Abstract
External fixation is a method of osteosynthesis currently used in traumatology and orthopedic surgery. Pin tract infection is a common problem in clinical practice. Infection occurs after bacterial colonization of the pin due to its contact with skin and the local environment. One way to prevent such local contamination is to create a specific coating that could be applied in the medical field. In this work, we developed a surface coating for external fixator pins based on the photocatalytic properties of titanium dioxide, producing a bactericidal effect with sufficient mechanical strength to be compatible with surgical use. The morphology and structure of the sol-gel coating layers were characterized using, respectively, scanning electron microscopy and X-ray diffraction. The resistance properties of the coating were investigated by mechanical testing. Photodegradation of acid orange 7 in aqueous solution was used as a probe to assess the photocatalytic activity of the titanium dioxide layers under ultraviolet irradiation. The bactericidal effect induced by the process was evaluated against two strains, ie, Staphylococcus aureus and multiresistant Staphylococcus epidermidis. The coated pins showed good mechanical strength and an efficient antibacterial effect after 1 hour of ultraviolet irradiation.
Keywords: hybrid sol-gel, external pin fixation, titanium dioxide, antibacterial effect, mechanical strength, ultraviolet photoactivity
Introduction
External fixation is a method of osteosynthesis commonly used by surgeons in the fields of orthopedic surgery and trauma. The main feature of this method is that the implant of the assembly, called the tube fixator, is located outside the body and connected to bone by transcutaneous pins.1–3 Complications associated with these pins, mainly pin tract infection, are an important source of morbidity that increase the complexity of care, with additional surgical procedures, resulting in longer hospital stays4 and higher socioeconomic costs.5
Although pin tract infection is a common problem in clinical practice, there is no consensus regarding its definition, which is reflected in the wide ranges of incidence (0%–80%) reported in the literature.6–9 Infection occurs after bacterial colonization of the pin, due to its contact with the skin and local environment, and phenomena brought about by surgery, such as a decrease in the local immune response, soft tissue trauma, and bone necrosis.10,11 Infection can develop locally, but may also spread to adjacent soft tissue and bone, leading to osteomyelitis and loss of mechanical anchorage of the pin.
Diagnosis of pin tract infection should be based on nonspecific clinical arguments such as local inflammation, presence of discharge or pus, and pain as described in the Checketts–Otterburn classification.12 Unfortunately, there is no objective test or standardized tool to confirm the diagnosis, and only evolution and time will do so. In a study of 214 implanted pins in 42 patients, Mahan et al13 found a bacterial colonization rate of 74.8%, mainly involving three species, ie, Staphylococcus epidermidis (90.6%), Staphylococcus aureus (37.5%), and Escherichia coli (9.4%). S. aureus was more frequently associated with local infection and osteolysis than S. epidermidis. Consequently, since the 1970s, clinicians have tried to solve the problem of pin tract infection using a number of methods,14 including use of alloys to manufacture pins or coating the pin with antibiotics or chemical substances (such as hydroxyapatite), silver particles, and zinc or titanium oxide, and applying external electromagnetic fields. However, none of the latter experiments settled the issue.
Since a publication by Matsunaga et al15 in 1985, the bactericidal effect induced by titanium dioxide (TiO2) exposed to ultraviolet irradiation has been used successfully in many areas16–19 like disinfection of water and textiles and in other cleaning processes. TiO2 is indeed one of the most effective photocatalysts currently in use due to its strong oxidizing power, lack of toxicity, and long-term physical and chemical stability. It has been widely used for the decomposition of organic compounds and to destroy microbial organisms, such as cancer cells, viruses, and bacteria, and has potential application in the sterilization of medical devices and air-conditioning filters because of its self-sterilizing ability.20–23 When irradiated with near ultraviolet light, TiO2 has strong bactericidal activity.24
Other antibacterial materials available include silver-based compounds, which have been widely investigated as antibacterial agents because of their potent broad-spectrum antibacterial activity.25–29 However, the antibacterial mechanism of silver species raises concerns about their potential cytotoxicity.30–32 It is suggested that diffusing silver may act as a Trojan horse by entering cells and then releasing silver ions that damage intracellular function.32 Development of inorganic antibacterial agents with high antibacterial activity, biosafety, and hopefully osteoconductivity is necessary. TiO2 is a good candidate because of its efficient photoactivity and lack of toxicity.
In this work, we hypothesized that the photoactive properties of TiO2 could be used to prevent pin tract infection. Scanning electron microscopy and X-ray diffraction were used to characterize the morphology and structure of the coating layers. Photodegradation tests of acid orange 7 (AO7) in aqueous solution were used to assess the photocatalytic activity of TiO2 coatings exposed to ultraviolet irradiation. Mechanical tests were performed using a fresh bovine femur to evaluate the strength of the coating. Finally, we evaluated the bactericidal effect of the coating using S. aureus and S. epidermidis strains.
Materials and methods
External fixator pin, metal disk, and quartz substrates
Stainless steel pins (FeCrNiMo18-15-3; ~ AISI 316 low carbon vacuum melt), 150 mm in length and 4 mm in diameter, and produced by L Klein (Bienne, Switzerland) were provided by Biomet, Inc (Warsaw, IN, USA). For technical reasons, it was necessary to use substrates of a suitable format. Stainless steel disks (AISI 316 FeCrNiMo18-10-3) 15 mm in diameter and 0.25 mm in thickness were provided by Goodfellow SARL (Lille, France). The coatings on the quartz substrates were specifically designed for use in X-ray analysis.
