Abstract
Neural crest is a population of multipotent progenitor cells that form at the border of neural and non-neural ectoderm in vertebrate embryos, and undergo epithelialmesenchymal transition and migration. According to the traditional view, the neural crest is specified in early embryos by signaling molecules including BMP, FGF and Wnt proteins. Here we identify a novel signaling pathway leading to neural crest specification, which involves Rho-associated kinase (ROCK) and its downstream target non-muscle Myosin II. We show that ROCK inhibitors promote differentiation of human embryonic stem cells into neural crest-like progenitors (NCPs) that are characterized by specific molecular markers and ability to differentiate into multiple cell types, including neurons, chondrocytes, osteocytes and smooth muscle cells. Moreover, inhibition of Myosin II was sufficient for generating NCPs at high efficiency. Whereas Myosin II has been previously implicated in the self-renewal and survival of human pluripotent ES cells, we demonstrate its role in neural crest development during ES cell differentiation. Inhibition of this pathway in Xenopus embryos expanded neural crest in vivo, further indicating that neural crest specification is controlled by ROCK-dependent Myosin II activity. We propose that changes in cell morphology in response to ROCK and Myosin II inhibition initiate mechanical signaling leading to neural crest fates.
Keywords: human embryonic stem cells, Xenopus, ROCK, neural crest, Myosin II
INTRODUCTION
Neural crest consists of multipotent ectoderm-derived progenitor cells, which are transiently specified at the border of the neural plate in all vertebrate embryos. Neural crest cells delaminate from the neural plate in a process equivalent to the epithelial-mesenchymal transition (EMT) [1-3], and migrate to various destinations in the body. After their migration, neural crest progenitors differentiate into melanocytes, mesenchymal cell types and the peripheral nervous system, including neurons and Schwann cells [4-6]. Although neural crest cells represent an important model for embryonic cell lineage determination, molecular and cellular mechanisms of neural crest specification are complex and remain poorly understood.
Canonical models of neural crest specification postulate that this cell population develops as a result of inductive signaling from adjacent embryonic tissues. Both neuroectoderm- and epidermis-derived signals contribute to neural plate boundary formation and the neural crest cell fate [7]. The underlying mesoderm is an additional source of secreted neural crest-promoting factors [8, 9]. Current experimental evidence indicates that several signaling pathways, including those triggered by BMP, FGF and Wnt proteins, initiate and maintain neural crest-specific transcription [6, 10, 11]. Manipulation of these signaling pathways in embryonic stem cells allows generation of neural crest-like progenitor (NCP) populations that can serve as useful models of neural crest development in vitro [12-15].
Neural crest specification might also involve pathways that are responsible for subsequent migratory properties of this cell population. Accumulating evidence suggests that this process is regulated by molecules that affect cell shape and cell-cell contacts, such as small Rho GTPases, junctional proteins and β-catenin-independent Wnt signaling [16-20]. One of the Rho GTPase effectors is Rho-associated protein kinase (ROCK), which modulates the activity of non-muscle Myosin II, a primary regulator of cell contractility and cell-cell adhesion [21-23]. The Rho/ROCK/Myosin II pathway has been implicated in cell responses to diverse mechanical forces [24, 25] and can influence progenitor differentiation [26, 27]. These observations raise a possibility that, in addition to the known growth-factor-mediated pathways, Rho-dependent signaling serves as an alternative regulator of neural crest specification.
To examine this putative alternative mechanism of neural crest specification, we differentiated human embryonic stem cells (hESCs) in the presence of pharmacological inhibitors of ROCK and Myosin II. We report that hESCs treated with ROCK antagonists readily convert into NCPs that are capable of differentiating into neurons and mesenchymal cells. Similar observations were made upon inhibition of Myosin II, a major downstream target of ROCK. Moreover, Myosin II inhibition promotes neural crest development in Xenopus embryos, suggesting a conserved function of Myosin II in neural crest specification in vivo. These findings support a hypothesis that ROCK/Myosin II pathway is a key regulator of neural crest development.
MATERIALS AND METHODS
Human embryonic stem cell culture and inhibitor treatment
The human embryonic stem cell lines H9 (WiCell, passages 43-48) and RUES1 (a gift of A. Brivanlou, passages 35-38) were cultured on Matrigel-coated plates (BD biosciences) in the mTeSR1 medium (Stem Cell technologies) without feeders for self-renewal [28] and passaged with Accutase (Stemgent) every 3-5 days. Neural crest differentiation was performed according to the published protocol with modifications [29]. In brief, the cells were seeded on Matrigel-coated plates at the density of 1×105 cells/cm2. Differentiation was initiated at about 70 % confluency (approximately 48 hrs after seeding) by changing the medium to DMEM/F12 with Knockout serum replacement (KSR, Supporting Information Materials and Methods). Starting at two days of culture, the KSR-containing medium was gradually replaced with the DMEM/F12-N2 medium (Supporting Information Materials and Methods) during the next 4 days. Cells were cultured in the DMEM/F12-N2 medium for 1 additional day and subjected to further analysis. Cell cultures were treated with Y-27632 (10 μM, Calbiochem or Stemgent), Rockout (12.5 μM, Calbiochem), Blebbistatin (10 μM, Sigma), SB431542 (20 μM, Stemgent) and BIO (0.1 μM, Sigma) for 7 days from the initiation of differentiation. NCP aggregates were enzymatically separated from the non-aggregated cells by treating the Y-27632 cultures with Accutase for 3- 5 min, resulting in the selective detachment of the NCP aggregates from the substrate. Isolated NCP aggregate cells were maintained on Matrigel in DMEM/F12-N2 medium containing bFGF (10 ng/ml, Life Technologies), EGF (10 ng/ml, R&D Systems) and Y-27632 (10 μM) and passaged every 3-4 days.
Differentiation into neural crest derivatives
For spontaneous neuronal differentiation, NCP cultures formed after 7 days differentiation were maintained in the DMEM/F12-N2 medium plus Y-27632 (10 μM) for 3-4 extra days (a total of 10-11 days differentiation). For mesenchymal differentiation, the isolated NCPs (passages 5-7) were used. These cells were cultured in the medium composed of DMEM (Mediatech) and 10% fetal bovine serum (FBS, Gibco) for 5 days [30], and CD44 and CD73 expression was examined by RT-PCR. Then, the cells were passaged 2-3 times in the same medium and analyzed for smooth muscle actin (SMA) expression by immunocytochemistry. MSCs were subjected to the differentiation into chondrocytes and osteocytes with StemPro Chondrogenesis and Osteogenesis kits (Invitrogen), according to the manufacturer's protocol. Mesenchymal cell lineages were defined by standard alcian blue, alkaline phosphatase and alizarin red staining methods.
BrdU incorporation and TUNEL assays
H9 cells were incubated with BrdU (10 μM) for 1 hour and replated on the coverslip for immunocytochemistry using the BrdU Labeling and Detection Kit (Roche). Apoptotic cells were detected using the In Situ Cell Death Detection Kit, Fluorescein (Roche).
