Summary
Purpose
Mesial temporal lobe epilepsy (mTLE) is a chronic disorder with spontaneous seizures recurring for years, or even decades. Many structural and functional changes have been detected in both the seizure focus and distal regions throughout the brain over this duration that may reflect the development of epileptogenic networks. Resting state functional Magnetic Resonance Imaging (fMRI) connectivity mapping has the potential to elucidate and quantify these networks. The network between the left and right hippocampus may very likely be one of the most susceptible to changes due to long term seizure propagation effects. Therefore, the objective of this study was to quantify cross hippocampal influence in mTLE using high temporal resolution fMRI, and to determine its relationship with disease duration.
Methods
Functional MRI images were acquired in the resting (interictal) state with 500 ms temporal resolution across the temporal lobes of 19 mTLE patients (13 left, 6 right). The left and right hippocampus was identified on each subject’s images using both structurally defined and functionally defined boundaries. The cross hippocampal influence was quantified in two ways for each pair of regions: (1) the non-directional hippocampal functional connectivity calculated as the Pearson’s correlation between the average time series in the left and the right hippocampus regions, and (2) the Granger causality (GC) laterality measure which implies directional influence by determining temporal precedence. Each of these measures was correlated with age, age of onset and disease duration across subjects to investigate relationship to disease progression.
Key Findings
The hippocampal connectivity was not significantly different between the left and right mTLE patients using either the structurally or the functionally defined regions. Across all patients, hippocampal connectivity was not significantly correlated with age of onset or duration of disease. However, as duration of disease increased after 10 years (9 patients), the hippocampal connectivity increased linearly.
Using the functionally defined regions, the GC laterality was increased in the right mTLE over the left mTLE, indicating that the left hippocampus was influencing the right hippocampus more than the right influencing left. This was also positively correlated with age of onset. Furthermore, like hippocampal connectivity, the relationship between GC laterality and duration of disease changes after 10 years duration of disease. After this duration, the GC laterality was positive in the 3 out of 3 right mTLE patients (left influencing right), while the GC laterality was negative in 5 of 6 left mTLE patients (right influencing left).
Significance
This study reveals a relationship between fMRI functional connectivity and causal influence of the left and right hippocampus and duration of disease in mTLE. During the interictal state, the interhemispheric hippocampal connectivity initially is disrupted and then linearly increases as the epilepsy progresses longer than 10 years. This increase in connectivity appears to be due to the hippocampus contralateral to the epileptogenic focus exerting more influence over the ipsilateral hippocampus. These findings may have implications in understanding the functional development of epileptic networks and possibly prediction of surgical outcome of mTLE.
Keywords: temporal lobe epilepsy, brain, functional MRI, connectivity, hippocampus
Introduction
Mesial temporal lobe epilepsy (mTLE) is a common epileptic syndrome characterized by seizures originating from one or both mesial temporal lobe structures including the hippocampus, often accompanied by hippocampal sclerosis (Margerison & Corsellis 1966). In a large percentage of these patients with seizures refractory to medical intervention (Engel 1993, Schachter & Schomer 1997), the seizures can continue and even progress over decades. The effects of repeated seizures over this long duration have not been fully characterized, but include mossy fiber sprouting (Sutula & Dudek 2007) accompanied by neuronal loss (Hattiangady & Shetty 2008), inflammation (Yang, et al. 2010) and loss of functional inhibition (Dudek & Sutula 2007) in ipsilateral, as well as contralateral, structures (Parekh, et al. 2010). These changes are believed to be at least partially responsible for the structural atrophy (Bernasconi, et al. 2005, Coan, et al. 2009), and glucose metabolism decreases (Akman, et al. 2010) that occur in ipsilateral temporal regions within and outside the hippocampus over time in mTLE. Furthermore, it has been that shown that these structural and functional changes can be seen in the contralateral hippocampus, as well (Araujo, et al. 2006, Jokeit, et al. 1999, Knake, et al. 2009). Therefore, while the evidence supports that mTLE is a widespread and bilateral disease that may progress over time, the implications of this network reorganization remain unclear.
Functional Magnetic Resonance Imaging (fMRI) is well suited to study brain networks using functional connectivity. This method quantifies connectivity between brain regions based on low-frequency (<0.1 Hz) linear correlations of series of temporal fMRI signals (Rogers, et al. 2007). In this study we use functional connectivity mapping to investigate resting-state (interictal) hippocampal network alterations and their relationship to disease duration in mTLE.
