Abstract
Background
The aim of this study was to detect the expression of cold-inducible RNA-binding protein in pituitary adenoma and to determine its effects on tumor recurrence.
Material/Methods
We collected a total of 60 post-op samples collected from pituitary adenoma patients (including 20 cases of invasive pituitary adenoma, 20 cases of non-invasive adenoma, and 20 cases of non-invasive recurrent adenoma) admitted in our hospital. Both protein and mRNA levels of CIRP in 3 types of pituitary adenoma samples were quantified by Western blotting and real-time PCR, respectively.
Results
Western blotting revealed significantly elevated CIRP expression levels in invasive pituitary adenoma compared to non-invasive tumors, with statistical significance (p<0.05). Recurrent pituitary adenoma expressed significantly higher CIRP levels compared to non-recurrent tumors (p<0.05). Real-time PCR for CIRP mRNA obtained consistent results: transcript levels were significantly higher in invasive pituitary adenoma compared to non-invasive adenoma (p<0.05); recurrent adenoma also had significantly higher CIRP mRNA levels compared to non-recurrent tumors (p<0.05). Among all 3 types of pituitary adenoma, recurrent tumors had the highest levels of CIRP mRNA and protein.
Conclusions
The expression of CIRP in pituitary adenoma is closely related with tumor proliferation and invasion, and its significantly elevated expression level indicates post-op recurrence.
Keywords: ACTH-Secreting Pituitary Adenoma, Neoplasm Invasiveness, RNA-Binding Protein EWS
Background
As a common intracranial benign tumor, pituitary adenoma accounts for about 10% of all intracranial tumors and has an increasing rate of incidence [1,2]. Pituitary adenoma stimulates hypophysis to over-secrete hormones, which can disrupt the body endocrine system, further causing damage to multiple tissues and organs. The tumor-induced pituitary hypo-function can also severely impair patient quality of life, endanger physical and mental heal, or even cause death [3]. Although pituitary adenoma is widely accepted as a benign intracranial tumor, it is often found to invade toward peripheral brain tissues, including cavernous sinus on both sides, sphenoid sinus, suprasellar and parasellar regions, or even damage brain tissues and structures such as those in the sellar region and vomer peripheral region [4]. Thus pituitary adenoma may have some biological features of malignant tumors, causing the lower rate of complete resection and higher post-op recurrence rate, even when being assisted with radio- or chemo-therapy [5]. Therefore, it is extremely difficult to completely cure pituitary adenoma, which is now a major health issue and attracts wide research attention. Pituitary adenoma recurrence has been well defined by studies on biological behaviors of pituitary tumors. Complicated mechanisms, including multiple steps and various factors related with tumor invasiveness and growth, are involved in the pathogenesis of recurrent pituitary adenoma [6]. Current opinions agree that pituitary adenoma’s biological behavior is difficult to evaluate objectively solely based on clinical diagnostic methods such as symptoms and radiological and histopathological examinations [7]. Therefore, current clinical research focuses on finding an accurate and simple method to detect the tumor type in order to provide theoretical evidence for the diagnosis of recurrent pituitary adenoma.
Cold-inducible RNA-binding protein (CIRP) was first identified from mouse testicular cells in 1997 and was later proved to be expressed in various cells, including recombinant cells of human and mammalians [8]. It can be over-expressed if accompanied with moderate cold stress [9]. As more knowledge has been obtained about CIRP, it is found to participate in various biological activities beyond the cold-induced response, suggesting its critical role in body physiological functions [10,11]. Changes in temperature, hypoxia, local ischemia, hydrogen peroxide, and osmotic pressures can induce the expression of CIRP, which participates in regulating endometrial cycles, embryonic development, oncogenesis, and hypothermia protecting tumor necrotic factor-induced cell apoptosis, in addition to the elevation of recombinant protein yields via its over-expression in recombinant cells [12–14]. The potential role of CIRP expression in pituitary adenoma as a tumor’s biomarker, however, has not been fully defined. This study therefore aims to measure the expression profile of CIRP in pituitary adenoma and to investigate its effects on tumor recurrence.
