Abstract
Characterization of the cellular participants in tissue immune responses is crucial to understanding infection, cancer, autoimmunity, allergy, graft rejection and other immunological processes. previous reports indicate that leukocytes in lung vasculature fail to be completely removed by perfusion. several studies suggest that intravascular staining may discriminate between tissue-localized and blood-borne cells in the mouse lung. Here we outline a protocol for the validation and use of intravascular staining to define innate and adaptive immune cells in mice. We demonstrate application of this protocol to leukocyte analyses in many tissues and we describe its use in the contexts of lymphocytic choriomeningitis virus and Mycobacterium tuberculosis infections or solid tumors. Intravascular staining and organ isolation usually takes 5–30 min per mouse, with additional time required for any subsequent leukocyte isolation, staining and analysis. In summary, this simple protocol should help enable interpretable analyses of tissue immune responses.
INTRODUCTION
Development of the protocol
Cells of the immune system act locally to ameliorate, prevent or exacerbate disease1–5. Thus, immune responses must be characterized directly within affected tissues. However, cells of the immune system also continuously recirculate throughout the vasculature, which includes an abundant capillary network that permeates every organ. Immune cells in blood, compared with those in tissues, exhibit vastly different phenotypes, functions and differentiation states5–11. Those within blood are typically (although not always) recirculating cells rather than participants in local immune responses. Thus, blood-borne cell contamination of tissues confounds the identification and purification of leukocytes that are truly participating in local immunological processes. A common solution to this problem in animal models is the putative removal of blood-borne leukocytes by perfusing tissues with a cell-free solution post mortem.
We recently used an intravascular staining approach12 and demonstrated that perfusion does not remove many CD8 T cells from the lung vasculature during the course of an antiviral response13. Rather unexpectedly, up to 97% of the CD8 T cells that were thought to be located within the lung of perfused mice were not in the tissue13, an observation that questions the interpretation of previous studies on CD8 T cell migration, differentiation, maintenance and correlates of protective immunity against respiratory infections.
This simple protocol enables the identification of major leukocyte lineages contained within the vasculature that might other-wise conflate interpretation of adaptive and innate immune responses in tissues. Importantly, this protocol eliminates the need to perform perfusion, which may have unintended consequences. The strengths and weaknesses of different staining strategies for different leukocyte subsets and for different tissues are documented, with an emphasis on sample data in which the inclusion of intravascular staining allows for a revised interpretation of the results. Typical results seen using this protocol strongly suggest that substituting intravascular staining for perfusion whenever possible provides considerable advantages for interpreting the anatomic distribution of immune responses.
Applications of the protocol
Discriminating between tissue-localized and blood-borne cells is relevant for studies examining cellular processes in nonlymphoid tissues. Our protocol outlines how to perform intravascular staining for the identification of vascular T and B cells, neutrophils and mononuclear phagocytes in mice. As cells in blood-borne compartments may also have important roles in immune responses14–18, intravascular staining may also be useful for those interested in including these populations of cells in analyses rather than eliminating them via perfusion.
Intravascular staining can be applied to studies of viral and bacterial infections, as well as to those of solid tumors in mice, but investigations of many other disease processes, such as models of graft-versus-host disease, allergy and autoimmunity, as well as studies of cell migration, may also greatly benefit from this type of analysis. Although our research was limited to immune responses in mice, this protocol may be adaptable to other species, and additional applications and refinements are likely to develop as intravascular staining is applied to different settings.
Comparison with other methods
Previous work examining neutrophil migration through the lung vasculature demonstrated that neutrophils transited the large vessels of the lung in ~3 min, but required 3 h if routed through the capillary bed19. As a result, the ratio of neutrophils: red blood cells (RBCs) in lung capillaries is 100-fold higher than in peripheral blood or large lung vessels (1:100 versus 1:10,000). Consequently, even though capillaries only make up 40% of the blood volume of the lung, they contain 99% of the lung blood-borne neutrophils. These data indicate that hemodynamic forces greatly influence the transit rate of leukocytes in the microvasculature. Such forces may sequester lymphocytes within pulmonary vasculature as well, perhaps owing to the expression of adhesion molecules or the fact that the volume of a lymphocyte is greater than an RBC (~290 fl versus ~90 fl)20,21.
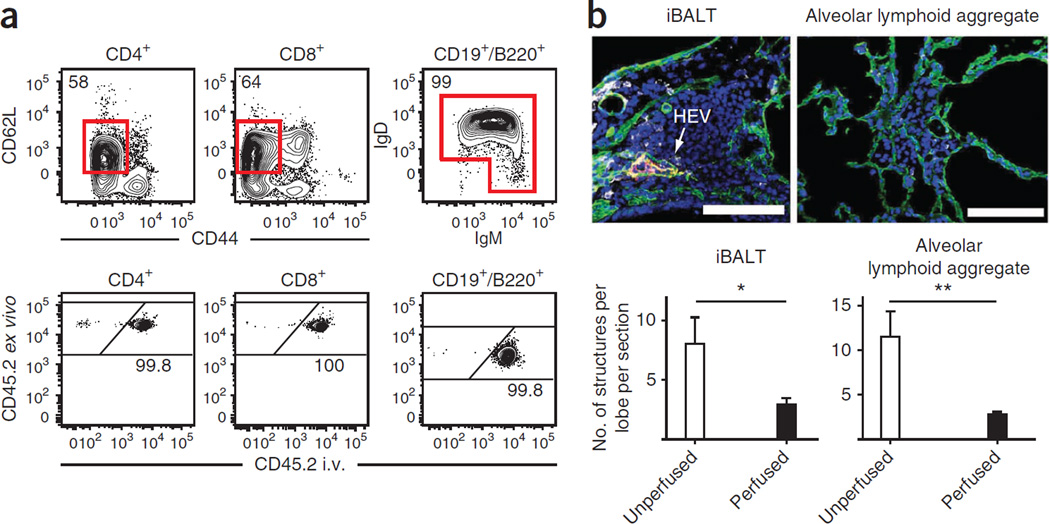
Perfusion, a method of flushing the pulmonary vasculature with buffer, has been used to remove RBCs and leukocytes from the lung vasculature. However, we recently showed that the vast majority of CD8 T cells isolated from the lung of perfused mice were likely trapped within the vasculature because they were rapidly stained with i.v.-injected monoclonal antibody (mAb)13. These results call into question several studies that report that naive lymphocytes are present within the lung tissue of specific pathogen–free (SPF) mice that lack inducible bronchus-associated lymphoid tissue (iBALT)22–25; these are observations that violate the central dogma that naive lymphocytes are excluded from nonlymphoid tissues (NLTs) and which were proposed to have major biological implications. Here we show how intravascular staining can be used to address these issues. Consistent with previous reports using this protocol13, we recovered B cells and T cells that expressed a naive phenotype (CD62L + and CD44lo for CD4 and CD8 T cells and IgD + and/or IgM + for B cells) from the lungs of uninfected mice that were perfused to remove blood from organs. When anti-CD45.2 mAb was injected i.v. 3 min before mice were killed and perfused (CD45 is expressed on the surface of all leukocytes), >99% of naive lymphocytes, regardless of lineage, became labeled (Fig. 1a). These results confirm that intravascular staining can be used to identify naive lymphocytes within the pulmonary vasculature.
Figure 1.
Evidence that perfusion should be avoided. (a) Anti-CD45.2 mAb was injected i.v. via the tail vein of naive C57BL/6 mice 3 min before perfusion and lymphocyte isolation. Naive CD4 + and CD8 + T cells and B cells were identified by flow cytometry, as indicated and examined for labeling with injected anti-CD45.2 mAb. Representative of 12 mice from two experiments. (b) At 15 d after i.t. LCMV infection, lungs from P14 chimeras were perfused or left unperfused. Representative immunofluorescence images of iBALT or aggregate structures in lungs stained with anti-collagen type IV (green), anti-PNAd (red), anti-Thy1.1 (gray) and DAPI (blue). Arrow designates PNAd + high endothelial venule (HEV). Scale bars, 100 µm. Representative of two experiments totaling 10 mice per condition. *P = 0.05, **P < 0.01, using a two-tailed Student’s t test with a 95% confidence interval. Error bars indicate s.e.m.
Perfusion has been suggested to have other consequences on lung architecture. Mice seeded with naive Thy1.1 + P14 CD8 T cells (referred to as ‘P14 chimeric mice’, which allows for tracking of a monoclonal CD8 T cell population specific for lymphocytic choriomeningitis virus (LCMV)) and infected via the intratracheal (i.t.) route with the mouse pathogen LCMV develop the transient tertiary lymphoid organ iBALT. iBALT has been well characterized, and it is classically defined as a collection of lymphocytes and dendritic cells that abuts a large airway, is typically adjacent to large blood vessels and contains both lymphatic vessels and high endothelial venules (HEV, as detected by peripheral lymph node adressin (PNAd) staining)26. Along with iBALT, i.t. LCMV infection induces abundant lymphocyte aggregates that are distinct from iBALT in that they lack HEVs, they are much smaller and they are not located near large airways. Consistent with the expectation that perfusion disrupts lung architecture, perfusion markedly reduces the abundance of iBALT and alveolar lymphoid aggregates detectable per lung lobe (Fig. 1b), and it reduces the cellularity of those that remained (data not shown). Indeed, alveolar lymphoid aggregates were difficult to detect after perfusion, which may help explain why they have rarely been noted previously27–31.