Immunoblotting, immunocytochemistry, RT-PCR
Immunoblot analysis was performed according to standard protocol [31] using antibodies against Myosin II light chain (MLC, Cell Signaling, #3672), phospho-MLC (Cell signaling, #3674), unphosphorylated β-catenin (Millipore, #05-665), β-catenin (Sigma, C2206) and Tubulin (Sigma, T5168). For immunofluorescence staining (Supporting Information Materials and Methods), antibodies against p75 (Promega, G323A), AP2α (Santa Cruz Biotechnology, SC-12726), FoxD3 (a gift from D. Kessler), SOX3 (a gift of M. Klymkowsky), PAX6 (Covance, PRB-278P), smooth muscle actin (BioGenex laboratories, MU128-UC), Tuj1 (Covance, MMS-435P), phospho-histone H3 (Cell signaling, #9706), YAP (Santa Cruz Biotechnology, sc-15407) and ZO-1 (Zymed, #61-7300) were used. F-actin was stained with Alexa Fluor® 568 Phalloidin (Life technologies). Fluorescence microscopy and statistical analysis were carried out as described [32].
For RT-PCR, total RNA was purified using Trizol (Ambion) or RNeasy Mini Kit (Qiagen) and reverse transcribed with the SuperScript first-strand synthesis system (Invitrogen). Same amount of RNA (0.1-1 μg) was used for reverse transcription in each group. PCR was performed with gene-specific primer sets (Supporting Information Table S1). For real-time quantitative PCR experiments, predesigned primers for GAPDH, p75, AP2α, SOX10, SLUG, OCT4 and CD44 (KiCqStart SYBR Green Primers, Sigma) were also used in combination with PerfeCTa SYBR Green FastMix (Quanta Biosciences). Gene expression was analyzed by iCycler Real-Time PCR system (BioRad) with 2-3 replicates per sample and normalized to GAPDH expression. The results from 2-4 independent experiments were statistically analyzed.
Xenopus embryos, injections and whole mount in situ hybridization
Eggs and embryos are obtained from Xenopus laevis and cultured in 0.1x Marc's modified Ringer's solution (MMR) [33]. For microinjection, embryos were transferred to 3% Ficoll in 0.5x MMR and unilaterally injected at the four to eight cell stage with 10 nl of a solution containing Y-27632 (50-100 μM), Blebbistatin (0.5-1 mM), Rok-C (0.1-0.25 ng) [34], ROCK (1 ng) [35], GFP-MLC (0.3 ng) [35], GFP-CAAX [32] and/or LacZ RNA. DMSO (1-2%) served as a control for drug injections. RNA was in vitro transcribed with mMessage mMachine kit (Ambion). Whole mount in situ hybridization and lineage tracing (X-Gal staining) of early neurulae [stage 14-16; 36] were carried out with FoxD3 [37], Sox8 [38], Sox9 [39], MyoD [40] or Sox2 [41] anti-sense probes as described [42]. Each group contained 10 to 35 embryos, with several independent experiments.
RESULTS
Human embryonic stem cells differentiate into neural crest-like progenitors in response to ROCK inhibitors
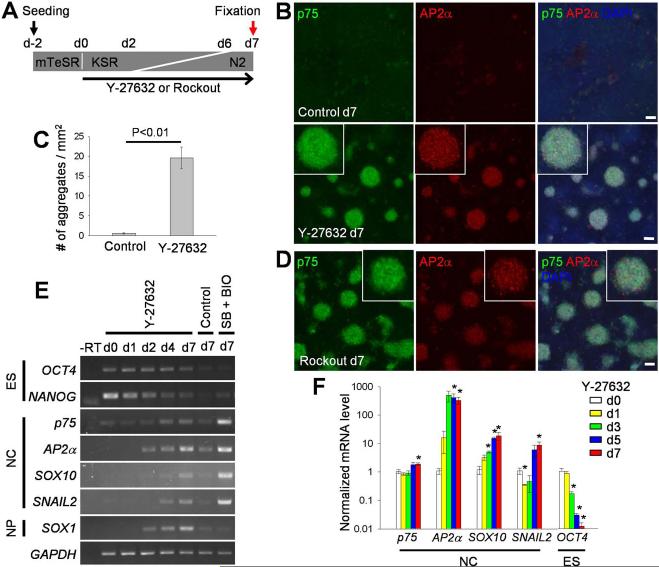
We examined a role of Rho signaling in H9 hESCs cultured in defined conditions without feeder cells using the ROCK inhibitor Y-27632 (Fig. 1A; Materials and Methods). After several days of culture in the presence of Y-27632, the neural crest markers p75 and AP2α [43-45] became gradually activated (Fig. 1A, 1 B). AP2α expression became detectable in some cells as early as day 3, followed by p75 expression (Supporting Information Fig. S1A). By day 7, the cells positive for p75 and AP2α formed conspicuous dense aggregates that were morphologically and immunochemically distinct from the surrounding cells (Fig. 1B, 1C; Supporting Information Fig. S1B) and were not detected in control cultures (Fig. 1B, 1C). Rockout, another inhibitor of ROCK, had similar effects (Fig. 1D; Supporting Information Fig. S1C). The same molecular markers were activated in our cultures by the combination of SB431542 and BIO, chemical inhibitors of Smad and GSK-3 (Supporting Information Fig. S1D, S1E), as reported previously [13]. We also found that Y-27632 upregulated neural crest marker expression in differentiating RUES1 cells (Supporting Information Fig. S1F), extending our findings to an independently derived hES cell line.
Figure 1. Inhibition of ROCK induces neural crest markers.
(A) Experimental scheme. H9 hES cells were seeded on a Matrigel-coated plate in the mTeSR self-renewal medium two days before differentiation (d-2). Differentiation was initiated by adding the KSR-containing medium on day 0 (d0), which was gradually replaced with the N2 medium from day 2 (d2), see Materials and Methods. ROCK inhibitors were added to the medium at d0. Cells were fixed on day 7 (d7) for immunofluorescence staining. (B and D) Cells cultured with no inhibitor (control) or Y-27632 (10 μM)(B), or with Rockout (12.5 μM)(D) were immunostained for p75 and AP2α. DNA was stained with DAPI. Insets represent magnified views of cell aggregates expressing neural crest markers. Scale bars, 100 μm. (C) Number of cell aggregates in control and Y-27632-treated day 7 cultures. Data are combined from three independent experiments. The means ± SEM are shown. P value was determined using the Student's t-test. (E) RT-PCR analysis showing time-dependent expression of pluripotency (ES), neural crest (NC) and neural progenitor (NP) markers. GAPDH is a ubiquitous control RNA. No reverse transcriptase (-RT). SB431542 (SB, 20 μM) and BIO (0.1 μM)-treated cells served as a positive control for neural crest marker expression. (F) Real-time quantitative PCR was carried out with samples from indicated time points in 3 to 4 independent experiments. Gene expression levels were normalized to GAPDH and the means ± SEM are shown. *, significant differences from day 0, p < 0.05.
To further define the spectrum of cell types present in the Y-27632-treated H9 cultures, we analyzed various cell fate markers using RT-PCR. Similar to p75 and AP2α, transcription of the neural crest markers SOX10 and SNAIL2/SLUG [43, 44, 46] was increased in a time-dependent manner (Fig. 1E, 1F). In real-time quantitative PCR (qPCR) experiments, AP2α was highly upregulated on day 3, followed by the gradual increases of p75, SOX10 and SNAIL2 (Fig. 1F). These results are consistent with the known neural crest gene regulatory network, in which AP2α is one of the earliest specifiers of the neural crest lineage [47]. By contrast, the pluripotency markers OCT4 and NANOG [48] steadily decreased both in control and Y-27632-treated cells (Fig. 1E, 1F). Besides neural crest markers, neural progenitor markers such asSOX1 and SOX3 [49, 50] were simultaneously upregulated (Figs. 1E, 3A), however, PAX6, another neural progenitor marker [50] was expressed both in the control and Y-27632-treated cultures (Fig. 3C; Supporting Information Fig. S3A). No significant expression of early mesoderm and endoderm markers was detected at any time point (Supporting Information Fig. S2), suggesting that the NCPs that form in response to ROCK inhibitors do not originate from mesendoderm. Together, these results show that ROCK inhibition promotes neural crest specification from hESCs.