Material and Methods
Research objects and sample collection
We selected 60 pituitary adenoma patients admitted to our Department of Neurosurgery, Shandong Provincial Hospital, Shandong University between January 2011 and December 2013. All patients had undergone surgical resections. There were 27 males and 33 females included in this study, with age between 24 and 58 years (average=33.4±15.3 years). In a pathological classification, there were 20 patients with invasive pituitary adenoma, 20 patients with non-invasive pituitary adenoma, and 20 patients with non-invasive recurrent adenoma. All patients presented clinical symptoms consistent with diagnostic criteria and were confirmed by radiology, or surgical and pathological examinations. Patients had no history of using bromocriptine or other growth hormone inhibitors. All included patients had no statistical significant difference in general information such as age or sex. Written consent was obtained from all research subjects. The protocol of this study was pre-approved by the ethics committee of our hospital. Tumor samples collected from surgical resection were divided into small cubes in a sterile environment and were stored in cryopreservation tubes, which were immediately stored in liquid nitrogen for further use.
Reagents
PVDF membrane was purchased from Pal Life Science. EDTA was a product of Hyclone (USA). Chemical reagents in Western blotting were purchased from Beyotime Biotech (Shanghai, China). ECL reagents were produced by Amersham Biosci. Trizol reagents, RNA extraction kit, RT-PCR primers, RT kits, and real time-PCR reagents were all purchased from Invitrogen (US). Rabbit anti-human CIRP antibody was a product of DPC Biermann (Germany). Mouse anti-rabbit IgG secondary antibody horseradish peroxidase conjugate was purchased from Cell Signaling (USA). β-actin antibody was produced by Santa Cruz (USA).
Detection of CIRP protein in pituitary adenoma by Western blotting
Total tissue proteins were extracted from pituitary adenoma. In brief, lysis buffer was added to tissues and kept on ice for 15~30 min, followed by ultrasonic cell rupture for 5 sec × 4 times. Tissue mixtures were centrifuged at 10 000 g for 15 min. Supernatants were then transferred to a new Eppendorf tube, in which proteins were quantified and kept at −20°C for Western blotting. Tissues lysis mixtures after processing were added with loading buffer, denatured at 95°C for 5 min, and separated using 10% SDS-PAGE protein electrophoresis. After separation, proteins were then transferred onto the PVDF membrane and blocked in defatted milk powder for 2 h at room temperature to decrease non-specific backgrounds. CIRP antibody diluted in 1:500 was incubated with the membrane at 4°C overnight and washed with PBST. Mouse anti-rabbit IgG secondary antibody (1:2 000) was added to incubate the membrane for 30 min at room temperature in the dark. After a general PBST washing, enhanced ECL chromogenic substrate was used to develop the membrane for 1 min, followed by X-ray exposure; images were scanned by Bio-Rad image analysis system and density of bands were measured by Quantity One software. Anti-actin antibody was used an internal reference to measure sample volume of all groups. All experiments were repeated 4 times (N=4) and data were collected and analyzed.
Quantification of CIRP mRNA in pituitary adenoma by real-time PCR
In a sterile environment, frozen tissue samples of pituitary adenoma were quickly ground on ice using a tissue-grinding rod. After complete homogenization, tissue mixtures were eluted repeatedly with 150 μl buffer to make a clear solution, which was then transferred to sterile centrifuged tubes. Using high-speed centrifugation at 20 000 rpm for 2 min, supernatants were transferred to new sterile tubes. Tissue mRNA was extracted by Trizol reagents. cDNA was synthesized using specific PCR primers. (Forward primer for CIRP: 5′-CAAGT ACGGA CAGAT CTCTG A-3′; Reverse primer for CIRP: 5′-CGGAT CTGCC GTCCA TCTA-3; Forward primer for GAPDH: 5′-AGTAC CAGTC TGTTG CTGG-3′; Reverse primer for GAPDH: 5′ –TAATA GACCC GGATG TCTGG T-3′). Real-time PCR used the following condition: 50°C for 1 min; 95°C denature 30 s, 56 or 60°C annealing for 50 s, plus 72°C extension for 35 sec, repeated for 35 cycles. Using GAPDH as an internal reference, data were collected based on CT numbers calculated from built-in software of the fluorescence quantitative PCR reactor. A standard curve was plotted from CT values of standard samples and acted as a basis for quantitative analysis using the 2−ΔCt method.
