Abstract
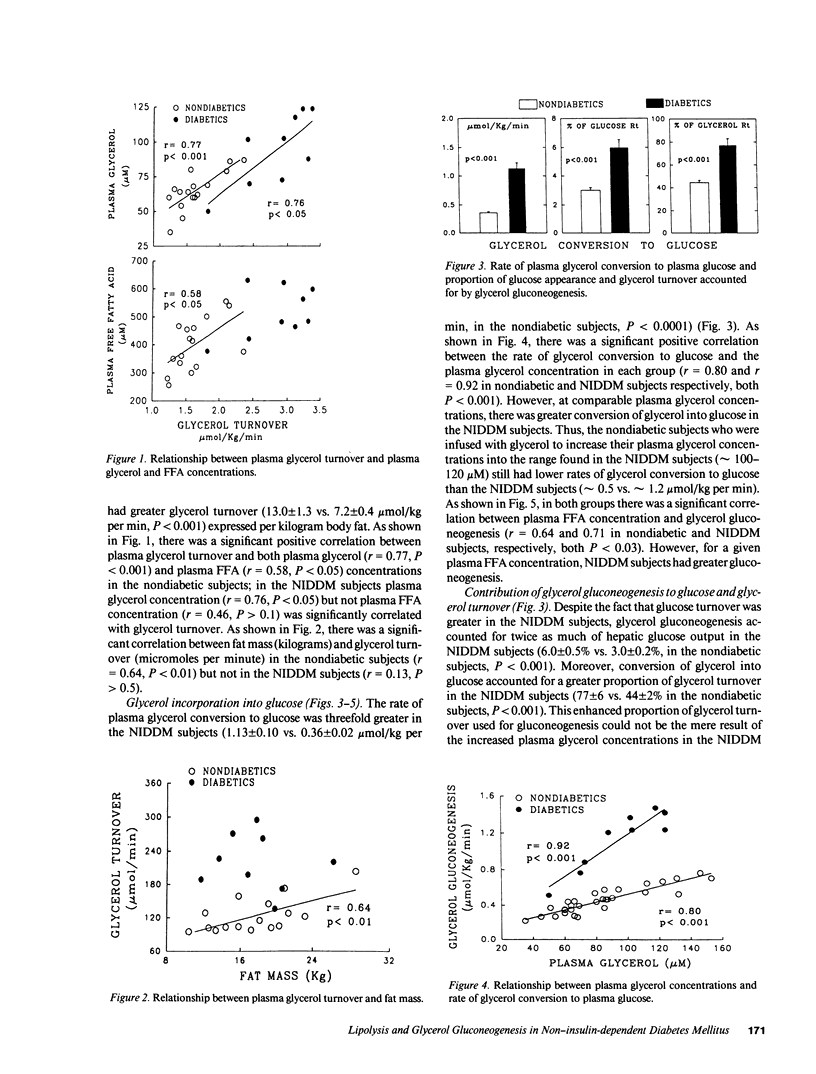
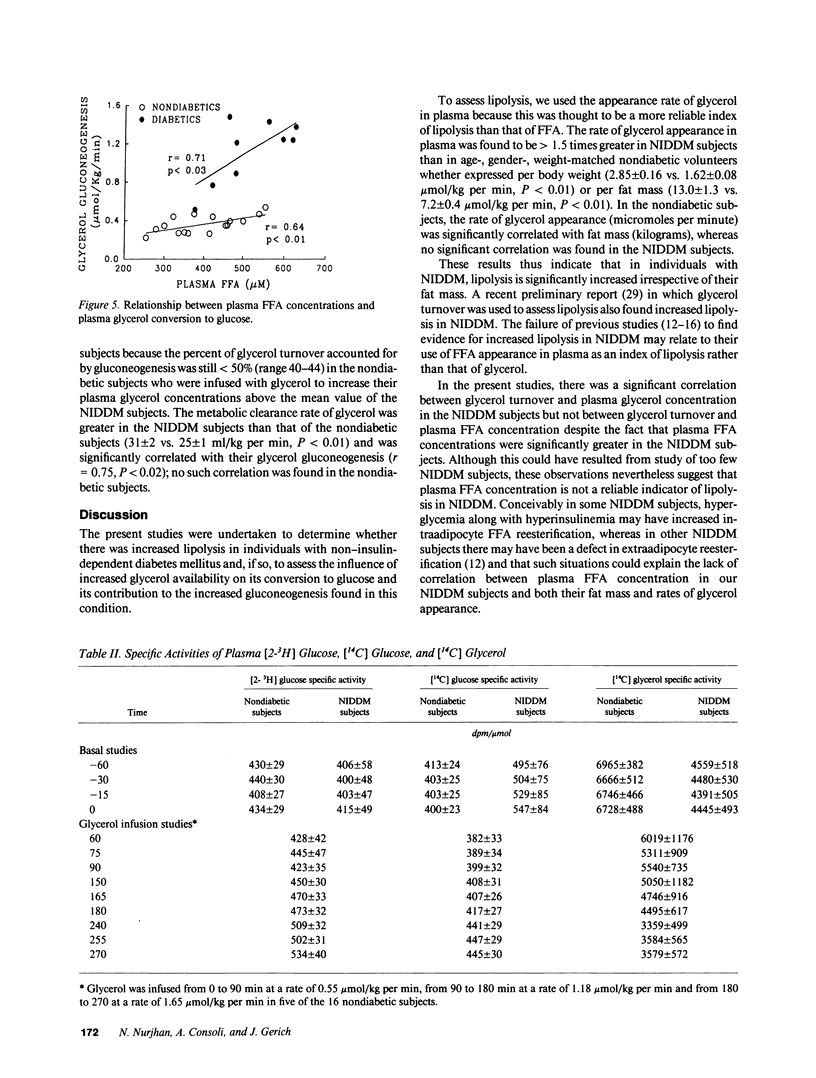
The present studies were undertaken to determine whether lipolysis was increased in non-insulin-dependent diabetes mellitus (NIDDM) and, if so, to assess the influence of increased glycerol availability on its conversion to glucose and its contribution to the increased gluconeogenesis found in this condition. For this purpose, we infused nine subjects with NIDDM and 16 age-, weight-matched nondiabetic volunteers with [2-3H] glucose and [U-14C] glycerol and measured their rates of glucose and glycerol appearance in plasma and their rates of glycerol incorporation into plasma glucose. The rate of glycerol appearance, an index of lipolysis, was increased 1.5-fold in NIDDM subjects (2.85 +/- 0.16 vs. 1.62 +/- 0.08 mumol/kg per min, P less than 0.001). Glycerol incorporation into plasma glucose was increased threefold in NIDDM subjects (1.13 +/- 1.10 vs. 0.36 +/- 0.02 mumol/kg per min, P less than 0.01) and accounted for twice as much of hepatic glucose output (6.0 +/- 0.5 vs. 3.0 +/- 0.2%, P less than 0.001). Moreover, the percent of glycerol turnover used for gluconeogenesis (77 +/- 6 vs. 44 +/- 2, P less than 0.001) was increased in NIDDM subjects and, for a given plasma glycerol concentration, glycerol gluconeogenesis was increased more than two-fold. The only experimental variable significantly correlated with the increased glycerol gluconeogenesis after taking glycerol availability into consideration was the plasma free fatty acid concentration (r = 0.80, P less than 0.01). We, therefore, conclude that lipolysis is increased in NIDDM and, although more glycerol is thus available, increased activity of the intrahepatic pathway for conversion of glycerol into glucose, due at least in part to increased plasma free fatty acids, is the predominant mechanism responsible for enhanced glycerol gluconeogenesis. Finally, although gluconeogenesis from glycerol in NIDDM is comparable to that of alanine and about one-fourth that of lactate is terms of overall flux into glucose, glycerol is probably the most important gluconeogenic precursor in NIDDM in terms of adding new carbons to the glucose pool.