Abstract
The current study is one of the first to prospectively examine longitudinal associations between observed father caregiving behaviors and child cortisol reactivity and regulation in response to emotional arousal at 7 and 24 months of child age. Observations of father and mother caregiving behaviors and child cortisol levels in response to challenges at 7 months and 24 months were collected. Analyses were based on a subsample of children from the Family Life Project who lived with both their biological mothers and fathers and for whom there was at least partial cortisol data (7 months: n=717; 24 months n= 579). At 7 months of child age the sample was 49.0% female, 25.8% African American, and 74.2% European American. At 24 months of child age the sample was 49.9% female, 24.7% African American and 75.3% European American. Analyses across assessment points were conducted simultaneously using mixed linear modeling for repeated measures data to test for differential effects of fathering across infancy and toddlerhood. Concurrent measures of father negativity were positively associated with greater increases in child cortisol levels in response to emotion challenge at 7 months (p = .01) and with higher overall levels of cortisol at 24 months (p < .001). However, there was no evidence that father caregiving during infancy independently predicted later cortisol activity during toddlerhood.
Keywords: Fathers, Parenting, Infancy, Toddlerhood, Cortisol
Sensitive and supportive parenting is one of the most consistent and robust predictors of multiple developmental outcomes. Although a majority of developmental studies have focused on the contributions of mothers’ caregiving, fathers also exert direct and indirect influences on children’s development above and beyond the effects of mothers (Cabrera, Tamis-LeMonda, Bradley, Hofferth, & Lamb, 2000; Grossman et al., 2002; Lamb & Tamis-LeMonda, 2004). The unique effects of fathers are likely due to the independent (although often correlated) qualities of parenting between mothers and fathers (Barnett, Deng, Mills-Koonce, Willoughby, & Cox, 2008), as well as the unique activities and styles of engagement for mothers and fathers (Marsiglio, Day, & Lamb, 2000; Roggman, Fitgerald, Bradley, & Raikes, 2002). For example, fathers may be warm and supportive even in the absence of such characteristics in the mother (Cabrera et al., 2000), whereas other differences between parents may emerge as more broadly defined stylistic differences between mother and father caregiving (Popenoe, 1996), such as greater encouragement of child risk-taking and independence among fathers.
To date, father caregiving has been shown to be associated with social functioning (Amato & Rivera, 1999), cognitive development (Yogman, Kindlon, & Earls, 1995), and emotional regulation and control (Gottman, Katz, & Hooven, 1997); however, there remains very limited research on father contributions to children’s psychobiological development, and no direct investigation into the effects of father parenting on child cortisol functioning. The limited inquiry into father effects on psychobiological development is particularly noteworthy given the ever-increasing roles of fathers as active caregivers in modern families, our awareness of the centrality of early caregiving on psychobiological development, and the subsequent effects of psychobiology on multiple domains of development. Prior studies demonstrate associations between low maternal sensitivity and child cortisol as seen in elevated baseline levels (Bugental, Martorell, & Barraza, 2003; Haley & Stansbury, 2003) as well as diminished cortisol responsivity to challenge (Blair et al., 2008). Maternal insensitivity may adversely affect the developing stress response by contributing to infant distress while simultaneously failing to provide the support that enables the infant to gain experience with regulated arousal. Sensitive caregiving may also facilitate levels of arousal that are within the limits of the child’s regulatory capabilities and buffer the child from excessive levels of negative arousal and stress reactivity. Given the increased role of fathers as caregivers coupled with the comparable variation in parenting behaviors across mothers and fathers (Cabrera et al., 2000; Sroufe, 1996), it is possible that father caregiving may likewise influence the development of core psychobiological functioning in young children via sensitive and non-negative caregiving.
Again, no studies of which we are aware have examined the associations between father caregiving and the development of cortisol responsivity in young children. However, Goslin, Booth, and Granger (2009) noted that self-reports of greater father-child relationship intimacy and lower father-child hostility were associated with greater father-child baseline cortisol attunement, thus supporting a biosocial model of family functioning (Booth, Carver, & Granger, 2000) that includes paternal influence on cortisol activity. Furthermore, father’s sensitive parenting, along with secure father-child attachments, predicts better emotional and behavioral correlates of cortisol functioning, including emotion regulatory abilities (Diener, Mangelsdorf, McHale, & Crosch, 2002; McDowell, Kim, O’Neil, & Parke, 2002), effortful control (Eiden, Edwards, & Leonard, 2004), and social inhibition (Belsky, Hsieh, & Crnic, 1998). Based on these findings, it is reasonable to hypothesize similar mother-child and father-child processes that may influence early psychobiological development. As such, the goal of this study is to examine the unique associations between father caregiving behaviors and children’s cortisol responses to challenge at 7 and 24 months of age. Hypothesis 1 proposes that fathers’ parenting will be associated with concurrent measures of child cortisol levels above and beyond any associations with mothers’ parenting. Hypothesis 2 proposes that there will be longitudinal effects of early father caregiving on later child cortisol levels. Each hypothesis will also test for moderating effects of child gender, race, and income.