Titanium sol formulation
In this work, the precursor solution was a mixture of titanium isopropoxide 97% (Sigma-Aldrich, St Louis, MO, USA) and ethyl alcohol 99.5% (Prolabo, VWR International, Radnor, PA, USA).
In order to moderate alkoxide reactivity, an organic ligand, acetylacetone (99.5%, Fluka, Sigma-Aldrich), was added to the precursor solution. A drop of deionized water was used to start the hydrolysis–condensation reactions. The chemical composition of the starting alkoxide sol was Ti(OC3H7)3:C5H8O2:C2H5OH:H2O (molar ratio 1:1:20:1). An organic spacer, polyethylene glycol (Sigma-Aldrich, number average molecular weight 600), was added to increase the porosity of the layer (volume equal to 20% of the weight).
Cleaning and dip coating of substrates
The substrates were degreased by successive sonication in trichloroethylene (98%, Prolabo), acetone (99%, Fisher Scientific, Waltham, MA, USA), and methanol (99.5%, UCB, Brussels, Belgium), followed by rinsing with deionized water and blowing dry with nitrogen. Samples were immersed in the sol for 1 minute. The withdrawing speed was set to 31 cm per minute to obtain a suitable coating thickness.
Annealing treatments
Annealing treatments were carried out in an RS 80/750/11 Nabertherm tubular furnace equipped with a P300 processor. Coatings were treated in air, using a ramp of 5°C per minute to 500°C and then isothermal treatment at 500°C for 1 hour. Samples were then allowed to cool down to room temperature.
X-ray diffraction analysis
The crystalline structure of the TiO2 layers was determined by X-ray diffraction, using a Philips X’pert pro diffractometer with Cu Kα radiation.
Field emission scanning electron microscopy
The morphology of the samples was characterized using a Zeiss Supra 55VP field emission scanning electron microscope (FESEM) equipped with a Gemini column. To limit the effects of charge, a low voltage (3 kV) was used. Pictures were obtained with the secondary in-lens electron detector. Samples were observed on a flat view or with a various tilt angle.
Photodegradation of AO7
Photodegradation of AO7 was used as a probe to assess the photoactivity of the TiO2 layers. Photocatalytic experiments were conducted using an aqueous solution of AO7 (also known as Orange II; Acros Organics, Geel, Belgium) at a concentration of 5.0×10−5 mol/L, placed in a cylindrical glass reactor equipped with a magnetic stirrer. The glass reactor was irradiated with a polychromatic fluorescent ultraviolet lamp (Philips TDL 8 W; total optical power, 1.3 W), in a configuration providing about 0.35 mW/cm2 at the sample surface. The photodegradation kinetics were recorded (in triplicate) by assaying the AO7 solution exposed to different ultraviolet irradiation times using a PerkinElmer Lambda 35 ultraviolet spectrophotometer. Quartz glass cells with an optical pathway of 1 cm were used. Deionized water was taken as the reference. After photodegradation of the dye, we monitored the decrease in absorbance of the solution at 483 nm (strong absorption band of AO7).
Mechanical tests
The mechanical tests were performed by an orthopedic surgeon, and consisted of screwing and unscrewing a coated pin in a fresh cow femur, precisely as it would be done in a human patient (with a dedicated surgical ancillary) when making a unilateral external fixation. The self-drilling and self-tapping pin was introduced using a T-handle with the chuck tightened at maximum strength. The pin was screwed into the bone to a depth of about 1 cm after threading. A tube was then connected to the pin with connecting rods. After these tests, the coating behavior on the pin was investigated by FESEM.
Bacterial culture
The bacterial strains used were S. aureus (ATCC 6538) and a clinical multiresistant strain of S. epidermidis (isolated in our university hospital). The strains were cultured for 18 hours in trypticase soy medium. Next, 50 μL of each inoculum with a concentration of 4×108 colony forming units (CFU)/mL were placed on each coated or uncoated disk, previously sterilized by autoclaving. The sample was incubated for 18 hours at room temperature in a dry atmosphere. Each disk with its bacterial load was then exposed to ultraviolet light. Irradiation was provided by a Philips polychromatic fluorescent ultraviolet lamp (Cleo Performance) with a total power of 40 W, in a configuration delivering about 5 mW/cm2 at the surface for 15, 30, 45, 60, and 90 minutes of single exposure. The viable bacteria count on the disks was measured after disintegration of the biofilm by sonication in 1 mL of saline solution. The number of CFU in the suspension was then determined after dilution and plating on trypticase soy agar, incubated at 37°C for 18 hours. Non-viable bacteria on the coated disks were counted after 90 minutes of ultraviolet irradiation. All tests were performed in triplicate.
Statistical analysis
Bacteriological data were subjected to the statistical analyses described below. As usual, bacterial concentrations were log-transformed to avoid the extreme skewness effect of the original concentration. The TiO2 coating and ultraviolet exposure were defined as binary variables. Bacterial concentrations were determined at six time points, ie, 0, 15, 30, 45, 60, and 90 minutes. For each observation, bacterial concentrations were counted on three different disks with a double reading. The reliability of the two bacterial concentration readings and the three disk counts was almost perfect, with all intraclass correlation coefficients being above 0.9. The effects of TiO2 coating, duration of ultraviolet exposure, and the staphylococcus strain used were tested in a generalized linear model, using bacterial concentration as the response variable, which included some first-order interactions, notably between the TiO2 coating and ultraviolet exposure and between time variable and each of the aforementioned factors. Finally, a second-order interaction was evaluated between TiO2 coating, ultraviolet exposure and time. On condition of significant effect, details were provided, performing Tukey’s post hoc multiple comparisons procedure. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA) and conducted setting the type I error rate (P) at 0.05.