While fMRI functional connectivity measurements can allow identification of regions making up a network of interest, these linear correlations do not provide information on the direction of influence between regions. The Granger causality mapping technique (Goebel, et al. 2003, Roebroeck, et al. 2005) was developed to use multivariate autoregressive models of time series data to investigate the amount of variance in one region explained by the signal history in another region. This method allows a means of quantifying the magnitude and direction of influence of one region time series on another.
The objectives of this study were: (1) to investigate resting-state hippocampal functional connectivity in mTLE using high temporal resolution fMRI acquisitions, (2) to quantify direction of influence between these structures using Granger causality analyses of these data, and (3) to determine whether these measures change with disease duration. We hypothesized that connectivity and magnitude of Granger causality between the left and right hippocampus will increase as disease duration increases. As a reflection of potential nodes in the epileptic ictal network, we also hypothesized that the ipsilateral hippocampus will have more influence on the contralateral hippocampus as the disease progresses.
Methods
Subjects
A total of 57 temporal lobe epilepsy patients were recruited from the Vanderbilt University Epilepsy Program. After video-EEG monitoring in the Vanderbilt University Epilepsy Monitoring Unit, 24 were identified as having pure left temporal interictal and ictal EEG and 12 as having pure right temporal EEG. These formed the initial subjects for this study. Of the 24 patients with pure left temporal EEG, 5 did not have all fMRI data collected, and 6 had lesions outside the temporal lobe. Of the 12 patients with pure right temporal EEG, 3 did not have all fMRI data collected, 1 had multifocal lesions or lesions outside the mesial temporal region, 1 had EEG activity localized to the right temporal and occipital regions, and 1 had EEG activity localized to the posterior temporal region. Therefore, 13 patients with pure left mesial temporal EEG (Left mTLE, 9F/4M, 1 left handed, 35.4± 9.4 years) and 6 patients with pure right mesial temporal EEG (Right mTLE, 4F/2M, 1 left handed, 35.8± 11.1 years) were included in this study. See Table 1 for patient characteristics.
Table 1. Patient Characteristics.
| ID | Gender/ Handedness |
Age (yrs) | Age of onset (yrs) |
Seizure types | Interictal and Ictal EEG |
MRI | PET Hypometabolism |
|---|---|---|---|---|---|---|---|
| 17 | F/R | 31 | 26 | SPS, CPS, SGTC | L TEMP | LMTS (body-mod) | LMT |
| 19 | F/R | 34 | 32 | SPS, CPS | L TEMP | Normal | LMT |
| 21 | F/L | 47 | 1.4 | SPS, CPS, SGTC | L TEMP | Normal | LMT |
| 27 | F/R | 45 | 39 | CPS, SGTC | L TEMP | Normal | Not done |
| 30 | M/R | 44 | 1.5 | SPS, CPS, SGTC | L TEMP | bilateral MTS, L>R (head & body–mod) |
LMT |
| 31 | F/R | 39 | 23 | SPS, CPS, SGTC | L TEMP | LMTS (head & body-sev) | LMT |
| 34 | F/R | 46 | 5 | SPS, CPS, SGTC | L TEMP | LMTS (body-mod) | LMT |
| 40 | M/R | 37 | 29 | SPS, CPS, SGTC | L TEMP | Normal | LMT |
| 45 | F/R | 32 | 31 | CPS, SGTC | L TEMP | Normal | Normal |
| 46 | M/R | 22 | 16 | CPS, SGTC | L TEMP | Normal | LMT |
| 53 | F/R | 19 | 1.4 | SPS, CPS, SGTC | L TEMP | LMTS (head & body-mod) | LMT |
| 56 | M/R | 41 | 17 | SPS, CPS, SGTC | L TEMP | Normal | Not done |
| 59 | F/R | 24 | 19 | SPS, SGTC | L TEMP | Normal | Not done |
| 16 | M/L | 53 | 28 | SPS, CPS, SGTC | R TEMP | Normal | RMT |
| 49 | F/R | 29 | 23 | CPS, SGTC | R TEMP | RMTS (head & body-sev) | RMT |
| 51 | F/R | 43 | 23 | SPS, CPS, SGTC | R TEMP | RMTS (body-mod) | RMT |
| 52 | M/R | 33 | 25 | CPS, SGTC | R TEMP | RMTS, encephalomalacia in the R insula (body-mod) |
RMT |
| 54 | F/R | 21 | 15 | CPS, SGTC | R TEMP | Normal | RMT |
| 58 | F/R | 36 | 0.6 | CPS, SGTC | R TEMP | RMTS (body-mild) | RMT |
F = female, M = male, SPS = simple partial seizures, CPS = complex partial seizures, SGTC= secondary generalized tonic clonic seizures, R = right, L = left, TEMP = temporal, MTS = mesial temporal sclerosis, head = atrophy in head of hippocampus, body = atrophy in body of hippocampus, mild = mild atrophy, mod = moderate atrophy, sev = severe atrophy, MT = mesial temporal lobe. First 13 patients listed were considered left mTLE, last 6 were considered right mTLE.