Method
Participants
The Family Life Project employed complex sampling procedures to recruit a representative sample of 1,292 families at the time that mothers gave birth in 3 target counties in eastern North Carolina and 3 target counties in central Pennsylvania. These regions are non-urban and often rural communities. Mothers were recruited in the hospital the day after giving birth. Mothers who lived in the target counties but gave birth outside of these counties (e.g., due to pregnancy complications) were identified by county birth records and contacted and recruited approximately one month after birth. To participate in this study mothers had to report that English was the primary language spoken in the home. Further details on the Family Life Project sampling plan and recruitment procedures are available in Burchinal, Vernon-Feagans, Cox, and the Family Life Project Investigators (2008). There were 717 families with participating and co-residential biological fathers at 7 months of age; there were 579 families with participating and co-residential biological fathers at 24 months of age. For these families, descriptions of father, child, and family information are presented in Table 1.
Table 1.
Family demographic information
| Variable | 7 Months (N = 717) | 24 Months (N = 579) |
|---|---|---|
| State (% NC)1 | 48.5% | 46.8% |
| Mother age (in years) | 28.0 (5.55) | 29.6 (5.32) |
| Father age (in years) | 30.6 (6.4) | 32.1 (6.5) |
| Child age (in months) | 7.35 (1.33) | 24.61 (1.63) |
| Income-to-needs ratio | 2.39 (1.85) | 2.34 (1.69) |
| Mother education (in years) | 13.2 (2.1) | 13.4 (2.1) |
| Father education (in years) | 12.9 (2.2) | 13.1 (2.2) |
| Child sex (% female) | 49.0% | 49.9% |
| Child ethnicity/race | ||
| African American | 25.8% | 24.7% |
| European American | 74.2% | 75.3% |
| Other2 | 0.0% | 0.0% |
Note:
As compared to PA.
The “other” ethnicities at 7 months included one Native American, one Guamanian, and one Vietnamese child. The “other” ethnicities at 24 months included only one Vietnamese child.
Procedures
At both the 7- and 24-month visits, mothers and fathers completed questionnaires concerning family demographics and engaged in a free-play interaction (at infancy) or puzzle task interaction (at toddlerhood) that was recorded for 10 min (Cox, Paley, Burchinal, & Payne, 1999). At each age mother and father interactions with the child occurred on separate visits approximately 2 weeks apart. The free-play interaction involved asking mothers and fathers to use a standardized set of toys (different sets for each parent) to play with their infant as they normally would if they had free time during the day. The puzzle task involved presenting the child with three jig-saw puzzles of increasing difficulty (different sets for each parent) and asking the parents to assist the child in any way they chose.
At the 7-month home visit children were presented with three procedures designed to elicit emotional reactivity; at the 24-month visit children were presented with 2 comparable tasks. The previously validated procedures (e.g., Buss & Goldsmith, 1998; Kochanska, Tjebkes, & Forman, 1998; Stifter & Braungart, 1995) at the 7-month assessment included a mask presentation challenge, followed by a barrier challenge, followed by an arm restraint challenge task. At the 24-month assessment, the barrier challenge was replaced with a toy removal challenge, which was followed by the mask presentation challenge. Challenge tasks were administered in these standard orders to all children. For the mask presentation task at each age, children were sequentially presented with four unusual masks. The experimenter wore each mask for 10 sec while calling the child’s name and moving slowly from side to side. For the barrier task at the 7-month assessment, children were presented with an attractive toy and encouraged to play with it for 30 sec. The experimenter then placed the toy behind a clear plastic barrier just beyond the child’s reach for 30 sec. The toy was then returned to the child, and the procedure was twice repeated. For the toy removal task at the 24-month assessment, the child was encouraged to play with an attractive toy for 60 sec. The child’s mother then removed the toy, engaged in conversation with the experimenter for 2 min, and then returned the toy to the child while continuing to be engaged in conversation with the experimenter for 1 min. For the arm restraint task at the 7-month assessment the experimenter crouched behind the child and gently restrained his or her arms for 2 min or until 20 sec of hard crying ensued. Mothers watched the infant during the task from a vantage point out of the infant’s line of sight.
