Abstract
In the present study, the effects of morphine were examined on tests of spatial memory, object exploration, locomotion, and anxiety in male ICR mice. Administration of morphine (15 or 30 mg/kg, intraperitoneally (i.p.)) induced a significant decrease in Y-maze alternations compared to saline vehicle-treated mice. The reduced Y-maze alternations induced by morphine were completely blocked by naloxone (15 mg/kg) or β-funaltrexamine (5 mg/kg) but not by norbinaltorphimine (5 mg/kg) or naltrindole (5 mg/kg), suggesting that the morphine-induced spatial memory impairment was mediated predominantly by μ-opioid receptors (MOPs). Significant spatial memory retrieval impairments were observed in the Morris water maze (MWM) in mice treated with morphine (15 mg/kg) or scopolamine (1 mg/kg), but not with naloxone or morphine plus naloxone. Reduced exploratory time was observed in mice after administration of morphine (15 mg/kg), in a novel-object exploration test, without any changes in locomotor activity. No anxiolytic-like behavior was observed in morphine-treated mice in the elevated plus maze. A significant reduction in buried marbles was observed in morphine-treated mice measured in the marble-burying test, which was blocked by naloxone. These observations suggest that morphine induces impairments in spatial short-term memory and retrieval, and reduces exploratory behavior, but that these effects are not because of overall changes in locomotion or anxiety.
Keywords: morphine, spatial memory, Y-maze task, Morris water maze, μ-opioid receptor
Introduction
Behavioral abnormalities associated with opiate addiction include memory and learning deficits,1–3 which are the result of some combination of acute and chronic actions of opiates. Abused opiate drugs are not specific for particular opioid receptor subtypes, leaving the role of the different opioid receptor subtypes in these impairments a matter of debate. Experiments in animal models of learning and memory functions have also used non-specific opiate agonists for the most part, so that there is little preclinical evidence into the precise opioid mechanisms that may underlie these impairments. Additionally, opiates produce a wide range of behavioral effects, and it is not known to what degree the impairments in cognitive processes that are observed either in human addicts or in animal models are purely mnemonic in origin or result indirectly from other behavioral effects of opiates. Acute or chronic administration of morphine induces a number of other behavioral abnormalities, such as hyperlocomotion,4,5 that may confound interpretation of many cognitive tests in rodents.
In animal models, acute opiate treatment is well known to produce a variety of deficits in tests of cognitive and mnemonic function, although the precise nature of these deficits has been a matter of some dispute (ie, Refs. 6 and 7). These authors suggested that morphine-induced learning impairments in the Morris water maze (MWM) were associated with reduced motivation to escape the maze rather than learning or memory impairments per se. Supporting this notion, morphine produced effects in a water maze discrimination task that were similar to warming the water,8 reductions in swim speed and still time, but not accuracy. On the other hand, another approach has been to use a visible platform version of the task to rule out motor or motivational deficits, and this approach tends to find no effects of morphine on learning or performance (eg, Ref. 9). Studies have consistently found that morphine impairs acquisition of learning in the MWM.9–11 One of these studies involved the daily repositioning of the platform,10 so perhaps addressed working memory more specifically, but another study showed that morphine treatment impairs acquisition of both spatial and working memory versions of the water maze.11 Effects of morphine on memory retrieval have been observed in some,11 but not all,12 studies.
Findings that morphine administration can produce working and spatial memory deficits in the MWM are consistent with observations in tests of spontaneous alternation. A single systemic administration of morphine or a single intracerebro-ventricular injection of DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin; a synthetic opioid peptide with high μ-opioid receptor (MOP) specificity) impairs spontaneous alternation.13,14 The effects of the specific MOP agonist suggest the possibility that morphine may also produce its effects in this model via the MOP receptor, but studies with specific opioid receptor antagonists have yet to confirm this idea.
The purpose of the present experiments was thus twofold: (1) to address the involvement of specific opioid receptors in the cognitive effects of acute morphine treatment and (2) to examine potential behavioral confounds. There is little research addressing behavioral or emotional alterations that may occur along with deficits of cognitive functions and may confound tests of cognitive abilities. To address this question, we first examined the effects of acute morphine on Y-maze and MWM tasks in mice in order to confirm that acute morphine treatment impairs short-term and long-term spatial memory functions. We then examined behavioral and emotional alterations with the same dose of morphine that caused cognitive impairment. The specificity of the effects of morphine on spontaneous alternation for MOP was further evaluated using selective MOP, δ-opioid receptor (DOP), and κ-opioid receptor (KOP) antagonists.
Materials and Methods
Subjects
Male ICR mice (10–11 weeks old; Japan SLC) were housed in groups of six (cage size: 37 × 22 × 15 cm; with fresh wood chips) in a temperature- (22 ± 2°C) and humidity-controlled environment (50 ± 10%) under a 12-hour light/dark cycle (lights on at 07:00) with food and water available ad libitum. Animal handling and care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (7th edition, Institute of Laboratory Animal Resources—National Research Council, National Academy Press, 1996), and all experiments were reviewed and approved by the Institutional Animal Research Committee of Hyogo College of Medicine. The mice were only used once after at least one-week habituation in the facility (n = 297, 11–13 weeks old at experimental day 1, 36–46 g body weight).