Collectively, these data confirm that perfusion not only leaves behind vascular-bound lymphocytes but also depletes leukocyte populations within lung compartments that may be of interest. An important advantage of intravascular staining is that it mitigates the need for perfusion.
Experimental design
The sample preparation and flow cytometry acquisition steps described in the following procedure are for CD8αβ T cells isolated from a spleen, as an example application.
Controls
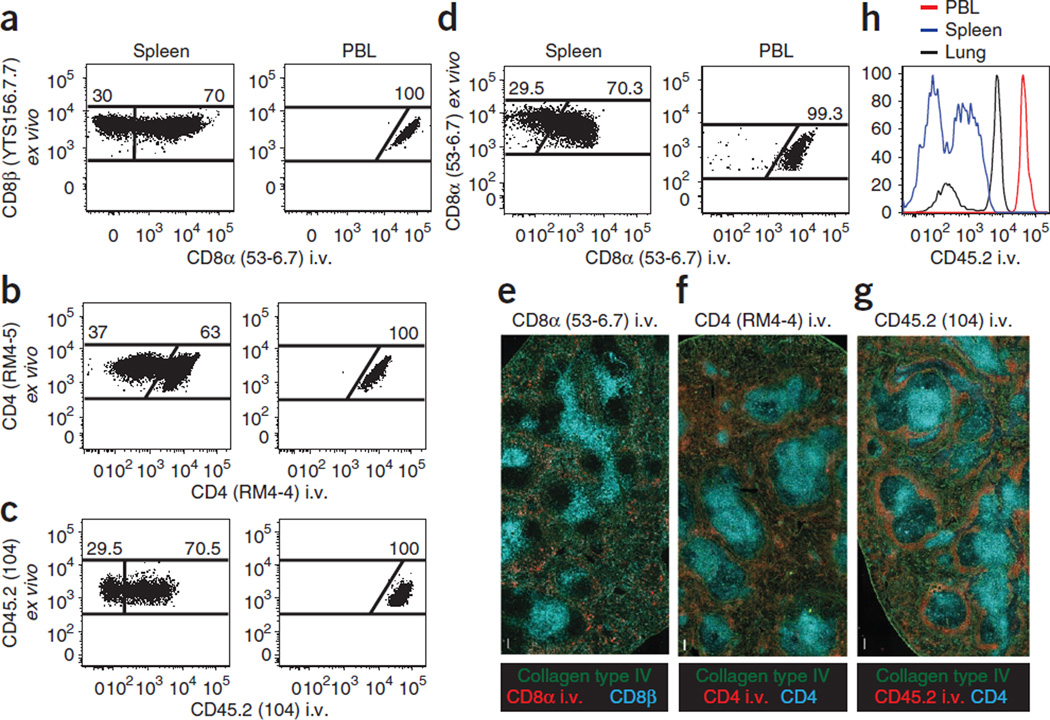
When using intravascular staining, critical staining controls must be included in every experiment. Peripheral blood (from retro-orbital or cardiac puncture) should be sampled from every mouse. One should observe that >99% of the cell population targeted by the intravascular Ab will stain positively within the peripheral blood. A negative staining control, such as lymph nodes, should also be sampled. One should observe less than 10% of cells isolated from lymph nodes staining positively with intravascular Abs. For a discussion of Ab selection, see Figure 2.
Figure 2.
Technical considerations for intravascular staining. (a–d) Thy1.1 + P14 CD8 T cell chimeras (a), CD45.1 + SMARTA CD4 T cell chimeras (b) or CD45.2 + C57BL/6 mice were infected with LCMV i.t. (c,d). (a–g) After 12–15 d, mice were injected i.v. with the indicated mAb (clones are in parentheses), killed 3 min later and lymphocytes were either isolated and further stained ex vivo for flow cytometric analysis (a–d) or tissue sections were examined by epifluorescence microscopy (e–g). (a–d) Plots are gated on CD8P+ and Thy1.1 + (a), CD4 (clone RM4–5) + and CD45.1 + (b), CD45.2 + (clone 104, stained ex vivo), CD8α+ and H2-Db/gp33–41 MHC I tetramer + (c) or CD8α+ (stained ex vivo) and H2-Db/gp33–41 MHC I tetramer + lymphocytes (d). Plots are representative of at least three experiments and nine mice per condition. (e–g) mAb specific for collagen type IV (green) and CD8β or CD4 (cyan, as indicated) stained ex vivo, and the indicated i.v. injected mAb (red) on spleen sections. Images are representative of at least three experiments and eight mice per condition. Scale bars, 100 µm. (h) At 30 d after i.t. LCMV infection of C57BL/6 mice, anti-CD45.2 i.v. mAb staining intensity was examined on CD8α + H2-Db/gp33–41 MHC I tetramer + lymphocytes isolated from blood (PBL, red), spleen (blue) and lung (black). Representative of three experiments totaling nine mice.
Limitations
We have used intravascular injection of anti-collagen type IV Ab to test for vascular leakage in all the models examined in this manuscript. Although the conditions we tested allow for reliable intravascular staining, there could be conditions under which vascular leakage may compromise the integrity of staining. For instance, there may be inflammatory contexts or angiogenic tumor models that allow rapid exudation of Ab into tissues, which could compromise interpretation of intravascular staining. Investigators examining unique conditions would be advised to perform appropriate validating controls, such as histological analysis after injection of anti-collagen Ab (Box 1) or injection of Evans Blue dye (EBD), as described13,32,33. A collagen basement membrane surrounds endothelial vessels in tissues such as the lung and intestine, and it is not exposed to intravascular Ab under conditions that we have tested. Collagen staining in the liver sinusoids, glomerular capillaries of the kidney and red pulp of the spleen can be used as positive staining controls, as these are exposed to vascular Ab under steady-state conditions (data not shown and Anderson et al.13). As an alternative approach, one could consider using EBD, as described. EBD stains the serum protein albumin, which is only found in blood vessels under conditions with intact endothelium, but which permeates into tissues when vascular permeability is increased. Histological analysis or a quantitative comparison of EBD levels in sample tissues compared with healthy controls can be used to validate the appropriateness of intravascular staining.
Box 1 | Sample preparation and acquisition steps for examining vascular permeability of the lung.
! CAUTION Microscopy should be performed by trained individuals and in accordance with all institutional biosafety regulations. Refer elsewhere for detailed overviews of immunofluorescence staining59 and microscopy60,61.
Follow PROCEDURE Steps 1–4 by using purified polyclonal goat anti-mouse collagen type IV (15 µg per mouse). As a control, include a sample slide for ex vivo anti-collagen type IV staining. One of the following should be included as a positive control for intravascular collagen type IV staining: liver sinusoids, glomerular capillaries of the kidney or red pulp of the spleen.
Embed the lung and spleen in OCT medium.
Snap-freeze the samples in liquid nitrogen or on dry ice.
Prepare 7-µm sections on microscope slides with a cryostat.
-
Fix the slides in cold acetone for 10 min.
■ PAUSE POINT Slides can be stored at −20 °C or −80 °C for at least 18 months.
Rehydrate the slides with 1× DPBS for 10 min.
Block the slides for 60–90 min at room temperature (or overnight in the refrigerator) with 5% (wt/vol) BSA blocking solution.
Stain the slides with mAb anti-CD31-AF488 for 60 min (dilute anti-CD31 Ab 1:100 in 5% (wt/vol) BSA blocking solution or PBS). The control slide should also be stained with purified polyclonal goat anti-mouse collagen IV (dilute anti-collagen IV Ab 1:200 in 5% (wt/vol) BSA blocking solution or PBS).
Wash the slides gently three times with 5% (wt/vol) BSA blocking solution or PBS.
Stain the slides with purified polyclonal donkey anti-goat-AF555 for 30 min (dilute anti-goat Ab 1:2,000 in 5% (wt/vol) BSA blocking solution or PBS).
Wash the slides gently three times with 5% (wt/vol) BSA blocking solution or PBS.
Stain the slides with DAPI for 10 min.
Wash the slides gently with 5% (wt/vol) BSA flocking solution or PBS.
Gently tap or blot off excess 5% (wt/vol) BSA flocking solution or PBS and mount the coverslips onto slides with Prolong Gold antifade mounting reagent.
Acquire images by using a Leica DM5500B epifluorescence microscope with Leica Acquisition Suite Advanced Fluorescence software.
MATERIALS
REAGENTS
Δ CRITICAL Reagents and equipment can be substituted with appropriate alternatives from other manufacturers.