To assess changes in cortisol indicative of the child’s HPA response to the emotion challenge tasks, three saliva samples were collected: A pre-task baseline collected before administration of the challenge tasks, a sample 20 min after the infant’s peak emotional arousal to the tasks, and a sample 40 min after peak arousal. The order of administration of the tasks was structured so that the most arousing task was presented last. Unstimulated whole saliva was collected by using either cotton or hydrocellulose absorbent material and expressing the sample into 2-ml cryogenic storage vials using a needleless syringe (cotton) or by centrifugation (hydrocellulose). Two prior studies have indicated no differences in cortisol concentrations associated with the two collection techniques (Granger et al., 2007; Harmon, Granger, Hibel, & Rumyantseva, 2007). After collection, samples were immediately placed on ice, transported to interviewers’ homes, and then stored frozen (−20 °C). They were stored frozen until batched and shipped on dry ice overnight to the Behavioral Endocrinology Laboratory at the Pennsylvania State University. Samples were then stored frozen at −80 °C until assay. On the day of testing, samples were brought to room temperature, centrifuged at 3,000 RPM for 15 min, and the clear top-phase of the sample was pipetted into appropriate test wells by robot.
The special characteristics of the sample (rurality, single-parent household, or economic disadvantage), repeated interview schedule, length of each interview protocol (2–4 hr), and age of the infants required that in-home assessments be scheduled when families were available. As such, the time of day for saliva collection for cortisol assaying occurred for approximately 80% of the sample between 10:00am and 6:00pm (range: 8:30am to 8:00pm) at infancy and between 10:00am and 7:00pm (range: 9:00am and 8:40pm) at toddlerhood. Therefore, time of the day varied and was used as a covariate in all analyses involving cortisol (as well as time-of-day2 given the nonlinearity of the diurnal rhythm of cortisol [Dokoumetzidis, Iliadis, & Macheras, 2002)]). Given the positive correlation between sickness, elevated body temperature, and cortisol levels (Economou, Andronikou, Challa, Cholevas & Lapatsanis, 1993), children’s body temperature was also collected and included as a control for cortisol levels at each time point.
Measures
Salivary cortisol
All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay U.S. Food and Drug Administration 510k cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, Pennsylvania). The test used 25 μl of saliva (for singlet determinations), had a range of sensitivity from 0.007 to 1.8 μg/dl, and had average intra- and inter-assay coefficients of variation of less than 10% and 15%, respectively. All samples were assayed in duplicate. The criterion for repeat testing was variation between duplicates greater than 20%, and the average of the duplicates was used in all analyses. The cortisol distributions were subject to log transformation to correct positive skew.
Observed parenting
The 10-min videotaped mother–child and father-child interactions at 7 and 24 months (the free play and puzzle tasks, respectively) were observed by four trained and reliable coders and rated globally on the following dimensions of parenting behavior: sensitivity, detachment, intrusiveness, stimulation, positive regard, negative regard, and animation (Cox & Crnic, 2002; see also NICHD ECCRN, 1999). A single rating for each code was made based on the overall quality of the entire interaction using Likert-type scales ranging from 1 (not at all characteristic) to 5 (highly characteristic) at the 7-month assessment and ranging from 1 to 7 at the 24-month assessment (these scores were rescaled to a 1–5 range for the current analyses). On the basis of the results of factor analyses conducted with an oblique rotation (Promax), two broad-based parenting factors emerged for both mothers and fathers: sensitivity (the average of sensitivity, detachment (reversed), stimulation, positive regard, and animation) and negativity (the average of intrusiveness and negative regard). At least 30% of all interactions were double coded for reliability. See Table 2 for details regarding the reliability and factor structure of the individual parenting dimensions and composites.
Table 2.