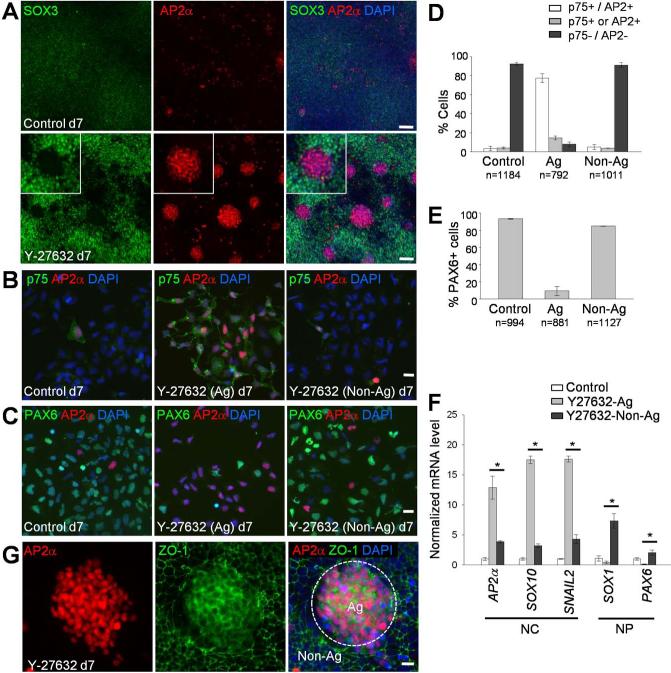
Figure 3. Neural crest progenitors form aggregates in Y-27632-treated cultures.
(A) Control and Y-27632-treated H9 cells were co-stained with antibodies to SOX3 and AP2α after 7 days of differentiation. Insets show magnified views of a cell aggregate. (B and C) In Y-27632-treated cultures, aggregate-forming cells (Ag) were enzymatically separated from non-aggregate cells (Non-Ag) and seeded on Matrigel-coated plates. Two hours after plating, the cells were fixed and immunostained for AP2α and p75 (B) or PAX6 (C). (D and E) Quantification of marker expression. Indicated numbers of cells were analyzed in each group from two independent experiments. The means ± SEM are shown. (F) Gene expression analysis in cell aggregates after hESC differentiation in the presence of Y-27632. Total RNA was prepared from aggregates (Ag) or non-aggregate cells (Non-Ag) in Y-27632-treated cultures. Cells cultured without the inhibitor served as a control. The expression level of neural crest (NC) and neural progenitor (NP) markers were examined by real-time qPCR. *, p <0.05. (G) Y-27632-treated day 7 cultures were immunostained for AP2α and the tight junction marker ZO-1. Dashed line, a boundary of AP2α-positive cell aggregate (Ag) surrounded by non-aggregate cells (Non-Ag). Scale bars, 100 μm (A) and 20 μm (B, C and G).
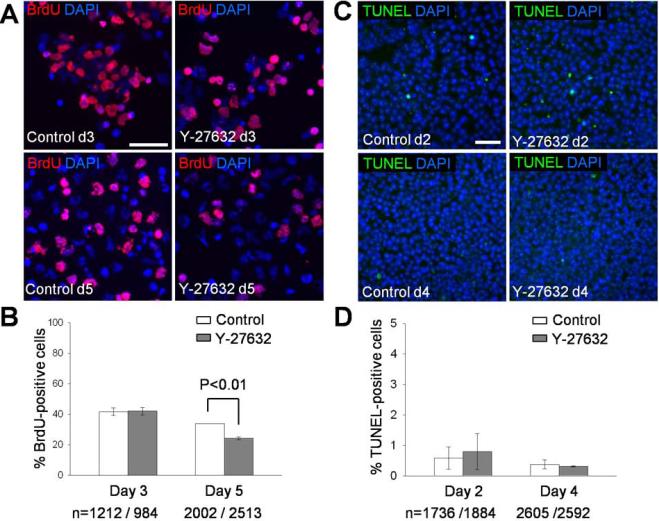
As ROCK inhibitor has been implicated in the survival and growth of hESCs [51-53], we tested the effect of Y-27632 on cell proliferation or apoptosis. The number of BrdU-positive S-phase cells was not affected after 3 days of treatment with Y-27632, but slightly decreased on day 5 (Fig. 2A, 2B). Also, cell death rate was similar in control and Y-27632-treated cultures (Fig. 2C, 2D). These results suggest that the ROCK inhibitor effect on neural crest development is not due to enhanced proliferation or survival of differentiating hESCs in our cultures.
Figure 2. Rates of cell proliferation and apoptosis during neural crest specification.
(A) On day 3 and day 5 of differentiation, H9 cells were incubated with the medium containing BrdU (10 μM) for 1 hour. The cells were replated on coverslips, fixed after 2 hours and immunostained with anti-BrdU antibody. (B) Quantification of data shown in (A). The means ± SEM are shown. N, the number of cells analyzed. Data are combined from 2 independent experiments. (C) Apoptotic cells identified by the TUNEL assay in day 2 and day 4 differentiation cultures. (D) TUNEL-positive cells were quantified from 2 independent experiments. Scale bars, 50 μm (A, C).
Y-27632 treatment triggers the formation of neural crest progenitor aggregates
We noticed that the Y-27632-treated cultures are composed of diverse cell populations with segregated staining for AP2α and SOX3 (Fig. 3A) or PAX6 (Supporting Information Fig. S3A). We therefore investigated the composition of the cell aggregates that form as a result of ROCK inhibition. We found that these aggregates do not adhere to the substrate as strongly as other cells (data now shown) and can be easily detached from the substrate by enzymatic treatment (see Materials and Methods). Using this approach, we separated cell aggregates from non-aggregated cells and recovered morphologically distinct cell populations (Supporting Information Fig. S3B). The comparison of the separated populations revealed that the aggregates were predominantly composed of cells that are double positive for p75 and AP2α (Fig. 3B, 3D). Consistently, PAX6-positive cells were present largely outside of the p75+/AP2α+ aggregates (Fig. 3C, 3E). Real-time qPCR analysis confirmed that the aggregates have higher expression of neural crest genes and lower expression of neural progenitor genes when compared to non-aggregate cells (Fig. 3F). The cell aggregates showed diffused localization of ZO-1, as compared to its junctional localization in the surrounding cells (Fig. 3G), consistent with EMT of neural crest cells. We conclude that our culture conditions promote the formation of NCP aggregates, which can be separated from Sox1- and Pax6-positive neural progenitors.
