Abstract
Background
Patients with composite bone non-union and soft tissue defects are difficult to treat. Vacuum-assisted closure (VAC) combined with open bone grafting is one of the most effective treatments at present. The aim of the present study was to preliminarily investigate the effect and mechanism of VAC combined with open bone grafting to promote rabbit bone graft vascularization, and to propose a theoretical basis for clinical work.
Material/Methods
Twenty-four New Zealand white rabbits were randomly divided into an experimental and a control group. Allogeneic bones were grafted and banded with the proximal femur with a suture. The experimental group had VAC whereas the control group had normal wound closure. The bone vascularization rate was compared based on X-ray imaging, fluorescent bone labeling (labeled tetracycline hydrochloride and calcein), calcium content in the callus, and expression of fibroblast growth factor-2 (FGF-2) in bone allografts by Western blot analysis at the 4th, 8th, and 12th week after surgery.
Results
At the 4th, 8th, and 12th week after surgery, the results of the tests demonstrated that the callus was larger, contained more calcium (p<0.05), and expressed FGF-2 at higher levels (p<0.05) in the experimental group than in the control group. Fluorescent bone labeling showed the distance between the two fluorescent ribbons was significantly shorter in the control group than in the experimental group at the 8th and 12th week after surgery.
Conclusions
VAC combined with open bone grafting promoted rabbit bone graft vascularization.
Keywords: Bone Regeneration; Bone Transplantation; Negative-Pressure Wound Therapy; Transplantation, Homologous
Background
It is complicated to treat patients with composite bone non-union and soft tissue defect caused by high-energy injuries, and the period of the treatment is long. Due to the lack of soft tissue surrounding it, the injured bone possesses worse ability to heal and fight infection than normal bone does. In order to solve the problem associated with soft tissue and bone healing in such injuries, Papineau introduced an open bone graft technique [1]. The technique grafts autogenous cancellous bone matched in shape to the bone defect after debridement, and, while maintaining the wound open, the wound is covered with petroleum jelly gauze and drained. This approach resulted in high efficacy. However, advancements in technology have led some researchers to combine vacuum-assisted closure (VAC) with open bone grafting to treat patients with osteomyelitis or bone non-union, and a better curative effect has been attained [2]. VAC technology uses a medical-grade porous foam material (aperture size 400–600 μm), called Vacuseal, packed into a drainage tube with several holes. Vacuseal is placed on the wound, and an adherent semipermeable membrane covering makes the wound airtight. This apparatus is then linked to a vacuum through a drainage bottle to create negative pressure [3]. In this study, we propose to verify the impact of VAC combined with open bone grafting in promoting rabbit bone graft vascularization; we also seek to provide a theoretical basis for its clinical application.
Material and Methods
Animals and experimental groups
Twenty-four male New Zealand white rabbits weighing 2.8 to 3.2 kg aged four to five months (from the WuHan WanQian JiaXing Bio-Technology Co., Ltd, China) were used in this study. The rabbits were fed with standard feed and housed in an ambient environment at constant temperature for at least one week prior to surgery. The rabbits were randomly divided into two groups: the experimental group and the control group. Rabbits in the experimental group were grafted with allogeneic bone (made from rabbit cancellous bone of the lower limbs provided by the Second Clinical College of Wuhan University) onto the proximal femur. The wound was covered with Vacuseal using continuous suction. Then, the rabbits were singly placed in a cage and fed in such a way that they could not turn around to bite the tube. The Vacuseal was replaced every five to seven days. Animals in the control group underwent a similar surgical procedure other than the fact that the surgical wound was closed by primary closure rather than with Vacuseal.