Mice: all mice were adult females from the following strains. We used SPF C57BL/6 mice (Jackson Laboratories) for analysis of immune responses to infection; SPF BALB/c mice (National Cancer Institute) were used for analysis of immune responses to solid tumors (see Reagent Setup for further details of how to set up these model systems) ! CAUTION Animal tissue should be collected in compliance with legislative and institutional requirements. ! CAUTION Experiments involving mice must conform to institutional and governmental regulations. The procedures described here were approved by the Institutional Animal Care and Use Committees (IACUC) at either the University of Minnesota or the NIH.
Dulbecco’s PBS (DPBS; Hyclone, cat. no. SH30028.03)
Ab for intravascular injection (see Table 1 for information about specific antibodies that we have used)
Ab for ex vivo staining and analysis (see Table 2 for information about specific antibodies that we have used)
Isoflurane, USP (Phoenix Pharmaceutical) ! CAUTION Isoflurane is a powerful anesthetic, and it should always be used inside a laminar flow hood and in accordance with institutional biosafety requirements.
Ethanol, 70% (vol/vol) (Decon Laboratories)
RPMI (Hyclone, cat. no. SH30027.02)
FBS (Atlas Biologicals, cat. no. FP-0500-A)
HEPES (Fisher Scientific, cat. no. BP310-1)
Sodium azide (optional; NaN3; Fischer Scientific, cat. no. BP922I-500). Dilute 10 g of NaN3 in 100 ml of distilled water and filter-sterilize the solution.
BSA (optional; Roche, cat. no. 03 116 964 001)
Potassium bicarbonate (optional; J.T. Baker Chemicals, cat. no. 2940-01)
Ammonium chloride (optional; Fischer, cat. no. A661-500)
EDTA, 0.5 M (optional; Calbiochem, cat. no. 324506)
Optimal cutting temperature (OCT) compound (optional; Tissue Tek, cat. no. 4583)
Acetone (optional; Sigma-Aldrich, cat. no. 320110-1L)
LIVE/DEAD fixable Aqua dead cell stain kit (Invitrogen, cat. no. L34957)
DAPI nucleic acid stain (DAPI dilactate; Invitrogen, cat. no. D3571)
ProLong Gold antifade reagent (Invitrogen, cat. no. P36934)
TABLE 1.
Antibodes we have used for intravascular injection.
| Analysis | Marker | Fluorochrome | Clone | Company |
|---|---|---|---|---|
| CD8 T cells | CD8α | APC | 53–6.7 | eBioscience |
| CD8α | PE | 53–6.7 | eBioscience | |
| CD4 T cells | CD4 | FITC | RM4-4 | eBioscience |
| Endothelium | CD31 | AF488 | MEC13.3 | BioLegend |
| Collagen basement membrane | Collagen IV | NA Purified Ab |
NA Polyclonal goat anti-mouse |
Millipore (cat. no. AB769) |
NA, not applicable.
TABLE 2.
Antibodies we have used for ex vivo staining for flow cytometry or immunofluorescence (optional).
| Analysis | Marker | Fluorochrome | Clone | Company |
|---|---|---|---|---|
| Epithelial cells | Cytokeratin 8 | NA Purified Ab |
NA Polyclonal rabbit anti-mouse |
Novus Biologicals |
| Cytokeratin 18 | NA Purified Ab |
NA Polyclonal rabbit anti-mouse |
Novus Biologicals | |
| HEV | PNAd | NA Purified Ab |
MECA-7 Rat anti-mouse |
BD Biosciences |
| Vascular endothelium | CD31 | AF488 | MEC13.3 | BioLegend |
| CD31 | AF647 | MEC13.3 | BioLegend | |
| T cells | CD4 | AF700 | RM4–5 | eBioscience |
| CD4 | AF647 | RM4–5 | BioLegend | |
| CD8α | PE-Cy7 | 53–6.7 | eBioscience | |
| CD8β | AF647 | YTS156.7.7 | BioLegend | |
| CD44 | APC-eF780 | IM7 | eBioscience | |
| CD45.2 | BV650 | 104 | BioLegend | |
| CD62L | PerCP-Cy5.5 | MEL-14 | BioLegend | |
| CD69 | eF450 | H1.2F3 | eBioscience | |
| CD90.1 (Thy1.1) | eF450 | HIS51 | eBioscience | |
| CD90.1 (Thy1.1) | AF647 | OX-7 | BioLegend | |
| CD103 | APC | 2E7 | eBioscience | |
| B cells | CD11c | APC-eF780 | N418 | eBioscience |
| CD19 | AF700 | eBio1D3 | eBioscience | |
| CD38 | PE-Cy7 | 90 | BioLegend | |
| CD45.2 | BV650 | 104 | BioLegend | |
| CD90.2 (Thy1.2) | APC-eF780 | 53–2.1 | eBioscience | |
| B220 | V500 | RA3–6B2 | BD Biosciences | |
| F4/80 | APC-AF750 | REF: MF48027 | Invitrogen | |
| GL7 | eF450 | GL-7 | eBioscience | |
| Ig (H + L) | AF350 | Cat: A11068 | Invitrogen | |
| IgD | PerCP-Cy5.5 | 11–26c.2a | BioLegend | |
| IgM | APC | II/41 | eBioscience | |
| Ly6G | APC-eF780 | RB6–8C5 | eBioscience | |
| Myeloid cells | CD11b | APC-eF780 | M1/70 | eBioscience |
| CD11c | PE-Cy7 | N418 | eBioscience | |
| CD45.2 | BV650 | 104 | BioLegend | |
| CD68 | PerCP-Cy5.5 | FA-11 | BioLegend | |
| CD103 | APC | 2E7 | eBioscience | |
| Ly6C | FITC | AL-21 | BD Biosciences | |
| Ly6G | AF700 | IA8 | BioLegend | |
| MHC II I-Ab | eF450 | M5/114.15.2 | eBioscience | |
| IHC | Goat anti-rabbit | AF488 | NA | Invitrogen |
| Goat anti-rabbit | AF555 | NA | Invitrogen | |
| Donkey anti-goat | AF488 | NA | Invitrogen | |
| Donkey anti-goat | AF555 | NA | Invitrogen | |
| Donkey anti-rat | AF488 | NA | Invitrogen | |
| Donkey anti-rabbit | AF647 | NA | Jackson ImmunoResearch Laboratories |
NA, not applicable.
EQUIPMENT
Syringe, 27 G, 0.5 inch, U-100 insulin syringe (Terumo, cat. no. SS10M2713)
Heat lamp Δ CRITICAL This is optional, but we find that warming the mice briefly causes vasodilation and facilitates easier tail-vein injections.
Restrainer Δ CRITICAL This is optional, but we find that stabilizing the mice facilitates easier tail-vein injections.
Euthanasia chamber
Timer
Forceps
Surgical scissors
Nylon cell strainer, 70 µm (BD Falcon, cat. no. 352350)
GentleMACS dissociator (optional; Miltenyi Biotec)
Multiple-well plates, 96 well, round bottom with lid (Sarstedt, cat. no. 82.1582.001)
LSRII (optional; Becton Dickinson Company)
Microscope slides (optional; Fischer Scientific, cat. no. 12–550-15)
Cryostat (optional; Leica Microsystems)
DM5500B epifluorescence microscope with Leica Acquisition Suite Advanced Fluorescence software (optional; Leica Microsystems)
SP5 laser scanning confocal microscope with Leica Acquisition Suite Advanced Fluorescence software (optional; Leica Microsystems)
Adobe Photoshop software (optional)
Imaris 7.6 (Bitplane) software (optional)
REAGENT SETUP
Mice for analysis after LCMV infection
We have used Thy1.1 + P14 chimeric mice generated as described ref. 13 and CD45.1 + SMARTA chimeric mice generated as described in ref. 34. This enables analysis of transgenic CD8 T cell (P14) or CD4 T cell (SMARTA) or endogenous lymphocytes after LCMV infection. Infect the C57BL/6 mice intratracheally (i.t.) with 1 × 105 plaque-forming units (p.f.u.) LCMV13.
Mice for myeloid and lymphoid analysis after M. tuberculosis infection
Infect C57BL/6mice with 100–150 colony-forming units (c.f.u.) of aerosolized M. tuberculosis (strain H37Rv), as described35.
Mice for myeloid and lymphoid analysis of solid tumors
We have used SPF BALB/c mice from the National Cancer Institute for analysis of immune responses to solid tumors. For intrarenal tumor challenge, make a skin incision on the left flank, and inject 2 × 105 renal adenocarcinoma (Renca) cells through the intact peritoneum into the left kidney36,37.
Ethanol, 70% (vol/vol)
Mix 300 ml of 100% ethanol with 700 ml of H2O. Store the solution at 20 °C in an airtight container.
RPMI + 5% (vol/vol) FBS
Combine 950 ml of RPMI with 50 ml of FBS and 2.4 g of HEPES. Store the solution at 4 °C for up to 2 weeks.
FACS buffer
(Optional) Combine 990 ml of 1× DPBS with 10 ml of NaN3 and 2.0 g of BSA. Store the buffer at 4 °C for up to 4 weeks.
Ack lysis buffer
(Optional) Combine 1 liter of distilled water with 1 g of potassium bicarbonate, 8.29 g of ammonium chloride and 200 ml of 0.5 M EDTA. Store the buffer at room temperature (25 °C) for up to 2 weeks.