Differentiation of neural crest progenitors derived from hESCs in response to Y-27632
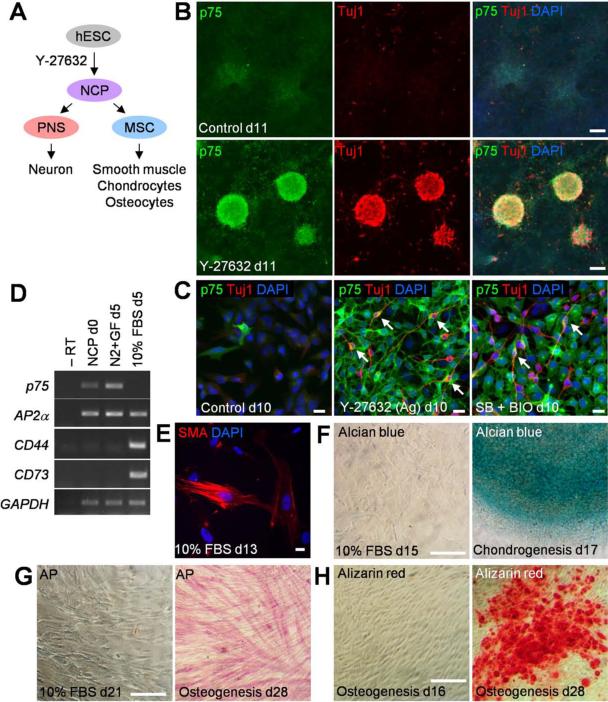
We wanted to examine the developmental potential of NCPs derived from hESCs and tested their ability to differentiate into neurons and mesenchymal cells [13, 43, 44] (Fig. 4A). When NCPs were maintained in the N2 medium for 3-4 extra days, they spontaneously formed Tuj1-positive neurons within the p75-positive aggregates (Fig. 4B). When the NCP aggregates were dispersed to visualize cell morphology, the Tuj1-positive cells revealed neurite-like protrusions that are characteristic of neurons (Fig. 4C). Half of these TuJ1-positive neurons coexpressed p75 (n=220), consistent with their neural crest origin. Upon further passaging of NCP aggregates in the N2 medium containing growth factors (bFGF and EGF, see Materials and Methods), the cultures lost this ability to differentiate into neurons but retained AP2α and p75 markers in 99 % of cells (Supporting Information Fig. S3C). When this pure NCP population was cultured for 5 days with 10 % fetal bovine serum (FBS), the mesenchymal stem cell (MSC) markers CD44 and CD73 [30, 43] were induced (Fig. 4D). These markers were absent from the initial culture and from NCPs maintained without FBS. Further differentiation of MSCs in the presence of FBS resulted in the appearance of 15 % of cells expressing smooth muscle actin (Fig. 4E). Directed differentiation of MSCs for 17-28 days in commercial media produced chondrocytes and osteocytes, confirming that the NCPs are capable of generating multiple mesenchymal cell lineages (Fig. 4F-4H). We conclude that the Y-27632-induced NCPs can differentiate into both neurons and mesenchymal cell types, and therefore, share the molecular characteristics and the differentiation potential with those of the embryo-derived neural crest.
Figure 4. Differentiation of neural crest progenitors (NCPs) in Y-27632-containing cultures.
(A) Scheme for NCP differentiation. PNS, peripheral nervous system; MSC, mesenchymal stem cell. (B) Spontaneous neuronal differentiation of NCPs. Y-27632-treated cultures were maintained in the N2 medium until day 11 and immunostained for Tuj1. (C) Aggregate-forming cells (Ag) in the Y-27632-treated group were passaged on day 7 and immunostained for p75 and Tuj1 on day 10. Arrows indicate cells double positive for p75 and Tuj1. The SB + BIO group (described in Figure 1 legend) is a positive control for neuronal differentiation. (D) Mesenchymal differentiation of NCPs (d0) obtained from Y-27632-treated cultures. RT-PCR analysis of neural crest markers and mesenchymal stem cell markers CD44 and CD73 after 5 day culture with the N2 medium containing growth factors (N2 + GF) or 10 % FBS. (E) MSCs obtained as in (D) were cultured with FBS for 8 more days and immunostained for smooth muscle actin (SMA). (F-H) MSCs were differentiated into chondrocytes and osteocytes as indicated and analyzed by alcian blue (F), alkaline phosphatase (AP, G) and alizarin red staining (H). Left panel of each figure serves as a negative control. Scale bars, 20 μm (C, E) and 100 μm (B, F-H).
Myosin II, a downstream target of ROCK, is involved in the pathway leading to neural crest specification
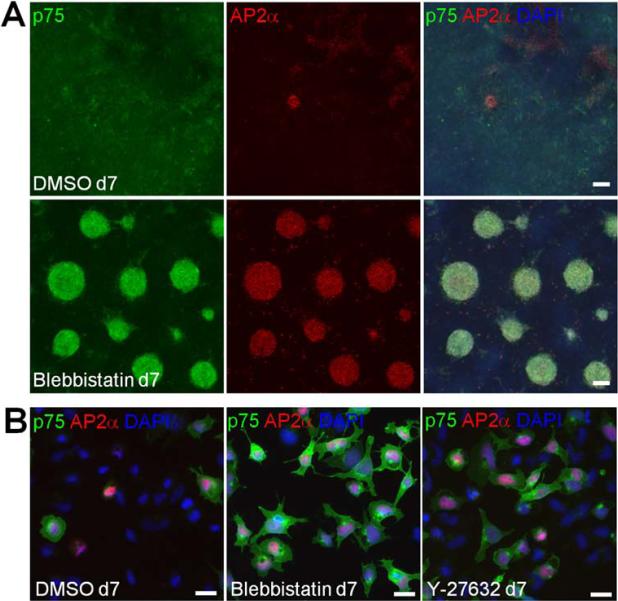
In non-muscle cells, ROCK controls actomyosin contractility by increasing Myosin II light chain (MLC) phosphorylation [21, 22]. Consistently, we observed the reduction of MLC phosphorylation and actomyosin cables in hESCs upon treatment with Y-27632 [52, 53] (Supporting Information Fig. S4A, S4B). Also, in NCP populations, we consistently observed more compact cells in the Y-27632-treated groups, as compared to the SB + BIO group (Fig. 4C). This effect is likely due to direct regulation of actomyosin contractility by Y-27632. To test whether Myosin II activity is involved in neural crest specification, hESCs were treated with Blebbistatin, a specific inhibitor of Myosin II [54]. Similar to Y-27632 and Rockout, Blebbistatin induced p75+/AP2α+ cell aggregates, suggesting that Myosin II acts downstream of ROCK to regulate neural crest specification from hESCs in vitro (Fig. 5A; Supporting Information Fig. S4C). Cell dissociation confirmed the double-positive staining for p75 and AP2α (Fig. 5B).
Figure 5. Myosin II inhibition promotes neural crest specification from hESCs.
(A) Activation of neural crest markers in hESCs by Blebbistatin. H9 cells were cultured with 10 μM of Blebbistatin as described in Figure 1A for ROCK inhibitors. DMSO is a negative control. Immunostaining for p75 and AP2α is shown. (B) Cells from day 7 cultures were dissociated and replated to show neural crest marker expression. Scale bars, 100 μm (A) and 20 μm (B).