Results
X-ray analysis
The X-ray characterizations of the TiO2 layers on the quartz substrate before and after annealing at 500°C in air for 1 hour are presented in Figure 1. The non-annealed TiO2 layer was amorphous. In order to convert the TiO2 layer to a crystalline phase, the sample was annealed in air at 500°C, using a ramp rate of 5°C per minute. We observed the characteristic line of anatase (1 0 1). Crystallite size was calculated using the Scherrer formula:
where λ is the wavelength of the CuKα1 line, θ is the Bragg diffraction angle, and β is the full-width at half maximum of a peak. We calculated the crystallite size by using the full-width at half maximum of the anatase (1 0 1). The average crystal size was about 64 nm.
Figure 1.
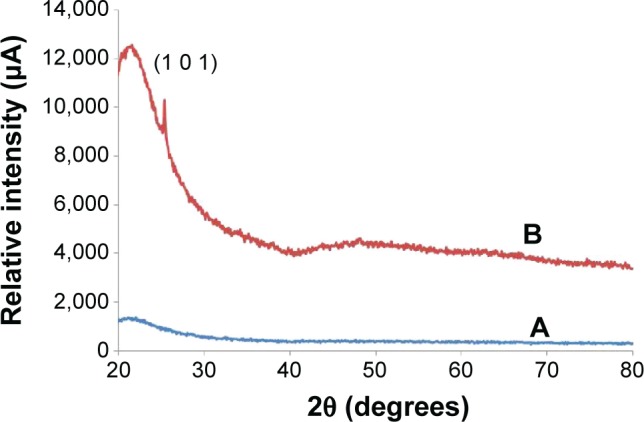
X-ray diffraction patterns of TiO2 layers on quartz substrate.
Notes: (A) As grown and (B) annealed at 500°C.
Sample surface morphology
Initial surface images of disk and pin fixator
FESEM images of the iron disk and pin fixator are presented in Figure 2. The surface of both samples was rough and scratched. There was no marked difference between the initial surface of the disk and pin fixer.
Figure 2.
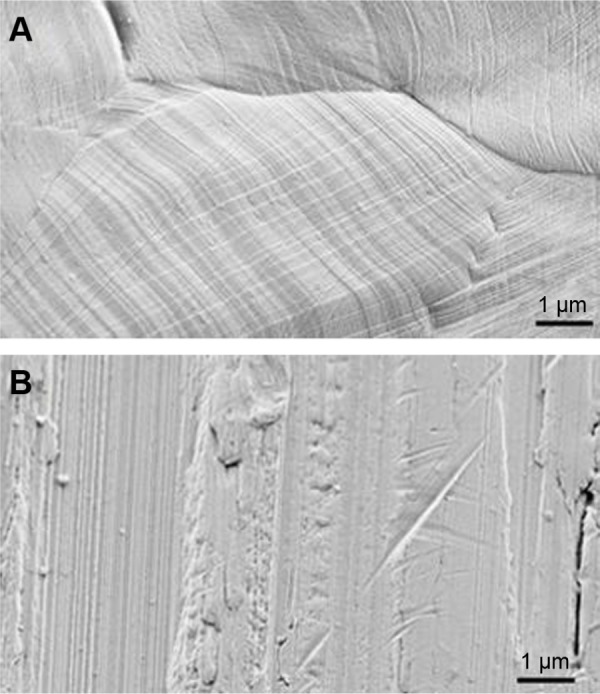
Field emission scanning electron micrographs of iron disk (A) and pin fixator (B).
Surface images of coated disk and pin fixator
The sol formula, the dip-coating process parameters, and the annealing treatment allowed full coverage of the substrates for both the iron disk and pin fixator. Some scratches were detected on the layer (Figure 3). These punctual defects did not affect the overall strength of the cover layer.
Figure 3.
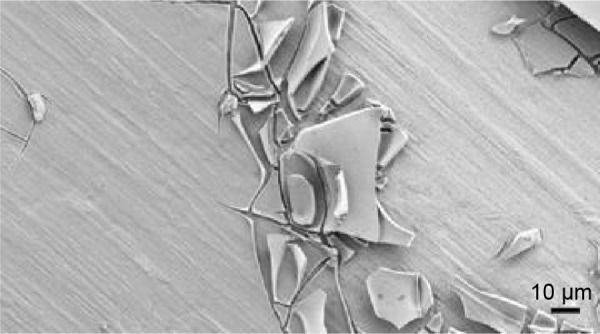
Field emission scanning electron micrograph of a punctual scratch on a cover layer.
The thickness of the oxide layer ranged between 300 and 500 nm (measured by FESEM) depending on the nature of the substrates. Using high magnification at the nanoscale, we highlighted the grain morphology of the layer (Figure 4).
Figure 4.