The age of onset and duration of disease were not significantly different between patient groups. Simultaneous scalp electroencephalography (EEG) was acquired during the fMRI using a Neuroscan EEG system (NeuroscanCompumedics, Charlotte, NC) with either a 64 channel cap or 25 individual gold leads (Ives EEG Solutions, Manotick, Ontario, Canada). The EEG data are not presented here. Finally, we also recruited 15 right handed healthy controls subjects for comparison (12F/3M, 31.5± 10.8 years). These subjects underwent the same imaging as the patients without the simultaneous EEG.
Imaging
All MRI imaging was performed using a Philips Achieva 3T MRI scanner (Philips Healthcare, Inc., Best, Netherlands) using an 8-channel head coil. Informed consent wasobtained prior to scanning per Institutional Review Board guidelines. The imaging parameters were as follows: 1) Three-dimensional, T1-weighted high-resolution image seriesacross the whole brain for inter-subject normalization (1 × 1 × 1 mm3), 2) Two-dimensional, T1-weighted high-resolution axial full brain image series in the same slice locations as the fMRI scan #3 for functional to 3D data registration (1 × 1 × 5 mm3), 3) fMRI Blood Oxygenation Level Dependent (BOLD) image series at rest with eyes closed – 64×64, FOV = 240 mm, 30 axial slices, TE=35 ms, TR = 2 sec, slice thickness = 4.5 mm/ 0.5 mm gap, 300 volumes, 4) fMRI BOLD image series at rest with eyes closed covering the temporal lobes (see Figure 1A) – same parameters as above except TR = 500 ms, 9 axial slices, and 400 volumes. 5) Two-dimensional, T1-weighted high-resolution 9 axial slice image series covering the temporal lobes for functional (scan #4) to 3D data registration (1 × 1 × 5 mm3). The 2 second TR fMRI data (scans 2 and 3) were not included in the present analyses. All subsequent references to fMRI data are referring to the 500 ms TR fMRI data covering the temporal lobes (scan 4) unless specified.
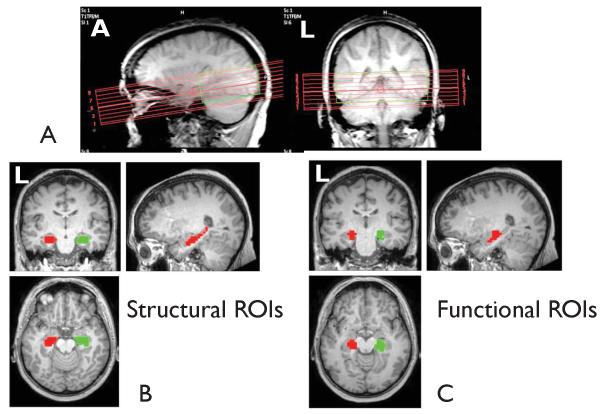
Figure 1.
(A) Sagittal and coronal structural MRI images showing the placement of the nine axial imaging slices for the fMRI acquisitions with TR=500 ms. (B) Location of the left hippocampus (red) and right hippocampus (green) structural ROIs determined using the Harvard-Oxford atlas. (C) Location of the left hippocampus (red) and right hippocampus (green) functional ROIs determined using 2dTCA and functional connectivity in a separate set of patients.
Image Preprocessing and Regions of Interest Identification
The images were analyzed using SPM8 image analysis software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The fMRI image sets were corrected for slice timing effects, motion corrected and spatially normalized to the Montreal Neurological Institute (MNI) template using co-registration to the three-dimensional (scan 1) and the nine slice, two-dimensional T1-weighted structural image set (scan 5) as intermediate steps. This resulted in functional image series of 46 × 55 × 46 voxels (4 × 4 × 4 mm3).
We used two methods individually to identify the left and right hippocampal regions of interest (ROI) in order to compare the effect of ROI definition on the subsequent analyses. First, structural ROIs were identified as the left and right hippocampus using the Harvard-Oxford atlas (http://web.mit.edu/mwaskom/pyroi/harvard_oxford_ref.html) set at 50% probability threshold in the FSL software package (http://www.fmrib.ox.ac.uk/fsl/). These regions are based on manually segmented T1-weighted MRI images of 37 healthy subjects (Figure 1B). The left hippocampus structural ROI volume was 4672 mm3 and the right was 4864 mm3.