Surgical procedure
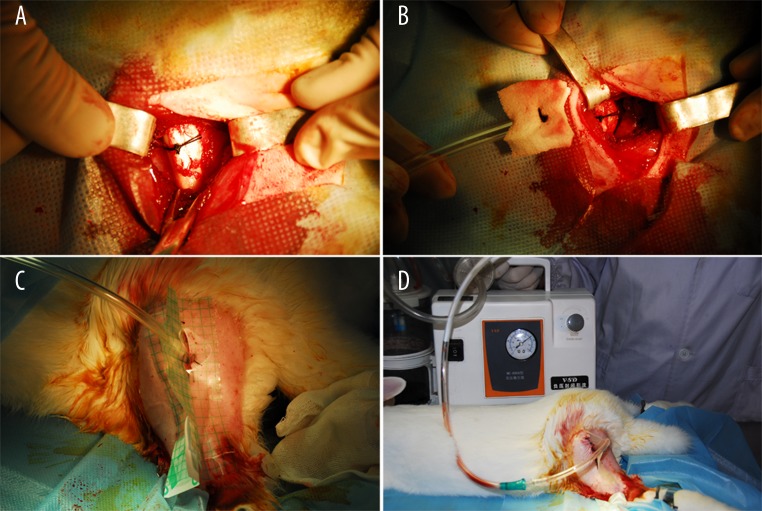
Animals in the experimental group were anesthetized by injecting a 3% solution of sodium pentobarbital via the ear vein (30 mg/kg). Following confirmation that the animals were anesthetized, the limbs were fixed in a prone position. Fur in the surgical area (hind leg) was removed and the skin was prepared using 8% Na2S solution. After regular disinfection and draping, a 3 cm long incision was performed posterior to the midline on the thigh to expose the proximal femur. Three allogeneic bones were grafted and banded with the proximal femur with a suture (Figure 1A). Vacuseal (Dragonbio Orthopedic Products Co., Ltd., China) was placed to cover the allogeneic bones (Figure 1B) and was fixed to the wound with sutures. Then, the semipermeable membrane (Smith & Nephew Co., England) was adhered to cover the Vacuseal outside the wound (Figure 1C), and the dressing was linked to a negative pressure aspirator to maintain continuous vacuum aspiration at a pressure of about −60 mmHg to −75 mmHg (Figure 1D). The animals were housed in a special cage and an intramuscular injection of penicillin (400,000 U/Ci) was given for three days after surgery. The Vacuseal was replaced every five to seven days. The difference in the surgical procedure between the control and experimental groups was that the wound was sutured with VAC in the experimental group but not in the control group. The postoperative procedures were similar in both groups. Four rabbits from each group were sacrificed with air embolism separately at the 4th, 8th, and 12th week after surgery.
Figure 1.
Surgical procedure. Allogeneic bones banded with proximal femur with a suture (A); a cover of Vacuseal on allogeneic bones (B); adherent semipermeable membrane (C); connection to negative pressure (D).
X-ray imaging
The femurs of the two groups were assessed by lateral X-ray imaging. Then, we estimated the state of bone allograft vascularization in the callus based on the imaging.
Fluorescent bone labeling
All the animals were injected with tetracycline hydrochloride (Aladdin Reagent Co., Ltd, China) (35 mg/kg) 15 d prior to sacrifice, and they were injected with calcein (Sinopharm Chemical Reagent Co., Ltd, China) (10 mg/kg) 3 d prior to sacrifice. After sacrificing the animals, the bone allografts of the two groups were obtained and were fixed in 10% paraformaldehyde, dehydrated, and defatted. The allografts were embedded with methyl methacrylate without decalcification over 24 h, and then the specimens were serial sectioned with slices of 5 micron thickness using a microtome (Jung type). The bone cortex and callus fluorescence staining for each slice was assessed by Leica TCS SP2 confocal microscopy (Leica Microsystems IR GmbH., Germany), and the distance between the two marking lines comprised of tetracycline hydrochloride and calcein was measured by Aim Image Examiner (Carl Zeiss MicroImaging GmbH., Germany).
Calcium content in the callus
The bone allografts were accurately weighed after being rinsed, defatted, and dried off, then the specimens were decalcified with 0.1 g/ml trichloroacetic acid, and the calcium content of each specimen was determined using back titration.
Expression of FGF-2 in bone allografts by Western blot analysis
Total protein was extracted from cancellous bone by hammer grinding and all protein samples were stored at −80°C. The protein of each group was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked in Tris-buffered saline with Tween (TBST) with 5% skim milk for 2 h and incubated overnight at 4°C with anti-FGF-2 antibody (Boster Reagent Co., Ltd, China) (1:200 dilution). After washing in TBST for 5 times, the membrane was incubated with horseradish-peroxidase (HRP)-coupled secondary antibody (1:50000 dilution) at room temperature for 2 h. Subsequently, the samples were washed with TBST again, and the membranes were incubated with an ECL detection kit (Beijing ComWin Biotech CO., LTD., China) for 5 min and exposed to medical X-ray film. BandScan was used to analyze the grey value of the film.
Statistical analysis
To compare the difference in each test between the groups, SPSS17.0 for Windows statistical software analysis was performed. Measurement data were presented as the mean ±SD and analyzed by Student’s t-test. Enumeration data were presented as percentages and were analyzed by the chi-square test. In all cases, a significance level of p<0.05 was used.