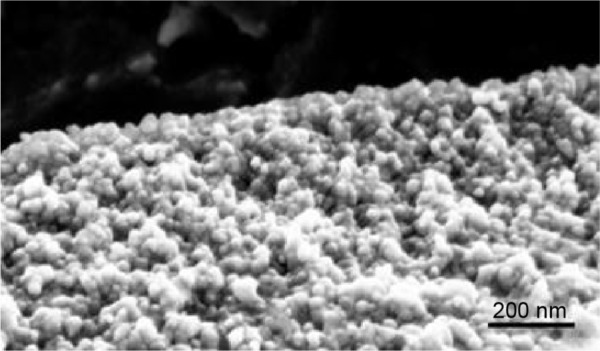
Field emission scanning electron micrograph showing the granular structure of the oxide layer at nanoscale.
Mechanical testing
Unilateral external fixation was performed on a fresh cow femur (Figure 5). Mechanical experiments were done in “extreme conditions” rather than classical clinical practice. There was no detectable change in the mechanical properties of the covered pin when compared to the uncovered pin. Thus, resistance of this layer to scratching would be preserved in the clinical setting.
Figure 5.
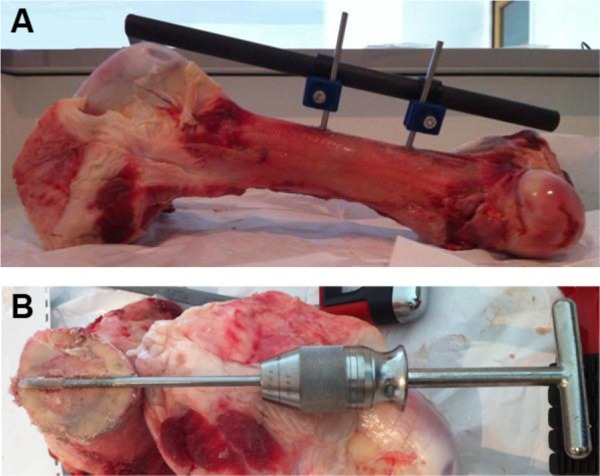
Illustrations of the mechanical test performed.
Notes: (A) Image of a unilateral fixator assembly performed on a cow femur. (B) Visualization of the insertion of the pin into the bone on a horizontal section.
An FESEM image showing the contact area after tightening of the pin with a T-handle and chuck used for the screwing and unscrewing experiments is given in Figure 6. Damaged areas on the coating can be seen, consisting of scratches and crunches in the area of contact between the grip and the coated pin.
Figure 6.
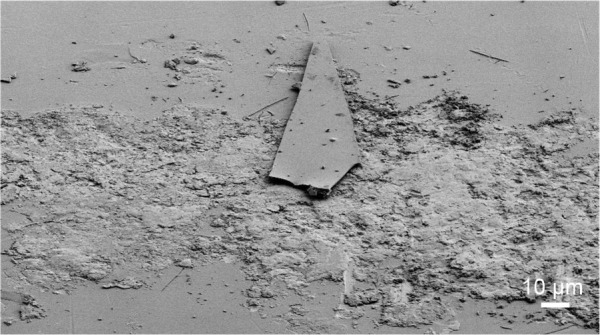
Field emission scanning electron micrograph of the contact area between the grip and the coated pin.
Concerning the area of contact between the coated pin and bone, simulating overscrewing, we observed a crushing effect on the layer in the area of contact, resulting in some scratches at the interface (Figure 7).
Figure 7.

Field emission scanning electron micrograph of the contact area between bone and the coated pin after explantation.
Kinetics of photodegradation
AO7, which contains an azo bond, is a model molecule commonly used in photocatalytic tests to simulate azo dyes in wastewater pollutants coming from industry.33 We did not find significant adsorption of the dye in the first step of the experiment, ie, immersion of the coated sample in AO7 solution under stirring in the dark. However, under ultraviolet irradiation, we observed a gradual discoloration of the solution, consistent with the decrease in absorbance recorded versus irradiation time.
Figure 8 shows the photodegradation kinetics of the organic dye versus irradiation time. After 8 hours of cumulative ultraviolet irradiation, about 64% of the initial AO7 molecular species was degraded. This result confirmed the photoactivity of the coated sample, which was the consequence of crystallization of the TiO2 layer during annealing. The anatase phase, previously detected in the X-ray experiments, was responsible for induction of ultraviolet-assisted degradation of the molecular dye species.
Figure 8.
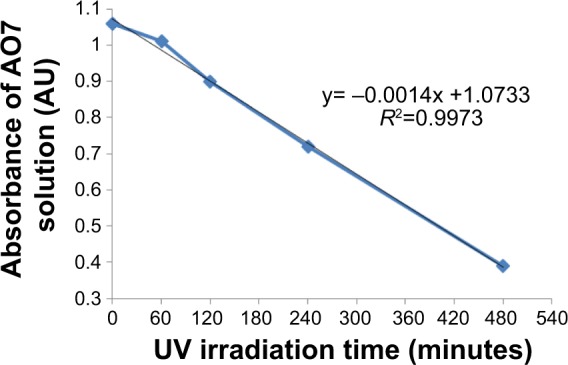
Photodegradation of AO7 solution in the presence of TiO2-coated disk versus UV irradiation time, as measured by the solution’s absorbance at λ =483 nm.
Abbreviations: AO7, acid orange 7; AU, absorbance units; UV, ultraviolet.