BSA blocking solution, 5% (wt/vol)
(Optional) Combine 40 ml of 1× DPBS and 2.0 g of BSA. Store the solution at 4 °C for up to 4 weeks.
PROCEDURE
-
1|
Prepare the Ab dilution in sterile 1× DPBS and keep it on ice. For most procedures, 3 µg of Ab in 300 µl of DPBS per mouse is sufficient. If the Ab is conjugated to a fluorescent molecule, protect it from light. Titration of untested Ab clones or fluorochromes is recommended before performing large experiments.
-
2|
! CAUTION Do not dilute the Ab in buffer containing sodium azide, as high azide concentrations are toxic.
Inject 300 µl of Ab dilution i.v. into a mouse via the tail vein.
-
3|
Kill the mouse 3 min after injection by using isoflurane. Euthanasia with inhaled isoflurane should be performed in a closed container with an absorbent material soaked in the anesthetic (the amount of isoflurane to use will vary with container size). Avoid contact between the mouse and the anesthetic.
! CAUTION Isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) is used for inhalational anesthesia. It is liquid at room temperature and vaporizes readily. Isoflurane is a powerful anesthetic, and it should always be used inside a laminar flow hood. Only approved containers should be used for euthanasia with inhaled anesthetics.
! CAUTION Isoflurane euthanasia should be approved by an IACUC and an institutional biosafety committee.
Δ CRITICAL STEP Longer wait times have not been validated.
? TROUBLESHOOTING
-
4|
Collect the tissues of interest into appropriate containers (Petri dishes, conical tubes or gentleMACS tubes) containing RPMI + 5% (vol/vol) FBS.
Δ CRITICAL STEP Mouse euthanasia and tissue collection should be performed quickly to improve cell viability.
-
5|
Immediately smash or mince tissues and wash them to rinse away or dilute any excess Ab. Store the tissues on ice in RPMI + 5% (vol/vol) FBS.
? TROUBLESHOOTING
■ PAUSE POINT Tissue pieces can be left on ice while additional mice are processed for up to 3 h. Nevertheless, empirically, faster processing yields better results.
-
6|
If desired, isolate leukocytes from tissues. Numerous protocols have been described, and they vary depending on the tissue and leukocyte population of interest38,39. These protocols will not be reviewed here. Each investigator should optimize this protocol for the tissue and cell type of interest. Alternatively, organs may be embedded in freezing medium such as OCT medium for subsequent immunohistochemical analysis38.
Sample preparation and analysis
-
7|
Smash the spleen and filter it through a 70-µm nylon cell strainer to remove clumps. In parallel, process the cells that are suitable for use as controls.
Δ CRITICAL STEP Compensation controls should be prepared by using either compensation particles or cells from an animal that did not receive intravascular antibody, because injected animals will already contain fluorescent Ab.
Δ CRITICAL STEP CD8αβ T cell isolation from spleen is described here as a typical example. Adapt this protocol to the cells and tissue of interest to you.
-
8|
Spin down the cells at 550g for 5 min at 4 °C. Pour off the supernatant.
-
9|
Resuspend the pellet of cells by vortexing. Lyse the RBCs with 2 ml of Ack lysis buffer for 2 min at room temperature. Add 10 ml of RPMI + 5% FBS to dilute Ack lysis buffer.
-
10|
Spin down the cells at 550g for 5 min at 4 °C. Remove and discard the supernatant. Resuspend the cells in a desired volume of FACS buffer and add the cells into 96-well round-bottom plates.
-
11|
Stain the cells with ex vivo Abs, diluted as indicated in Table 3 in FACS buffer, for 30 min on ice.
Δ CRITICAL STEP Do not add ex vivo Ab conjugated to the same fluorochrome as the Ab that was injected i.v.
-
12|
Dilute the stain with 150 µl of FACS buffer and spin the plate at 858g for 2 min at 4 °C to wash the cells. Discard the supernatant. Repeat the wash twice. Resuspend the pelleted cells in 200–300 µl of FACS buffer for acquisition.
■ PAUSE POINT Cells can be fixed in 2% (vol/vol) paraformaldehyde for 30 min, washed with FACS buffer (as in Step 12) and stored at 4 °C in FACS buffer for up to 48 h before acquisition.
TABLE 3.
Sample CD8αβ T cell ex vivo staining panel.
| Fluorochrome | Marker | Ab clone | Company | Dilution |
|---|---|---|---|---|
| PE | CD44 | IM7 | eBioscience | 2 µg ml−1 (1:100) |
| PerCP-Cy5.5 | CD8P | YTS156.7.7 | BioLegend | 2 µg ml−1 (1:100) |
| APC-eF780 | CD62L | MEL-14 | eBioscience | 2 µg ml−1 (1:100) |
| Aqua | LIVE/DEAD | - | Invitrogen | Per protocol |
| APC | CD8α i.v. | 53–6.7 | eBioscience | - |
Data acquisition and analysis procedure
-
13|
Prepare an acquisition template in the flow cytometry software (e.g., BD FACSDiva software) containing the fluorescent parameters in the staining panel.
! CAUTION Flow cytometry should be performed by trained individuals and in accordance with all institutional biosafety regulations. Refer elsewhere for detailed overviews of flow cytometry acquisition40 and data analysis41.
-
14|
Perform compensation by using an unstained control and single-stained samples.
-
15|
Prepare an acquisition layout containing the following plots: forward scatter (FSC)-A versus side scatter (SSC)-A— create a lymphocyte gate (‘lymphocyte’ population); SSC-A versus SSC-W—display ‘lymphocyte’ population and create a singlet gate (‘singlets’ population); CD8β versus live/dead—display’singlets’ population and create a CD8β+ live/dead-gate (‘CD8β’ population); CD8α i.v. versus CD8β—display ‘CD8β+’ population and create CD8α i.v.+ and CD8α i.v− gates.
-
16|
Acquire 10,000–20,000 CD8P+ events and export Flow Cytometry Standard format (FCS) files for analysis in flow cytometry analysis software (e.g., Tree Star FlowJo software).
-
17|
In Flow Jo, create lymphocyte, singlet, live CD8β+ and CD8α i.v.+ and CD8α i.v.− gates as in Step 15.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 4.
TABLE 4.
Troubleshootinq table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3 | Cell type of interest in peripheral blood staining control is not >99% stained with i.v. injected Ab |
Poor intravascular injection, Ab did not circulate long enough, or method of euthanasia disrupted circulation of Ab |
Ensure that tail vein injections are performed correctly Allow Ab to circulate slightly longer (3.5–4 min) before euthanasia Do not use cervical dislocation to euthanize mice |
| 5 | Cells isolated from peripheral blood have a higher staining intensity than the vascular fraction in non-lymphoid tissues |
Excess Ab was not diluted out immediately or too much Ab was injected |
Dilute peripheral blood immediately after removal Reduce concentration of Ab to inject |
| Tissues with low vasculature:tissue ratios have very high intravascular staining frequencies or MFI when compared with negative control samples |
Excess Ab was not rinsed off immediately |
Proceed to rinsing tissues faster during the harvesting process |
● TIMING
The time from Ab injection until organ isolation may take 5–30 min per mouse, depending on the number of organs harvested and the experience of the investigator. This does not include subsequent isolation of leukocytes, staining and analysis.
ANTICIPATED RESULTS
Technical considerations for intravascular staining
Practical combinations of mAb clones for intravascular staining are outlined here. When tracking adoptively transferred CD8+ T cells in mice, such as P14 T cells, vascular populations can be readily detected by i.v. injection of anti-CD8α mAb (clone 53–6.7), followed by ex vivo staining of all isolated populations via anti-CD8β mAb (clone YTS156.7.7, Fig. 2a). This approach takes advantage of the fact that most CD8+ T-cell populations express heterodimeric CD8 consisting of both a and β chains. For tracking CD4 T cells (CD4 consists of a single chain), two anti-CD4 mAb clones that do not compete for the same binding sites are optimal. For example, this can be achieved by i.v. injection of anti-CD4 mAb clone RM4-4 followed by ex vivo staining with clone RM4–5 (Fig. 2b). Alternatively, a mAb specific for CD45 (Fig. 2c), a pan-leukocyte marker, is useful if you are contemporaneously examining multiple leukocyte lineages in one mouse or if the use of anti-CD8β must be avoided; for instance, CD8β staining can block staining with peptide:major histocompatibility complex (MHC) I tetramers42. In this case, we have used the same mAb clone for both intravascular and ex vivo staining (anti-CD45.2 mAb clone 104). This strategy could be pursued for other markers, particularly when only one mAb clone is available for a marker of interest (Fig. 2d shows dual staining with the anti-CD8α clone 53–6.7 as an example). However, this approach may diminish the intensity of the ex vivo staining. Although other mAb combinations may suit investigators’ unique goals, it is important to avoid i.v. injection of complement-fixing mAb that may rapidly eliminate labeled cells. Although several antibodies with different fluorescent tags can be mixed and injected intravenously, this option will use up fluorescent channels, and it may reduce the number of markers an investigator can examine ex vivo. When possible, investigators are advised to use a single intravascular Ab specific for all cell types of interest, as this also may reduce the cost of reagents.