Results
VAC promotes callus formation
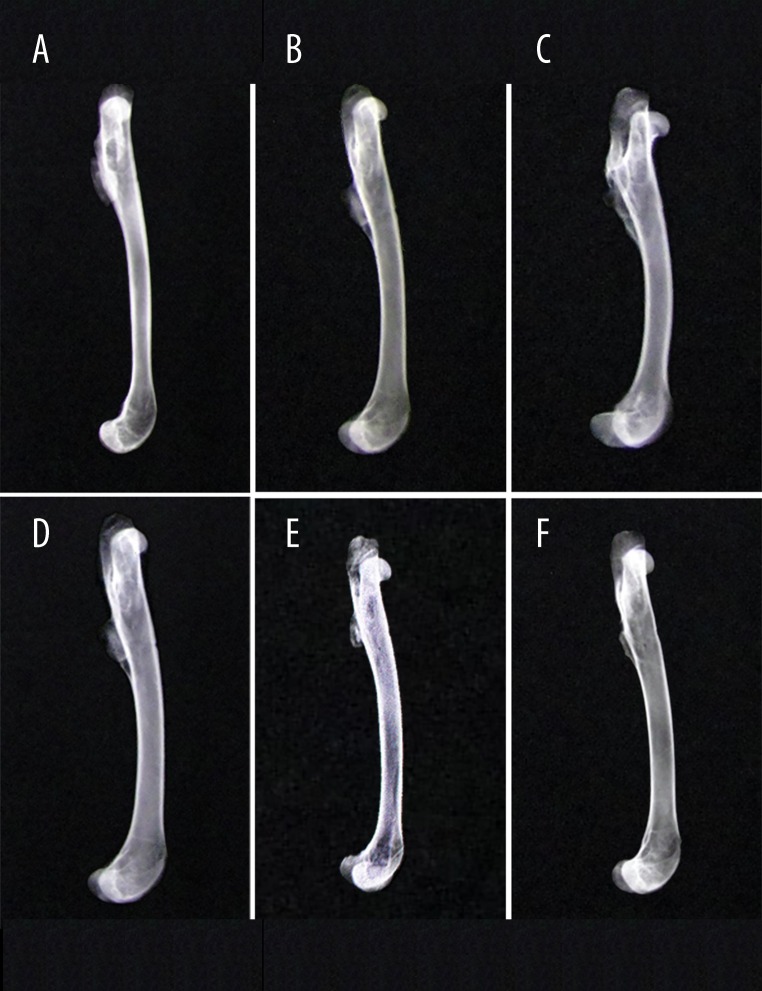
By general observation and X-ray imaging (Figure 2A–2F), some callus growths crawling from the femur to the bone graft were observed, and the dividing line could be seen at the 4th week after surgery. The femur connected a little more to the bone graft in the experimental group than in the control group. At the 8th week after surgery, more callus growth was observed around the bone graft, and the femur attached to the bone graft solidly in both groups. The dividing line was blurred in the experimental group while a part of the line was still visible in control group. At the 12th week after surgery, the bone graft connected to the femur entirely and a peripheral callus was beginning to form in both groups.
Figure 2.
X-ray imaging in different groups: control group at the 4th week after surgery (A); experimental group at the 4th week after surgery (D); control group at the 8th week after surgery (B); experimental group at the 8th week after surgery (E); control group at the 12th week after surgery (C); experimental group at the 12th week after surgery (F).