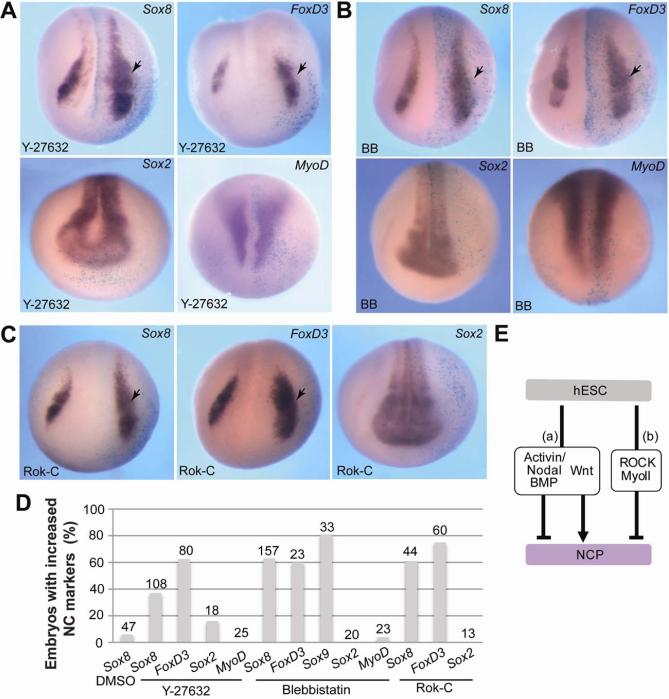
We next wanted to assess the role of the ROCK/Myosin II signaling pathway in neural crest specification in vivo. We chose to use Xenopus laevis embryos, a well-established system for vertebrate neural crest development. The efficiency of Y-27632 or Blebbistatin was confirmed in vivo, using ROCK-dependent morphological and molecular activity assays (Supporting Information Fig. S5A, S5B) and Myosin II-dependent blastopore formation assay [55, 56](Fig. S5C, S5D). Four-to-eight cell embryos were unilaterally injected into presumptive ectoderm with Y-27632 or Blebbistatin, and allowed to develop until neurula stages (Fig. 6). In situ hybridization revealed an expansion of the neural crest markers Sox8 and FoxD3 at the injected side, as compared to the uninjected side (Fig. 6A, 6B, 6D). The same effects were observed after the injection of RNA encoding a dominant negative ROCK [Rok-C; 34] (Fig. 6C, 6D). The interference with ROCK and Myosin II did not significantly change the pan-neural marker Sox2 (Fig. 6A-6D) and the mesoderm marker MyoD (Fig. 6A, 6B, 6D). The number of mitotic FoxD3-positive cells was not altered by Y-27632 or Blebbistatin, suggesting that expanded neural crest is not due to the increased rate of cell division (Supporting Information Fig. S6A, S6B). Together, these results support our model that ROCK/Myosin II signaling has an inhibitory role in neural crest-specific transcription (Fig. 6E).
Figure 6. Inhibition of ROCK and Myosin II expands neural crest territory in Xenopus embryos.
(A-C) Albino embryos were injected unilaterally with Y-27632 (A), Blebbistatin (BB, B) or Rok-C RNA (C) at the 4-8-cell stage, cultured until stages 14-16. The neural crest genes Sox8 andFoxD3, the pan-neural marker Sox2 and the somitic marker MyoD were analyzed by in situ hybridization as indicated. Dorso-anterior views of representative embryos are shown. Arrows point to altered gene expression on the injected side, which is marked by β-galactosidase staining (light blue). (D) Quantification of the effect shown in (A-C) as percentage of embryos with increased marker expression. Total number of embryos pooled from several independent experiments is indicated at the top of each bar. (E) Pathways that convert hESC into NCPs. (a) Simultaneous inhibition of Activin/Nodal/BMP and upregulation of the Wnt pathway promotes neural crest development. (b) Inhibition of ROCK or Myosin II is sufficient to trigger NCP formation.
DISCUSSION
Although fundamental roles of the Rho/ROCK/Myosin pathway in cell division, migration, adhesion and apoptosis have been well established [57-59], the function of this pathway in neural crest development has remained largely unknown. Of note, both positive and negative effects of Rho pathway modulation on neural crest specification, migration and differentiation have been reported in different vertebrate models [16, 60-62]. These diverse outcomes are likely due to stage- or context-dependent cell responses, which might be mediated by different Rho effectors. Our analysis of hESCs differentiation in vitro reveals that one branch of Rho signaling, the ROCK/Myosin II pathway, functions as a negative regulator of neural crest specification. This mechanism appears to be conserved in vivo, since the treatments that are effective in hESCs lead to an expansion of the neural crest territory in Xenopus embryos. Notably, the same pathway plays critical roles in other contexts, including hESC self-renewal [51-53, 63-65], endothelial differentiation of mesodermal precursors [66], and the directed differentiation of mesenchymal stem cells [26, 27]. Based on the primary role of Myosin II in regulating actin polymerization and contractility, we propose that actomyosin-dependent tension suppresses neural crest specification.
Neural crest cells have been previously derived from hESCs using an intermediate step of neural rosette or neurosphere formation [15, 43, 44, 67, 68] or by altering signaling pathways that affect neural induction [12, 13, 69-71]. Relevant to our work, Hotta et al. previously reported that Y-27632 promotes neural crest generation from ESC-derived neurospheres in the presence of mouse embryo fibroblasts, after stimulation with Noggin, EGF and FGF [68]. By contrast, we obtained NCPs, starting directly from hES cells in the absence of feeders or exogenously added growth factors (Fig. 6E). Complementing the previous findings, our study raises a possibility that the neural crest fate can be acquired independently of known growth factor signaling through the modulation of the ROCK/Myosin II pathway.
Besides neural crest markers, we observe that ROCK inhibition upregulates the neural progenitor markers Sox1 and Sox3, suggesting that the treatment promotes differentiation of more than one cell lineage. The appearance of at least two different cell types in response to ROCK inhibition might be due to heterogeneity of the hESC population [72-74]. For example, self-renewing hESCs are heterogeneous with respect to the level of endogenous Wnt signaling [74]. Thus, the intrinsic status of each hESC may affect the initial response to ROCK inhibitors and subsequent lineage commitment. As in our study, both neural progenitors and NCPs were induced from hESCs by Smad inhibitors [12]. More work is needed to define the characteristics of hESC that are critical for the specification of neural and neural crest lineages.
Our culture conditions promoted the appearance of large aggregates of loosely adherent NCPs. While the significance of this observation is unclear, neural crest cells are known to express homophilic cell adhesion molecules, such as N-cadherin, Cadherin 6 and Cadherin 11 [75], which might promote aggregate formation. Alternatively, NCPs may be excluded from the monolayer of the non-neural crest population, which are connected by tight junctions. Supporting the latter possibility, the NCP aggregates lose junctional ZO-1, possibly due to epithelialmesenchymal transition.
It is currently unclear how the generated NCPs correspond to those previously obtained by in vitro differentiation of ES cells or to neural crest progenitors in vivo. At early stages, the NCPs induced by the ROCK/Myosin II pathway inhibition readily differentiated into neurons and mesenchymal cell types (data not shown). However, with passaging, the NCPs lost the potential for neurogenesis, while becoming prone to mesenchymal differentiation. Nevertheless, the major neural crest markers p75 and AP2α have been maintained. Therefore, these cells might have become ‘frozen’ in one out of several progenitor states, in which their differentiation capacity is restricted to particular cell types. This is consistent with the idea of progressive restriction of the developmental potential of neural crest cells observed in vivo [76, 77]. Similarly, hESC-derived neural crest cells were described as a heterogeneous population with limited differentiation ability [43, 78]. Thus, different experimental conditions may produce neural crest progenitors with different developmental potential, as has been shown for melanocyte specification [12, 69].