Next, ROIs were identified functionally as regions found to be active interictally in a separate set of left mTLE patients. The functional ROIs were taken from a previously published study (Morgan, et al. 2010). The subjects in that study were a homogeneous group of five left mesial mTLE patients (3F/2M, mean age 34.8 ± 7.4 yrs). All had left mesial temporal epileptogenic zones based on the presurgical evaluation results and freedom from disabling seizures for at least one year after left selective amygdalohippocampectomy. Four had left mesial temporal sclerosis confirmed by tissue pathology, and one had a left hippocampal lesion of increased T2 signal on MRI. The 2dTCA analysis (Morgan & Gore 2009, Morgan, et al. 2008) was used to detect fMRI BOLD changes interictally in the left hippocampal region across the group. This was used as the functionally defined left hippocampal ROI. Next, a seed based functional connectivity analysis using the activated left hippocampus ROI as the seed region and time courses of motion and cerebral spinal fluid as confounds was performed. This identified several regions functionally connected to the left hippocampus region (FWE p<0.001, cluster 10). One region in the right hippocampus was identified and used as the right hippocampal ROI. These functionally derived ROIs were applied to all subjects in this study and are shown in Figure 1C. The volumes of the functional ROIs were 2432 mm3 and 2624 mm3 for the left and right hippocampus, respectively.
Hippocampal Connectivity
The same procedure was used to calculate the connectivity between the left and right hippocampal structural ROIs, and the left and right hippocampal functional ROIs, separately. The ROIs were identified on the fMRI image sets. The time course of each voxel in the brain was high pass filtered at a cutoff period of 150 s to remove low frequency drifts. Then the average time course in each ROI and the average time course in the whole brain volume (global time course) were determined. The global time course was used to correct for physiological noise effects, as other corrections such as cerebral spinal fluid time courses could not be consistently identified on all datasets due to the small imaging volume. The global time course, the six motion time courses determined from the motion correction, and the six first derivatives of the motion time courses were linearly regressed from each ROI time course. Each ROI time course was then low pass filtered (<0.1Hz) (Cordes, et al. 2001) and the correlation coefficient between the preprocessed left and right hippocampal ROI time courses was determined. This value was transformed to a Z value using the Fisher Z transformation and used as the measure of hippocampal connectivity. The statistical significance of the linear correlation between hippocampal connectivity and age, age of onset and disease duration across patients was assessed with IBM SPSS version 19 (IBM Corp., Somer, NY,USA). Hippocampal connectivity was also calculated in the healthy controls using the same procedure and ROIs.
Granger Causality Analysis
A Granger causality analysis (Goebel, et al. 2003, Roebroeck, et al. 2005) of temporal precedence was performed using both sets of ROIs as described in (Rogers, et al. 2010a) and briefly outlined in the Supplementary Appendix S1. This analysis resulted in a Granger causality laterality index (GC laterality) which quantifies the direction and magnitude of influence between the left and right hippocampus ROIs. This index is zero when the influence between left and right hippocampus is equal. It is positive when the left hippocampus is influencing the right hippocampus more than the right hippocampus is influencing the left, and is negative when the opposite is true.
The GC laterality was compared between the left mTLE and the right mTLE patients using an unpaired T-test. Pearson’s correlations were used to determine significant linear correlations between GC laterality and age, age of onset and duration of disease. The GC laterality indices were also determined for the healthy controls.
Results
Hippocampal Connectivity
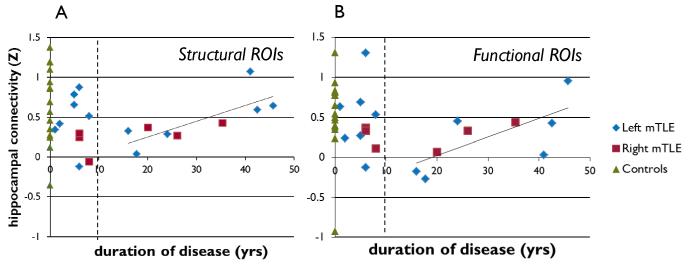
Using the structural ROIs, the hippocampal connectivity in the left mTLE patients was (mean ± stdev) 0.49 ± 0.33, and was 0.26 ± 0.17 in the right mTLE patients. Across all patients, hippocampal connectivity was not significantly correlated with age (Pearson’s correlation coefficient (cc) = 0.020, p = 0.935), age of onset (cc = −0.380, p = 0.108) or duration of disease (cc = 0.316, p = 0.187). However, a relationship between this hippocampal connectivity and duration of disease is apparent in Figure 2A. When duration of disease is less than 10 years (10 patients), hippocampal connectivity is highly variable. However, as duration of disease increases after 10 years (9 patients), the hippocampal connectivity increased linearly (cc = 0.770, p = 0.015). The average hippocampal connectivity for the healthy controls was 0.66 ± 0.48.