Antibacterial effect
The inactivation kinetics for the two bacterial strains were found to be similar overall, and are presented in Figure 9 (S. aureus) and Figure 10 (S. epidermidis). For this reason, the results are analyzed and interpreted together. After incubation (for 18 hours), the inoculum concentration was recorded to be about 106 CFU/mL for S. aureus, but was significantly (P<0.001) higher for S. epidermidis (about 107 CFU/mL), without any significant difference between the coated and uncoated disks (P=0.979). Concerning disks kept in the dark (control group), no significant changes (P=0.981) in bacterial concentration were noted throughout the procedure.
Figure 9.
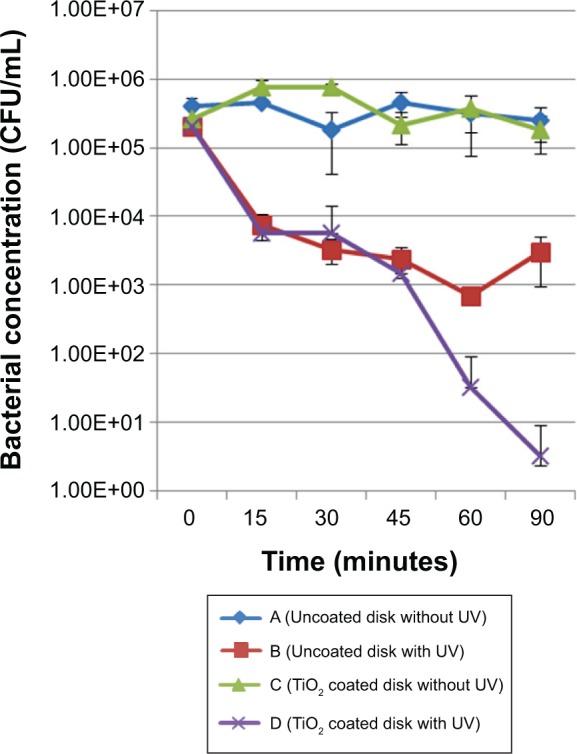
Inactivation kinetics for the Staphylococcus aureus strain. Comparison between coated and uncoated samples submitted to the same UV treatment duration.
Abbreviations: CFU, colony forming units; UV, ultraviolet.
Figure 10.
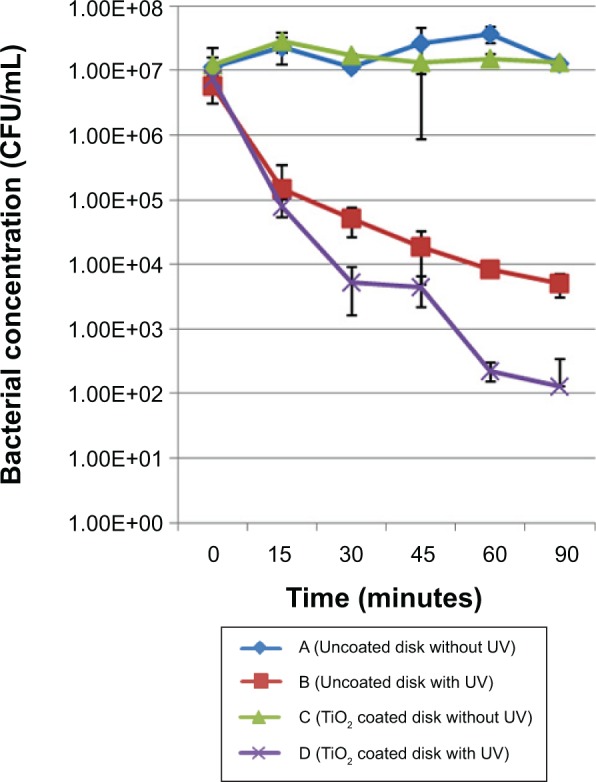
Inactivation kinetics for the Staphylococcus epidermidis strain. Comparison between coated and uncoated samples subjected to the same UV treatment duration.
Abbreviations: CFU, colony forming units; UV, ultraviolet.
After 15 minutes of ultraviolet irradiation, we noticed a significant decrease (P<0.003) in bacterial concentration (about 2 log CFU/mL) for both the coated and uncoated disks. Until the end of the experiment, ultraviolet irradiation alone proved to have no more effect on bacterial concentration (“uncoated disk with ultraviolet” group); however, after 60 minutes of ultraviolet irradiation, an additional significant decrease (P=0.0014) was noticed with the TiO2-coated disks. This observation was confirmed and amplified after 90 minutes of ultraviolet irradiation (P=0.001; about 2 log CFU/mL).
Our results indicate a rapid and lethal effect of ultraviolet light on bacteria during the first step of the experiment. Importantly, they show a further cumulative detrimental effect of the photoactive TiO2 coating and ultraviolet irradiation on survival of bacteria. The role of TiO2 is to promote and accelerate inactivation of bacteria at lower levels of ultraviolet exposure.
Discussion
The use of TiO2 coating on orthopedic implants has been well researched.34,35 However, in this study, we used sol-gel and dip-coating processes for coating rather than the plasma ion implantation techniques used in other studies. Our study entailed application of a TiO2 coating on a specific stainless steel (FeCrNiMo18-15-3; ~ AISI 316 low carbon vacuum melt), which is used commercially to create the pin. This technique has the advantages of being simple and cost-effective for production on an industrial scale. The novelty of our work is that mechanical tests were done to assess the feasibility of including this layer in real-life conditions, which has rarely been included in other studies. Finally, we found comparable results for bacterial inactivation, including resistant nosocomial strains that are problematic in everyday practice.