The molecular mechanism underlying the roles of ROCK and Myosin II in neural crest specification is currently unclear. Although Y-27632 was reported to promote hESC survival and growth [51-53], we did not observe enhanced cell proliferation or reduced apoptosis in our differentiating cultures and in Xenopus embryos. The ROCK/Myosin II pathway is a key regulator of cell contractility and cell junctions [22, 58], and plays a role in mechanotransduction [79-81]. Of note, Myosin II-dependent cell responses to mechanical signals have been implicated in directed in vitro differentiation of mesenchymal stem cells [26, 27, 82]. Changes in actin cytoskeleton and cell-cell adhesion could mechanically alter subcellular localization of some transcriptional regulators, which can shuttle between the cell junctions and the nucleus. Several candidate proteins, such as β-catenin and YAP, are known to translocate into the nucleus in response to mechanical forces [83-86] and can activate neural crest-specific transcription [6, 87]. Early morphological changes observed in human and mouse ESCs after ROCK and Myosin inhibitor treatment [88, 89 , 90] may be relevant to the above mechanism leading to neural crest lineage specification. Also, we detected changes in actomyosin cables and nuclear enrichment of YAP in Y-27632- and Blebbistatin-treated cells (Supporting Information Fig. S7A and S7B), further supporting the hypothesis that mechanical signals specify neural crest through YAP. Notably, YAP has been recently implicated in neural differentiation of hESCs in response to substrate rigidity [91]. Another possible mechanism relates to Ajuba proteins that were shown to modulate neural crest in complex with Snail transcription factors [20]. This transcriptional complex might respond to mechanical signals and ROCK/Myosin II inhibition, as has been recently demonstrated for Ajuba in a Drosophila model [92]. Alternatively, modulating ROCK and Myosin II activity might change Wnt, BMP or FGF signaling pathways that are known to control neural crest development [6, 10, 11]. We did not detect the stabilization of β-catenin after 24 hrs in our differentiating cultures (Supporting Information Fig. S7C), suggesting that ROCK and Myosin inhibitors might affect NC specification independently of the Wnt/β-catenin pathway. Finally, the ROCK/Myosin pathway may suppress neural crest genes by modulating actin- or Myosin II-dependent transcription [93, 94]. Future studies are warranted to discriminate between these alternative models and explain how changes in cell contractility or tension lead to neural crest-specific gene expression.
Supplementary Material
Figure S1. Responses of H9 and RUES1 hESCs to Y-27632.
(A) Dynamics of H9 cell differentiation in response to ROCK inhibitors treated as in Figure 1A. Gradual increase of neural crest markers was observed in differentiating hESC cultures at indicated time points. (B) Y-27632-treated cultures form cell aggregates (arrows) after 7-day differentiation. (C) The complete data set, including the corresponding control panel, which is relevant to Figure 1C. (D) Activation of p75 and AP2α in H9 cells treated with SB431542 (SB, 20 μM) and BIO (0.1 μM). (E) Number of cell aggregates in control and (SB+BIO)-treated day 7 cultures. Data are presented as means ± SEM from two independent experiments. (F) Differentiation of RUES1 cells in the presence of Y-27632 (10 μM) and Blebbistatin (BB, 10 μM) (as described in Figure 1A) caused upregulation of neural crest markers. Scale bars, 100 μm.
Figure S2. Lack of mesendoderm differentiation in the presence of Y-27632.
RT-PCR analysis for markers of mesoderm and endoderm was carried out for total RNA prepared from the cells at indicated time points. No reverse transcriptase (-RT). GAPDH is a ubiquitous control RNA. Mesendoderm differentiation was performed as described in Supplemental Materials and Methods.
Figure S3. Y-27632 treatment promotes the formation of neural crest progenitor-enriched aggregates.
(A) NCP aggregates forming in the Y-27632 cultures. Day 7 cultures were coimmunostained for AP2α and PAX6, a neural progenitor marker. Insets show magnified views of AP2α-positive cell aggregates. (B) Brightfield images of dissociated cells from control or Y-27632-treated cultures. Separated aggregate-forming cells (Ag) and non-aggregate cells (Non-Ag) were replated for morphological examination. (C) Isolated NCP aggregates were maintained and passaged in DMEM/F12 containing N2 supplement and growth factors (see Materials and Methods). NCPs (passage 5) were immunostained for AP2α and p75. Scale bars, 100 μm (A and B) and 50 μm (C).
Figure S4. Inhibition of Myosin II activity stimulates neural crest specification.
(A) hESC colonies were dissociated into single cells and replated with or without Y-27632. Myosin II light chain (MLC) phosphorylation was analyzed in cell lysates prepared 1 hr after replating with anti-MLC and anti-phospho-MLC antibodies. Tubulin is a loading control. (B)Altered actomyosin cables in cultures treated with ROCK or Myosin II inhibitors. H9 cells were incubated in the KSR medium with Y-27632 (10 μM) or Blebbistatin (10 μM) for 16 hours. The cells were immunostained for phospho-MLC. F-actin was visualized with phalloidin. (C) Dose-dependent effect of Blebbistatin (BB) on neural crest specification in H9 hESC cultures. Scale bars, 10 μm (B) and 100 μm (C).
Figure S5. Efficiency of ROCK and Myosin II inhibitors.
(A) ROCK RNA (1 ng) and 10 nl of Y-27632 (125 μM) were injected into animal blastomeres of 4-8 cell stage Xenopus embryos. uni st 10, control stage 10 uninjected embryos. Animal pole view is shown. ROCK gain-of-function phenotype (dark pigmentation, white arrowheads) is suppressed by Y-27632. (B) Y-27632 decreases MLC phosphorylation. Animal cap lysates were prepared from the uninjected (uni) or GFP-myosin light chain (MLC) injected embryos and subjected to immunoblotting. GFP-MLC RNA (0.3 ng) was coinjected with ROCK RNA (1 ng) and 10 nl of Y-27632 (125 μM) as indicated. β-catenin is a loading control. (C) Y-27632 and Blebbistatin block blastopore closure. Indicated drugs were dorsally injected into 4-8 cell stage Xenopus embryos together with β-galactosidase RNA as lineage tracer (blue staining). Vegetal view is shown, the dorsal (D)-ventral (V) axis is indicated. (D) Frequencies of blastopore closure defects are shown for embryos pooled from 2-3 independent experiments. Total number of embryos used for quantification is indicated at the top of each bar.
Figure S6. Frequencies of mitotic cells during neural crest specification.
(A) Ten nanoliter of Y-27632 (50 μM) and Blebbistatin (500 μM) were unilaterally injected into 4-8 cell stage Xenopus embryos, along with GFP-CAAX RNA as a tracer. The embryos were fixed at neurula stage (st 14-15) and immunostained for phospho-histone H3 (pHH3) and the neural crest markers FoxD3. White arrows mark mitotic neural crest cells. Scale bar, 10 μm. (B) Quantification of mitotic neural crest cells. N indicates the number of FoxD3-positive cells analyzed in two independent experiments.
Figure S7. ROCK and Myosin inhibitors promote nuclear retention of YAP during hESC differentiation.
(A and B) Immunostaining of differentiated H9 cells with anti-YAP antibody. (A) Cultures differentiated for 0, 4 and 24 hours reveal progressive reduction of YAP nuclear staining. (B) Twenty four hour treatment with Y-27632 (10 μM), Blebbistatin (5 μM), but not SB431542 (SB, 20 μM) + BIO (1 μM) promotes the retention of nuclear YAP in confluent cultures. This result was observed in two independent experiments. Cells with nuclear (white arrow) and cytoplasmic (green arrow) YAP are indicated. Insets, magnified views of single cells with outlined nuclei. Scale bars, 10 μm (A, B). (C) H9 cells were treated with DMSO (vehicle), Y27632 (10 μM) or Blebbistatin (10 μM) for 24 hours in the KSR medium, and cell lysates were immunoblotted with antibodies to unphosphorylated β-catenin and total β-catenin. Cells treated with SB431542 (20 μM) and BIO (1 μM) were used as a positive control. Relative ratios of unphosphorylated β-catenin to total β-catenin are shown. α-tubulin, a loading control.
