Abstract
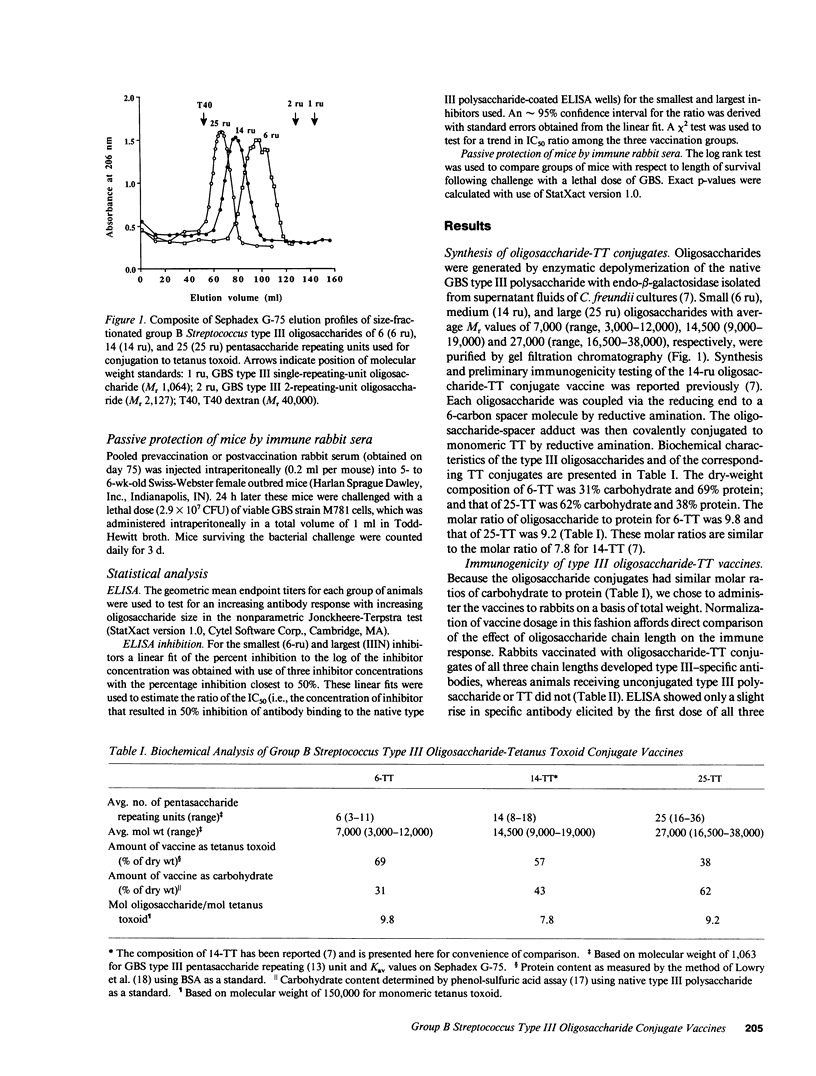
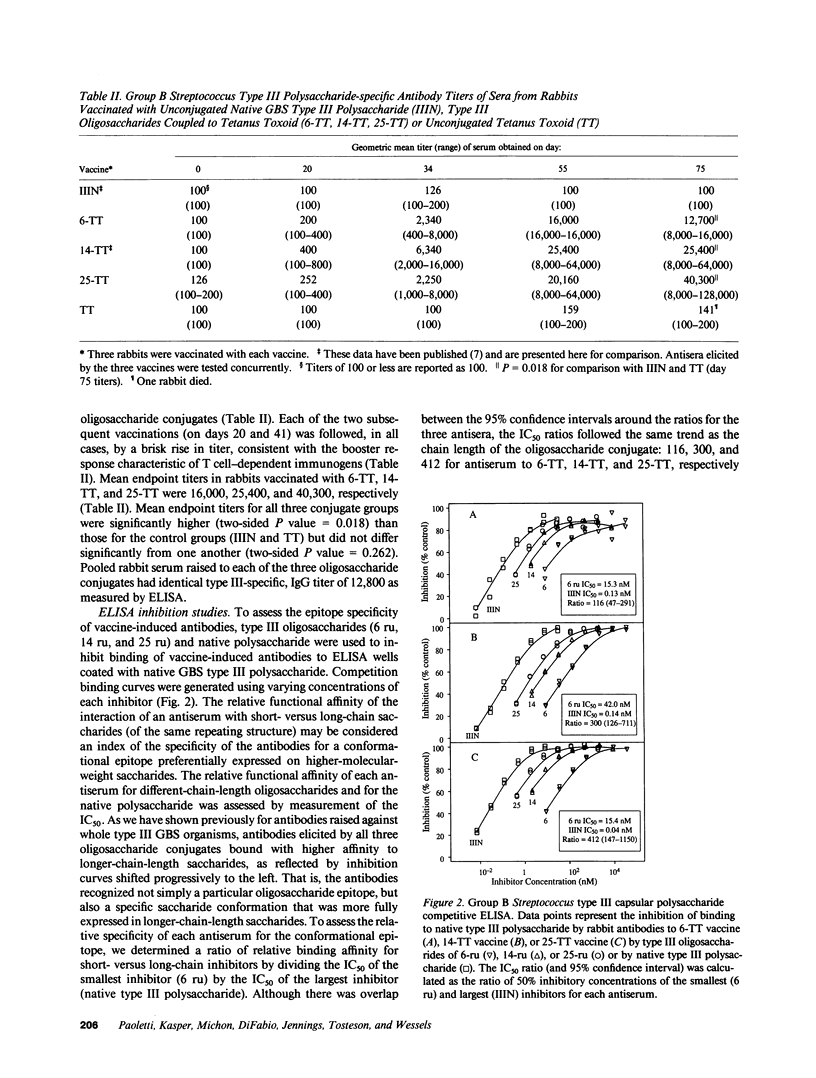
One method to improve the immunogenicity of polysaccharide antigens is the covalent coupling of the native polysaccharide or a derivative oligosaccharide to a carrier protein. In general, T cell-dependent properties are enhanced in conjugates of smaller saccharides, but a conformational epitope of the native polysaccharide may be better expressed in conjugates of larger saccharides. We have reported previously the synthesis and immunogenicity in animals of an oligosaccharide-tetanus toxoid conjugate vaccine against type III group B Streptococcus. In this study, we sought to determine the optimal size of group B Streptococcus type III oligosaccharide for use in a conjugate vaccine by evaluating the relative immunogenicity of conjugate vaccines containing oligosaccharides that were twofold smaller (7,000 Mr) or larger (27,000 Mr) than that reported previously (14,500 Mr). All three type III oligosaccharide conjugate vaccines were immunogenic in rabbits, in contrast to native, uncoupled group B Streptococcus type III polysaccharide. However, with respect to eliciting specific antibodies that were protective in vivo, the vaccine containing the intermediate-size oligosaccharide was superior to the smaller or larger conjugate vaccine. Analysis of opsonic activity of vaccine-induced antibodies demonstrated a predominance of IgG antibodies, thought to reflect T cell dependence, in response to shorter chain length conjugates, while the conformational epitope of the native polysaccharide was maximally expressed on longer chain length conjugates. These opposing trends may account for the optimal immunogenicity of an intermediate-size group B Streptococcus type III oligosaccharide conjugate vaccine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Stein E. C., Porcelli S., Betts R. F., Connuck D. M., Korones D., Insel R. A., Zahradnik J. M., Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol. 1989 Apr 1;142(7):2464–2468. [PubMed] [Google Scholar]
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pichichero M. E., Insel R. A. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Invest. 1985 Jul;76(1):52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Fukuda M. N., Matsumura G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J Biol Chem. 1976 Oct 25;251(20):6218–6225. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Kasper D. L. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochemistry. 