As shown previously43, when examining the spleen, intravascular staining discriminates between cells occupying red pulp (i.v. stain positive) and white pulp (i.v. stain negative, Fig. 2e–g). When injecting anti-CD45 mAb, the marginal zone is stained particularly brightly. However, among T cells, CD45 staining in splenic red pulp is less intense than the vascular compartments of other tissues (lung, for example) or peripheral blood (Fig. 2h).
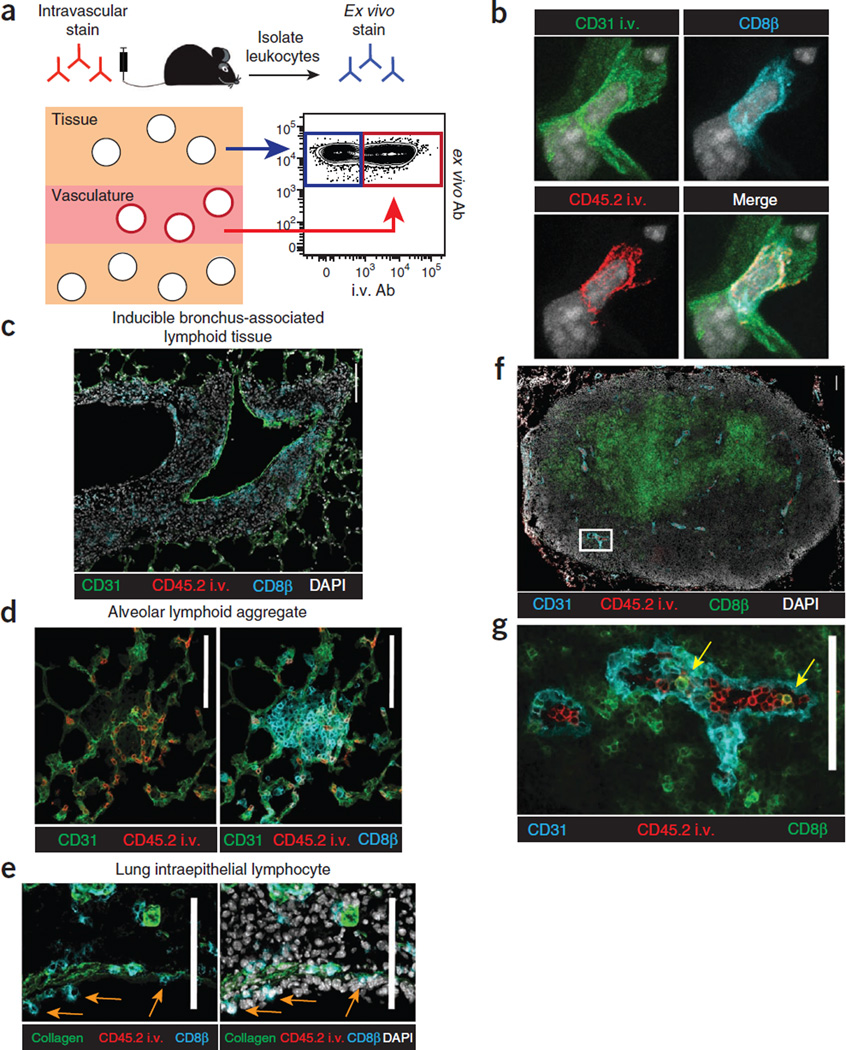
Intravascular staining is confined to vascular cells
In the lung, confocal and epifluorescence microscopy shows that lymphocytes stained with intravascular Ab are present within the vasculature (Fig. 3). Cells stained with i.v. mAb were truly confined to CD31+ vessels, whereas cells protected from the i.v. mAb were present outside of capillaries, including the pulmonary epithelium, iBALT, airway lamina propria, surrounding large blood vessels, as well as pericapillary and lung airway compartments (Fig. 3b–e, Supplementary Videos 1 and 2 and data not shown). These data demonstrate, as expected, that CD8 T cells stained with intravascular Ab within the lung are confined to vasculature, and CD8 T cells protected from intravascular staining are outside of vasculature.
Figure 3.
Intravascular staining is confined to vascular cells. (a) Schematic of intravascular staining. (b–g) Anti-CD31 and anti-CD45.2 (b) or anti-CD45.2 mAb alone (c–g) was injected i.v. into C57BL/6 mice 12 d after i.t. LCMV infection. (b) Confocal imaging of lung, anti-CD31 i.v. (green), anti-CD45.2 i.v. (red), anti-CD8β (cyan) and DAPI (gray). Representative of three experiments totaling four mice. (c–e) iBALT (c), alveolar lymphoid aggregates (d) and intraepithelial lymphocytes (IEL) (e) in the lung 12 d after i.v. LCMV infection. Orange arrows indicate sample IELs. (f) Inguinal lymph node sections showing anti-CD45.2 i.v. mAb staining (red) and ex vivo anti-CD31 (cyan), anti-CD8β (green) and DAPI (gray). (g) White inset from f without DAPI displayed. Yellow arrows indicate anti-CD45.2 i.v. and anti-CD8β mAb co-staining. Scale bars, 100 µm. (c–g) Data are representative of three experiments totaling nine mice.
Although perfusion fails to completely remove ‘contaminating’ vascular lymphocytes from many tissues (Fig. 1), it should also be noted that vascular lymphocyte populations are also often of interest15–18. For example, histological examination of the lymph nodes of mice that were not perfused confirms that i.v. mAb-labeled CD8 T cells are largely contained within HEVs (Fig. 3f,g), and they probably represent marginated cells preparing for lymph node entry. Thus, intravascular staining may be a useful approach to identify this population for further characterization. Permissiveness to intravascular Ab labeling in additional tissues will be shown in Figure 4. In summary, perfusion is unnecessary if intra-vascular staining is incorporated, and neglecting to perfuse has advantages.
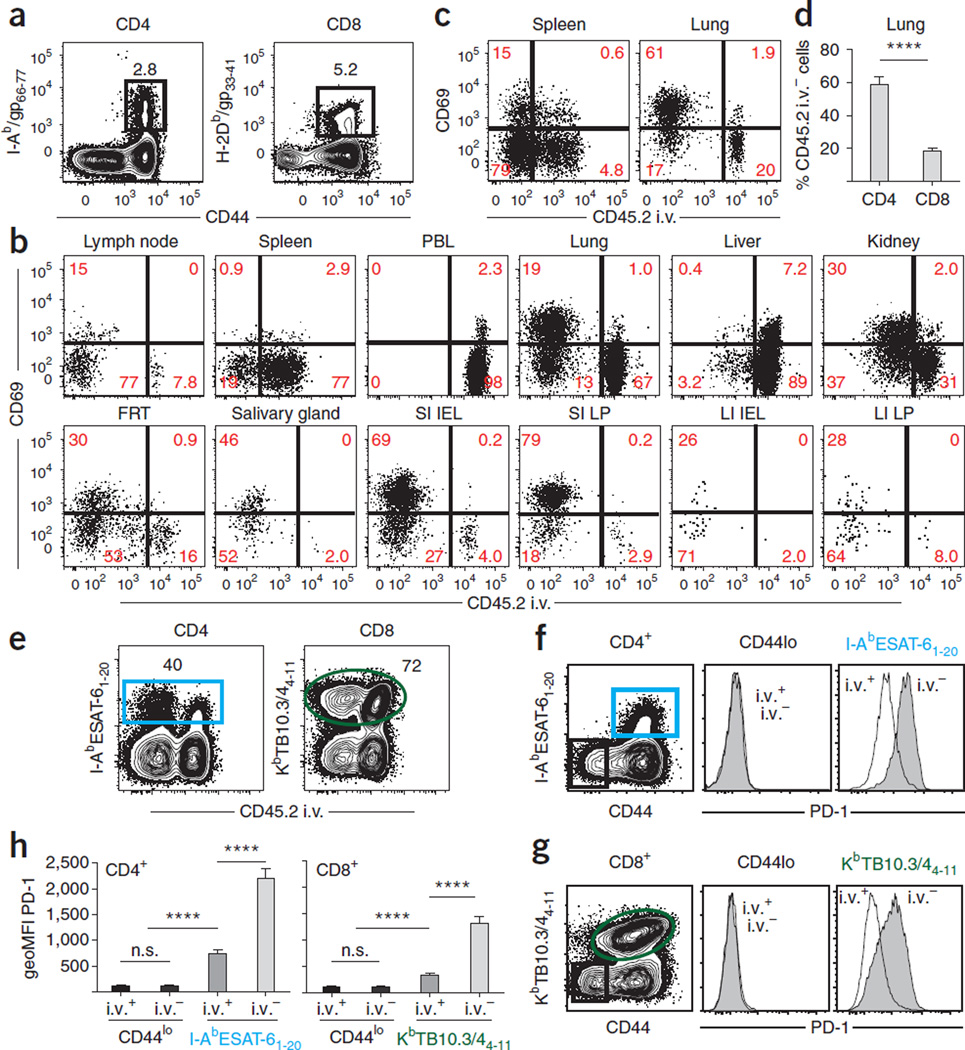
Figure 4.