Psychometric information on parenting composites
| Composite | Dimensions | 7 month
|
24 months
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Reliability1
|
Factor Structure2
|
Reliability1
|
Factor Structure2
|
||||||
| Dimension | Composite | Loadings | α | Dimension | Composite | Loadings | α | ||
| Mother sensitivity | Sensitivity | .75 | .87 | .68 | .89 | .85 | .93 | .34 | .87 |
| Detachment3 | .74 | .87 | .83 | .64 | |||||
| Stimulation | .75 | .81 | .89 | .76 | |||||
| Positive regard | .78 | .87 | .87 | .87 | |||||
| Animation | .79 | .68 | .82 | .65 | |||||
|
| |||||||||
| Mother negativity | Intrusiveness | .71 | .80 | .82 | .63 | .83 | .87 | .91 | .70 |
| Negative regard | .76 | .55 | .87 | .63 | |||||
|
| |||||||||
| Father sensitivity | Sensitivity | .64 | .85 | .78 | .83 | .83 | .92 | .25 | .83 |
| Detachment* | .74 | .88 | .82 | .62 | |||||
| Stimulation | .74 | .78 | .91 | .73 | |||||
| Positive regard | .80 | .81 | .89 | .86 | |||||
| Animation | .79 | .68 | .85 | .51 | |||||
|
| |||||||||
| Father negativity | Intrusiveness | .63 | .73 | .68 | .59 | .82 | .86 | .91 | .64 |
| Negative regard | .70 | .44 | .85 | .55 | |||||
Notes:
Reliability scores were calculated using intraclass correlations.
Factor analyses were conducted using an oblique rotation (Promax).
Detachment was reverse coded for compositing.
Results
Missing Data
Because father co-residency and father participation varied systematically by family income, ethnicity/race, and marital status, missing parenting data was not random. In contrast, missing cortisol data did vary at random across demographic and caregiving variables. As such, the sample used in subsequent analyses includes imputed values for child cortisol levels at the 7 and 24 month assessments dependent on having concurrent father parenting data and at least one cortisol sample from either time point. For the 7-month assessment, this required the imputation of 2.0% of baseline, 11.0% of the post-20 min, and 16.6% of the post-40 min values. For the 24-month assessment, this required the imputation of 7.4% of baseline, 6.2% of the post-20 min, and 9.0% of the post-40 min values. Under the ignorable missing assumption, missing data were imputed five times by multiple imputations (Rubin, 1987; Schafer, 1997). Analyses were conducted five times and results were combined using the recommended procedures from Schafer (1997). All analyses were performed by SAS (9.1, Cary NC).
Data Analyses
Means and standard deviation for predictor and outcome variables are presented in Table 3. Data analyses involved multilevel modeling of predictors of children’s cortisol levels. To address Hypothesis 1, we modeled child’s age of assessment (infancy versus toddlerhood) and time of saliva sampling for cortisol (baseline, 20-min post, 40-min post). The variable age defined whether the observations and cortisol levels were at 7 or 24 months of age. The variable trial serves as a linear contrast to examine the change in cortisol from baseline to 20-min post-peak arousal; the variable trial x trial serves as a quadratic contrast term used to examine the decline in cortisol levels from 20-min to 40-min post-peak arousal (trial was centered at baseline with the assumption of constant increments over trials such that 20-min = 1 and 40-min = 2). The nesting structure of this model was trial within age within family, which allowed us to investigate the cross-sectional associations between parenting and cortisol levels at the two child ages. To address Hypothesis 2, we examined the effects of parenting at 7 months on cortisol levels at 24 months to test for unique longitudinal effects of early fathering on later child cortisol levels. Each model included controls for child demographics (including age, race, sex, and ethnicity/race), family demographics (income-to-needs ratios, father education level, marital status, and state of residence), time of day for the first saliva collection, and child body temperature. All linear, quadratic, and moderation effects by model variables were examined. Below is the basic equation (minus control variables) used for the final analyses addressing Hypothesis 1. Note that i represents the ith subject, j represents the jth age of the child, and k represents the kth trial.
Table 3.
Means and standard deviations for control, predictor, and outcome variables
| Variable | 7 Months | 24 Months |
|---|---|---|
| Time of day | 13:47 (3:01) | 14:57 (3:27) |
| Body temperature | 98.39 (0.71) | 98.26 (0.68) |
| Mother sensitivity | 9.85 (3.59) | 10.05 (3.94) |
| Mother negativity | 4.47 (1.37) | 4.33 (1.59) |
| Father sensitivity | 8.02 (3.79) | 8.64 (3.46) |
| Father negativity | 4.38 (1.33) | 4.61 (1.36) |
| Raw cortisol values | ||
| Baseline (μg/dL) | .204 (.304) | .150 (.172) |
| 20-min post-peak (μg/dL) | .239 (.256) | .160 (.230) |
| 40-min post-peak (μg/dL) | .223 (.267) | .153 (.213) |
Hypothesis 1: Testing within-age effects of father parenting on child cortisol levels
The statistics from the final model are presented in Table 4. There were significant associations between father negativity and child cortisol levels that were moderated by age and by trial. Probing these effects revealed that father negativity was not associated with baseline levels of cortisol at 7 months of age but was positively associated with greater increases in cortisol following the challenge procedure, β = 0.159, t= 2.52, p = .01 (Figure 1). There was a marginal associations (p = .09) between father negativity and the quadratic change in cortisol that suggested a greater return to baseline for children of low-negative fathers at 7 months of age. At 24 months of age, father negativity was positively associated with baseline levels of cortisol, β = 0.314, t= 3.73, p < .001, but there were no associations with any linear or quadratic changes in cortisol levels at this age (Figure 2). No moderating effects of child gender, race, or income were found.