1981 Aug 4;20(16):4511–4518. doi: 10.1021/bi00519a001. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985 Apr;134(4):2651–2657. [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Osserman E. F., Gamian A., Michon F., Jennings H. J. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. 1988 Aug 1;168(2):699–711. doi: 10.1084/jem.168.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Michon F., Brisson J. R., Jennings H. J. Conformational differences between linear alpha (2----8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry. 1987 Dec 15;26(25):8399–8405. doi: 10.1021/bi00399a055. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Péterfy F., Outschoorn I. G., Richter A. W., Seppälä I. Immunogenic properties of alpha (1----6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand J Immunol. 1984 Jun;19(6):541–550. doi: 10.1111/j.1365-3083.1984.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Paoletti L. C., Kasper D. L., Michon F., DiFabio J., Holme K., Jennings H. J., Wessels M. R. An oligosaccharide-tetanus toxoid conjugate vaccine against type III group B Streptococcus. J Biol Chem. 1990 Oct 25;265(30):18278–18283. [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä I., Mäkelä O. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989 Aug 15;143(4):1259–1264. [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Mäkelä O., Mattila P., Eskola J., Käyhty H. Human antibody responses to two conjugate vaccines of Haemophilus influenzae type B saccharides and diphtheria toxin. Scand J Immunol. 1988 Oct;28(4):471–479. doi: 10.1111/j.1365-3083.1988.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines. Prog Allergy. 1983;33:120–143. doi: 10.1159/000407424. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wessels M. R., Kasper D. L. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989 Jun 1;169(6):2121–2131. doi: 10.1084/jem.169.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Muñoz A., Kasper D. L. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9170–9174. doi: 10.1073/pnas.84.24.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Paoletti L. C., Kasper D. L., DiFabio J. L., Michon F., Holme K., Jennings H. J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990 Nov;86(5):1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Pozsgay V., Kasper D. L., Jennings H. J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987 Jun 15;262(17):8262–8267. [PubMed] [Google Scholar]
- Wessels M. R., Rubens C. E., Benedí V. J., Kasper D. L. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]