VAC promotes callus development
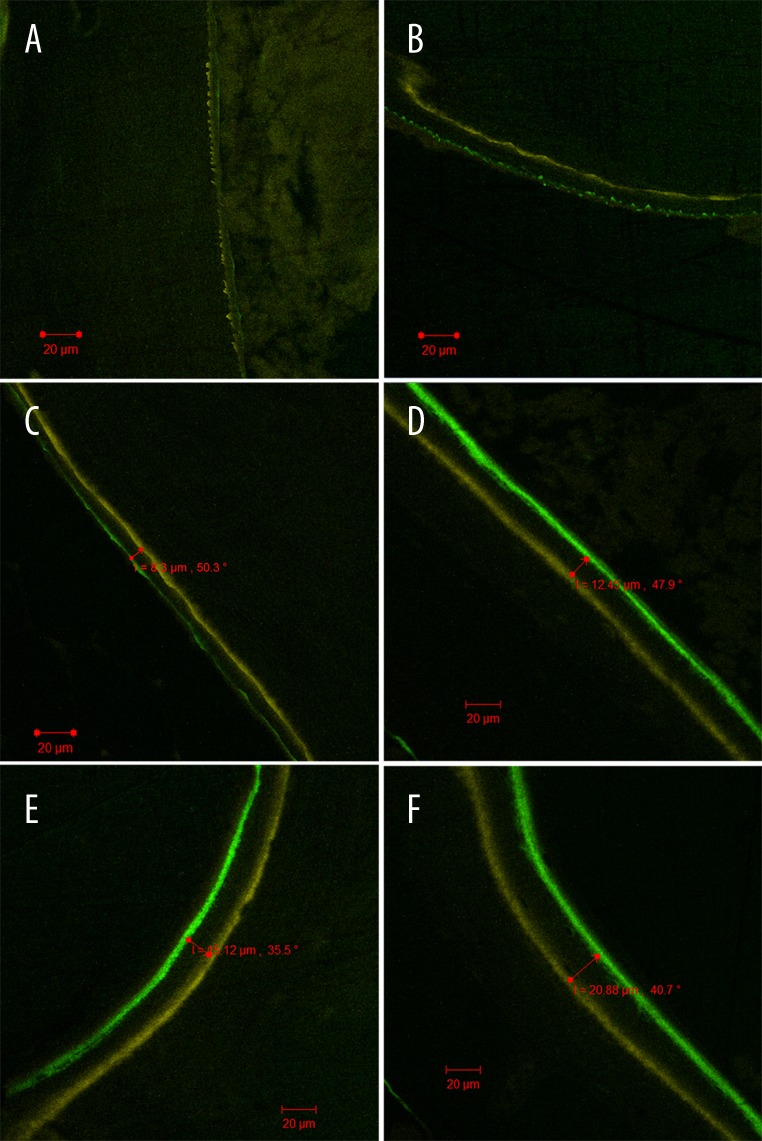
Fluorescent bone labeling demonstrated a discrete yellow fluorescent line (tetracycline hydrochloride) arranged around the bone graft along with scattered punctiform green fluorescence (calcein) present in the experimental group, while there was only scattered punctiform yellow fluorescence in the control group at the 4th week after surgery (Figure 3A, 3B). At the 8th week after surgery, two fluorescent ribbons with clear boundaries could be observed around the bone graft in the experimental group, while the corresponding ribbons were discontinuous and uneven in thickness in the control group (Figure 3C, 3D). At the 12th week after surgery, two annular-shaped fluorescent ribbons could be found in both groups, but the distance between the colored ribbons was significantly shorter in the control group than in the experimental group (P<0.05) (Figure 3E, 3F, Table 1).
Figure 3.
Fluorescent bone labeling in different groups: scattered punctiform yellow fluorescence in the control group at the 4th week after surgery (A); discrete linear yellow fluorescence and scattered, punctiform green fluorescence in the experimental group at the 4th week after surgery (B); two discontinuous fluorescent ribbons in the control group at the 8th week after surgery (C); two fluorescent ribbons with clear boundaries in the experimental group at the 8th week after surgery (D); two annular fluorescent ribbons in the control groups at the 12th week after surgery (E); two annular fluorescent ribbons in the experimental groups at the 12th week after surgery (F).
Table 1.
Distance between the two fluorescent ribbons (μm), mean ±SD.
P<0.05, versus experimental at the 8th and 12th week after surgery.
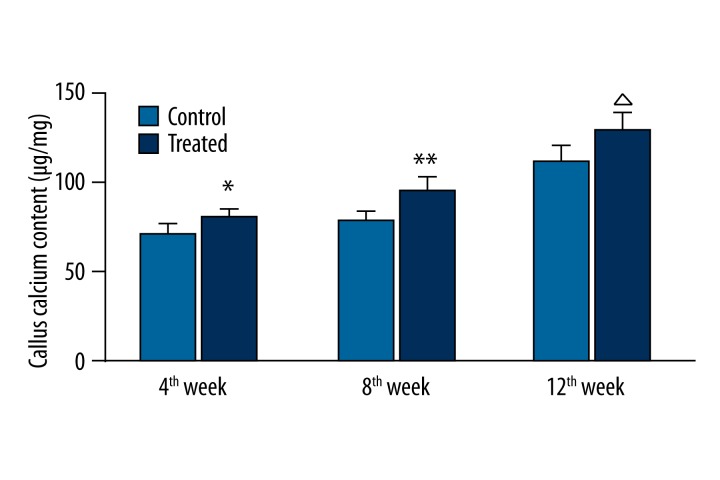
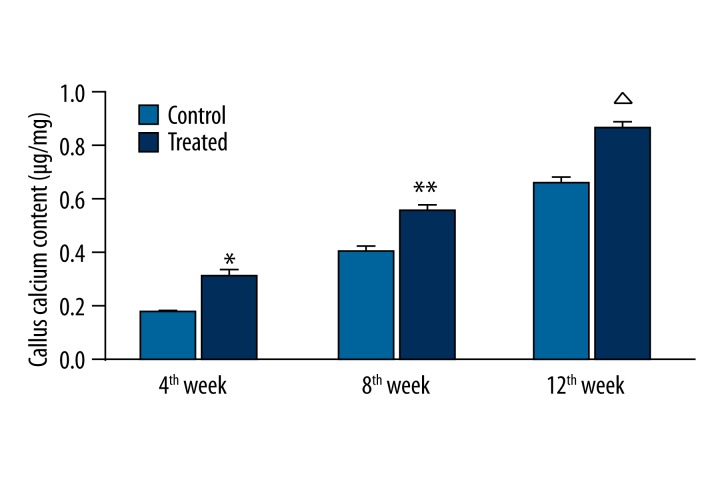
VAC increases calcium content in the callus
The calcium content in the callus in each group is shown in Figure 4. The calcium content in the callus at the 4th, 8th, and 12th week after surgery was significantly different between the control and experimental group (P<0.05). The calcium content in the callus in the experimental group was significantly higher than in the control group at each time point after surgery (P<0.05).