Table 4.
Mixed model of father caregiving correlates of cortisol levels across 7 and 24 month assessments
| Variable | B | SE | 95% C.I. | P-value |
|---|---|---|---|---|
| Intercept | −2.98 | 0.52 | (−3.99, −1.95) | .001 |
| Child sex (female) | −0.02 | 0.05 | (−0.11,0.07) | .710 |
| Child ethnicity/race | 0.01 | 0.07 | (−0.13,0.16) | .862 |
| Father education | 0.00 | 0.01 | (−0.02,0.02) | .717 |
| Father cohabiting | 0.06 | 0.06 | (−0.07,0.17) | .393 |
| State (NC vs. PA) | 0.04 | 0.06 | (−0.07,0.16) | .463 |
| Family income | 0.00 | 0.01 | (−0.03,0.03) | .827 |
| Child body temperature | 0.01 | 0.02 | (−0.03,0.06) | .636 |
| Age (7 vs. 24 months) | −0.01 | 0.08 | (−0.18,0.15) | .852 |
| Time of day | 0.21 | 0.07 | (0.07,0.36) | .003 |
| Time of day2 | −0.01 | 0.00 | (−0.02, −0.01) | .001 |
| Trial | −0.04 | 0.08 | (−0.20,0.13) | .679 |
| Trial x trial | −0.02 | 0.04 | (−0.09,0.05) | .620 |
| Trial x age | 0.45 | 0.12 | (0.20,0.69) | .001 |
| Trial x trial x age | −0.15 | 0.06 | (−0.26, −0.04) | .009 |
| Mother sensitivity | −0.03 | 0.06 | (−0.14,0.11) | .671 |
| Mother negativity | 0.00 | 0.05 | (−0.09, 0.10) | .956 |
| Father sensitivity | 0.05 | 0.05 | (−0.05, 0.15) | .330 |
| Father negativity | −0.31 | 0.08 | (−0.48, −0.15) | .001 |
| Father negativity x age | 0.33 | 0.11 | (0.14,0.56) | .001 |
| Father negativity x trial | 0.07 | 0.04 | (−0.01,0.15) | .099 |
| Father negativity x trial x age | −0.31 | 0.11 | (−0.53, −0.09) | .007 |
| Father negativity x trial x trial x age | 0.09 | 0.05 | (−0.01,0.19) | .089 |
Figure 1.
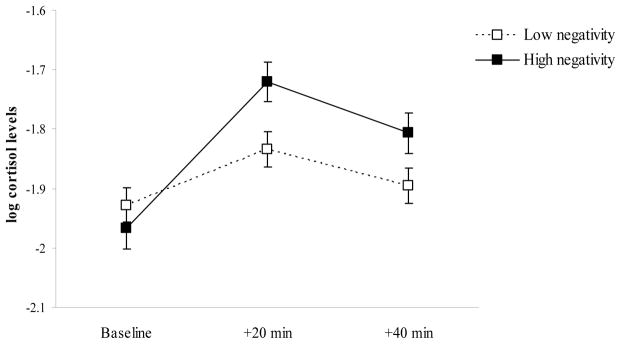
Baseline, reactivity, and regulation levels of child cortisol at 7 months of age as a function of father negativity. Dashed line = low father negativity; solid line = high father negativity. Error bars represent two times the standard error of the estimated mean.
Figure 2.
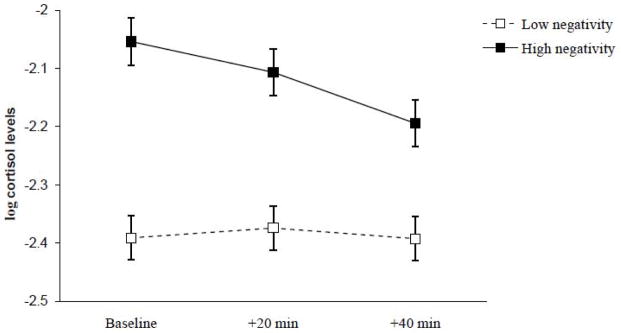
Baseline, reactivity, and regulation levels of child cortisol at 24 months of age as a function of father negativity. Dashed line = low father negativity; solid line = high father negativity. Error bars represent two times the standard error of the estimated mean.
