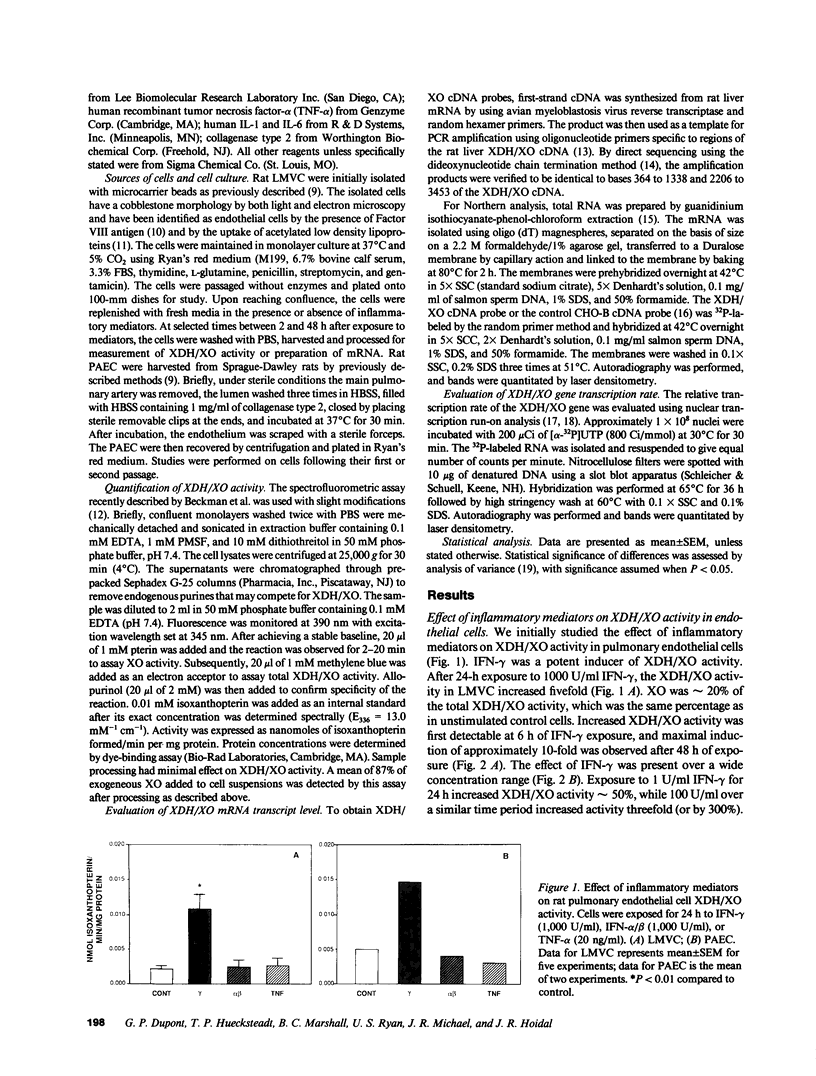
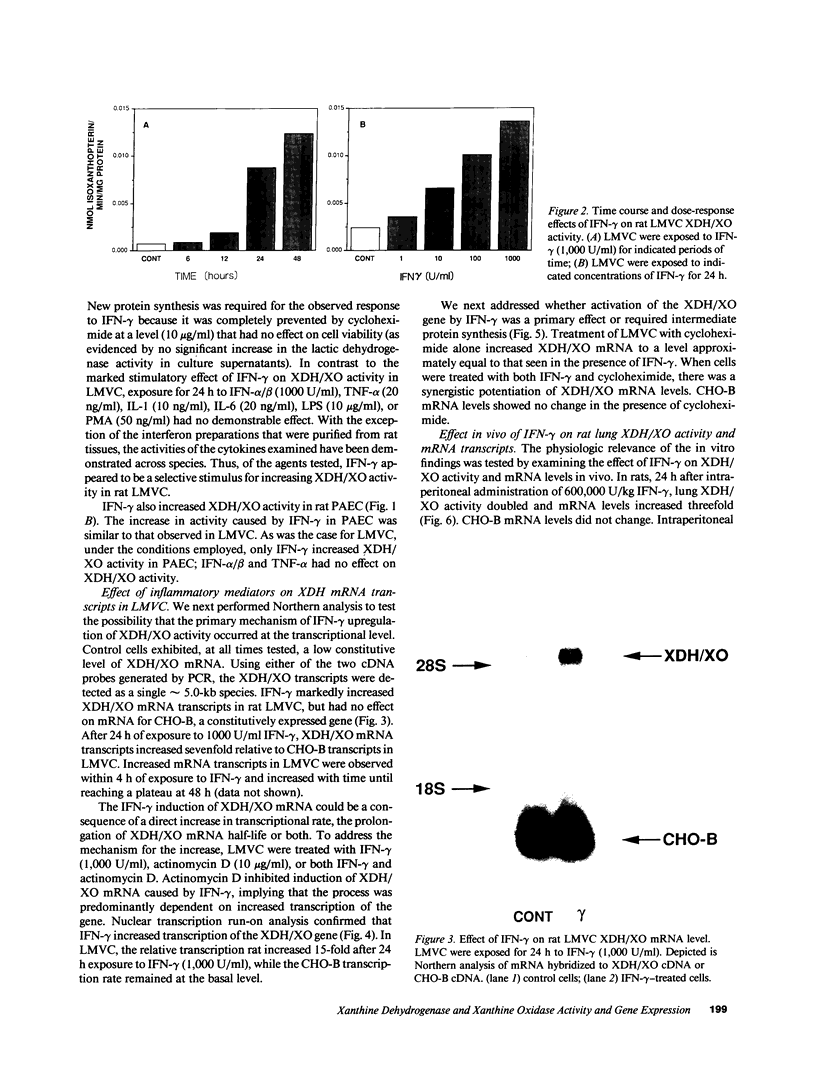
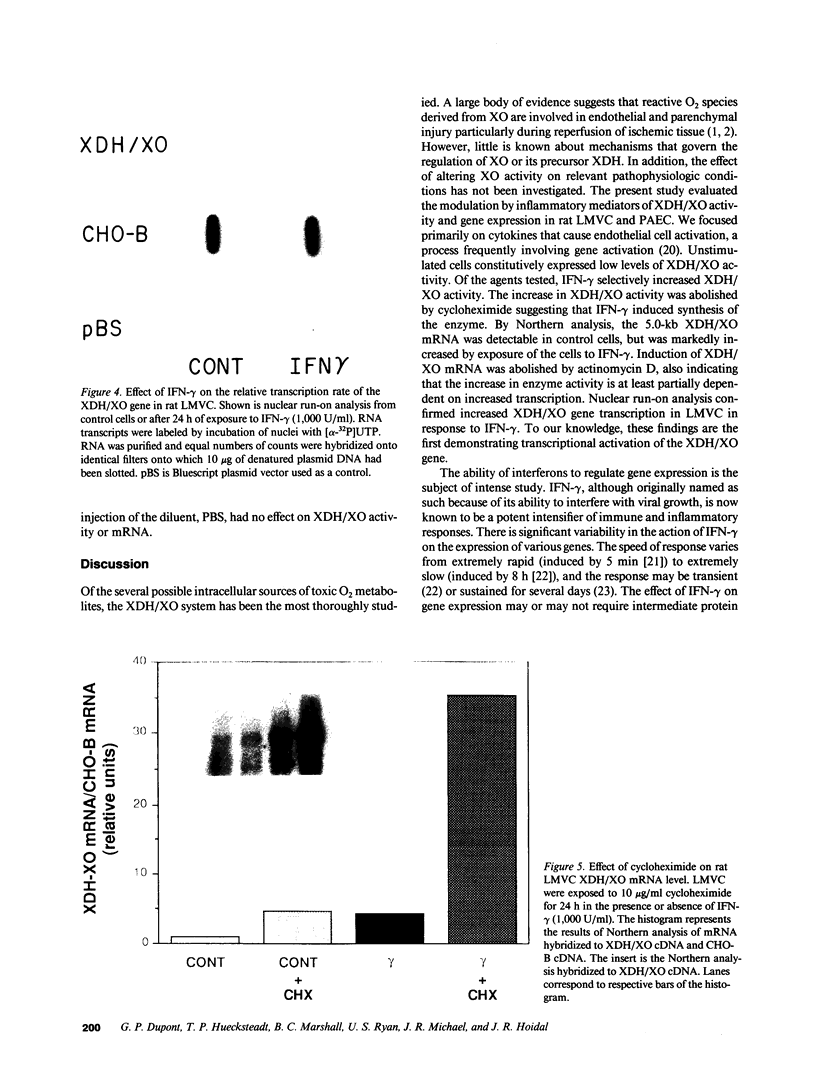
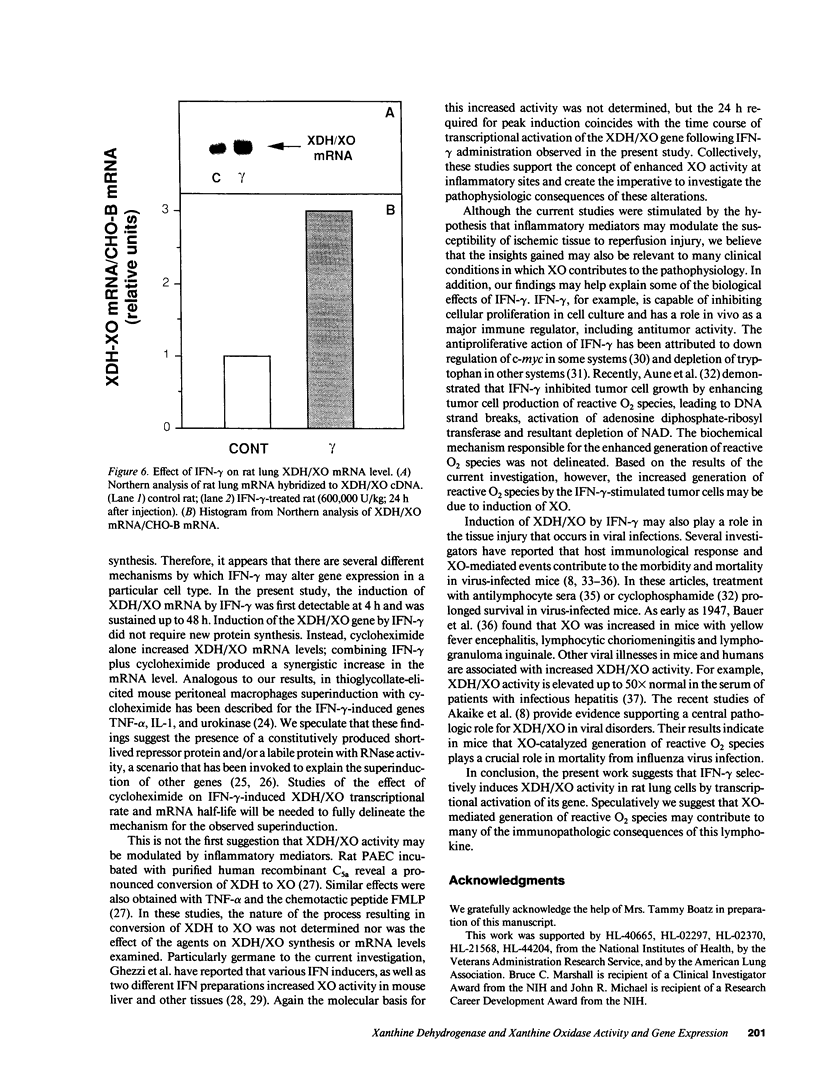
Abstract
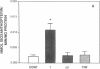
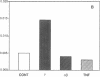
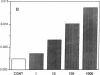
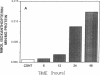
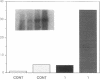
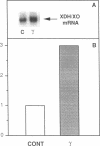
The central importance of xanthine dehydrogenase (XDH) and xanthine oxidase (XO) in the pathobiochemistry of a number of clinical disorders underscores the need for a comprehensive understanding of the regulation of their expression. This study was undertaken to examine the effects of cytokines on XDH/XO activity and gene expression in pulmonary endothelial cells. The results indicate that IFN-gamma is a potent inducer of XDH/XO activity in rat lung endothelial cells derived from both the microvasculature (LMVC) and the pulmonary artery. In contrast, interferon-alpha/beta, tumor necrosis factor-alpha, interleukin-1 or -6, lipopolysaccharide and phorbol myristate acetate have no demonstrable effect. The increase in XDH/XO activity requires new protein synthesis. By Northern analysis, IFN-gamma markedly increases the level of the 5.0-kb XDH/XO mRNA in LMVC. The increase is due, in part, to increased transcription rate of the XDH/XO gene. Transcriptional activation does not require new protein synthesis. The physiologic relevance of these observations was evaluated by administering IFN-gamma to