Statistical analysis
SPSS 12.0 software package (IBM Corp., USA) was used to process all collected data. Enumeration data were analyzed in chi-square test, while measurement data are presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) was utilized to compare means among multiple groups. Statistical significance was defined as p<0.05.
Results
General information on patients
An analysis of clinical records of pituitary adenoma patients, including invasive adenoma, non-invasive adenoma, and non-invasive recurrent adenoma, showed that the majority of patients in all 3 tumor types were young people, with an average age of 33.4±15.3 years. The incidence of pituitary adenoma has no sex bias. Patients among all 3 types had no statistically significant differences in age or sex, as shown in Table 1. There was no statistical difference in general information across all included patients (p>0.05).
Table 1.
General information of patients.
| Type of pituitary adenoma (N) | Sex (M/F) | Age (year) | Tumor size (mm) |
|---|---|---|---|
| Non-invasive pituitary adenoma (20) | 11/9 | 32.1±12.35 | 19.2±6.38 |
| Invasive pituitary adenoma (20) | 8/12 | 34.5±13.16 | 21.3±5.17 |
| Recurrent pituitary adenoma (20) | 9/11 | 33.7±14.66 | 20.6±5.22 |
Expressional profiles of CIRP protein in pituitary adenoma
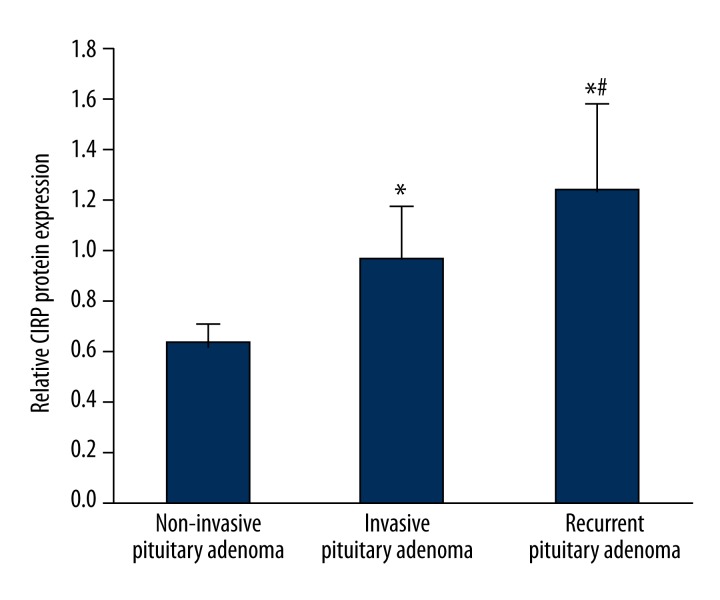
Tissue proteins were extracted from samples of invasive pituitary adenoma, non-invasive adenoma, and non-invasive recurrent adenoma, and were semi-quantitatively analyzed by Western blotting. Results showed that CIRP protein was expressed in all 3 types of pituitary adenoma tissues, but with different levels: non-invasive adenoma had the lowest CIRP proteins; invasive pituitary adenoma had significantly higher CIRP protein levels compared to non-invasive tumors with statistical significance (p<0.05); and CIRP protein expression was further elevated for recurrent pituitary adenoma with statistically significant difference compared to non-recurrent tumors (p<0.05) as shown in Figures 1 and 2. These results suggest that CIRP is directly related with tumor oncogenesis and progression, and may be an important biomarker.
