Flood AP, Jaeb MA, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Preventive Medicine. 2009;49(5):384–389. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Research design in clinical psychology. 2. Boston, MA: Allyn and Bacon; 1992. [Google Scholar]
- Kiernan M, Goldberg JH, Cooper L, Lee R, Olson S, Raymond P. Efficacy of taste-based goal setting: Results of the Stanford Healthy Weight Project. Annals of Behavioral Medicine. 2005;29:S18. [Google Scholar]
- Kiernan M, Goldberg JH, Durkin L. Having a specified weight range promotes weight stability. Annals of Behavioral Medicine. 2003;25:S23. [Google Scholar]
- Kiernan M, Goldberg JH, Kirkpatrick S, Raymond P. Cognitive strategies used to prevent weight gain. Annals of Behavioral Medicine. 2000;22:S48. [Google Scholar]
- King AC, Castro CM, Pruitt LA, Ahn DK, Prosak C, Buman M, et al. Optimizing diet and exercise changes in chronically stressed adults: Results of the CALM trial. Annals of Behavioral Medicine. 2009;37:S112. [Google Scholar]
- King AC, Friedman R, Marcus BH, Castro C, Forsyth L, Napolitano M, et al. Harnessing motivational forces in the promotion of physical activity: The Community Health Advice by Telephone (CHAT) project. Health Education Research. 2002;17(5):627–636. doi: 10.1093/her/17.5.627. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: The conceptual foundation. Eating Disorders. 2010;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- Kruglanski AW, Mayseless O. Classic and current social comparison research: Expanding the perspective. Psychological Bulletin. 1990;108(2):195–208. doi: 10.1037/0033-2909.108.2.195. [DOI] [Google Scholar]
- Langer EJ. The illusion of control. Journal of Personality and Social Psychology. 1975;32(2):311–328. doi: 10.1037/0022-3514.32.2.311. [DOI] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, Illinois: Human Kinetics Books; 1988. [Google Scholar]
- Lutes LD, Winett RA, Barger SD, Wojcik JR, Herbert WG, Nickols-Richardson SM, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: 3-month evidence from the ASPIRE randomized trial. Annals of Behavioral Medicine. 2008;35(3):351–357. doi: 10.1007/s12160-008-9033-z. [DOI] [PubMed] [Google Scholar]
- Meyers C, Jones TB. Promoting active learning: Strategies for the college classroom. San Francisco: Jossey-Bass Publishers; 1993. [Google Scholar]
- Moher D, Hopewell S, Schultz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. British Medical Journal. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics. 2009;78(5):275–284. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of obesity treatment. New York: Guildford Press; 2002. pp. 357–379. [Google Scholar]
- Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: The treatment of obesity in rural settings (TOURS) randomized trial. Archives of Internal Medicine. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. Journal of Consulting and Clinical Psychology. 2001;69(4):722–726. doi: 10.1037/0022-006X.69.4.722. [DOI] [PubMed] [Google Scholar]
- Perri MG, Nezu AM, Viegener BJ. Improving the long-term management of obesity: Theory, research, and clinical guidelines. New York: John Wiley and Sons; 1992. [Google Scholar]
- Pull CB. Current empirical status of acceptance and commitment therapy. Current Opinions in Psychiatry. 2008;22(1):55–60. doi: 10.1097/YCO.0b013e32831a6e9d. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychology. 2000;19(1 Suppl):64–69. doi: 10.1037//0278-6133.19.1. [DOI] [PubMed] [Google Scholar]
- Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: Preliminary results of a cognitive-behavioral decision-based treatment for obesity. Journal of Consulting and Clinical Psychology. 1999;67(2):260–266. doi: 10.1037/0022-006X.67.2.260. [DOI] [PubMed] [Google Scholar]
- Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, et al. Practices associated with weight loss versus weight-loss maintenance. American Journal of Preventive Medicine. 2011;41(2):159–166. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage; 1999. [Google Scholar]
- Spring B, Doran N, Pagoto S, Schneider K, Pingitore R, Hedeker D. Randomized controlled trial for behavioral smoking and weight control treatment: Effect of concurrent versus sequential intervention. Journal of Consulting and Clinical Psychology. 2004;72(5):785–796. doi: 10.1037/0022-006X.72.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VJ, Obarzanek E, Cook R, Lee I, Appel LJ, West DS, et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Annals of Internal Medicine. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychological Assessment. 2000;12(2):123–131. doi: 10.1037/1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis J, Loria CM, et al. Comparison of strategies for sustaining weight loss: The Weight Loss Maintenance randomized controlled trial. Journal of the American Medical Association. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionniare (PAR-Q) Canadian Journal of Sports Science. 1992;17(4):338–345. [PubMed] [Google Scholar]
- Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2005;28(1):151–170. doi: 10.1016/S0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- West DS, Gorin AA, Subak LL, Foster G, Bragg C, Hecht J, et al. A motivation-focused weight loss maintenance program is an effective alteranative to a skill-basesd approach. International Journal of Obesity. 2011;35(2):259–269. doi: 10.1038/ijo.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Wilfrey DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Archives of General Psychiatry. 2010;67(1):94–101. doi: 10.1001/archgenpsychiatry.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: Are there differences between men and women or between weight loss and maintenance? International Journal of Obesity. 1995;19(1):67–73. [PubMed] [Google Scholar]
- Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. New England Journal of Medicine. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]