ACKNOWLEDGMENTS
We thank Sunita D'souza and the Mount Sinai human ES cell Core Facility for helping us start this project, A. Brivanlou for the RUES1 cell line, M. Klymkowsky and D. Kessler for antibodies, Valerie Gouon-Evans and Andriani Ioannou for critical reading of the manuscript and members of the Sokol laboratory for discussion. This study was supported by NIH grants to S. Y. S.
Footnotes
Author contribution:
Kyeongmi Kim: Conception and design, Collection and assembly of data, Data analysis and interpretation, Manuscript writing
Olga Ossipova: Conception and design, Collection and assembly of data, Data analysis and interpretation
Sergei Y. Sokol: Conception and design, Data analysis and interpretation, Manuscript writing
REFERENCES
- 1.Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos Trans R Soc Lond B Biol Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- 6.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 7.Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- 8.Marchant L, Linker C, Ruiz P, et al. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- 9.Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Prasad MS, Sauka-Spengler T, LaBonne C. Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Dev Biol. 2012;366:10–21. doi: 10.1016/j.ydbio.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menendez L, Yatskievych TA, Antin PB, et al. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milet C, Monsoro-Burq AH. Embryonic stem cell strategies to explore neural crest development in human embryos. Dev Biol. 2012;366:96–99. doi: 10.1016/j.ydbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Bajpai R, Chen DA, Rada-Iglesias A, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broders-Bondon F, Chesneau A, Romero-Oliva F, et al. Regulation of XSnail2 expression by Rho GTPases. Dev Dyn. 2007;236:2555–2566. doi: 10.1002/dvdy.21273. [DOI] [PubMed] [Google Scholar]
- 17.Guemar L, de Santa Barbara P, Vignal E, et al. The small GTPase RhoV is an essential regulator of neural crest induction in Xenopus. Dev Biol. 2007;310:113–128. doi: 10.1016/j.ydbio.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Ossipova O, Sokol SY. Neural crest specification by noncanonical Wnt signaling and PAR-1. Development. 2011;138:5441–5450. doi: 10.1242/dev.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CY, Jhingory S, Taneyhill LA. The tight junction scaffolding protein cingulin regulates neural crest cell migration. Dev Dyn. 2011;240:2309–2323. doi: 10.1002/dvdy.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer EM, Feng Y, Zhaoyuan H, et al. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 22.Levayer R, Lecuit T. Biomechanical regulation of contractility: spatial control and dynamics. Trends Cell Biol. 2011;22:61–81. doi: 10.1016/j.tcb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Menke A, Giehl K. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch Biochem Biophys. 2012;524:48–55. doi: 10.1016/j.abb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Eyckmans J, Boudou T, Yu X, et al. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark K, Langeslag M, Figdor CG, et al. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Sordella R, Jiang W, Chen GC, et al. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 27.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 29.Lee G, Chambers SM, Tomishima MJ, et al. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- 30.Barberi T, Willis LM, Socci ND, et al. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Lake BB, Haremaki T, et al. Rab11 regulates planar polarity and migratory behavior of multiciliated cells in Xenopus embryonic epidermis. Dev Dyn. 2012;241:1385–1395. doi: 10.1002/dvdy.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 34.Marlow F, Topczewski J, Sepich D, et al. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 35.Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci. 2014;127:2542–2553. doi: 10.1242/jcs.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieuwkoop PD. The “organization centre”. 3. Segregation and pattern formation in morphogenetic fields. Acta Biotheor. 1967;17:178–194. doi: 10.1007/BF01601987. [DOI] [PubMed] [Google Scholar]
- 37.Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell M, Hong CS, Huang X, et al. Functional analysis of Sox8 during neural crest development in Xenopus. Development. 2006;133:3817–3826. doi: 10.1242/dev.02558. [DOI] [PubMed] [Google Scholar]
- 39.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 40.Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuseki K, Kishi M, Matsui M, et al. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- 42.Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Methods Cell Biol. Academic Press Inc.; San Diego: 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- 43.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 44.Curchoe CL, Maurer J, McKeown SJ, et al. Early acquisition of neural crest competence during hESCs neuralization. PLoS One. 2010;5:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betters E, Liu Y, Kjaeldgaard A, et al. Analysis of early human neural crest development. Dev Biol. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieto MA, Sargent MG, Wilkinson DG, et al. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 47.de Croze N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci U S A. 2011;108:155–160. doi: 10.1073/pnas.1010740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 49.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Huang CT, Chen J, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 52.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Chen G, Hou Z, Gulbranson DR, et al. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straight AF, Cheung A, Limouze J, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 55.Skoglund P, Rolo A, Chen X, et al. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JY, Harland RM. Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev Biol. 2007;311:40–52. doi: 10.1016/j.ydbio.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 58.Vicente-Manzanares M, Ma X, Adelstein RS, et al. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Street CA, Bryan BA. Rho kinase proteins--pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31:3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips HM, Papoutsi T, Soenen H, et al. Neural crest cell survival is dependent on Rho kinase and is required for development of the mid face in mouse embryos. PLoS One. 2012;7:e37685. doi: 10.1371/journal.pone.0037685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groysman M, Shoval I, Kalcheim C. A negative modulatory role for rho and rho-associated kinase signaling in delamination of neural crest cells. Neural Dev. 2008;3:27. doi: 10.1186/1749-8104-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berndt JD, Clay MR, Langenberg T, et al. Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev Biol. 2008;324:236–244. doi: 10.1016/j.ydbio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–581. doi: 10.1016/j.jbiosc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21:274–282. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Walker A, Su H, Conti MA, et al. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joo HJ, Choi DK, Lim JS, et al. ROCK suppression promotes differentiation and expansion of endothelial cells from embryonic stem cell-derived Flk1(+) mesodermal precursor cells. Blood. 2012;120:2733–2744. doi: 10.1182/blood-2012-04-421610. [DOI] [PubMed] [Google Scholar]
- 67.Cimadamore F, Fishwick K, Giusto E, et al. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotta R, Pepdjonovic L, Anderson RB, et al. Small-molecule induction of neural crest-like cells derived from human neural progenitors. Stem Cells. 2009;27:2896–2905. doi: 10.1002/stem.208. [DOI] [PubMed] [Google Scholar]
- 69.Mica Y, Lee G, Chambers SM, et al. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013;3:1140–1152. doi: 10.1016/j.celrep.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noisa P, Lund C, Kanduri K, et al. Notch signaling regulates neural crest differentiation from human pluripotent stem cells. J Cell Sci. 2014 doi: 10.1242/jcs.145755. [DOI] [PubMed] [Google Scholar]
- 71.Kreitzer FR, Salomonis N, Sheehan A, et al. A robust method to derive functional neural crest cells from human pluripotent stem cells. Am J Stem Cells. 2013;2:119–131. [PMC free article] [PubMed] [Google Scholar]
- 72.Enver T, Pera M, Peterson C, et al. Stem cell states, fates, and the rules of attraction. Cell Stem Cell. 2009;4:387–397. doi: 10.1016/j.stem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Singh AM, Chappell J, Trost R, et al. Cell-cycle control of developmentally regulated transcription factors accounts for heterogeneity in human pluripotent cells. Stem Cell Reports. 2013;1:532–544. doi: 10.1016/j.stemcr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blauwkamp TA, Nigam S, Ardehali R, et al. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- 77.Lo L, Anderson DJ. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron. 1995;15:527–539. doi: 10.1016/0896-6273(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 78.Jiang X, Gwye Y, McKeown SJ, et al. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yim EK, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 2012;3:41. doi: 10.1186/scrt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519–542. doi: 10.1146/annurev-biophys-042910-155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 83.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 84.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 85.Brunet T, Bouclet A, Ahmadi P, et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat Commun. 2013;4:2821. doi: 10.1038/ncomms3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 87.Gee ST, Milgram SL, Kramer KL, et al. Yes-associated protein 65 (YAP) expands neural progenitors and regulates Pax3 expression in the neural plate border zone. PLoS One. 2011;6:e20309. doi: 10.1371/journal.pone.0020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang TC, Chen YC, Yang MH, et al. Rho kinases regulate the renewal and neural differentiation of embryonic stem cells in a cell plating density-dependent manner. PLoS One. 2010;5:e9187. doi: 10.1371/journal.pone.0009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D, Zhou J, Wang L, et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191:631–644. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y, Yong KM, Villa-Diaz LG, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater. 2014;13:599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rauskolb C, Sun S, Sun G, et al. Cytoskeletal Tension Inhibits Hippo Signaling through an Ajuba-Warts Complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Q, Sarna SK. Nuclear myosin II regulates the assembly of preinitiation complex for ICAM-1 gene transcription. Gastroenterology. 2009;137:1051–1060. 1060, e1051–1053. doi: 10.1053/j.gastro.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Responses of H9 and RUES1 hESCs to Y-27632.