Figure 1.
Expressional profiles of CIRP protein in pituitary adenoma by Western blotting. 1, non-invasive pituitary adenoma; 2, invasive pituitary adenoma; 3, non-invasive recurrent pituitary adenoma.
Figure 2.
Relative expression levels of CIRP protein I pituitary adenoma. * p<0.05 compared to non-invasive pituitary adenoma; # p<0.05 compared to invasive pituitary adenoma.
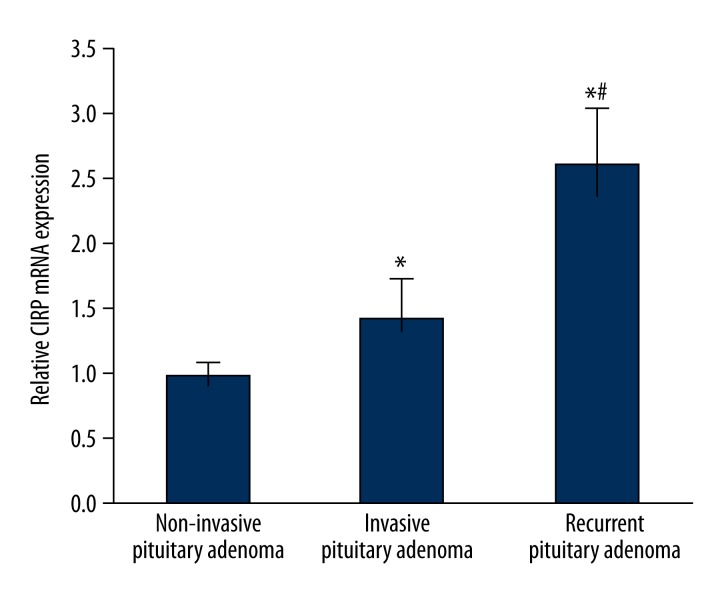
Assay of CIRP mRNA levels in pituitary adenoma by real-time PCR
To further confirm the expressional profile of CIRP mRNA in pituitary adenoma, mRNA was extracted from samples of invasive pituitary adenoma, non-invasive adenoma, and non-invasive recurrent adenoma and was semi-quantitatively analyzed by real-time PCR. Results showed that CIRP mRNA existed in all 3 types of adenoma, but with different levels. Non-invasive pituitary adenoma has the lowest CIRP mRNA level, which was significantly increased in invasive pituitary adenoma (p<0.05 compared to non-invasive adenoma). The highest level of CIRP was found in recurrent pituitary adenoma, with a significant difference (p<0.05 compared to non-recurrent tumors) (Figure 3). These results are consistent with those of CIRP protein levels.
Figure 3.
Analysis of CIRP mRNA levels in pituitary adenoma by real-time PCR. * p<0.05 compared to non-invasive pituitary adenoma; # p<0.05 compared to invasive pituitary adenoma.
Discussion
Derived mainly from monoclonal adenoma of adenohypophysis gland cells, pituitary adenoma is a common intracranial tumor with a high incidence, which shows a younger trend, as most patients are young men between 20 and 50 years of age, with no sex bias [15]. This study randomly selected pituitary adenoma patients, most of which were young people with an average age of 33.4±15.3 years. Patients had no sex bias or no statistical difference of general clinical information such as age or sex among all 3 types, consistent with results of a previous study [16]. Due to these critical physiological functions, pituitary adenoma leads to various neural and endocrine symptoms, including optic nerve impairment, hypothyroidism, underdevelopment of sexual characters, growth hormone secretion insufficiency, adrenal cortex dysfunction, and hyperprolactinemia [17–19]. The invasive nature of pituitary adenoma makes it impossible to completely remove tumor tissues by surgical resection, causing the high recurrence rate of pituitary adenoma. Studies have revealed a complicated mechanism underlying pathogenesis of invasive pituitary adenoma, which is induced by multiple factors involved in the neural-endocrine-immune regulatory network [20]. Currently, there are few effective and easy methods for clinical diagnosis of pituitary adenoma and prediction of tumor prognosis.