Hypothesis 2: Testing longitudinal effects of father parenting on child cortisol levels
There was no evidence indicating that early father caregiving at 7 months of child age was uniquely associated with later child cortisol levels at 24 months of age.
Discussion
This study prospectively examined associations between father caregiving and young children’s cortisol reactivity during infancy and toddlerhood. Consistent with a previous report from this sample regarding cortisol responses at this age (Blair et al., 2008), infants exhibited increases in response to the emotion challenge, as well as a return to baseline following the increase. There were no linear or quadratic changes in cortisol levels in response to the emotion challenge in toddlerhood, a finding consistent with previous studies that suggests the potential of a hyporesponsive period for cortisol activation (at least in response to mild laboratory stressors) for children at this age (Gunnar & Quevedo, 2007). As proposed in Hypothesis 1, concurrent father parenting was associated with child cortisol levels, but this association was limited to negative caregiving. Interestingly, and perhaps due to the correlation between fathering variables across time points, there was no unique longitudinal association between early fathering and later child cortisol levels as was proposed in Hypothesis 2.
Fathers’ Parenting and Children’s Cortisol Responses at Infancy
At 7 months of child age, high father negativity was associated with greater increases in child cortisol following peak arousal to an emotional challenge. There was not an association between father negativity and baseline cortisol levels, and the association between father negativity and the quadratic change in cortisol following challenge was marginal. There were no correlations between father sensitivity and any dimension of cortisol activity, which is particularly interesting given previous analyses with this sample demonstrating significant associations between mothers’ sensitivity (but not negativity) and children’s cortisol reactivity and regulation at the same time point (Blair et al., 2008). It is possible that harsh and controlling caregiving by fathers leads to exacerbated cortisol reactivity in infancy, which would suggest that fathers’ negativity may be uniquely capable of priming the child’s response to stressful situations and undermining the child’s ability to regulate such physiological arousal.
Fathers’ Parenting and Children’s Cortisol Responses at Toddlerhood
At 24 months of child age, father negativity was positively associated with overall levels of cortisol. In contrast to findings at 7 months of age, there was no significant association between father caregiving and any linear or quadratic changes in cortisol levels. This may be due to the overall insignificant increase in cortisol in response to the challenge at 24 months of age. Although this finding may be a methodological artifact due to mother involvement during the 24-month challenge (she was not directly involved at 7 months) blunting the child’s reactivity to the task, the finding is also consistent with previous research noting increased difficulty in provoking a cortisol response in toddlers by means of mild stress tasks (Gunnar & Donzella, 2002). Gunnar (2003) also suggests that by toddlerhood children in “supportive caregiving relationships appear to have entered the human functional equivalent of the rodent stress-hyporesponsive period” (p. 155). By contrast, children from unsupportive relationships with insensitive or negative caregivers may not exhibit this pattern. The positive association between father negativity and child baseline cortisol levels in toddlerhood suggests that there are relevant father effects on cortisol functioning at this age. At least two interpretations of this finding are possible. First, as with the 7-month assessment, the initial assessment of cortisol at 24 months of age occurred in the child’s home. Children of highly negative fathers may be more sensitive to the introduction of unfamiliar adults in their home due in part to an inadequate caregiver support system. Given the lack of an adequate caregiver support system to assist in regulating their distress, the child may automatically mount a cortisol response with the arrival of the data collectors. Based on this scenario, the initial measure of cortisol should more accurately be interpreted as an initial stress response measure, which suggests that father negativity is associated with increased HPA reactivity (or decreased hyporesponsiveness following Gunnar’s (2003) proposal). An alternative interpretation would be that the initial value is indeed representative of baseline levels, and although there is no significant change in level across the visit, negative fathering is associated with elevated overall levels of cortisol.