Intravascular staining without perfusion is sufficient to reveal unique lymphocyte subsets in tissues. (a–d) C57BL/6 mice were injected with anti-CD45.2 mAb i.v. 12 d after i.t. LCMV infection. Lymphocytes were isolated and LCMV-specific CD4 + and CD8 + T cells were identified with I-Ab/gp66–77 and H2-Db/gp33–41 MHC tetramers, respectively. (a) Representative tetramer staining in spleen. (b,c) Anti-CD45.2 i.v. and anti-CD69 mAb staining of H2-Db/gp33–41-gated CD8 (b) or I-Ab/gp66–77 CD4 (c) T cells isolated from the indicated compartments. (d) Frequency of tetramer-positive cells protected from i.v. mAb staining in lung. Plots are representative of two experiments totaling nine mice. ****P < 0.0001, Student’s t test. Error bars indicate s.e.m. (e–h) M. tuberculosis–specific CD4 + and CD8 + T cells isolated from lung 24 d after infection with 100–150 c.f.u. of aerosolized M. tuberculosis (strain H37Rv) were identified by staining with I-AbESAT-61–20 and KbTB10.3/44–11 MHC tetramers, respectively. (e) Representative anti-CD45.2 i.v. mAb and MHC tetramer staining. (f,g) PD-1 expression on CD4 + (f) and CD8 + (g) T cells from lungs. Shaded histograms are anti-CD45.2 i.v. mAb −, and black line indicates anti-CD45.2 i.v. mAb + . (h) Geometric mean fluorescence intensity (geoMFI) of PD-1 expression on naive (CD44lo) or M. tuberculosis MHC tetramer + CD4 and CD8 T cells. Plots are representative of two experiments totaling nine mice. ****P < 0.0001, Student’s t test. n.s., not significant. Error bars indicate s.e.m.
Intravascular staining reveals unique lymphocyte subsets in tissues
Intravascular staining can also be used in the context of polyclonal endogenous CD4 and CD8 T-cell responses. For example, 12 d after i.t. LCMV infection, H-2Db/gp33 MHC I tetramers and I-Ab/gp66 MHC II tetramers were used to identify LCMV-specific CD8 and CD4 T cell responses, respectively (Fig. 4a). In this case, vascular T cells were identified via injection of anti-CD45.2 mAb, but mice were not perfused. All examined tissues contained LCMV-specific CD8 and CD4 T cells that were labeled by i.v.-injected mAb (Fig. 4b,c), and immunohisto-chemical analysis confirmed that T cells exposed to i.v. mAb were associated with CD31 + blood vessels in NLT (supplementary Fig. 1). Consistent with reports that CD69 expression on T cells is a marker of tissue residence9,10,38,47,48, CD69 + T cells were only found in the compartment of NLT protected from the i.v. mAb, with the exception of cells isolated from the liver. These results are in keeping with published reports suggesting that liver may contain a fraction of sinusoidal resident CD8 T cells9,14. CD69 expression profiles in tissues were similar 12 and 30 d after infection (data not shown). The proportion of i.v. protected LCMV-specific CD4 T cells in tissues is typically greater than that observed for CD8 T cells, particularly in the lung (Fig. 4d and supplementary Fig. 2).
In the context of M. tuberculosis infection, intravascular staining also discriminates between the function and phenotype of local T cells truly within the lung as compared with those in vasculature (Fig. 4e and data not shown). M. tuberculosis causes a chronic lung infection that is contained, but not eliminated, by local immune responses. Twelve-week-old C57BL/6 mice were infected with 100–150 c.f.u. of aerosolized M. tuberculosis (strain H37Rv); anti-CD45.2 mAb was injected i.v. 24 d later, and leukocytes from the lungs, bronchioalveolar lavage (BAL) of the lung airways and blood were evaluated. Intravascular staining confirms that I-Ab ESAT-61–20 and KbTB10.3/44–11 tetramer + T cells (which identify M. tuberculosis– specific CD4 and CD8 T cells, respectively) have varied PD-1 expression, a receptor that inhibits T cell function and is regulated by antigen stimulation (Fig. 4f,g). Among both antigen-specific CD4 and CD8 T cells, PD-1 is more highly expressed on cells protected from intravascular staining compared with i.v. labeled cells present in vasculature (Fig. 4h). These data reinforce that intravascular staining has the potential to refine our understanding of local immune control over M. tuberculosis.
Intravascular staining indicates anatomic localization of B cells
Intravascular staining can also be applied for refining the anatomic compartmentalization of different B-cell subsets43. A 13-parameter flow cytometry panel was used to phenotypically identify Ab-secreting (intracellular Ig high), B1 (B220low, CD43 + ), germinal center (B220 + , CD43low, CD38low, GL7 + ), switched memory (CD19 + , B220 + , CD38 + , IgM/IgD − ) or naive and unswitched memory (CD19 + , B220 + , CD38 + , IgM/IgD + ) B cells after LCMV infection (Fig. 5).
Figure 5.
Intravascular staining indicates anatomic localization of B cells. Anti-CD45.2 mAb was injected i.v. into C57Bl/6 mice 12 d after i.t. LCMV infection. (a) Gating strategy (the letters in panel a correspond to panels b–f). (a–f) Lymphocytes were isolated from spleen, and anti-CD45.2 mAb staining was examined on the following B cell lineages that were identified by 13-parameter flow cytometry (defining markers indicated in parentheses): Ab-secreting cells (intracellular Ig + , IgM − , IgD − ) (b), B1 B cells (intracellular Ig + , CD19 + , B220 , CD43 + ) (c), germinal center B cells (intracellular Ig + , CD19 + , B220 + , CD43 , CD38 , GL7 + ) (d), unswitched naïve and memory B cells (intracellular Ig + , CD19 + , B220 + , CD43 , CD38 + , GL7 − ) (e) or isotype-switched memory B cells (intracellular Ig + , CD19 + , B220 + , CD43 − , CD38 + , GL7 − , IgM − , IgD − ) (f). Plots are representative of three experiments totaling nine mice.
In the spleen, Ab-secreting and germinal center B cells were protected from i.v. anti-CD45.2 mAb (12 d after i.t. challenge with LCMV, Fig. 5b,d), consistent with preferential localization to white pulp follicles44–46. Although B1, naive, unswitched memory and switched memory B cells were also predominantly protected from i.v. mAb staining, ~30% of each subset was exposed to i.v. mAb (Fig. 5c,e,f). These data demonstrate how intravascular staining approaches can be applied to examine B cells.
Intravascular staining during Mtb infection reveals distinct myeloid subsets
Intravascular staining can also delineate differences between vascular and tissue cells of the myeloid lineage. Fourteen-parameter flow cytometry can be used to measure markers characteristic of neutrophils (Ly6G, clone IA8) and mononuclear phagocytes (intracellular CD68 + ; these include monocytes, macrophages and dendritic cells, DCs) (Fig. 6). Neutrophils can be isolated from the lungs of naive mice, regardless of perfusion (Fig. 6b), even though neutrophils are not prototypically present in healthy tissues. However, the use of intravascular staining demonstrates that all of the isolated neutrophils from the naive lung were labeled with the i.v. mAb, confirming that they are not in the pulmonary tissue of uninfected mice and that perfusion failed to remove them from vasculature (Fig. 6c). Both i.v. mAb-labeled and unlabeled CD68 + mononuclear phagocytes were recovered, consistent with the fact that certain myeloid cells are present both in blood and in tissue parenchyma and airways at steady state49 (Fig. 6d).