CIRP was the first-discovered cold-stress or cold-shock protein in mammalian cells. Recent studies found highly-conserved structures of CIRP in terms of its nucleotide or amino acid sequence, accompanied by high homogeneity. Tissue specificity possibly exists in either of body cell localization or physiological function. Although first discovered under cold-induced cell growth inhibition, CIRP was later found to participate in multiple stress responses, including those induced by ultraviolet radiation, osmotic alternation, or hypoxia/ischemia, in addition to critical roles in embryonic development, reproduction, and tumors; therefore, having valuable prospective clinical applications. Previous studies have reported CIRP as a novel oncogene that can facilitate the proliferation of tumor cells via its up-regulation of expression in various tumor cells and further inhibition of oxidative-induced tissue damage, thereby exerting a trophic role in oncogenesis [21,22]. Thus, this study focused on CIRP to determine if it can work as an index for pathogenesis and progression of pituitary adenoma. Results showed that CIRP protein was expressed in all 3 types of pituitary adenoma, but with different expression levels. Non-invasive pituitary adenoma had the lowest expression of CIRP protein, which was significantly elevated when adenoma was invading peripheral neural tissues. CIRP protein was further increased for recurrent adenoma after surgery. It is important that both mRNA and protein of CIRP have the highest levels in recurrent pituitary adenoma, suggesting a close relationship between invasion and proliferation of pituitary adenoma and CIRP expression, whose significant elevation clearly indicates post-op recurrent of pituitary adenoma. The mechanism of CIRP in tumor proliferation and invasion has not yet been reported, making it necessary to further elucidate the role of CIRP in pathogenesis of pituitary adenoma. Due to the relatively small sample size involved in this study, further research should be conducted in an expanded sample to further validate the expressional profile of CIRP in pituitary adenoma tissues.
Conclusions
CIRP can work as an index for evaluating invasion and proliferation of pituitary adenoma, and may indicate the possibility of post-op tumor recurrence. The inhibition of CIRP expression can be a treatment strategy against recurrent pituitary adenoma, although the detailed functional mechanism of CIRP in pathogenesis of pituitary adenoma remains to be fully defined.
Footnotes
Source of support: Departmental sources
References
- 1.Manara R, Gabrieli J, Citton V, et al. Intracranial internal carotid artery changes in acromegaly: a quantitative magnetic resonance angiography study. Pituitary. 2014;17(5):414–22. doi: 10.1007/s11102-013-0516-y. [DOI] [PubMed] [Google Scholar]
- 2.Oklu R, Deipolyi AR, Wicky S, et al. Identification of small compound biomarkers of pituitary adenoma: a bilateral inferior petrosal sinus sampling study. J Neurointerv Surg. 2014;6(7):541–46. doi: 10.1136/neurintsurg-2013-010821. [DOI] [PubMed] [Google Scholar]
- 3.Babbo A, Kalapurakal GT, Liu B, et al. The presence of a pituitary tumor in patients with prostate cancer is not a contraindication for leuprolide therapy. Int Urol Nephrol. 2014;46(9):1775–78. doi: 10.1007/s11255-014-0708-z. [DOI] [PubMed] [Google Scholar]
- 4.Kwancharoen R, Blitz AM, Tavares F, et al. Clinical features of sellar and suprasellar meningiomas. Pituitary. 2014;17(4):342–48. doi: 10.1007/s11102-013-0507-z. [DOI] [PubMed] [Google Scholar]
- 5.Ding D, Yen CP, Starke RM, et al. Unyielding progress: recent advances in the treatment of central nervous system neoplasms with radiosurgery and radiation therapy. J Neurooncol. 2014;119(3):513–29. doi: 10.1007/s11060-014-1501-7. [DOI] [PubMed] [Google Scholar]