Reagents
Morphine hydrochloride was purchased from Takeda Chemical Industries (Osaka, Japan) and was dissolved in sterile saline (Otsuka Pharmaceutical Co. Ltd.). Naloxone hydrochloride dihydrate (a relatively non-selective opioid receptor antagonist), β-funaltrexamine hydrochloride ((E)-4-[[(5α,6β)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-morphinan-6-yl]amino]-4-oxo-2-butenoic acid methyl ester hydrochloride (a highly selective MOP antagonist), norbinaltorphimine dihydrochloride (a highly selective KOP antagonist), naltrindole hydrochloride (a highly selective DOP antagonist), and (−)-scopolamine hydrochloride (a muscarinic antagonist) were purchased from Sigma-Aldrich. Doses of all drugs refer to the weight of the salt. All reagents were dissolved in sterile saline. Drug solutions were prepared in such a way that the necessary dose could be injected in a volume of 0.1 mL/10 g of body weight by an intraperitoneal (i.p.) route. The doses of the drugs were chosen based on the literature.15–20 In the present study, no reduced body weight in mice treated with naloxone, compared with mice treated with saline, was observed (data not shown).
Test protocol
Y-maze test
Y-maze testing was conducted as reported previously.19 The Y-maze used in this study was made of three gray acrylic arms (7.5 × 30 cm) linked by a common central platform (triangular neutral zone, 7.5 × 7.5 × 7.5 cm) and enclosed by transparent acrylic walls (15 cm height). Mice were injected with drugs at the indicated doses (Figs. 1A and 4) and then returned to their home cages. After 30 minutes, the mice were put into the neutral zone of the Y-maze, and arm entries were recorded for 8 minutes. Alternation behavior was defined as consecutive entries into all three arms without repeated entries and was expressed as percentage of the total arm entries. For instance, if the three arms are labeled A, B, and C, the mouse enters the arms in the following sequence:
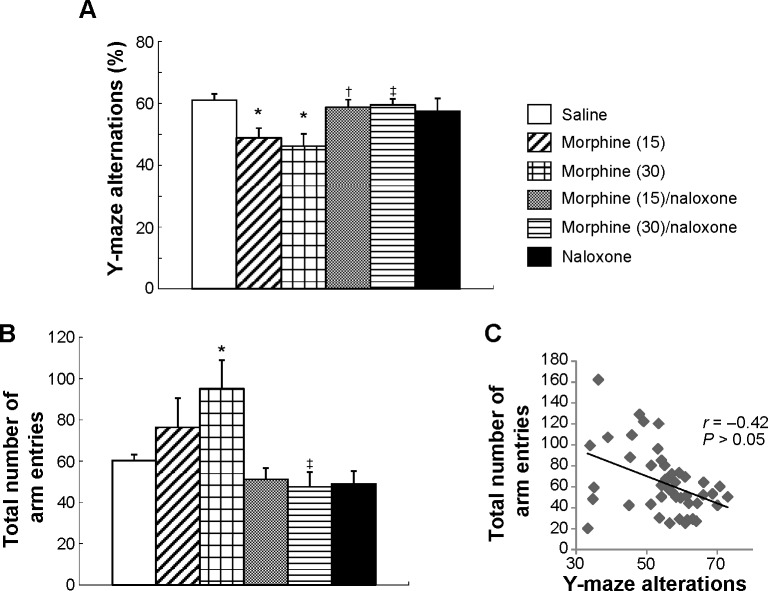
Figure 1.
The effects of morphine on spontaneous alternation behavior (A) and the total number of arm entries (B) in mice tested in a Y-maze. Mice (n = 48) were randomly divided into six treatment conditions (n = 8/group): saline, 15 mg/kg morphine, 30 mg/kg morphine, 15 mg/kg morphine plus 15 mg/kg naloxone, 30 mg/kg morphine plus 15 mg/kg naloxone, and 15 mg/kg naloxone. Thirty minutes after these injections, the mice were placed in a Y-maze. Values are shown as means ± SEM (n = 8/group). *P < 0.05, compared with saline-treated mice. *P < 0.05, compared with saline-treated mice. †P < 0.05, compared with 15 mg/kg morphine-treated mice. ‡P < 0.05, compared with 30 mg/kg morphine-treated mice. (C) Relationship between the total number of arm entries and Y-maze alterations (correlation coefficient = −0.42, P > 0.05; n = 48).
Figure 4.
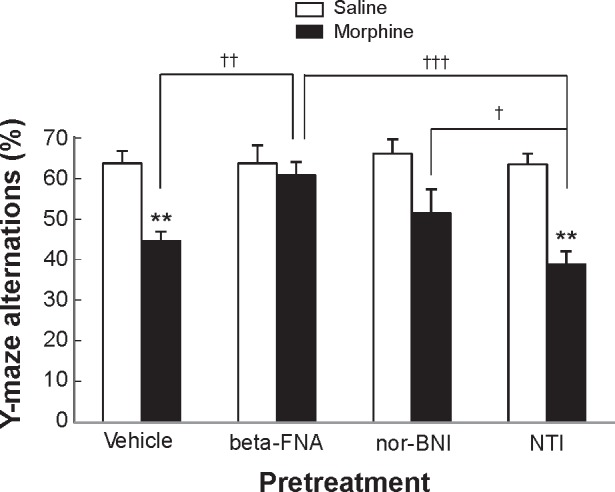
Effect of selective opioid receptor antagonists on the morphine-induced decrease in Y-maze alternations in mice. Values are shown as mean ± SEM (n = 10). beta-FNA, β-funaltrexamine (a μ-selective opioid receptor antagonist); nor-BNI, norbinaltorphimine (a k-selective opioid receptor antagonist); and NTI, naltrindole (a δ-selective opioid receptor antagonist). **P < 0.01, compared with a corresponding group treated with saline (one-way ANOVA followed by a post hoc Bonferroni–Dunn test). †P < 0.05, ††P < 0.01, †††P < 0.001; significant difference between the groups is shown by horizontal bars (one-way ANOVA followed by a post hoc Bonferroni–Dunn test).