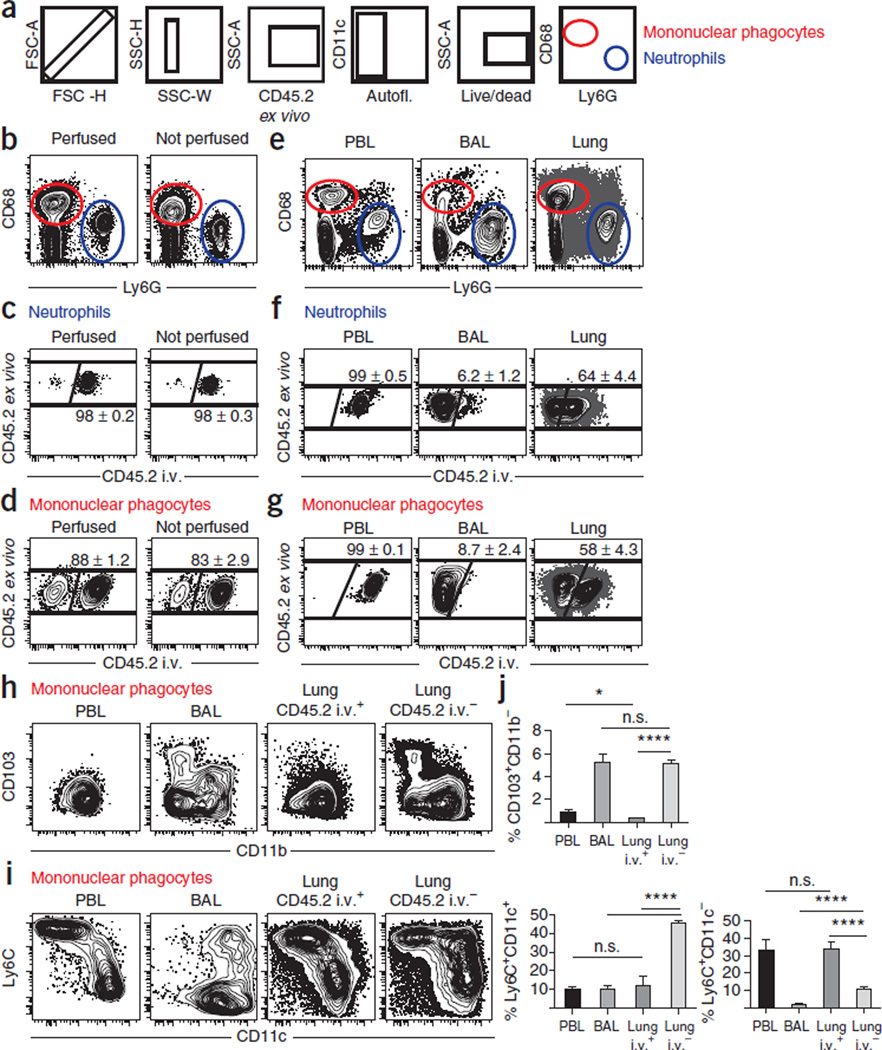
Figure 6.
Intravascular staining during Mtb infection reveals tissue-specific myeloid cell subsets. (a) Gating strategy for myeloid neutrophils and mononuclear phagocytes. (b) Neutrophils and mononuclear phagocytes were recovered from the lungs of naive C57BL/6 mice with or without perfusion. (c,d) Intravascular staining of neutrophils (c) or mononuclear phagocytes (d) from lungs of perfused (left plots) or unperfused (right plots) naive mice. Representative of nine mice per condition from two independent experiments. (e–j) Intravascular staining of myeloid and lymphoid cells isolated from PBL, BAL or lungs of C57BL/6 mice 24 d after M. tuberculosis infection without perfusion. (f,g) Intravascular staining of neutrophils (f) or mononuclear phagocytes (g). (h,i) CD11b and CD103 (h) or CD11c and Ly6C staining (i) on mononuclear phagocytes isolated from the indicated compartments. (j) Summary of CD68 + mononuclear phagocyte subsets as defined in h and i. Plots are representative of two experiments totaling nine mice. *P < 0.05. ****P < 0.0001, Student’s t test. n.s., not significant. Error bars indicate s.e.m.
To illustrate that bona fide pulmonary neutrophils are protected from intravascular staining, we evaluated the intravascular staining technique in M. tuberculosis infection, a situation in which neutrophils localize to lung tissue. Unlike in uninfected mice, approximately half of the neutrophils recovered from the lung are protected from i.v. mAbs (PBL and BAL served as positive and negative staining controls, respectively, Fig. 6e,f). Similarly, and consistent with enhanced recruitment of myeloid cells to the lung between 3 and 4 weeks after M. tuberculosis infection35,50, the proportion of unlabeled CD68 + mononuclear phagocytes also increases after M. tuberculosis infection compared with naive mice (Fig. 6g). CD68 + cells can further discriminate various pulmonary myeloid subsets, such as inflammatory monocytes/macrophages (Ly6C + , CD11b + , CD11c −), conventional DCs (cDCs; CD11b + /CD11b − and CD11c + ), tissue-resident CD103 + cDCs (CD103 + , CD11c + , CD11b − ) and inflammatory monocyte-derived DCs (moDCs; Ly6C + , CD11c + CD11b + )11,51. As expected, CD103 + cDCs isolated from the lung were indeed protected from intravascular mAb staining (Fig. 6h,j). Ly6C + , CD11c− monocytes/macrophages were largely contained in the i.v. labeled fraction (Fig. 6i,j) consistent with the idea that the majority of this subset represents true monocytes present in pulmonary blood vasculature, whereas only a minority were tissue-localized bona fide macrophages. It should be noted that previously the Ly6C + , CD11c − population was found to be seemingly homogeneous with uniform expression of over 50 phenotypic markers of interest35 (data not shown), highlighting the crucial importance of the intravascular staining technique in distinguishing pulmonary Ly6C + inflammatory monocytes from macrophages.
Moreover, inflammatory moDCs (CD11b + , CD11c + , Ly6C + ) represent the major myeloid cell type truly present in the lungs of M. tuberculosis–infected mice, as they were completely protected from i.v. mAb labeling (Fig. 6j). These inflammatory moDCs have recently been shown to be highly multifunctional, producing interleukin (IL)-1α, IL-1β, tumor necrosis factor (TNF)α, inducible nitric oxide synthase (iNOS) and IL-10, and they are phenotypically characterized by high toll-like receptor 2 (TLR2), CD13, Ly6C and CD64 expression35. Thus, the use of the intravascular staining technique uniquely enabled the enrichment and recovery of inflammatory moDCs as myeloid effector cells in lung tissue in response to M. tuberculosis infection, another observation other-wise obscured by the presence of large numbers of myeloid cells in the lung blood vasculature.
Intravascular staining reveals distinct leukocyte subsets in tumor-bearing tissue
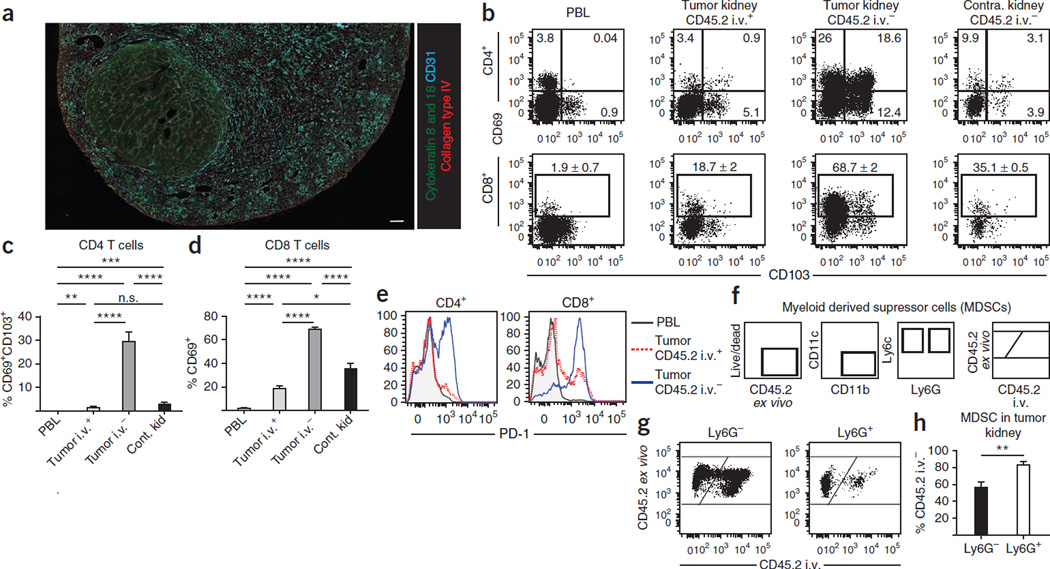
Intravascular staining can also distinguish cells in a noninfectious disease model. As an illustration, we phenotypically analyzed T cells and myeloid cells in a solid tumor model of renal cancer36,37. Mouse Renca cells were injected into the left kidneys of 8-week-old BALB/c mice. Tumor nodules were detectable histologically in the left kidneys 14 d later (Fig. 7a). When a polyclonal Ab against collagen was injected i.v., it labeled glomerular basement membrane, but it did not infiltrate the tumor (data not shown), illustrating that i.v.-injected Ab staining was limited to vascular-localized epitopes. In separate mice, anti-CD45.2 mAb was injected i.v., and then cells isolated from blood, tumor-bearing kidneys and non-tumor-bearing contralateral kidneys were examined. As expected, intravascular staining reveals striking phenotypic differences among T cells within tumor-bearing kidney compared with those in control kidney tissue and also blood (Fig. 7b–e). For instance, ~30% of CD4 T cells protected from i.v. mAb staining isolated from tumor-bearing tissue coexpressed CD69 and CD103, a pheno-type suggestive of regulatory T cells that may interfere with tumor immune responses. The inhibitory molecule PD-1, which is expressed upon cognate Ag stimulation, was expressed by most CD45.2 unlabeled T cells in tumor-bearing kidneys. This observation provides further evidence that intravascular staining may be used to identify tumor-infiltrating lymphocytes.
Figure 7.