Assessing the Unique Effects of Fathers: Limitations and Conclusions
There are limitations to this study that must also be considered when interpreting and generalizing these findings. First, there are methodological limitations that should be considered when interpreting the cortisol analyses, such as potential confounds that were not measured at one (or both) time points in the current study. These omitted variables include the child’s time of waking (from night sleep and most recent nap), elapsed time since last meal or snack, and current medication use. Second, this study did not include a measure of father involvement to determine whether the associations with father caregiving quality are dependent on the quantity of time or type of time spent with the child. Also, father negativity may be a marker for other potentially disruptive family events that may cause elevations in cortisol independent of fathers’ caregiving behaviors. Naturalistic observations of families suggest that traumatic family events (such as conflict, punishment, shaming, and fighting) are associated with elevated cortisol levels in children during the proximal timing of the trauma (Flinn & England, 1995). Similarly, using data from the Family Life Project, Hibel et al. (2009) reported that inter-parental conflict was positively associated with concordant elevations in child and mother cortisol levels. Because adult trauma and conflict have been associated with insensitive and harsh parenting behavior, it is possible that observed violence and conflict may also be responsible for correlations between father negativity and children’s cortisol levels. Furthermore, the inability to detect associations between child cortisol levels and sensitive fathering (as comparable to previous findings with sensitive mothering) may stem from restricting the measurement of father caregiving to the same dimensions of parenting traditionally used for mothers. In doing so we may have failed to capture some of the unique aspects of fathers’ sensitive caregiving. Such a possibility is important to note given the relatively small factor loading of our sensitivity ratings onto the father sensitivity composite at 24 months. Finally, the fathers in this study did not participate in the challenge tasks or post-challenge soothing sessions. Future research should consider the relevance of father presence at these tasks on reactivity levels, as well as any effects of father involvement in the soothing of the child on regulation levels of cortisol post-challenge.
Despite these limitations, the current findings are consistent with both psychobiological and family systems perspectives on child development. Paternal negativity was associated with greater child cortisol reactivity at 7 months and greater overall levels of cortisol at 24 months of child age. It is possible that fathers’ harsh and controlling caregiving may push children’s cortisol responses beyond optimal ranges of reactivity to distress, and continued exposure to such a caregiving environment may contribute to a potential allostatic load for these children as they maintain elevated cortisol levels during toddlerhood. It is also important to note that these associations were present above and beyond any associations with mothers’ caregiving. Interestingly, there was no association between father caregiving at infancy and later cortisol levels above and beyond the associations with concurrent fathering at toddlerhood, which is possibly due to the strong correlation in paternal caregiving from 7 to 24 months of child age.
The current research is generalizable only to residential fathers living in non-urban settings. Furthermore, it should be noted that families with participating residential fathers typically have higher SES households than families with non-participating residential fathers and families with nonresidential fathers. As such, although these findings are consistent with conceptual models of family and father functioning, further research is needed to replicate and extend this line of research, including investigation into families characterized by significant change in father caregiving across the first two years, as well as the effects of non-residential fathering and other non-maternal caregiving scenarios. Future research on these topics should continue to shed light on the intersection of psychobiological development and family systems.
Acknowledgments
We would like to thank the many families and research assistants who made this study possible. We would also like to thank Eva Lefkowitz and three anonymous reviewers for their insightful comments on earlier drafts of this manuscript. Support for this research was provided by National Institute of Child Health and Human Development Grant P01 HD39667, with cofunding from the National Institute on Drug Abuse. The Family Life Project key investigators include Lynne Vernon-Feagans, Martha Cox, Clancy Blair, Peg Burchinal, Linda Burton, Keith Crnic, Ann Crouter, Patricia Garrett-Peters, Mark Greenberg, Stephanie Lanza, Roger Mills-Koonce, Debra Skinner, Cynthia Stifter, Emily Werner, and Michael Willoughby. In the interest of full disclosure, Douglas A. Granger is president and founder of Salimetrics, LLC, State College, Pennsylvania.