The total alternation opportunities would be 18 (total entries minus 2), and the alternation behavior is 12 (for instance, BCB means repetitive), so that the percent alternation behavior would be 66.7% (12 out of 18). The Y-maze was wiped clean between trials with 10% ethanol.
MWM test
The procedure followed methods described previously in the literature.21 Briefly, the MWM for mice consisted of a circular pool (60 cm in diameter, 30 cm in height) filled to a depth of 20 cm with water maintained at 25 ± 1°C. The tank was divided into four equal quadrants with the help of two threads, fixed at right angles to each other on the rim of the pool. A submerged platform (with top surface 6 × 6 cm) was placed inside one of the target quadrants of the pool 1 cm below the surface of the water. The platform was consistently maintained in the same position throughout the training sessions. Each animal was subjected to four consecutive trials each day with a gap of five minutes between trials for four consecutive days, during which they were allowed to escape onto the hidden platform and to remain there for 20 seconds. During each training session, the mouse was gently placed in the water between quadrants, facing the wall (10 cm from the wall) of the pool with the drop location changing for each trial, and allowed 120 seconds to locate the submerged platform. If the mouse failed to find the platform within 120 seconds, it was guided gently onto the platform and allowed to remain there for 20 seconds. Escape latency was measured as the time taken by the animal to move from the starting position to the hidden platform in the target quadrant. Each animal was subjected to training trials for four consecutive days. The target quadrant remained constant throughout all sessions. The starting position was changed with each trial in the following pattern:
On the fifth day, a probe test was conducted. The platform was removed, and each mouse was placed in one of the three non-target quadrants and allowed to explore the maze for 120 seconds. The mean time spent in the target quadrant in search of the missing platform was noted as index of retrieval memory. The observers always stood at the same position. Care was taken not to disturb the relative location of the water maze with respect to other objects in the laboratory, which operated as extra-maze cues.
Novel-object exploration
The mice were placed in a transparent acrylic box (30 × 30 × 35 cm) with sawdust bedding (approximately 25 g) and were allowed to explore freely for 30 minutes. Mice were injected with drugs as described above and then returned to the acrylic box. After 30 minutes, a novel object (a small metal binder clip) was placed at the center of the acrylic box. The duration of exploration of the novel object was recorded by an observer, while horizontal movement (ie, horizontal locomotor activity) was recorded automatically using Animex Auto (Muromachi Kikai Co., Ltd)18 for five minutes. The object was wiped clean between trials with 10% ethanol.
Elevated plus maze test
Testing was carried out as previously described22 using a standard method. The apparatus was made of four acrylic arms (two enclosed arms of 30 × 5 × 15 cm that formed a cross shape with the two open arms of 30 × 5 × 0.5 cm, which were linked by a common central platform (neutral zone), 5 × 5 cm). The maze was raised 50 cm above the floor and illuminated by a dim light above the apparatus (10–12 lux). Mice were injected with drugs as described above and then returned to their home cages. After 30 minutes, the mice were placed on the central platform facing an open arm and allowed to explore the apparatus for 5 minutes. The placement of all four paws on an arm qualified as an entry. Entries into the open and closed arms and the cumulative time spent in each arm type and the neutral zone were measured. The maze was wiped clean between trials with 10% ethanol.
Marble-burying test
The transparent acrylic box described in novel-object recognition test was used in this test. In this case, the box contained extra amounts (2 cm high) of sawdust bedding and had 25 marbles placed evenly on the bedding. The subject was treated with drugs 30 minutes prior to the test as described above. The subject was then placed in the box for 30 minutes. At the end of the period, animals were removed from the cage and the number of marbles covered (at least two out of three) was counted.
Statistics
Data were presented as mean ± the standard error of the mean (SEM). Statistical analysis was performed using Student’s t-test or mixed factor analysis of variance (ANOVA), with or without repeated-measures as appropriate, followed by Bonferroni–Dunn post hoc analyses of individual means for significant factors from ANOVA (StatView 5.0 for Apple Macintosh, SAS Institute, Inc.). Statistical significance was set at P < 0.05.
Results
Effects of morphine on spontaneous alternation behavior in the Y-maze
Mice (n = 48) were randomly divided into six treatment conditions (n = 8/group): saline, 15 mg/kg morphine, 30 mg/kg morphine, 15 mg/kg morphine plus 15 mg/kg naloxone, 30 mg/kg morphine plus 15 mg/kg naloxone, and 15 mg/kg naloxone. Thirty minutes after these injections, the mice were placed in a Y-maze as described in the Materials and Methods section.
Figure 1 shows the percent alternation behavior (A) and the total number of arm entries (B). One-way ANOVA (treatment) applied to the data in Figure 1A indicated that there was a significant main effect of treatment (F(5,42) = 4.1, P < 0.01). Post hoc comparisons of the significant drug effect found that Y-maze alternations were significantly decreased in mice treated with morphine (15 and 30 mg/kg) compared with the control mice treated with saline (P < 0.05). In addition, post hoc comparisons also indicated that coadministration of naloxone with morphine antagonized the effect of morphine on Y-maze alternations. Naloxone treatment alone had no effect on Y-maze alternations.
ANOVA applied to the data in Figure 1B indicated that there was a significant main effect of treatment (F(5,42) = 4.1, P < 0.01). Post hoc comparisons of the significant drug effect found that the total number of arm entries was significantly increased in mice treated with morphine (30 mg/kg) compared with the control mice treated with saline (P < 0.05). In addition, post hoc comparisons also indicated that coadministration of naloxone antagonized the effect of morphine on the total number of arm entries. Naloxone alone had no effect on Y-maze alternations. There was no correlation between the total number of arm entries and Y-maze alterations (Fig. 1C; correlation coefficient = −0.42, P > 0.05; n = 48).