(A) Dynamics of H9 cell differentiation in response to ROCK inhibitors treated as in Figure 1A. Gradual increase of neural crest markers was observed in differentiating hESC cultures at indicated time points. (B) Y-27632-treated cultures form cell aggregates (arrows) after 7-day differentiation. (C) The complete data set, including the corresponding control panel, which is relevant to Figure 1C. (D) Activation of p75 and AP2α in H9 cells treated with SB431542 (SB, 20 μM) and BIO (0.1 μM). (E) Number of cell aggregates in control and (SB+BIO)-treated day 7 cultures. Data are presented as means ± SEM from two independent experiments. (F) Differentiation of RUES1 cells in the presence of Y-27632 (10 μM) and Blebbistatin (BB, 10 μM) (as described in Figure 1A) caused upregulation of neural crest markers. Scale bars, 100 μm.
Figure S2. Lack of mesendoderm differentiation in the presence of Y-27632.
RT-PCR analysis for markers of mesoderm and endoderm was carried out for total RNA prepared from the cells at indicated time points. No reverse transcriptase (-RT). GAPDH is a ubiquitous control RNA. Mesendoderm differentiation was performed as described in Supplemental Materials and Methods.
Figure S3. Y-27632 treatment promotes the formation of neural crest progenitor-enriched aggregates.
(A) NCP aggregates forming in the Y-27632 cultures. Day 7 cultures were coimmunostained for AP2α and PAX6, a neural progenitor marker. Insets show magnified views of AP2α-positive cell aggregates. (B) Brightfield images of dissociated cells from control or Y-27632-treated cultures. Separated aggregate-forming cells (Ag) and non-aggregate cells (Non-Ag) were replated for morphological examination. (C) Isolated NCP aggregates were maintained and passaged in DMEM/F12 containing N2 supplement and growth factors (see Materials and Methods). NCPs (passage 5) were immunostained for AP2α and p75. Scale bars, 100 μm (A and B) and 50 μm (C).
Figure S4. Inhibition of Myosin II activity stimulates neural crest specification.
(A) hESC colonies were dissociated into single cells and replated with or without Y-27632. Myosin II light chain (MLC) phosphorylation was analyzed in cell lysates prepared 1 hr after replating with anti-MLC and anti-phospho-MLC antibodies. Tubulin is a loading control. (B)Altered actomyosin cables in cultures treated with ROCK or Myosin II inhibitors. H9 cells were incubated in the KSR medium with Y-27632 (10 μM) or Blebbistatin (10 μM) for 16 hours. The cells were immunostained for phospho-MLC. F-actin was visualized with phalloidin. (C) Dose-dependent effect of Blebbistatin (BB) on neural crest specification in H9 hESC cultures. Scale bars, 10 μm (B) and 100 μm (C).
Figure S5. Efficiency of ROCK and Myosin II inhibitors.
(A) ROCK RNA (1 ng) and 10 nl of Y-27632 (125 μM) were injected into animal blastomeres of 4-8 cell stage Xenopus embryos. uni st 10, control stage 10 uninjected embryos. Animal pole view is shown. ROCK gain-of-function phenotype (dark pigmentation, white arrowheads) is suppressed by Y-27632. (B) Y-27632 decreases MLC phosphorylation. Animal cap lysates were prepared from the uninjected (uni) or GFP-myosin light chain (MLC) injected embryos and subjected to immunoblotting. GFP-MLC RNA (0.3 ng) was coinjected with ROCK RNA (1 ng) and 10 nl of Y-27632 (125 μM) as indicated. β-catenin is a loading control. (C) Y-27632 and Blebbistatin block blastopore closure. Indicated drugs were dorsally injected into 4-8 cell stage Xenopus embryos together with β-galactosidase RNA as lineage tracer (blue staining). Vegetal view is shown, the dorsal (D)-ventral (V) axis is indicated. (D) Frequencies of blastopore closure defects are shown for embryos pooled from 2-3 independent experiments. Total number of embryos used for quantification is indicated at the top of each bar.
Figure S6. Frequencies of mitotic cells during neural crest specification.
(A) Ten nanoliter of Y-27632 (50 μM) and Blebbistatin (500 μM) were unilaterally injected into 4-8 cell stage Xenopus embryos, along with GFP-CAAX RNA as a tracer. The embryos were fixed at neurula stage (st 14-15) and immunostained for phospho-histone H3 (pHH3) and the neural crest markers FoxD3. White arrows mark mitotic neural crest cells. Scale bar, 10 μm. (B) Quantification of mitotic neural crest cells. N indicates the number of FoxD3-positive cells analyzed in two independent experiments.
Figure S7. ROCK and Myosin inhibitors promote nuclear retention of YAP during hESC differentiation.
(A and B) Immunostaining of differentiated H9 cells with anti-YAP antibody. (A) Cultures differentiated for 0, 4 and 24 hours reveal progressive reduction of YAP nuclear staining. (B) Twenty four hour treatment with Y-27632 (10 μM), Blebbistatin (5 μM), but not SB431542 (SB, 20 μM) + BIO (1 μM) promotes the retention of nuclear YAP in confluent cultures. This result was observed in two independent experiments. Cells with nuclear (white arrow) and cytoplasmic (green arrow) YAP are indicated. Insets, magnified views of single cells with outlined nuclei. Scale bars, 10 μm (A, B). (C) H9 cells were treated with DMSO (vehicle), Y27632 (10 μM) or Blebbistatin (10 μM) for 24 hours in the KSR medium, and cell lysates were immunoblotted with antibodies to unphosphorylated β-catenin and total β-catenin. Cells treated with SB431542 (20 μM) and BIO (1 μM) were used as a positive control. Relative ratios of unphosphorylated β-catenin to total β-catenin are shown. α-tubulin, a loading control.