Our hypothesis was that the photoactive properties of TiO2 could be used to prevent pin tract infection, and our study results show that the TiO2 coating had bactericidal effects relevant to current problematic strains. Thus, we are able to confirm the feasibility of using this new coating for external fixator pins. However, a lot of additional work will have to be done to be able to transfer our findings to clinical practice.
TiO2 seems to be safe or at least have controllable side effects, with the only potential risk being diffusion of free TiO2 molecules within the body.36–39 However, in this case, free TiO2 particles cannot diffuse into the body because the pin thread is not coated. There is no contact between the coating and the patient’s tissues. However, toxicity studies (eg, fibroblast cultures in presence of the substrate) are needed to confirm the safety of this technique. Ultraviolet irradiation is limited to the TiO2 coating (ie, the patient’s skin would not be exposed to ultraviolet rays). Exposure of patients to ultraviolet irradiation is indeed the key issue, and should be kept to a minimum. In our opinion, chemical and physical protection (eg, a custom-made cover applied all around the external fixator) would be necessary to avoid the adverse effects of ultraviolet irradiation, principally on the skin.
Sol-gel and dip-coating processes were used to create the coating because they are simple and cost-effective. The FESEM analysis of morphology showed full coverage of the substrate, with two superimposed layers showing cracks and scales. Mechanical tests confirmed the limitations of the under extreme conditions; nevertheless, the deeper layer was almost intact. To prevent this weakness, optimization of the layer could entail exchanging the sol formulation for a hardening element like zirconium, or using a modified annealing protocol.
Photodegradation of an AO7 solution confirmed the photocatalytic activity of the coating. However, the reaction was slow, with a 64% decrease in absorbance after 8 hours of cumulative ultraviolet irradiation. This was possibly due to the low power (0.35 mW/cm2) and the nonspecific spectrum (350–400 nm) of the ultraviolet lamp. Although our experimental conditions are different, our results are similar to those found in the literature.40,41
The bacterial parameters were chosen to simulate pin tract infection involving the most common causative organism (S. aureus)13 and the most common source of hospital-acquired infection (multiresistant S. epidermidis).42 The conditions chosen (ie, concentration, trypticase soy medium, incubation phase) allowed us to carry out bacterial survival and monitor development of biofilm.
The concentration of S. epidermidis was significantly higher (P<0.001) than that of S. aureus after the incubation phase, probably because of the greater ability of S. epidermidis to colonize biomaterial and produce biofilm.43,44 There was no significant difference (P=0.979) in this regard between the uncoated and coated disks.
After 15 minutes of ultraviolet irradiation, the concentrations of S. aureus and S. epidermidis decreased significantly (P<0.003) in every group exposed to UV irradiation, indicating the lethal DNA damage produced by ultraviolet irradiation in cells.45,46 However, it should be noted that our ultraviolet lamp power (5 mW/cm2) was within the range commonly found in the literature (0.5–20 mW/cm2).47 The duration of ultraviolet exposure is a real problem. Such long exposure is not compatible with everyday practice. We are attempting to dope the titanium sol with a metallic compound in order to expand the layer absorbance spectrum to an ultraviolet-visible wavelength. Another way to improve antibacterial efficiency is to work on the crystallinity of the layer.
We found a bactericidal effect due to the photoactive TiO2 coating after 1 hour of ultraviolet irradiation. This was indicated by a significant 5 log decrease in bacterial concentration for the two strains at the end of the test (P<0.001). Complete bacterial inactivation was not achieved, probably because of the high initial bacterial concentration (4×108 CFU/mL) chosen for the study. Our results confirm that this process is also efficient for multiresistant bacteria,19,48–50 with only small differences between the two tested strains.
A number of facts could account for the delayed effect:
The incubation phase enhanced the formation of biofilm, and in that state bacteria are less sensitive to reactive oxygen species.51
The trypticase soy medium that promotes staphylococcus survival and inhibits bactericidal TiO2 photoactive process by competitive interaction between free radical and organic materials, by absorption of ultraviolet radiation, and by decreasing the amount of direct contact between bacteria and TiO2 molecules.36,52–55
The high initial bacterial concentration (4×108 CFU/mL) may decrease the catalytic effect56,57 because agglomeration of numerous bacterial cells with TiO2 particles takes place.
The tested bacterial strains were Gram-positive bacteria, which are known to be more resistant to the photoactive effect of TiO2 than Gram-negative bacteria, due to their thick and complex cell wall.58,59
Inadequate photocatalytic properties of our TiO2 photoactive coating in regard to degradation tests using AO7 solution.
Conclusion
Our study enabled us to develop an antibacterial coating for stainless steel commonly used in surgical practice. The process using photoactive TiO2 exposed to ultraviolet irradiation is actually well known and applied widely in disinfection procedures. Our model was effective against the two main bacterial strains involved in pin tract infections. Mechanical tests confirmed the ability of the coating to resist important stresses. Moreover, this type of coating is created by sol-gel dip-coating techniques that are not expensive and easily scalable for industrial application. We hope that this new option could prevent pin tract infection, even if heavy optimization work is yet to be done in order to amplify its bactericidal properties. The future research plan for this project is to improve the mechanical properties of the coating (eg, by adding of zirconium alkoxide) and to increase the bactericidal properties with adjunction of metallic ions (TiO2 layer dopping) in order to expand the layer absorbance spectrum to an ultraviolet-visible wavelength. Work will then be done on the annealing parameters in order to create a crack-free layer with suitable crystallinity to ensure better antibacterial efficiency. Finally, if the results are positive, in vivo tests would be envisaged.