Effect of morphine on memory retrieval in the MWM
Figure 2A shows escape latencies recorded during trials of the training sessions on days 1–4 prior to drug treatments (n = 74) and in a post-treatment assessment of memory retention on day 14 (n = 5, mice selected from those treated with saline alone (ie, vehicle) on day 5) in mice tested in an MWM. Two-way repeated-measures ANOVA (trial × day) applied to the data in Figure 2A identified significant main effects of trial (F(3,1184) = 3.2, P < 0.05) and day (F(4,1184) = 46.1, P < 0.001). ANOVA with repeated-measures also indicated a significant trial × day interaction (F(12,1184) = 6.7, P < 0.001). Post hoc comparisons found that escape latencies recorded on day 1 were significantly longer than those observed on the other four days (P < 0.05) and that the escape latencies observed on day 2 were significantly longer than those observed on day 4 (P < 0.05). Post hoc comparisons within day 1 also found that escape latencies recorded during trial 1 were significantly longer than those observed in the other three trials (P < 0.05) and that the escape latencies observed in trial 2 were significantly longer than those observed in trial 4 within a day (P < 0.05).
Figure 2.
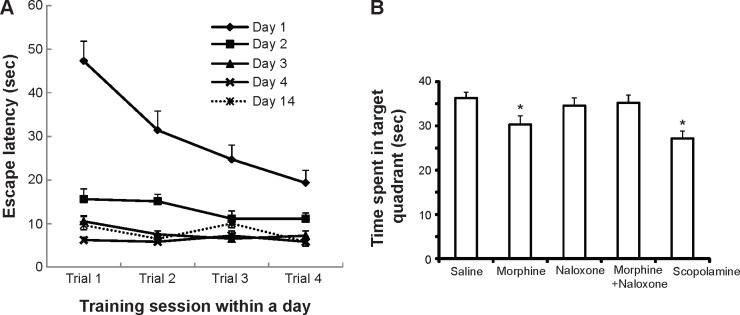
Escape latencies recorded during trials of the training sessions on days 1–4 prior to drug treatments and in a post-treatment assessment of memory retention on day 14 (n = 5, mice selected from those treated with saline alone (ie, vehicle) on day 5) in mice tested in an MWM (A). Values are shown as mean ± SEM (n = 74). Alterations in the mean time spent in the target quadrant during 120 seconds on day 5 (B). On day 5, 24 hours after the final training session (day 4), the mice (n = 67 out of 74 mice shown in Fig. 2A) were randomly divided into five treatment groups: saline (n = 15), 15 mg/kg morphine (n = 15), 15 mg/kg naloxone (n = 15), 15 mg/kg morphine plus 15 mg/kg naloxone (n = 15), and 1 mg/kg scopolamine (n = 7). Thirty minutes after these injections, the mice were exposed to the maze for the probe trial. Values are shown as mean ± SEM (n = 7–15). *P < 0.05, compared with saline (post hoc Bonferroni–Dunn test).
On day 5, 24 hours after the final training session (day 4), the mice (n = 67 out of 74 mice shown in Fig. 2A) were randomly divided into five treatment groups: saline (n = 15), 15 mg/kg morphine (n = 15), 15 mg/kg naloxone (n = 15), 15 mg/kg morphine plus 15 mg/kg naloxone (n = 15), and 1 mg/kg scopolamine (n = 7). Thirty minutes after these injections, the mice were exposed to the maze for the probe trial. Figure 2B shows the effect of morphine and other treatments on the time spent in the target quadrant during the probe trial of MWM.
One-way ANOVA (treatment) was applied for the time spent in the target quadrant. The ANOVA applied to the data represented in Figure 2B showed a significant main effect of treatment (F(4,62) = 3.7, P < 0.01). Post hoc comparisons found that mice treated with morphine and scopolamine showed significantly decreased times spent in the target quadrant compared with the control mice treated with saline (P < 0.05). Pretreatment of morphine-treated mice with naloxone reversed this effect, while naloxone alone was without effect. Post hoc comparisons found that morphine-/naloxone-treated mice or mice treated with naloxone alone spent significantly more time in the target quadrant compared with morphine-treated mice (P < 0.05), and were not significantly different from control mice treated with saline.
Effect of morphine on exploration of a novel object
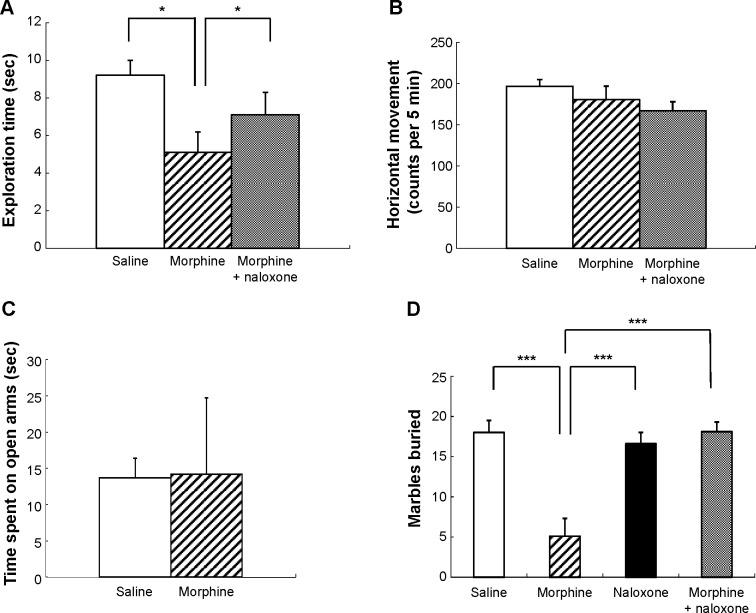
Mice (n = 39) were randomly divided into three groups (n = 13 mice/group) that received the following treatments: saline vehicle, 15 mg/kg morphine, or 15 mg/kg morphine plus 15 mg/kg naloxone. The mice were placed in the empty apparatus for 30 minutes and then exposed to a novel object. Figure 3A and B shows the effect of treatments on the duration of exploration and horizontal movement, respectively.