Figure 4.
Calcium content in the callus in each group.
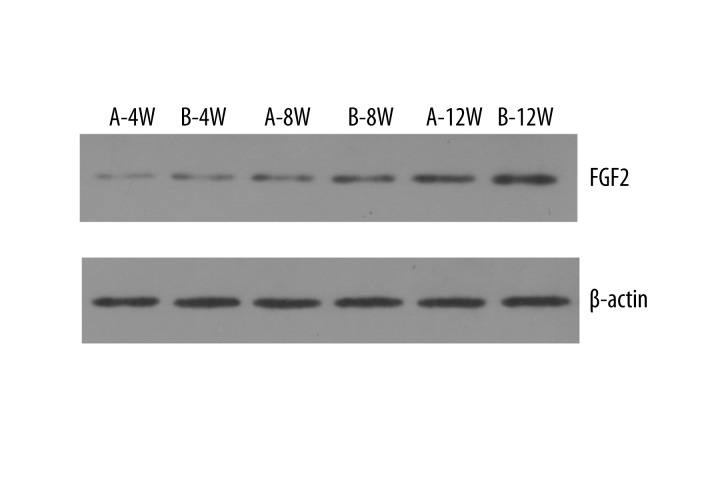
VAC increases FGF-2 expression in the bone graft
Typical imaging of the expression of FGF-2 by Western blot analysis in each group is shown in Figure 5. The grey value of each film in the experimental group was significantly higher than in the control group (P<0.05) (Figure 6).
Figure 5.
Expression of FGF-2 by Western blot analysis.
Figure 6.
Grey value of each film in the different groups
Discussion
For the high operation failure rates of former treatments of open, non-union bone fractures [4–10], Papineau introduced open bone graft technology to solve this problem [1]. Open bone grafting is also called the Rhinelander-Papineau or the Papineau technique, and its characteristic benefits are conferred by the facts that the microporous structure of cancellous bone is advantageous to osteoblast agglomeration and growth, and that osteoblasts can obtain nutrition from surrounding tissue exudates for survival. New vessels grow easily and quickly to establish a blood supply because of the unique clearance of each bone graft and such vessels bring more nutrients including osteogenesis factors [11–16]. Draining wound exudates through gauze is better at preventing infection when applied with open bone grafts compared with traditional bone grafts [17–19]. Furthermore, the blood from the soft tissue bed or cancellous bone marrow cavity can seep out through the gap and solidify. Afterwards, granulation tissue forms and covers the bone graft, and this is followed by capillary ingrowth, which provides the foundation for free skin grafting [20,21]. All of these characteristics confirm that open bone grafting is applicable to patients with a poor state of local soft tissue and infectious non-union bone [22]; however, potential problems include needing treatment more frequently, and spending a longer period of time in hospital [23].
Fortunately, the emergence of VAC greatly mitigates the said problems of open bone grafting. The basic working principle of VAC is to use a Vacuseal connected to a drainage tube between the wounds, which is collectively connected to negative pressure, enabling for the whole wound to drain and allow for clearance of exudate expeditiously [24,25]. VAC also contacts the wound closely and uses sustained active force provided by the negative pressure, enabling clearance of not only wound exudate, but also of pus and necrotic tissue in a complete and timely manner [26,27]. Vacuseal containing a porous structure has increased water permeability and ability to adsorb solutions better than petroleum jelly gauze. In addition, when exudate is eliminated, VAC can inhibit bacterial growth and prevent the absorption of toxins and spread of infection [28,29]. Therefore, the combination of VAC with open bone grafting was a natural fit.