Figure 2.
Relationship between hippocampal connectivity and duration of disease across left and right mTLE patients. (A) Results using structurally defined ROIs. (B) Results using functional ROIs. Both analyses show linear increase in hippocampal connectivity with increasing duration of disease after 10 years depicted by the linear trend line.
Using the functional ROIs the hippocampal connectivity was 0.38 ± 0.46 and 0.27 ± 0.15 in the left mTLE and right mTLE patients, respectively. A similar relationship to duration of disease was found using the functional ROIs. There was no significant correlation between hippocampal connectivity and age (cc = −0.147 p = 0.549), age of onset (cc = −0.149, p = 0.543) or duration of disease (cc = 0.022, p = 0.927) across all subjects. However, after 10 years duration, hippocampal connectivity increased linearly (cc = 0.707, p = 0.033) (Figure 2B). In the healthy controls, the average hippocampal connectivity was 0.53 ± 0.49.
Granger Causality Analysis
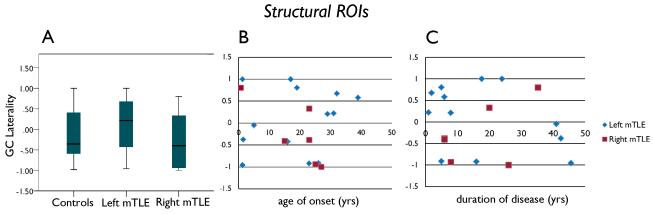
Using the structural ROIs, the GC laterality between the left and right mTLE patients were not significantly different (mean ± stdev: left mTLE = 0.062 ± 0.73, right mTLE = −0.266 ± 0.70; p = 0.369) (Figure 3A). In addition, using the structural ROIs there was no significant linear correlation between the GC laterality and age, age of onset or duration of disease (Figure 3B,C). The GC laterality in the control subjects varied greatly with an average of −0.09 ±0.69. This is shown by the shaded area in all parts of Figure 3.
Figure 3.
GC laterality results determined using the structural ROIs. (A) GC laterality in controls, left TLE and right TLE patients. (B) GC laterality vs. age of onset. (C) GC laterality vs. duration of disease. There were no differences between controls, left and right mTLE patients, and no relationships between GC Laterality and age of onset or duration of disease. Positive GC laterality indicates left hippocampus influences right. Negative GC laterality indicates right hippocampus influences left.
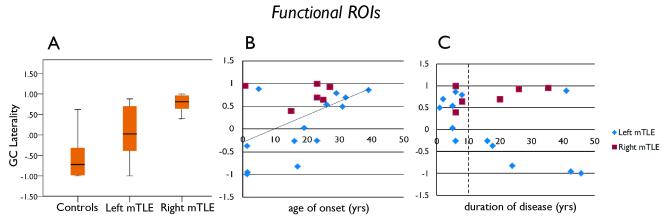
However, using the functional ROIs, the GC laterality was increased in the right mTLE over the left mTLE, indicating that in right mTLE the left hippocampus was influencing the right hippocampus more than the right was influencing the left (left mTLE = 0.045 ± 0.70, right mTLE = 0.767 ± 0.23; p = 0.004; 95% confidence interval of the difference = −1.18 to −0.26) (Figure 4A). The functional ROI based GC laterality was also positively correlated with age of onset (cc = 0.514, p = 0.024) (Figure 4B), but not age. This relationship may be further explained when looking at GC laterality vs. duration of disease (Figure 4C). There is no linear correlation between these measures across all subjects, however, like hippocampal connectivity, the relationship changes after 10 years duration of disease. After 10 years, the GC laterality was positive (left influencing right) in the 3 out of 3 right mTLE patients, while the GC laterality was negative (right influencing left) in 5 of 6 left mTLE patients. The GC laterality in the healthy controls was negative (−0.53 ± 0.55) indicating the right hippocampus influencing the left. This value is significantly lower than both the left and right mTLE patient groups.
Figure 4.
GC laterality results determined using the functional ROIs. (A) GC laterality in controls, left mTLE and right mTLE patients. (B) GC laterality vs. age of onset. (C) GC laterality vs. duration of disease. GC laterality is greater in right mTLE than in left mTLE (p = 0.004), and is greater in left mTLE than in controls (p = 0.026), and GC laterality increases as age of onset increases (indicated by linear trend line). Positive GC laterality indicates left hippocampus influences right. Negative GC laterality indicates right hippocampus influences left.