Figure 3.
(A and B) Effect of morphine on the novel-object exploration test and horizontal locomotor activity during the test. Numbers of novel-object (a small binder clip with fold-back arms) exploration were recorded (A) with a simultaneous measurement of horizontal movement by Animex Auto (B) for five minutes. Values are shown as mean ± SEM (n = 13). *P < 0.05, compared with saline- or morphine plus naloxone-treated mice (post hoc Bonferroni–Dunn test). (C) Effects of morphine on the time spent in open arms in the elevated plus maze. Values are shown as mean ± SEM (n = 8). (D) Effects of morphine on marble-burying behavior in mice. Values are expressed as number of marbles buried by mice for 30 minutes (mean ± SEM, n = 10). ***P < 0.001, compared with saline-, naloxone-, or morphine plus naloxone-treated mice (post hoc Bonferroni–Dunn test).
One-way ANOVA (treatment) applied to the data represented in Figure 3A identified a significant main effect of treatment (F(2,36) = 3.6, P < 0.05). Post hoc comparisons found that mice treated with morphine had significantly decreased exploration time compared with the control mice treated with saline (P < 0.05) and with the mice treated with morphine in combination with naloxone (P < 0.05). One-way ANOVA (treatment) applied to the horizontal movement data represented in Figure 3B showed no significant main effect of treatment (F(2,36) = 1.5, P = 0.24).
Effect of morphine in the elevated plus maze
Mice (n = 16) were randomly divided into two groups (n = 8 mice/ group) and were treated with either saline vehicle or 15 mg/kg morphine. Thirty minutes later, the mice were placed in the elevated plus maze. Figure 3C shows the effect of morphine on the time spent in the open arms. There was no significant difference between morphine- and saline-treated mice (t = 0.053, P = 0.96).
Effect of morphine in the marble-burying test
Mice (n = 40) were randomly divided into four groups (n = 10 mice/group) and were treated with saline vehicle, 15 mg/kg morphine, 15 mg/kg naloxone, or 15 mg/kg morphine plus 15 mg/kg naloxone. Thirty minutes later, the mice were placed in the apparatus for the marble-burying test. Figure 3D shows the effect of the treatments on the marble-burying behavior.
One-way ANOVA (treatment) applied to the data represented in Figure 3D identified a significant main effect of treatment (F(3,36) = 15.3, P < 0.001). Post hoc comparisons found that mice treated with morphine buried significantly fewer marbles compared with the control mice treated with saline (P < 0.001), with the mice treated with morphine in combination with naloxone (P < 0.001), and with the mice treated with naloxone alone (P < 0.001).
Effects of subtype-selective opioid receptor antagonists on morphine-induced decreases in spontaneous alternation behavior
Mice (n = 80) were randomly divided into four groups (n = 20 mice/group) and were pretreated with four different injections: vehicle, 5 mg/kg β-funaltrexamine, 5 mg/kg norbinaltorphimine, and 5 mg/kg naltrindole. Thirty minutes later, half of each group (n = 10) was injected with 15 mg/kg morphine, while the other half of each group (n = 10) was injected with saline. Thirty minutes after morphine (or saline) injection, the mice were exposed to the Y-maze.
Two-way ANOVA (opioid receptor antagonist pretreatment × morphine treatment) applied to the data represented in Figure 4 indicated that there were significant main effects of opioid receptor antagonist pretreatment (F(3,72) = 3.7, P < 0.05) and morphine treatment (F(1,72) = 34.4, P < 0.0001). ANOVA also indicated a significant opioid receptor antagonist pretreatment × morphine treatment interaction (F(3,72) = 3.1, P < 0.05). Post hoc comparisons found that Y-maze alternations were significantly decreased in mice treated with saline vehicle or naltrindole followed by morphine compared with the control mice treated with saline vehicle or naltrindole followed by saline (P < 0.01). Post hoc comparisons also found that the morphine-induced decreases in Y-maze alternation were reversed by pretreatment with β-funaltrexamine (P < 0.01, compared with saline-morphine-treated mice and P < 0.001, compared with naltrindole–morphine-treated mice, but not significantly different from saline/saline-treated mice). The norbinaltorphi-mine-pretreated mice showed no recovery of morphine-induced decrease in Y-maze alteration compared with the saline-treated mice (but P < 0.05, compared with the naltrindole–morphine-treated mice).