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Bolinder J., Engfeldt P., Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981 Aug;30(8):753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Bolzano K., Sandhofer F., Sailer S., Braunsteiner H. The effect of oral administration of sucrose on the turnover rate of plasma free fatty acids and on the esterification rate of plasma free fatty acids to plasma triglycerides in normal subjects, patients with primary endogenous hypertriglyceridemia, and patients with well controlled diabetes mellitus. Horm Metab Res. 1972 Nov;4(6):439–446. doi: 10.1055/s-0028-1094002. [DOI] [PubMed] [Google Scholar]
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. J., Mandarino L. J., Gerich J. E. Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism. 1988 Jan;37(1):15–21. doi: 10.1016/0026-0495(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Carlson C. W., Baxter R. C., Ulm E. H., Pogell B. M. Role of oleate in the regulation of "neutral" rabbit liver fructose 1,6-diphosphatase activity. J Biol Chem. 1973 Aug 25;248(16):5555–5561. [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am J Physiol. 1990 Nov;259(5 Pt 1):E677–E684. doi: 10.1152/ajpendo.1990.259.5.E677. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csorba T. R., Matsuda I., Kalant N. Effects of insulin and diabetes on flux rates of plasma glucose and free fatty acids. Metabolism. 1966 Mar;15(3):262–270. doi: 10.1016/0026-0495(66)90024-2. [DOI] [PubMed] [Google Scholar]
- Darmaun D., Matthews D. E., Bier D. M. Physiological hypercortisolemia increases proteolysis, glutamine, and alanine production. Am J Physiol. 1988 Sep;255(3 Pt 1):E366–E373. doi: 10.1152/ajpendo.1988.255.3.E366. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. M., Roche A. F., Chumlea W. C., Miles D. S., Pohlman R. L. Body composition predictions from bioelectric impedance. Hum Biol. 1987 Apr;59(2):221–233. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Perez G., Vranic M. Turnover and precursor-product relationships of nonlipid metabolites. Physiol Rev. 1983 Apr;63(2):606–667. doi: 10.1152/physrev.1983.63.2.606. [DOI] [PubMed] [Google Scholar]
- Houtkooper L. B., Lohman T. G., Going S. B., Hall M. C. Validity of bioelectric impedance for body composition assessment in children. J Appl Physiol (1985) 1989 Feb;66(2):814–821. doi: 10.1152/jappl.1989.66.2.814. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Pennington L. F., Boshell B. R. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970 Jan;19(1):53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- Lewis B., Mancini M., Mattock M., Chait A., Fraser T. R. Plasma triglyceride and fatty acid metabolism in diabetes mellitus. Eur J Clin Invest. 1972 Nov;2(6):445–453. doi: 10.1111/j.1365-2362.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Digirolamo M., Krotkiewski M., Smith U. Insulin binding and responsiveness in fat cells from patients with reduced glucose tolerance and type II diabetes. Diabetes. 1983 Aug;32(8):748–754. doi: 10.2337/diab.32.8.748. [DOI] [PubMed] [Google Scholar]