Acknowledgments
The authors thank Joel Cellier for the X-ray diffraction measurements and Lemlih Ouchchane for the statistical analysis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Quintin J, Schuind F. Plus de trente ans d’utilisation du fixateur externe de Hoffmann® en Orthopédie-Traumatologie pédiatrique [Over thirty years of use of Hoffmann® external fixator in pediatric orthopedics-traumatology] Rev Med Brux Nouv Ser. 2011;32(6):S46. French. [PubMed] [Google Scholar]
- 2.Schuind F. Technique de pose d’un fixateur externe unilatéral des membres [Technique for setting a unilateral external fixator members] Techniques Chirurgicales Orthopédie-Traumatologie. 2012;7(4):1–9. French. [Google Scholar]
- 3.Dell’Oca AF. External fixation. In: Ruedi TP, Murphy WM, editors. AO Principles of Fracture Management. Stuttgart, Germany: Georg Thieme Verlag; 2000. [Google Scholar]
- 4.Vincent JL. Nosocomial infections in adult intensive-care units. Lancet. 2003;361(9374):2068–2077. doi: 10.1016/S0140-6736(03)13644-6. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 6.Jennison T, McNally M, Pandit H. Prevention of infection in external fixator pin sites. Acta Biomater. 2014;10(2):595–603. doi: 10.1016/j.actbio.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Koseki H, Shida, Yoda, et al. Clinical and histomorphometrical study on titanium dioxide-coated external fixation pins. Int J Nanomedicine. 2013;8:593–599. doi: 10.2147/IJN.S39201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green SA. Complications of external skeletal fixation. Clin Orthop. 1983;(180):109–116. [PubMed] [Google Scholar]
- 9.Parameswaran AD, Roberts CS, Seligson D, Voor M. Pin tract infection with contemporary external fixation: how much of a problem? J Orthop Trauma. 2003;17(7):503–507. doi: 10.1097/00005131-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira N, Marais LC. Prevention and management of external fixator pin track sepsis. Strateg Trauma Limb Reconstr. 2012;7(2):67–72. doi: 10.1007/s11751-012-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 12.Checketts RG, MacEachem AG, Otterburn M. Pin track infection and the principles of pin site care. In: Bastiani GA, Apley G, Goldberg A, editors. Orthofix External Fixation in Trauma and Orthopaedics. London, UK: Springer; 2000. [Google Scholar]
- 13.Mahan J, Seligson D, Henry SL, Hynes P, Dobbins J. Factors in pin tract infections. Orthopedics. 1991;14(3):305–308. [PubMed] [Google Scholar]
- 14.Bajpai I, Basu B. Strategies to prevent bacterial adhesion on biomaterials. In: Ramalingam M, Wang X, Chen G, Ma P, Cui FZ, editors. Biomimetics: Advancing Nanobiomaterials and Tissue Engineering. Hoboken, NJ, USA: John Wiley & Sons Inc; 2013. [Google Scholar]
- 15.Matsunaga T, Tomoda R, Nakajima T, Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29(1–2):211–214. [Google Scholar]
- 16.Belapurkar AD, Sherkhane P, Kale SP. Disinfection of drinking water using photocatalytic technique. Curr Sci. 91(1):73–76. [Google Scholar]
- 17.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 18.Fretwell R, Douglas P. An active, robust and transparent nanocrystalline anatase TiO2 thin film preparation, characterisation and the kinetics of photodegradation of model pollutants. J Photochem Photobiol Chem. 2001;143(2):229–240. [Google Scholar]
- 19.Kangwansupamonkon W, Lauruengtana V, Surassmo S, Ruktanonchai U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomedicine. 2009;5(2):240–249. doi: 10.1016/j.nano.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Sunada K, Watanabe T, Hashimoto KJ. Studies on photokilling of bacteria on TiO2 thin film. J Photochem Photobiol A. 2003;156:227–233. [Google Scholar]
- 21.Blake DM, Maness PC, Huang Z, Wolfrum EJ, Huang J. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep Purif Methods. 1999;28(1):1–50. [Google Scholar]
- 22.Nonami T, Hase H, Funakoshi K. Apatite-coated titanium dioxide photocatalyst for air purification. Catal Today. 2004;96(3):113–118. [Google Scholar]
- 23.Sunda K, Kikuchi Y, Hashimoto K, Fujishima A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ Sci Technol. 1998;32:726–728. [Google Scholar]
- 24.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C. 2000;1(1):1–21. [Google Scholar]
- 25.Agarwal A, Weis TL, Schurr MJ, et al. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31(4):680–690. doi: 10.1016/j.biomaterials.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasilev K, Sah V, Anselme K, et al. Tunable antibacterial coatings that support mammalian cell growth. Nano Lett. 2010;10(1):202–207. doi: 10.1021/nl903274q. [DOI] [PubMed] [Google Scholar]
- 27.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Liu X, Cao C, Dong Y, Ding C. Biological and antibacterial properties of plasma sprayed wollastonite/silver coatings. J Biomed Mater Res B Appl Biomater. 2009;91(2):596–603. doi: 10.1002/jbm.b.31434. [DOI] [PubMed] [Google Scholar]
- 29.Necula BS, Fratila-Apachitei LE, Zaat SAJ, Apachitei I, Duszczyk J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009;5(9):3573–3580. doi: 10.1016/j.actbio.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Asharani PV, Lian Wu Y, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19(25):255102. doi: 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- 32.Park EJ, Yi J, Kim Y, Choi K, Park K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol In Vitro. 2010;24(3):872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol. 2001;56(1–2):69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- 34.Koseki H, Asahara T, Shida T, et al. Clinical and histomorphometrical study on titanium dioxide-coated external fixation pins. Int J Nanomedicine. 2013;8:593–599. doi: 10.2147/IJN.S39201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haenle M, Fritsche A, Zietz C, et al. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants In vitro effectiveness against MRSA and mechanical properties. J Mater Sci Mater Med. 2011;22(2):381–387. doi: 10.1007/s10856-010-4204-4. [DOI] [PubMed] [Google Scholar]
- 36.Agence Française de Sécurité Sanitaire des Produits de Santé French Agency for the Safety of Health Products AFSSAPS. Titanium dioxide nanoparticles of zinc oxide in cosmetics: state of knowledge on skin penetration, genotoxicity and carcinogenesis. Paris, France: National Agency for the Safety of Medicines and Health Products. 2011. [Accessed: June 1, 2014]. Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/af86f9684f0e2810a7cf1d5b0cefb0d5.pdf. French.