Discussion
In the present study, we demonstrated that acute administration of morphine to mice induces impairments of short-term spatial memory and retrieval in the Y-maze and MWM tasks. These morphine-induced spatial memory impairments were further shown to be mediated by opioid receptors as they were completely blocked by naloxone, a relatively non-selective opioid receptor antagonist in the Y-maze. These observations of an effect of naloxone to block the action of morphine in the Y-maze alternations13,14 as well as spatial memory impairments observed in the eight-arm radial maze task are consistent with previous reports.23 Further evidence for MOP involvement in spontaneous alternation comes from the effects of intracerebroventricular injections of endomorphin-1 and endomorphin-2.24 These endogenous MOP agonists produced effects that were similar to morphine. Selective DOP agonists did not affect spontaneous alternation, but did affect passive avoidance learning.25
The possible opioid receptor subtype-mediated impairment of spatial memory function was further specified by an experiment using antagonists selective for opioid receptor subtypes in the Y-maze task: the selective MOP antagonist β-funaltrexamine, but neither norbinaltorphimine (a KOP-selective antagonist) nor naltrindole (a DOP-selective antagonist), completely blocked the effect of morphine, suggesting the involvement of MOP in the morphine-induced spatial memory impairments. Some of the effects of morphine in this experiment appeared to reflect working memory deficits, which was consistent with some other reports using other procedures.10,11 The findings in the MWM suggest that morphine also produces spatial memory retrieval deficits. Thus, despite the impairment of performance by morphine during the probe trial, spatial memory in the MWM task was retained on day 14. The impairment by acute morphine in memory retrieval was of the same degree as that induced by scopolamine, suggesting that the morphine-induced spatial memory impairment resembles scopolamine-induced deficits.26 Indeed, effects of morphine on initial acquisition in the MWM can be ameliorated by either opioid or cholinergic antagonists,9,27 suggesting that interactions between opioidergic and cholinergic systems are involved in those effects. The effects of morphine on spontaneous alternation appear to involve the septum28,29 and septal projections to the hippocampus.30 There is a possibility that morphine affects animal’s ability to swim in the water. As shown in Figure 3A and B, morphine significantly reduced a novel-object exploration time, while the horizontal locomotion was not changed after morphine administration, suggesting that morphine at a dose of 15 mg/kg may affect mood state(s) without alterations in locomotor activity.
In the literature, it has been debated whether apparent morphine-induced spatial memory impairments result from other morphine-induced behavioral alterations, and thus represent behavioral confounds.6–8 Regarding this point, a possible positive association of morphine-induced, conditioned place preference with morphine-induced spatial learning was reported,31 although there is a contradictory report as well.32 In addition, a possible association of morphine-induced hyperlocomotion in mice with morphine-induced spatial memory deficits was reported.33 Of course, these could just represent non-causal relationships related to morphine dose. To investigate the possible association of apparent spatial memory impairments produced by morphine with other behavioral effects of morphine, the effects of acute morphine treatment on emotional behavior and exploration were examined in the present experiments. Reduced exploratory behavior was observed in mice after acute morphine treatment in a novel-object exploration test. This suggests that (1) morphine may reduce anxiogenic-like responses to unfamiliar objects, (2) curiosity toward novel objects may be attenuated by morphine, or (3) short-term recognition memory is increased by morphine treatment.
Regarding emotional alterations, no anxiolytic behavior was observed in mice after acute morphine administration in the elevated plus maze test, suggesting that morphine did not affect anxiety-like behaviors at these doses in this mouse strain. Systemic morphine injections or intracerebral injections into the dorsal periaqueductal gray area can reduce anxiety in the elevated plus maze,34 although higher doses of morphine injected intracerebrally were anxiogenic. However, this was not the case in the present experiments, although a reduction in what is most often characterized as an obsessive-compulsive disorder type of anxiolytic-like behavior was observed in the marble-burying test in mice after morphine administration. Of course, this could be interpreted more simply as a reduction in interaction with the novel objects or a reduction in curiosity in this task as well. This type of reduced interaction with objects in this task is also apparent in dopamine transporter knockout mice,35 which suggests the possibility that hyperactivity induced by morphine might also be a confound in this task. However, no changes in horizontal locomotion were observed in this experiment (Fig. 3B). Morphine has been reported to reduce exploratory behavior36 and has previously been suggested to reduce marble burying.37 These authors suggested that the effects of morphine on marble burying are non-specific effects resulting from other behavioral changes, but marble burying is reduced at doses that do not affect locomotion.38 Furthermore, morphine withdrawal increases marble-burying behavior, effects that can be ameliorated by morphine treatment,38 suggesting that there is a more primary role of opioids in this behavior.
Returning to the differences in spatial memory observed in the previous tests, it is unlikely that general reductions in exploration account for the results as increases in the number of arms entered were observed in the Y-maze. In any case, the primary behavioral measures in the Y-maze and the MWM reflect the distribution of behavior rather than the amount of behavior. Nonetheless, reduced exploratory tendencies, particularly exploration evoked by novelty (the less familiar arms in the Y-maze and the unexpected absence of the platform in the water maze), may account for the pattern of results observed in these experiments.
Overall, the results of the present study suggest that morphine induces impairments in spatial short-term memory and retrieval and reduces exploratory behavior, but that these effects are not because of overall changes in locomotion or anxiety. With regard to learning deficits in opiate-dependent individuals, this would suggest that at least some effects are because of ongoing opiate use. So saying, learning in the MWM is also impaired by morphine withdrawal39 and the effect of morphine on acquisition in the MWM can be reversed by repeated morphine treatments,40 which would be consistent with impairments being observed in withdrawal. At least some of the effects of morphine withdrawal have a different basis than those related to acute morphine treatment and may primarily result from activation of stress systems during withdrawal.41 Morphine can have a number of different types of effects on hippocampal plasticity depending on the context. Acutely morphine produces synaptic potentiation, but it enhances stress-induced induction of long-term depression.42 Chronic morphine treatment reduces long-term potentiation43 and blocks the effects of both stress and acute morphine on synaptic plasticity.42 Glucocorticoid-induced impairments in memory retrieval in the MWM can be blocked by intrahippocampal naltrexone.44
The present results suggest that the effects of acute morphine on memory function are mediated by MOP receptors. These effects are likely to be primarily the result of effects on memory (working memory and memory retrieval) and not the result of competing behavioral effects, although an influence of reduced exploratory tendencies (or curiosity) may be hard to exclude as an explanation for some effects. The memory effects of acute opiates appear to have a different basis from those of chronic opiates, so it will be important to determine the relative contributions of acute and chronic opiate actions in opiate-dependent individuals.