Discussion
This study represents one of the first attempts to quantify resting-state cross hippocampal connectivity and direction of influence in mTLE in relation to disease duration. In order to do this, we utilized higher temporal resolution fMRI acquisitions than conventionally used for fMRI studies of epilepsy (Bettus, et al. 2010, Pereira, et al. 2010, Waites, et al. 2006). Using these measures we were able to identify relationships between hippocampal connectivity and influence and disease duration across this group of left and right mTLE patients.
Hippocampal Connectivity
We found that resting-state cross hippocampal functional connectivity measured with high temporal resolution fMRI increases linearly as duration of disease increases after 10 years. Figures 2A and 2B indicate that the initial effect of spontaneous unilateral hippocampal seizures is the apparent disruption of the interhemispheric hippocampal network, while the long term effect is a rebuilding of the connection. Both structurally defined and functionally defined ROIs detected this relationship. These changes may reflect the brain’s plasticity over time as it is “learning” to use this aberrant network (Lewisa, et al. 2009, Ma, et al. 2010). These changes may also be responsible for the progressive memory impairments associated with chronic mTLE (Helmstaedter, et al. 2003). However, it is not clear if the progressive neuronal death and formation of recurrent excitatory circuits found in the studies of humans and animal models (Dudek & Sutula 2007) are the mechanism for these functional connectivity changes, and why the changes occur in a biphasic manner with differences occurring before and after 10 years duration.
In a previous report of hippocampal connectivity in mTLE, Pereira, et al. (2010) detected decreases in hippocampal functional connectivity in mTLE compared to controls. The duration of disease was not reported in this study. We found that connectivity in the patients using the functional ROIs is initially similar in comparison with controls; but in patients with about 8 to 20 years duration of disease, the connectivity may be less than controls. However, we did not have enough subjects to determine this statistically.
The results of this study should also be interpreted in relation to previous work investigating the interhemispheric functional connectivity in mTLE during a spatial memory task (Frings, et al. 2008). During the performance of this task, which utilizes the cross hippocampal network, the connectivity was decreased as duration of disease increased. These findings suggest that the repeated seizures or longer duration of damage reduced the ability of the hippocampus to engage in this task, which is then manifested in spatial memory dysfunction in these patients. The current study was acquired in the resting-state and showed the opposite effect, with connectivity increasing with disease progression. It is possible that the change in connectivity is not related to interictal spiking frequency which increases at rest (Mendez & Radtke 2001), but rather to physiological interaction between the two hippocampi. There are methodological differences between the two studies which may account for some differences, as well. The spatial memory task was performed and analyzed in a series of blocks of 26 to 28 seconds in which the subject performed either memory encoding, memory retrieval or a control condition. The connectivity measure was then determined across all of these conditions. Also, the fMRI acquisition temporal resolution of the spatial memory task was 4 sec using a 1.5T MRI scanner which may not provide the temporal signal to noise required to detect higher frequency signal correlations as in this study.
Granger Causality Analysis
The results of the Granger causality analyses using the functional ROIs showed an increase in GC laterality in right mTLE but not left mTLE patients. This increase in GC laterality indicates that the left hippocampus influences the right hippocampus more in right mTLE. While the GC laterality increases as age of onset increases, this does not explain the differences in these two patient groups, as the age of onset is not significantly different between them.
The GC laterality may be explained further by correlating it with duration of disease (Figure 4C). Similar to the hippocampal connectivity, the values before 10 years duration show a different relationship than after 10 years. In the initial 10 years of seizures, the GC laterality is generally positive indicating that the left hippocampus influences the right. It might be supposed that this is the “normal” direction for this circuit; but it is the opposite of what we found in the controls, where the GC laterality was negative. The controls were all right handed and all but one in each patient group was right-handed. Other factors such as language dominance or memory function, may play a role in the direction of this circuit. Further studies including cognitive assessments may help validate and elucidate these findings.
After 10 years, the GC laterality was positive in right mTLE (left influencing right) and was generally negative in left mTLE (right influencing left), in all but one patient. This suggests that the contralateral hippocampus increases in its influence on the ipsilateral hippocampus as the disease progresses. We had hypothesized that the ipsilateral hippocampus would influence the contralateral hippocampus as the ictal propagation network strengthens with disease duration. The results suggest the opposite may be true. We speculate that this may be a reflection of the extent of neuronal damage of the ipsilateral hippocampus, with the contralateral hippocampus acquiring dominance (Dudek & Sutula 2007).