- 37.Sayes CM, Wahi R, Kurian PA, et al. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92(1):174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 38.Yanagisawa R, Takano H, Inoue K, et al. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Biol Med. 2009;234(3):314–322. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 39.Landsiedel R, Ma-Hock L, Kroll A, et al. Testing metal-oxide nanomaterials for human safety. Adv Mater. 2010;22(24):2601–2627. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- 40.Hajkova P, Spatenka P, Horsky J, Horska I, Kolouch A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process Polym. 2007;4(S1):S397–S401. [Google Scholar]
- 41.Shiraish K, Koseki H, Tsurumoto T, et al. Antibacterial metal implant with a TiO2 – conferred photocatalytic bactericidal effect against Staphylococcus aureus. Surf Interface Anal. 2009;41(1):17–22. [Google Scholar]
- 42.Schoenfelder SM, Lange C, Eckart M, Hennig S, Kozytska S, Ziebuhr W. Success through diversity – how Staphylococcus epidermidis establishes as a nosocomial pathogen. Int J Med Microbiol. 2010;300(6):380–386. doi: 10.1016/j.ijmm.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Katsikogianni, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 44.Rupp ME, Ulphani JS, Fey PD, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67(5):2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagger J. Near-UV radiation effects on microorganisms. Photochem Photobiol. 1981;34(6):761–768. [PubMed] [Google Scholar]
- 46.Markowska-Szczupak A, Ulfig K, Morawski AW. The application of titanium dioxide for deactivation of bioparticulates: an overview. Catal Today. 2011;169(1):249–257. [Google Scholar]
- 47.Žvab U, Lavrenčič Štangar U, Bergant Marušič M. Methodologies for the analysis of antimicrobial effects of immobilized photocatalytic materials. Appl Microbiol Biotechnol. 2014;98(5):1925–1936. doi: 10.1007/s00253-013-5464-y. [DOI] [PubMed] [Google Scholar]
- 48.Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011;6(8):933–940. doi: 10.2217/fmb.11.78. [DOI] [PubMed] [Google Scholar]
- 49.Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65(13–14):1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Tsai TM, Chang HH, Chang KC, Liu YL, Tseng CC. A comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J Chem Technol Biotechnol. 2010;85(12):1642–1653. [Google Scholar]
- 51.Polo A, Diamanti MV, Bjarnsholt T, et al. Effects of photoactivated titanium dioxide nanopowders and coating on planktonic and biofilm growth of Pseudomonas aeruginosa. Photochem Photobiol. 2011;87(6):1387–1394. doi: 10.1111/j.1751-1097.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 52.Rincon A. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: implications in solar water disinfection. Appl Catal B Environ. 2004;51(4):283–302. [Google Scholar]
- 53.Alrousan DMA, Dunlop PSM, McMurray TA, Byrne JA. Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res. 2009;43(1):47–54. doi: 10.1016/j.watres.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Foster HA, Sheel DW, Evans P, et al. Antimicrobial activity against hospital-related pathogens of dual layer CuO/TiO2 coatings prepared by CVD. Chemical Vapor Deposition. 2012;18(4–6):140–146. [Google Scholar]
- 55.Nir Baram DS. Photocatalytic inactivation of microorganisms using nanotubular TiO2. Appl Catal B Environ. 2011;101:212–219. [Google Scholar]
- 56.Ireland JC, Klostermann P, Rice EW, Clark RM. Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl Environ Microbiol. 1993;59(5):1668–1670. doi: 10.1128/aem.59.5.1668-1670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65(9):4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Z, Maness PC, Blake DM, Wolfrum EJ, Smolinski SL, Jacoby WA. Bactericidal mode of titanium dioxide photocatalysis. J Photochem Photobiol Chem. 2000;130(2):163–170. [Google Scholar]
- 59.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34(4):415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]