Acknowledgments
The authors are grateful to Ms. A. Yoshioka of the Department of Pharmacology, Hyogo College of Medicine, for preparing the animal study proposal. They also thank Ms. C. Sakashita and Mr. T. Nakajima of Joint-Use Research Facilities, Hyogo College of Medicine, for offering and operating computer technology resources and constructing the acrylic test boxes, the elevated plus mazes, and the Y-mazes.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
FUNDING: This research was supported, in part, by Grants-in-Aid for Researchers, Hyogo College of Medicine (2011 and 2013 to NK; 2012 and 2014 to JK) and intramural funding from the National Institute on Drug Abuse, USA (GRU). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JK, NK, FSH, MF, AG, YK, AK, HK, SM, YM, SO, MS, MT. Analyzed the data: JK, NK, FSH, MF, AG, YK, AK, HK, SM, YM, SO, MS, KT, MT. Wrote the first draft of the manuscript: JK, NK, FSH, KT, MT. Contributed to the writing of the manuscript: JK, NK, FSH, KT, NN, GRU, MT. Agree with manuscript results and conclusions: JK, NK, FSH, MF, AG, YK, AK, HK, SM, YM, SO, MS, KT, NN, GRU, MT. Jointly developed the structure and arguments for the paper: FSH, KT, NN, GRU. Made critical revisions and approved final version: JK, NK, FSH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17(3):317–336. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu G, Zhou QX, Kang S, et al. Chronic morphine treatment impaired hippocampal long-term potentiation and spatial memory via accumulation of extracellular adenosine acting on adenosine A1 receptors. J Neurosci. 2010;30(14):15058–5070. doi: 10.1523/JNEUROSCI.0148-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenois F, Le Moine C, Cador M. The motivational component of withdrawal in opiate addiction: role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Rev Neurosci. 2005;16(3):255–276. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12(11):4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology. 1983;22(10):1193–1196. doi: 10.1016/0028-3908(83)90080-1. [DOI] [PubMed] [Google Scholar]
- 6.Mcnamara RK, Skelton RW. Pretraining morphine impairs acquisition and performance in the Morris water maze—motivation reduction rather than amnesia. Psychobiology. 1991;19(4):313–322. [Google Scholar]
- 7.McNamara RK, Skelton RW. Pharmacological dissociation between the spatial learning deficits produced by morphine and diazepam. Psychopharmacology. 1992;108(1–2):147–152. doi: 10.1007/BF02245300. [DOI] [PubMed] [Google Scholar]
- 8.Kikusui T, Tonohiro T, Kaneko T. Simultaneous evaluation of spatial working memory and motivation by the allocentric place discrimination task in the water maze in rats. J Vet Med Sci. 1999;61(6):673–681. doi: 10.1292/jvms.61.673. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Wu CF, Pei G, Xu NJ. Reversal of morphine-induced memory impairment in mice by withdrawal in Morris water maze: possible involvement of cholinergic system. Pharmacol Biochem Behav. 2001;68(3):507–513. doi: 10.1016/s0091-3057(01)00456-7. [DOI] [PubMed] [Google Scholar]
- 10.Galizio M, Keith JR, Mansfield WJ, Pitts RC. Repeated spatial acquisition: effects of NMDA antagonists and morphine. Exp Clin Psychopharmacol. 2003;11(1):79–90. doi: 10.1037//1064-1297.11.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Zhu F, Yan CX, Zhao Y, Zhao Y, Li PP, Li SB. Effects of pre-training morphine on spatial memory acquisition and retrieval in mice. Physiol Behav. 2011;104(5):754–760. doi: 10.1016/j.physbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Kahveci N, Gulec G, Ozluk K. Effects of intracerebroventricularly-injected morphine on anxiety, memory retrieval and locomotor activity in rats: involvement of vasopressinergic system and nitric oxide pathway. Pharmacol Biochem Behav. 2006;85(4):859–867. doi: 10.1016/j.pbb.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Stone WS, Walser B, Gold SD, Gold PE. Scopolamine- and morphine-induced impairments of spontaneous alternation performance in mice: reversal with glucose and with cholinergic and adrenergic agonists. Behav Neurosci. 1991;105(2):264–271. doi: 10.1037//0735-7044.105.2.264. [DOI] [PubMed] [Google Scholar]
- 14.Itoh J, Ukai M, Kameyama T. Dynorphin A-(1–13) potently improves the impairment of spontaneous alternation performance induced by the mu-selective opioid receptor agonist DAMGO in mice. J Pharmacol Exp Ther. 1994;269(1):15–21. [PubMed] [Google Scholar]
- 15.Brocardo PS, Budni J, Lobato KR, Santos AR, Rodrigues AL. Evidence for the involvement of the opioid system in the antidepressant-like effect of folic acid in the mouse forced swimming test. Behav Brain Res. 2009;200(1):122–127. doi: 10.1016/j.bbr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Campillo A, Cabanero D, Romero A, Garcia-Nogales P, Puig MM. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol. 2011;657(1–3):89–96. doi: 10.1016/j.ejphar.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Ward SJ, Portoghese PS, Takemori AE. Pharmacological characterization in vivo of the novel opiate, β-funaltrexamine. J Pharmacol Exp Ther. 1982;220(3):494–498. [PubMed] [Google Scholar]
- 18.Kitanaka N, Kitanaka J, Takemura M. Modification of morphine-induced hyperlocomotion and antinociception in mice by clorgyline, a monoamine oxidase- A inhibitor. Neurochem Res. 2006;31(6):829–837. doi: 10.1007/s11064-006-9087-x. [DOI] [PubMed] [Google Scholar]