- 6.Broder MS, Neary MP, Chang E, et al. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary. 2014;17(4):333–41. doi: 10.1007/s11102-013-0506-0. [DOI] [PubMed] [Google Scholar]
- 7.Yedinak CG, Fleseriu M. Self-perception of cognitive function among patients with active acromegaly, controlled acromegaly, and non-functional pituitary adenoma: a pilot study. Endocrine. 2014;46(3):585–93. doi: 10.1007/s12020-013-0106-9. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama H, Itoh K, Kaneko Y, et al. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137(4):899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiyama H, Danno S, Kaneko Y, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152(1):289–96. [PMC free article] [PubMed] [Google Scholar]
- 10.Xue JH, Nonoguchi K, Fukumoto M, et al. Effects of ischemia and H2O2 on the cold stress protein CIRP expression in rat neuronal cells. Free Radic Biol Med. 1999;27(11–12):1238–44. doi: 10.1016/s0891-5849(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 11.van Venrooy S, Fichtner D, Kunz M, et al. Cold-inducible RNA binding protein (CIRP), a novel XTcf-3 specific target gene regulates neural development in Xenopus. BMC Dev Biol. 2008;8:77. doi: 10.1186/1471-213X-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Leeuw F, Zhang T, Wauquier C, et al. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res. 2007;313(20):4130–44. doi: 10.1016/j.yexcr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Hong JK, Kim YG, Yoon SK, Lee GM. Down-regulation of cold-inducible RNA-binding protein does not improve hypothermic growth of Chinese hamster ovary cells producing erythropoietin. Metab Eng. 2007;9(2):208–16. doi: 10.1016/j.ymben.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Yoon SK, Hong JK, Choo SH, et al. Adaptation of Chinese hamster ovary cells to low culture temperature: cell growth and recombinant protein production. J Biotechnol. 2006;122(4):463–72. doi: 10.1016/j.jbiotec.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhan X, Desiderio DM, Wang X, et al. Identification of the proteomic variations of invasive relative to non-invasive non-functional pituitary adenomas. Electrophoresis. 2014;35(15):2184–94. doi: 10.1002/elps.201300590. [DOI] [PubMed] [Google Scholar]
- 16.Greenberger BA, Pulsifer MB, Ebb DH, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89(5):1060–68. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 17.Goel A. Challenge of giant pituitary tumors. World Neurosurg. 2014;82(1–2):e121–24. doi: 10.1016/j.wneu.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Marques P, Mafra M, Calado C, et al. Aggressive pituitary lesion with a remarkably high Ki-67. Arq Bras Endocrinol Metabol. 2014;58(6):656–60. doi: 10.1590/0004-2730000003116. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ye H, Wang X, et al. Evidence of brain tumor stem progenitor-like cells with low proliferative capacity in human benign pituitary adenoma. Cancer Lett. 2014;349(1):61–66. doi: 10.1016/j.canlet.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Ferone D, Pivonello C, Vitale G, et al. Molecular basis of pharmacological therapy in Cushing’s disease. Endocrine. 2014;46(2):181–98. doi: 10.1007/s12020-013-0098-5. [DOI] [PubMed] [Google Scholar]
- 21.Ren WH, Zhang LM, Liu HQ, et al. Protein overexpression of CIRP and TLR4 in oral squamous cell carcinoma: an immunohistochemical and clinical correlation analysis. Med Oncol. 2014;31(8):120. doi: 10.1007/s12032-014-0120-7. [DOI] [PubMed] [Google Scholar]
- 22.Lleonart ME. A new generation of proto-oncogenes: cold-inducible RNA binding proteins. Biochim Biophys Acta. 2010;1805(1):43–52. doi: 10.1016/j.bbcan.2009.11.001. [DOI] [PubMed] [Google Scholar]