As the study of VAC increases, more advantages to this technology have been discovered [30–33]: (1) VAC can promote vascular endothelial growth factor (VEGF) generation in damaged tissue; (2) The expression of CD34 significantly increases after VAC treatment; therefore, one can deduce that VAC apparently promotes the proliferation of vascular endothelial cells and fibroblasts in the tissue around the wound and also increase microvascular density, thus promoting wound granulation tissue growth; (3) The concentration of transforming growth factor-beta 1 (TGF-β1) and platelet-derived growth factor (PDGF) in wounds is significantly increased by VAC; (4) VAC can decrease the expression of matrix metalloproteinase (MMP)-1, 2, 9, 13 mRNA in wounds, and inhibit the degradation of gelatin and collagen by adjusting the balance of MMPs to tissue inhibitors of metalloproteinase (TIMP) to promote wound healing. The role of active substances, including VEGF and CD34, TGF-β1, PDGF, and TIMP-1 in promoting bone revascularization has been confirmed [34–38].
FGF2 is a member of the fibroblast growth factor family, which is widely distributed within human tissue, and it plays an important role in the repair of bone defects or fractures [39–41]. In this experiment, we detected FGF2 in allografts by Western blot analysis and found that the expression in the experimental group was significantly higher, which indicated that VAC can promote the expression of FGF2, and this may suggest that heightened FGF2 is probably one of the reasons behind VAC promoting bone vascularization.
VAC in combination with open bone grafting is currently used in clinical settings mainly for complex open tibial bone non-union with soft tissue defects. Because it can shorten fracture healing time, and the time for granulation tissue to cover the wound, it is recognized by a vast number of scholars. We had successfully treated 15 cases of complex tibial bone non-union using this technology since 2007 in our hospital, and the treatment efficacy has been satisfactory [42].
In this experiment, the results of X-ray imaging, fluorescent bone labeling and calcium content in the callus indicated that VAC plays a role in promoting bone allografts vascularization in rabbit, while the increased expression of FGF-2 in bone allografts may be the reason for this effect. However, there are some limitations to this study. We could not replace Vacuseal every five to seven days strictly, but rather deciding on replacement time based on practical circumstances. In addition, this study only confirmed that VAC could promote bone vascularization, but the effect was not quantified. Quantification of increased bone vascularization would need to be verified in later experiments.
Conclusions
This study showed that VAC facilitates bone allografts vascularization in rabbit. An increase in FGF-2 may be one of the mechanisms by which this occurs.
Acknowledgments
We thank Uni-edit Co. Ltd. for editing and proofreading this manuscript.
Footnotes
Source of support: The study was funded by the key grant from Hubei Provincial Natural Science (No. 2014CFA063)
References
- 1.Papineau LJ. Excision-graft with deliberately delayed closing in chronic osteomyelitis. Nouv Presse Med. 1973;2:2753–75. [PubMed] [Google Scholar]
- 2.Karargyris O, Polyzois VD, Karabinas P, et al. Papineau debridement, Ilizarov bone transport, and negative-pressure wound closure for septic bone defects of the tibia. Eur J Orthop Surg Traumatol. 2014;24:1013–17. doi: 10.1007/s00590-013-1279-x. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg. 1993;96:488–92. [PubMed] [Google Scholar]
- 4.Brighton CT, Friedenberg ZB, Zemsky LM, Pollis PR. Directcurrent stimulation of non-union and congenital pseudarthrosis. Exploration of its clinical application. J Bone Joint Surg Am. 1975;57:368–77. [PubMed] [Google Scholar]
- 5.Minami A, Kaneda K, Itoga H. Treatment of infected segmental defect of long bone with vascularized bone transfer. J Reconstr Microsurg. 1992;8:75–82. doi: 10.1055/s-2007-1006688. [DOI] [PubMed] [Google Scholar]