References
- Amato PR, Rivera F. Paternal involvement and children’s behavior. Journal of Marriage and the Family. 1999;61:375–384. [Google Scholar]
- Barnett M, Deng M, Mills-Koonce WR, Willoughby M, Cox M. Interdependence of parenting of mothers and fathers of infants. Journal of Family Psychology. 2008;22:561–573. doi: 10.1037/0893-3200.22.3.561. [DOI] [PubMed] [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3 years: Differential susceptibility to rearing experience? Journal of Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Kivlighan K, Mills-Koonce R, Willoughby M, Greenberg M, Hibel LC, Fortunado CK. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Booth A, Carver K, Granger DA. Biosocial perspectives on the family. Journal of Marriage and the Family. 2000;62:1018–1034. [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Burchinal P, Vernon-Feagans L, Cox M the Family Life Project Investigators. Cumulative social risk and infant development in rural low-income communities. Parenting: Science and Practice. 2008;8:41–82. doi: 10.1080/15295190701830672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss K, Goldsmith H. Fear and anger regulation in infancy: Effects on the temporal dynamics of affective expression. Child Development. 1998;69:359–374. [PubMed] [Google Scholar]
- Cabrerea NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Development. 2000;71:127–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Chang L, Schwartz D, Dodge KA, McBride-Chang C. Harsh parenting in relation to child emotion regulation and aggression. Journal of Family Psychology. 2003;17:598–606. doi: 10.1037/0893-3200.17.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M, Crnic K. Qualitative ratings for parent-child interaction at 3 – 12 months of age. Department of Psychology, University of North Carolina; Chapel Hill: 2002. Unpublished manuscript. [Google Scholar]
- Cox M, Paley B, Burchinal M, Payne C. Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and the Family. 1999;61:611–625. [Google Scholar]
- Diener M, Mangelsdorf C, McHale J, Frosch C. Infants’ behavioral strategies for emotion regulation with fathers and mothers: Associations with emotional expressions and attachment quality. Infancy. 2002;5:151–172. doi: 10.1207/S15327078IN0302_3. [DOI] [PubMed] [Google Scholar]
- Dokoumetzidis A, Iliadis I, Macheras P. Nonlinear dynamics in Clinical Pharmacology: the paradigm of cortisol secretion and suppression. British Journal of Clinical Pharmacology. 2002;54(1):21–29. doi: 10.1046/j.1365-2125.2002.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou G, Andronikou S, Challa A, Cholevas V, Lapatsanis PD. Cortisol secretion in stressed babies during the neonatal period. Hormone Research. 1993;40:217–221. doi: 10.1159/000183798. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress and family environment. Journal of Current Anthropology. 1995;36:854–866. [Google Scholar]
- Foster PA, Reese-Weber M, Kahn JH. Fathers’ parenting and coping: Associations with emotional expressiveness and their sons’ socioemotional competence. Journal of Infant and Child Development. 2007;16:277–293. [Google Scholar]
- Goslin MC, Booth A, Granger DA. Baseline cortisol attunement in family dyads: Interpersonal, individual, and contextual moderators. Poster session presented at the biannual meeting of the Society for Research in Child Development; Denver, CO. 2009. [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Metaemotion. Hillsdale, NJ: Erlbaum; 1997. [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:580–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Grossmann K, Grossmann KE, Fremmer-Bombik E, Kindler H, Scheuerer-Englisch H, Zimmerman P. The uniqueness of the child-father attachment relationship: Fathers’ sensitive and challenging play as the pivotal variable in a 16-year longitudinal study. Social Development. 2002;11:307–331. [Google Scholar]
- Gunnar M. Integrating neuroscience and psychological approaches in the study of early experiences. Roots of Mental Illness in Children. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Haley DW, Stansbury K. Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Development. 2003;74:1534–1546. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Harmon A, Granger DA, Hibel LC, Rumyantseva O. Measuring salivary cortisol in studies of child development: Watch out- What goes in may not come out of commonly used saliva collection devices. Developmental Psychobiology. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DG, Blair C, Cox MJ The Family Life Project Key Investigators. Intimate partner violence moderates the association between mother-infant adrenocortical activity across an emotional challenge. Journal of Family Psychology. 2009;23:615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Tjebkes T, Forman D. Children’s emerging regulation of conduct: Restraint, compliance, and internalization from infancy to the second year. Child Development. 1998;69:1378–1389. [PubMed] [Google Scholar]
- Lamb ME, Tamis-LeMonda CS. The role of the father: An introduction. In: Lamb ME, editor. The role of the father in child development. 4. Hoboken, NJ: John Wiley & Sons; 2004. pp. 1–31. [Google Scholar]
- Marsiglio W, Day R, Lamb ME. Exploring fatherhood diversity: Implications for conceptualizing father involvement. Marriage and the Family Review. 2000;29:269–203. [Google Scholar]
- McDowell D, Kim M, O’Neil R, Parke R. Children’s emotional regulation and social competence in middle childhood: The role of maternal and paternal interactive style. Journal of Marriage and Family Review. 2002;34:345–364. [Google Scholar]
- NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35:1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- Popenoe D. Life without father. New York: Free Press; 1996. [Google Scholar]
- Roggman LA, Fitzgerald AE, Bradley RH, Raikes H. Methodological, measurement, and design issues in studying fathers: An interdisciplinary perspective. In: Tamis-LeMonda CS, Cabrera N, editors. Handbook of father involvement: Multidisciplinary perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 1–30. [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. New York: Capman & Hall; 1997. [Google Scholar]
- Sroufe L. Emotional development: The organization of emotional life in the early years. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- Stifter CA, Braungart J. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Yogman MW, Kindlon D, Earls F. Father involvement and cognitive/behavioral outcomes of preterm infants. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:58–66. doi: 10.1097/00004583-199501000-00015. [DOI] [PubMed] [Google Scholar]