- 19.Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology. 1988;94(4):491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- 20.Kitanaka J, Kitanaka N, Hall FS, et al. The selective μ opioid receptor antagonist β-funaltrexamine attenuates methamphetamine-induced stereotypical biting in mice. Brain Res. 2013;1522:88–98. doi: 10.1016/j.brainres.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Dhingra D, Kumar V. Memory-enhancing activity of palmatine in mice using elevated plus maze and Morris water maze. Adv Pharmacol Sci. 2012;2012:357–368. doi: 10.1155/2012/357368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitanaka N, Kitanaka J, Tatsuta T, et al. Withdrawal from fixed-dose injection of methamphetamine decreases cerebral levels of 3-methoxy-4-hydroxyphenylglycol and induces the expression of anxiety-related behavior in mice. Neurochem Res. 2010;35(5):749–760. doi: 10.1007/s11064-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 23.Braida D, Gori E, Sala M. Relationship between morphine and etonitazene-induced working memory impairment and analgesia. Eur J Pharmacol. 1994;271(2–3):497–504. doi: 10.1016/0014-2999(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 24.Ukai M, Watanabe Y, Kameyama T. Effects of endomorphins-1 and -2, endogenous mu-opioid receptor agonists, on spontaneous alternation performance in mice. Eur J Pharmacol. 2000;395(3):211–215. doi: 10.1016/s0014-2999(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 25.Ukai M, Takada A, Sasaki Y, Kameyama T. Stimulation of delta1- and delta2-opioid receptors produces amnesia in mice. Eur J Pharmacol. 1997;338(1):1–6. doi: 10.1016/s0014-2999(97)01310-1. [DOI] [PubMed] [Google Scholar]
- 26.El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary Eel D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav. 2003;76(3–4):525–533. doi: 10.1016/j.pbb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Zheng XG, Li XW, Yang XY, Sui N. Effects of scopolamine and physostigmine on acquisition of morphine-treated rats in Morris water maze performance. Acta Pharmacol Sin. 2002;23(5):477–480. [PubMed] [Google Scholar]
- 28.Ragozzino ME, Hellems K, Lennartz RC, Gold PE. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci. 1995;109(6):1074–1080. doi: 10.1037//0735-7044.109.6.1074. [DOI] [PubMed] [Google Scholar]
- 29.Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68(4):981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- 30.McNay EC, Canal CE, Sherwin RS, Gold PE. Modulation of memory with septal injections of morphine and glucose: effects on extracellular glucose levels in the hippocampus. Physiol Behav. 2006;87(2):298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhai HF, Zhang ZY, Zhao M, Qiu Y, Ghitza UE, Lu L. Conditioned drug reward enhances subsequent spatial learning and memory in rats. Psychopharmacology. 2007;195(2):193–201. doi: 10.1007/s00213-007-0893-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu N, Wang L, Wu C, Pei G. Spatial learning and morphine-rewarded place preference negatively correlates in mice. Pharmacol Biochem Behav. 2001;68(3):389–394. doi: 10.1016/s0091-3057(00)00479-2. [DOI] [PubMed] [Google Scholar]
- 33.Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007;147(4):1059–1065. doi: 10.1016/j.neuroscience.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Motta V, Brandao ML. Aversive and antiaversive effects of morphine in the dorsal periaqueductal gray of rats submitted to the elevated plus-maze test. Pharmacol Biochem Behav. 1993;44(1):119–125. doi: 10.1016/0091-3057(93)90288-5. [DOI] [PubMed] [Google Scholar]
- 35.Fox MA, Panessiti MG, Hall FS, Uhl GR, Murphy DL. An evaluation of the serotonin system and preservative, compulsive, stereotypical, and hyperactive behaviors in dopamine transporter (DAT) knockout mice. Psychopharmacology. 2013;227(4):685–695. doi: 10.1007/s00213-013-2988-x. [DOI] [PubMed] [Google Scholar]
- 36.van Abeelen JH, van den Heuvel CM. Behavioural responses to novelty in two inbred mouse strains after intrahippocampal naloxone and morphine. Behav Brain Res. 1982;5(2):199–207. doi: 10.1016/0166-4328(82)90053-5. [DOI] [PubMed] [Google Scholar]
- 37.Nicolas LB, Kolb Y, Prinssen EP. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547(1–3):106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Umathe SN, Mundhada YR, Bhutada PS. Differential effects of acute morphine, and chronic morphine-withdrawal on obsessive-compulsive behavior: inhibitory influence of CRF receptor antagonists on chronic morphine-withdrawal. Neuropeptides. 2012;46(5):217–221. doi: 10.1016/j.npep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty KD, Walsh TJ, Bailey S, Schlussman S, Grasing K. Acquisition of a Morris water maze task is impaired during early but not late withdrawal from morphine. Pharmacol Biochem Behav. 1996;55(2):227–235. doi: 10.1016/s0091-3057(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 40.Farahmandfar M, Karimian SM, Naghdi N, Zarrindast MR, Kadivar M. Morphine-induced impairment of spatial memory acquisition reversed by morphine sensitization in rats. Behav Brain Res. 2010;211(2):156–163. doi: 10.1016/j.bbr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Rabbani M, Hajhashemi V, Mesripour A. Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal in mice. Stress. 2009;12(5):451–456. doi: 10.1080/10253890802659612. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Zheng X, Wang Y, et al. Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. J Neurosci. 2004;24(10):2412–2420. doi: 10.1523/JNEUROSCI.5544-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22(5):1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajadi AA, Samaei SA, Rashidy-Pour A. Blocking effects of intra-hippocampal naltrexone microinjections on glucocorticoid-induced impairment of spatial memory retrieval in rats. Neuropharmacology. 2007;52(2):347–354. doi: 10.1016/j.neuropharm.2006.08.021. [DOI] [PubMed] [Google Scholar]