- 6.Patzakis MJ, Scilaris TA, Chon J, et al. Results of bone grafting for infected tibial nonunion. Clin Orthop Relat Res. 1995;315:192–98. [PubMed] [Google Scholar]
- 7.Bumbasirević M, Tomić S, Lesić A, et al. War-related infected tibial nonunion with bone and soft-tissue loss treated with bone transport using the Ilizarov method. Arch Orthop Trauma Surg. 2010;130:739–49. doi: 10.1007/s00402-009-1014-6. [DOI] [PubMed] [Google Scholar]
- 8.Brighton CT, Black J, Friedenberg ZB, et al. A multicenter study of the treatment of non-union with constant direct current. J Bone Joint Surg Am. 1981;63:2–13. [PubMed] [Google Scholar]
- 9.Cattaneo R, Catagni M, Johnson EE. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop Relat Res. 1992;280:143–52. [PubMed] [Google Scholar]
- 10.Parrett BM, Matros E, Pribaz JJ, Orgill DP. Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg. 2006;117:1315–22. doi: 10.1097/01.prs.0000204959.18136.36. [DOI] [PubMed] [Google Scholar]
- 11.Papineau LJ. Excision-graft with deliberately delayed closing in chronic osteomyelitis. Nouv Presse Med. 1973;2:2753–55. [in French] [PubMed] [Google Scholar]
- 12.Papineau LJ, Alfageme A, Dalcourt JP, Pilon L. Chronic osteomyelitis: open excision and grafting after saucerization. Int Orthop. 1979;3:165–76. doi: 10.1007/BF00265708. [in French] [DOI] [PubMed] [Google Scholar]
- 13.Panda M, Ntungila N, Kalunda M, Hinsenkamp M. Treatment of chronic osteomyelitis using the Papineau technique. Int Orthop. 1998;22:37–40. doi: 10.1007/s002640050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emami A, Mjöberg B, Larsson S. Infected tibial nonunion. Good results after open cancellous bone grafting in 37 cases. Acta Orthop Scand. 1995;66:447–51. doi: 10.3109/17453679508995585. [DOI] [PubMed] [Google Scholar]
- 15.Tulner SA, Schaap GR, Strackee SD, et al. Long-term results of multiple-stage treatment for posttraumatic osteomyelitis of the tibia. J Trauma. 2004;56:633–42. doi: 10.1097/01.ta.0000112327.50235.0a. [DOI] [PubMed] [Google Scholar]
- 16.Saleh M, Kreibich DN, Ribbans WJ. Circular frames in the management of infected tibial non-union: a modification of the Papineau technique. Injury. 1996;27:31–33. doi: 10.1016/0020-1383(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 17.Kneser U, Stangenberg L, Ohnolz J, et al. Evaluation of processed bovine cancellous bone matrix seeded with syngenic osteoblasts in a critical sizecalvarial defect rat model. J Cell Mol Med. 2006;10:695–707. doi: 10.1111/j.1582-4934.2006.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueng WN, Shih CH. Semiopen cancellous bone grafting. A 2 step method for closing small infected tibial bone defects. Clin Orthop Relat Res. 1994;306:175–82. [PubMed] [Google Scholar]
- 19.Petty W, Spanier S, Shuster JJ, Silverthorne C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J Bone Joint Surg Am. 1985;67:1236–44. [PubMed] [Google Scholar]
- 20.Polyzois VD, Galanakos S, Zgonis T, et al. Combined distraction osteogenesis and Papineau technique for an open fracture management of the distal lower extremity. Clin Podiatr Med Surg. 2010;27:463–67. doi: 10.1016/j.cpm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Yang C, Su C, et al. Reconstruction of orbital defects by implantation of antigen-free bovine cancellous bone scaffold combined with bone marrow mesenchymal stem cells in rats. Graefes Arch Clin Exp Ophthalmol. 2013;251:1325–33. doi: 10.1007/s00417-013-2300-0. [DOI] [PubMed] [Google Scholar]
- 22.Hou Z, Irgit K, Strohecker KA, et al. Delayed flap reconstruction with vacuum-assisted closure management of the open IIIB tibial fracture. J Trauma. 2011;71:1705–8. doi: 10.1097/TA.0b013e31822e2823. [DOI] [PubMed] [Google Scholar]
- 23.Deng K, Yu AX, Xia CY, et al. Combination of negative pressure wound therapy with open bone grafting for bone and soft tissue defects. Mol Med Rep. 2013;8:468–72. doi: 10.3892/mmr.2013.1536. [DOI] [PubMed] [Google Scholar]
- 24.Müllner T, Mrkonjic L, Kwasny O, Vécsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealingtechnique. Br J Plast Surg. 1997;50:194–99. doi: 10.1016/s0007-1226(97)91369-2. [DOI] [PubMed] [Google Scholar]