Studies have investigated resting-state fMRI hippocampal connectivity to other intrahemispheric structures in mTLE (Bettus, et al. 2010, Bettus, et al. 2009). They concluded that increases in contralateral hippocampal connectivity may be a compensatory mechanism in response to the decreases in hippocampal connectivity in the ipsilateral hemisphere as compared to controls. This compensation of regions in the contralateral hemisphere may also be responsible in part for our GC laterality findings.
The anatomic ROIs did not reveal the same relationships of Granger causality that the functional ROIs did. The anatomic ROIs overlapped with the functional ROIs, but were larger in volume than the functional ROIs and incorporated both anterior and posterior sections of the hippocampus, while the functional ROIs covered mostly the anterior portion. Other studies have determined differing connectivity between these two regions (Bettus, et al. 2010, Bettus, et al. 2009). In addition, surgical treatment for mTLE usually involves resection of the anterior portion of the hippocampus which assumes that this region is responsible for generating the ictal activity (Siegel 2004, Wiebe, et al. 2001). The fact that we identified these regions as being activated interictally in a small homogeneous group of left mTLE patients (Morgan, et al. 2010), supports this.
Methodological Considerations
The most notable advantage of this study is the use of an increased temporal sampling rate for the fMRI acquisitions. In calculating functional connectivity, faster imaging allows for more accurate sampling of respiratory (Birn, et al. 2006) and cardiac (Bhattacharyya & Lowe 2004) induced noise fluctuations which are known to confound these measures. This leads to better modeling of these effects for more accurate artifact removal. In calculating Granger causality, it has been shown (Deshpande, et al. 2010, Roebroeck, et al. 2005, Rogers, et al. 2010b) that increased sampling rate improves sensitivity of this measure to detect neuronal signal lags on the order of milliseconds.
One primary limitation of this work is the small number of left and right mTLE patients. Our intention was to maintain as much uniformity as possible across the patients, even if sacrificing sample size. Incorporating patients with normal interictal EEG or EEG epileptiform activity outside the unilateral temporal lobe may have reduced detectability of the relationship between connectivity and duration of disease.
This work focused only on connectivity between the left and right hippocampus. We were not able to examine other intrahemispheric or interhemispheric connections due to the limited volume coverage afforded by the increased temporal resolution.
In addition, we applied the same regions of interest on all subjects after spatial normalization to a template. However, it is known that many subjects had mesial temporal sclerosis which may affect how accurately the ROIs define the actual anatomic and functional regions. Qualitative visual review of coronal T2-weighted MRI images indicated that all subjects with mesial temporal sclerosis showed atrophy in the body of the hippocampus, with some also including the head (see Table 1). This pattern of atrophy would affect both types of ROIs similarly, but the differing levels of atrophy may introduce variation in the accuracy of these regions between subjects.
We used the average time course across all voxels in these ROIs to calculate the connectivity and Granger causality. It is possible that this average may not represent the ROIs across subjects equally. We attempted to measure this by computing the correlation between this averaged time course and each voxel’s time course in each ROI in each subject. This value was higher in the functional ROIs than in the structural ROIs determined by a 2-way ANOVA, but was not different between the groups. This increase in the functional ROIs may be due to their smaller number of voxels, or may indicate that they define a more functionally homogeneous region.
The patients had greater motion during the scanning than the healthy controls (mTLE = 1.016 ± 0.926 mm, controls = 0.371 ± 0.292 mm; p = 0.009, unpaired T-test). This may introduce more variation in the measures across patients than controls. No controls and only two patients showed more than 2mm motion. One of these patients is the right mTLE patient with 35 years duration, and the other one is the left mTLE patient with 41 years duration. No subjects were excluded for motion because we only had a few with such long duration of disease.
Conclusions
This study reveals a relationship between fMRI functional connectivity and causal influence of the left and right hippocampus and duration of disease in mTLE. During the interictal state the interhemispheric hippocampal connectivity initially is disrupted and then linearly increases with the epilepsy duration longer than 10 years. This increase in connectivity appears to be due to the hippocampus contralateral to the epileptogenic focus exerting more influence over the ipsilateral hippocampus. These findings have implications in understanding the functional development of epileptic networks and prediction of surgical outcome of mTLE.
Supplementary Material
Acknowledgements
This work was supported in part by NIH R01 NS055822.
Footnotes
Disclosure
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
Appendix S1. Description of Granger causality analysis of functional connectivity data.