rats. Intraperitoneal administration leads to an increased XDH/XO activity and XDH/XO mRNA level in rat lungs. In sum, IFN-gamma is a potent and biologically relevant inducer of XDH/XO expression; the major site of upregulation occurs at the transcriptional level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya Y., Yamazaki K., Sato M., Noda K., Nishino T., Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990 Aug 25;265(24):14170–14175. [PubMed] [Google Scholar]
- Athar M., Elmets C. A., Bickers D. R., Mukhtar H. A novel mechanism for the generation of superoxide anions in hematoporphyrin derivative-mediated cutaneous photosensitization. Activation of the xanthine oxidase pathway. J Clin Invest. 1989 Apr;83(4):1137–1143. doi: 10.1172/JCI113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune T. M., Pogue S. L. Inhibition of tumor cell growth by interferon-gamma is mediated by two distinct mechanisms dependent upon oxygen tension: induction of tryptophan degradation and depletion of intracellular nicotinamide adenine dinucleotide. J Clin Invest. 1989 Sep;84(3):863–875. doi: 10.1172/JCI114247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Parks D. A., Pearson J. D., Marshall P. A., Freeman B. A. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med. 1989;6(6):607–615. doi: 10.1016/0891-5849(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Boettger E. C., Flavell R. A. Transcriptional activation of HLA-DR alpha by interferon gamma requires a trans-acting protein. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4672–4676. doi: 10.1073/pnas.85.13.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda D., Németh I., Hencz P., Dénes K. Effect of allopurinol treatment in premature infants with idiopathic respiratory distress syndrome. Dev Pharmacol Ther. 1984;7(6):357–367. doi: 10.1159/000457187. [DOI] [PubMed] [Google Scholar]
- Caplen H. S., Gupta S. L. Differential regulation of a cellular gene by human interferon-gamma and interferon-alpha. J Biol Chem. 1988 Jan 5;263(1):332–339. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. D., Stark G. R., Bloom B. R. Molecular cloning of a gene selectively induced by gamma interferon from human macrophage cell line U937. Mol Cell Biol. 1989 May;9(5):1922–1928. doi: 10.1128/mcb.9.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Mantovani A., Spreafico F., Salmona M. Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun. 1984 Feb 29;119(1):144–149. doi: 10.1016/0006-291x(84)91630-9. [DOI] [PubMed] [Google Scholar]
- Ghezzi P., Saccardo B., Bianchi M. Induction of xanthine oxidase and heme oxygenase and depression of liver drug metabolism by interferon: a study with different recombinant interferons. J Interferon Res. 1986 Jun;6(3):251–256. doi: 10.1089/jir.1986.6.251. [DOI] [PubMed] [Google Scholar]
- Giler S., Sperling O., Brosh S., Urca I., De Vries A. Serum xanthine oxidase in jaundice. Clin Chim Acta. 1975 Aug 18;63(1):37–40. doi: 10.1016/0009-8981(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988 Dec;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hurd J., Heath R. B. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975 May;11(5):886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Rodell T. C., Cheronis J. C., Ohnemus C. L., Piermattei D. J., Repine J. E. Xanthine oxidase mediates elastase-induced injury to isolated lungs and endothelium. J Appl Physiol (1985) 1987 Nov;63(5):2159–2163. doi: 10.1152/jappl.1987.63.5.2159. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura E., Suzuki F., Ishida N. Characterization of cells infiltrating the lungs of x-irradiated and nude mice after influenza virus infection. Microbiol Immunol. 1982;26(2):129–138. doi: 10.1111/j.1348-0421.1982.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Bovine aortic endothelial cells display macrophage-like properties towards acetylated 125I-labelled low density lipoprotein. Biochim Biophys Acta. 1980 Dec 5;620(3):631–635. doi: 10.1016/0005-2760(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Ward P. H., Günther B., Vivaldi E. Superoxide radical involvement in the pathogenesis of burn shock. Circ Shock. 1984;12(4):229–239. [PubMed] [Google Scholar]
- Visner G. A., Dougall W. C., Wilson J. M., Burr I. A., Nick H. S. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990 Feb 15;265(5):2856–2864. [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M. Dependence of the in vitro antiproliferative activity of recombinant human gamma-interferon on the concentration of tryptophan in culture media. Cancer Res. 1988 Jan 15;48(2):346–350. [PubMed] [Google Scholar]