- 25.Li RG, Ren GH, Tan XJ, et al. Free flap transplantation combined with skin grafting and vacuum sealing drainage for repair of circumferential or sub-circumferential soft-tissue wounds of the lower leg. Med Sci Monit. 2013;19:510–17. doi: 10.12659/MSM.883963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh BH, Lee SH, Nam KA, et al. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. Br J Dermatol. 2013;168:333–38. doi: 10.1111/bjd.12099. [DOI] [PubMed] [Google Scholar]
- 27.Argenta PA, Rahaman J, Gretz HF, et al. Vacuum-assisted closure in the Treatment of complex gynecologic wound failures. Obstet Gynecology. 2002;99:497–501. doi: 10.1016/s0029-7844(01)01752-5. [DOI] [PubMed] [Google Scholar]
- 28.Dohmen PM, Markou T, Ingemansson R, et al. Use of incisional negative pressure wound therapy on closed median sternal incisions after cardiothoracicsurgery: clinical evidence and consensus recommendations. Med Sci Monit. 2014;20:1814–25. doi: 10.12659/MSM.891169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weed T, Ratlitt C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: Does the wound VAC enhance bacterial clearance. Ann Plast Surg. 2004;52:279–80. doi: 10.1097/01.sap.0000111861.75927.4d. [DOI] [PubMed] [Google Scholar]
- 30.Streubel PN, Stinner DJ, Obremskey WT. Use of negative-pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg. 2012;20:564–74. doi: 10.5435/JAAOS-20-09-564. [DOI] [PubMed] [Google Scholar]
- 31.Sahin I, Eski M, Acikel C, et al. The role of negative pressure wound therapy in the treatment of fourth-degree burns. Trends and new horizons. Ann Burns Fire Disasters. 2012;25:92–97. [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Topaz M, Tan H, et al. Treatment of infected soft tissue blast injury in swine by regulated negative pressure wound therapy. Ann Surg. 2013;257:335–44. doi: 10.1097/SLA.0b013e318269d1ca. [DOI] [PubMed] [Google Scholar]
- 33.Chiummariello S, Guarro G, Pica A, Alfano C. Evaluation of negative pressure vacuum-assisted system in acute and chronic wounds closure: our experience. G Chir. 2012;33:358–62. [PubMed] [Google Scholar]
- 34.Blum A, Zarqh O, Peleg A, et al. Vascular inflammation and endothelial dysfunction in fracture healing. Am J Orthop (Belle Mead NJ) 2012;41:87–91. [PubMed] [Google Scholar]
- 35.Kawakami Y, Ii M, Alev C, et al. Local transplantation of ex vivo expanded bone marrow-derived CD34 positive cells accelerates fracture healing. Cell Transplant. 2012;21:2689–709. doi: 10.3727/096368912X654920. [DOI] [PubMed] [Google Scholar]
- 36.Sarahrudi K, Thomas A, Mousavi M, et al. Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury. 2011;42:833–37. doi: 10.1016/j.injury.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bordei P. Locally applied platelet-derived growth factor accelerates fracture healing. J Bone Joint Surg Br. 2011;93:1653–59. doi: 10.1302/0301-620X.93B12.27244. [DOI] [PubMed] [Google Scholar]
- 38.Lienau J, Schmidt-Bleek K, Peters A, et al. Differential regulation of vessel formation between standard and delayed bone healing. J Orthop Res. 2009;27:1133–40. doi: 10.1002/jor.20870. [DOI] [PubMed] [Google Scholar]
- 39.Fei Y, Gronowicz G, Hurley MM. Fibroblast growth factor-2, bone homeostasis and fracture repair. Curr Pharm Des. 2013;19:3354–63. doi: 10.2174/1381612811319190002. [DOI] [PubMed] [Google Scholar]
- 40.Yoon WJ, Cho YD, et al. Prolyl Isomerase Pin1-mediated Conformational Change and Subnuclear Focal Accumulation of Runx2 Are Crucial for Fibroblast Growth Factor 2 (FGF2)-induced Osteoblast Differentiation. J Biol Chem. 2014;289:8828–38. doi: 10.1074/jbc.M113.516237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao L, Ueno D, Catros S, et al. Fibroblast growth factor-2 isoform (low molecular weight/18 kDa) overexpression in preosteoblast cells promotesbone regeneration in critical size calvarial defects in male mice. Endocrinology. 2014;155:965–74. doi: 10.1210/en.2013-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Z, Cai L, Jin W, et al. One-stage reconstruction with open bone grafting and vacuum-assisted closure for infected tibial non-union. Arch Med Sci. 2014;10:764–72. doi: 10.5114/aoms.2013.34411. [DOI] [PMC free article] [PubMed] [Google Scholar]