References
- Akman CI, Ichise M, Olsavsky A, Tikofsky RS, Van Heerturn RL, Gilliam F. Epilepsy duration impacts on brain glucose metabolism in temporal lobe epilepsy: Results of voxel-based mapping. Epilepsy Behav. 2010;17:373–380. doi: 10.1016/j.yebeh.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo D, Santos AC, Velasco TR, Wichert-Ana L, Terra-Bustamante VC, Alexandre V, Carlotti CG, Assirati JA, Machado HR, Walz R, Leite JP, Sakamoto AC. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia. 2006;47:1354–1359. doi: 10.1111/j.1528-1167.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy - Differential atrophy in mesial temporal structures. Neurology. 2005;65:223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2010;81:1147–1154. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Human Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya PK, Lowe MJ. Cardiac-induced physiologic noise in tissue is a direct observation of cardiac-induced fluctuations. Magn Reson Imaging. 2004;22:9–13. doi: 10.1016/j.mri.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73:834–842. doi: 10.1212/WNL.0b013e3181b783dd. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X. Effect of hemodynamic variability on Granger causality analysis of fMRI. Neuroimage. 2010;52:884–896. doi: 10.1016/j.neuroimage.2009.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Dentate Gyrus: A Comphrehensive Guide to Structure, Function, and Clinical Implications. Elsevier Science Bv; Amsterdam: 2007. Epileptogenesis in the dentate gyrus: a critical perspective; pp. 755–773. [DOI] [PubMed] [Google Scholar]
- Engel J. Surgical Treatment of the Epilepsies. In: Engel J, Shewmon D, editors. Who should be a surgical candidate? Raven Press; New York: 1993. pp. 23–34. [Google Scholar]
- Frings L, Schulze-Bonhage A, Spreer J, Wagner K. Reduced interhemispheric hippocampal BOLD signal coupling related to early epilepsy onset. Seizure. 2008;18:153–157. doi: 10.1016/j.seizure.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49:26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A, Arnold S, Schuller M, Antke C, Huang YX, Steinmetz H, Seitz RJ, Witte OW. Bilateral reductions of hippocampal volume, glucose metabolism, and Wada hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. J Neurol. 1999;246:926–933. doi: 10.1007/s004150050484. [DOI] [PubMed] [Google Scholar]
- Knake S, Salat DH, Halgren E, Halko MA, Greve DN, Grant PE. Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic Disord. 2009;11:244–250. doi: 10.1684/epd.2009.0272. [DOI] [PubMed] [Google Scholar]
- Lewisa CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. ProcNatlAcadSci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JAN. Epilepsy and the Temporal Lobes: A clinical electroencephalographic and neuropathological study of brain in epilepsy with particular reference to temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Mendez M, Radtke RA. Interactions between sleep and epilepsy. J Clin Neurophysiol. 2001;18:106–127. doi: 10.1097/00004691-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Gore JC. Detection of irregular, transient fMRI activity in normal controls using 2dTCA: Comparison to event-related analysis using known timing. Human Brain Mapp. 2009;30:3393–3405. doi: 10.1002/hbm.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Li Y, Abou-Khalil B, Gore JC. Development of 2dTCA for the detection of irregular, transient BOLD activity. Human Brain Mapp. 2008;29:57–69. doi: 10.1002/hbm.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh MB, Carney PR, Sepulveda H, Norman W, King M, Mareci TH. Early MR diffusion and relaxation changes in the parahippocampal gyrus precede the onset of spontaneous seizures in an animal model of chronic limbic epilepsy. Exp Neurol. 2010;224:258–270. doi: 10.1016/j.expneurol.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FRS, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HFB, Castellano G, Covolan RJM, Damasceno BP, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Katwal SB, Morgan VL, Asplund CL, Gore JC. Functional MRI and multivariate autoregressive models. Magn Reson Imaging. 2010a;28:1058–1065. doi: 10.1016/j.mri.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Katwal SB, Morgan VL, Asplund CL, Gore JC. Functional MRI and multivariate autoregressive models. Magn Reson Imaging. 2010b;28:1058–1065. doi: 10.1016/j.mri.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter SC, Schomer DL. The Comprehensive Evaluation and Treatment of Epilepsy. In: Schlaug G, Patel M, editors. Radiographic assessment of patients with epilepsy. Academic Press; San Diego: 1997. pp. 37–60. [Google Scholar]
- Siegel AM. Presurgical evaluation and surgical treatment of medically refractory epilepsy. Neurosurg Rev. 2004;27:1–18. doi: 10.1007/s10143-003-0305-6. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Dentate Gyrus: A Comphrehensive Guide to Structure, Function, and Clinical Implications. Elsevier Science Bv; Amsterdam: 2007. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system; pp. 541–563. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Yang TH, Zhou D, Stefan HM. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: Uncontrolled inflammation drives disease progression? J Neurol Sci. 2010;296:1–6. doi: 10.1016/j.jns.2010.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.