Intravascular staining reveals myeloid and lymphoid tissue–specific subsets in a mouse renal adenocarcinoma model. Renca cells were injected into the kidney of BALB/c female mice in order to establish solid tumors. (a) After 14 d, kidneys were removed and sections were stained ex vivo for cytokeratin 8 and 18 (green), collagen type IV (red), CD31 (cyan) and DAPI (gray). Scale bar, 250 µm. Representative of five mice analyzed from three experiments. (b–f) Anti-CD45.2 mAb was injected i.v. 14 d after intrarenal Renca cell injection. (b) CD69 and CD103 expression on CD4 and CD8 T cells isolated from PBL, tumor-bearing kidney and contralateral kidney. (c,d) Frequency of CD69 + /CD103 + CD4 T cells (c) and CD69 + CD8 T cells (d) from tumor-bearing mice. (e) PD-1 expression on CD4 and CD8 T cells isolated from PBL (gray) and tumor-bearing kidney (anti-CD45.2 i.v. mAb + in red and anti-CD45.2 i.v. mAb− in blue). (f) Gating strategy for MDSCs. (g) MDSCs were isolated from tumor-bearing kidneys 14 d after Renca cell injection. CD45.2 i.v. mAb staining of Ly6G and Ly6G + MDSCs are shown. (h) Percentage of Ly6G − and Ly6G + MDSCs from tumor-bearing kidneys protected from CD45.2 i.v. mAb staining. All plots are representative of 8–11 mice from three experiments. *P < 0.05, **P < 0.01, ***P < 0.0005, ****P < 0.0001, Student’s t test. Error bars indicate s.e.m.
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that can both suppress antitumor immune responses and promote cancer52. The presence and subsequent suppressive capacity of MDSCs have been reported in both human renal cell patients and Renca murine models53,54. Two main subsets of MDSCs exist on the basis of their Ly6G expression52, which have been shown to be phenotypically and functionally distinct55,56. Therefore, understanding where they reside within the tumor-bearing kidney can shed light on their functional capabilities and suppressive mechanisms. Anti-CD45.2 i.v. mAb staining on both Ly6G + and Ly6G− MDSC subsets confirms that these subsets are differentially distributed between vasculature and tumor-bearing kidney tissue in a Renca solid tumor model (Fig. 7f). Ly6G + MDSCs were predominantly found in Renca tumors, whereas Ly6G − MDSCs were isolated from both tumor tissue and vasculature (Fig. 7g,h). Together, these data demonstrate that intravascular staining is also a useful tool to refine our understanding of antitumor immune responses.
Concluding remarks
Intravascular staining has previously been used to distinguish between single populations of leukocytes in the vascular circulation and those in the tissue of the lung12,13,32,48,57,58 or the red and white pulp of the spleen43. Moreover, perfusion was shown to inefficiently remove CD8 T cells from the lung vasculature13. The typical results shown here demonstrate the true scope of this issue by showing that cells of both the adaptive and innate immune system accumulate in the vasculature of many tissues and in the disease models examined. Perfusion not only fails to remove many vascular-bound leukocytes but it may also disrupt novel tissue populations such as alveolar lymphoid aggregates. No specific problems with intravascular staining were encountered in any of the models presented.
Findings from numerous studies13,32,57 in addition to the sample data presented here invite reassessment of the widely used practice of perfusion. Moreover, they provide a compelling rationale for the incorporation of intravascular staining when possible, without perfusion, when studying any tissue leukocyte population. In fact, this approach may be absolutely necessary to capture an accurate representation of the local participants in immune-mediated protection and disease.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by US NIH grant nos. AI084913-01 and DP2 OD006467 (D.M.), the Arnold and Mabel Beckman Foundation (D.M.), grant no. T90DE022732 from the National Institute of Dental and Craniofacial Research (K.G.A.), the National Institute of Allergy and Infectious Diseases Intramural Program (K.M.-B. and D.L.B.) and US NIH grant no. CA109446 (T.S.G.). We thank the University of Minnesota Center for Immunology Imaging Core, the University of Minnesota Imaging Center and the Flow Cytometry Core.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTION K.G.A., K.M.-B., H.S., L.B., B.R.J., J.J.T., T.S.G., V.V., D.L.B. and D.M. designed the experiments. K.G.A., K.M.-B., H.S., L.B., B.R.J., L.Q. and D.L.B. performed the experiments and analyzed data. K.G.A. and D.M. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Andrian von UH, Mackay CR. T-cell function and migration Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat. Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefrançois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu. Rev. Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat. Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 10.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 12.Galkina E, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KG, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissmann F, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W-Y, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SY, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanlon ST, et al. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J. Exp. Med. 2011;208:2113–2124. doi: 10.1084/jem.20110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlin LM, et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg JC, et al. Erythrocyte and polymorphonuclear cell transit time and concentration in human pulmonary capillaries. J. Appl. Physiol. 1994;77:1795–1800. doi: 10.1152/jappl.1994.77.4.1795. [DOI] [PubMed] [Google Scholar]
- 20.Segel GB, Cokelet GR, Lichtman MA. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood. 1981;57:894–899. [PubMed] [Google Scholar]
- 21.Berlin-Rufenach C, et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cose S, Brammer C, Khanna KM, Masopust D, Lefrançois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 23.Harp JR, Onami TM. Naïve T cells re-distribute to the lungs of selectin ligand deficient mice. PLoS ONE. 2010;5:e10973. doi: 10.1371/journal.pone.0010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inman CF, Murray TZ, Bailey M, Cose S. Most B cells in non-lymphoid tissues are naive. Immunol. Cell Biol. 2012;90:235–242. doi: 10.1038/icb.2011.35. [DOI] [PubMed] [Google Scholar]
- 25.Caucheteux SM, Torabi-Parizi P, Paul WE. Analysis of naive lung CD4 T cells provides evidence of functional lung to lymph node migration. Proc. Natl. Acad. Sci. USA. 2013;110:1821–1826. doi: 10.1073/pnas.1221306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Structure, development and function of tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart BA, Harmsen AG, Low RB, Emerson R. Biochemical, cytological, and histological alterations in rat lung following acute beryllium aerosol exposure. Toxicol. Appl. Pharmacol. 1984;75:454–465. doi: 10.1016/0041-008x(84)90182-0. [DOI] [PubMed] [Google Scholar]
- 28.Woodland DL, Randall TD. Anatomical features of anti-viral immunity in the respiratory tract. Semin. Immunol. 2004;16:163–170. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyron-Quiroz J, Rangel-Moreno J, Carragher DM, Randall TD. The function of local lymphoid tissues in pulmonary immune responses. Adv. Exp. Med. Biol. 2007;590:55–68. doi: 10.1007/978-0-387-34814-8_4. [DOI] [PubMed] [Google Scholar]
- 30.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin. Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randall TD. Chapter 7—bronchus-associated lymphoid tissue (BALT): structure and function. synthetic vaccines. In: Fagarasan S, Cerutti A, editors. Advances in Immunology. Vol. 107. Elsevier; 2010. pp. 187–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barletta KE, et al. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J. Immunol. Methods. 2012;375:100–110. doi: 10.1016/j.jim.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J. Vis. Exp. 2013;16:e50062. doi: 10.3791/50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer-Barber KD, et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norian LA, et al. Eradication of metastatic renal cell carcinoma after adenovirus-encoded TNF-related apoptosis-inducing ligand (TRAIL)/CpG immunotherapy. PLoS ONE. 2012;7:e31085. doi: 10.1371/journal.pone.0031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James BR, et al. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J. Immunol. 2012;189:1311–1321. doi: 10.4049/jimmunol.1100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 40.Sharrow SO. Overview of flow cytometry. Curr. Protoc. Immunol. 2002;5 doi: 10.1002/0471142735.im0501s50. 5.1. [DOI] [PubMed] [Google Scholar]
- 41.Roederer M. Multiparameter FACS analysis. Curr. Protoc. Immunol. 2002;5 doi: 10.1002/0471142735.im0508s49. 5.8. [DOI] [PubMed] [Google Scholar]
- 42.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnon TI, Horton RM, Grigorova IL, Cyster JG. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2013;493:684–688. doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allman D, Pillai S. Peripheral B cell subsets. Curr. Opin. Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol. 2010;22:413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 47.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 48.Teijaro JR, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin. Immunol. 2009;21:147–155. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Wolf AJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo . J. Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 51.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finke J, et al. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietlin TA, et al. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J. Leukoc. Biol. 2007;81:1205–1212. doi: 10.1189/jlb.1006640. [DOI] [PubMed] [Google Scholar]
- 56.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 57.Reutershan J. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 58.Kang SS, et al. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J. Exp. Med. 2011;208:747–759. doi: 10.1084/jem.20101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donaldson JG. Immunofluorescence staining. Curr. Protoc. Cell Biol. 2001;4.3:1–4. doi: 10.1002/0471143030.cb0403s69. 3.6. [DOI] [PubMed] [Google Scholar]
- 60.Combs CA. Fluorescence Microscopy: A Concise Guide to Current Imaging Methods. John Wiley & Sons; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman B. Fluorescence microscopy. Curr. Protoc. Immunol. 2002;48:21.2.1–21.2.10. doi: 10.1002/0471142735.im2102s48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.