- Malmendier C. L., Delcroix C., Berman M. Interrelations in the oxidative metabolism of free fatty acids, glucose, and glycerol in normal and hyperlipemic patients. A compartmental model. J Clin Invest. 1974 Aug;54(2):461–476. doi: 10.1172/JCI107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely P., El-Maghrabi M. R., Pilkis S. J., Claus T. H. Effect of diabetes, insulin, starvation, and refeeding on the level of rat hepatic fructose 2,6-bisphosphate. Diabetes. 1981 Dec;30(12):1062–1064. doi: 10.2337/diab.30.12.1062. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Campbell P. J., Kennedy F. P., Miles J. M., Gerich J. E. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986 Dec;35(12):1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Kennedy F., Consoli A., Martin C., Miles J., Gerich J. Quantification of the glycolytic origin of plasma glycerol: implications for the use of the rate of appearance of plasma glycerol as an index of lipolysis in vivo. Metabolism. 1988 Apr;37(4):386–389. doi: 10.1016/0026-0495(88)90140-0. [DOI] [PubMed] [Google Scholar]
- SHAPIRO B., CHOWERS I., ROSE G. Fatty acid uptake esterification in adipose tissue. Biochim Biophys Acta. 1957 Jan;23(1):115–120. doi: 10.1016/0006-3002(57)90292-5. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tani Y., Yamada H., Tabata M., Murachi T. Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem. 1980 Sep 1;107(1):193–198. doi: 10.1016/0003-2697(80)90511-4. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Williams P. E., Liljenquist J. E., Lacy W. W., Keller U., Cherrington A. D. Effect of hyperglycemia independent of changes in insulin or glucagon on lipolysis in the conscious dog. Metabolism. 1980 Apr;29(4):317–320. doi: 10.1016/0026-0495(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962 Nov;237:3354–3358. [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Kubo K., Zawadzki J., Lillioja S., Young A., Abbott W., Foley J. E. Dissociation of in vitro sensitivities of glucose transport and antilipolysis to insulin in NIDDM. Am J Physiol. 1987 Sep;253(3 Pt 1):E300–E304. doi: 10.1152/ajpendo.1987.253.3.E300. [DOI] [PubMed] [Google Scholar]



