Abstract
Carbon disulfide (CS2) has been historically associated with the production of rayon, cellophane, and carbon tetrachloride. This study identifies multiple mechanisms by which CS2 contributes to the formation of CO2 in the atmosphere. CS2 and other associated sulfide compounds were found by this study to be present in emissions from unconventional shale gas extraction and processing (E&P) operations. The breakdown products of CS2; carbonyl sulfide (COS), carbon monoxide (CO), and sulfur dioxide (SO2) are indirect greenhouse gases (GHGs) that contribute to CO2 levels in the atmosphere. The heat-trapping nature of CO2 has been found to increase the surface temperature, resulting in regional and global climate change. The purpose of this study is to identify five mechanisms by which CS2 and the breakdown products of CS2 contribute to atmospheric concentrations of CO2. The five mechanisms of CO2 formation are as follows:
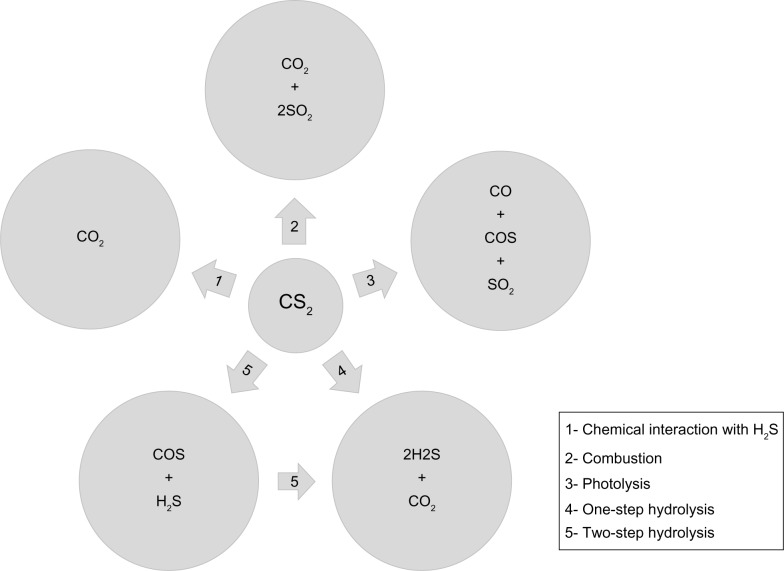
Chemical Interaction of CS2 and hydrogen sulfide (H2S) present in natural gas at high temperatures, resulting in CO2 formation;
Combustion of CS2 in the presence of oxygen producing SO2 and CO2;
Photolysis of CS2 leading to the formation of COS, CO, and SO2, which are indirect contributors to CO2 formation;
One-step hydrolysis of CS2, producing reactive intermediates and ultimately forming H2S and CO2;
Two-step hydrolysis of CS2 forming the reactive COS intermediate that reacts with an additional water molecule, ultimately forming H2S and CO2. CS2 and COS additionally are implicated in the formation of SO2 in the stratosphere and/or troposphere. SO2 is an indirect contributor to CO2 formation and is implicated in global climate change.
Keywords: global climate change, carbon disulfide, GHG, carbon dioxide
Introduction
Carbon disulfide (CS2) is a chemical intermediate best known for its historical use in the production of rayon, cellophane, and carbon tetrachloride.1–3 Industrial emissions of CS2 to the atmosphere have declined as textile manufacturing has shifted from the US to Asia, and the use of CS2 in carbon tetrachloride production has been phased out.4 With energy extraction expanding across the US, emission of CS2 may be on the rise and its contribution in formation of greenhouse gas (GHGs) is underreported.
CS2 is a component of sour natural gas and occurs naturally in the environment from the degradation of organic material, including geologic deposits of oil and natural gas; it is a waste gas emitted from the processing of sour natural gas.5,6 In natural gas extraction, CS2 is used as a paraffin solvent for sulfur, phosphorous, selenium, bromine, resins, and rubber.7 It is also a component of mercaptan, an odorant added to natural gas.8 Mercaptans may also be removed from a gas stream by oxidation to disulfides, which are then easily separated from the gas stream by absorption.9 It can be produced from the interaction of natural gas with hydrogen sulfide at high temperatures. CS2 is also known to be released in fossil fuel combustion, including natural gas combustion. During the extraction of natural gas, venting and flow-back operations release large amounts of gases, including CS2, directly to the atmosphere.10,11
Recent ambient air monitoring studies have identified CS2 and other associated sulfide compounds present in emissions emanating from natural gas exploration and production (E&P).12–15 As CS2 is present in many geologic formations and aspects of energy extraction, residential communities experiencing the trend of “urban drilling” – ie, extraction and processing of natural gas in populated urban communities – may be exposed to higher concentrations of CS2 in ambient air when compared to other residential areas not experiencing extraction and processing.16
CS2 is categorized as a non-methane volatile organic compound (NMVOC) and classified, along with carbonyl sulfide, as a hazardous air pollutant (HAP) according to the Clean Air Act Amendment of 1990 (CAAA). NMVOCs are a category of chemicals commonly used as solvents in industrial processes with the ability to vaporize at room temperature.17 NMVOCs may be classified as a direct or indirect GHG, and can be involved in indirect radiative reactions that form CO2.14 NMVOCs indirectly contribute to global climate change by producing GHGs, such as CO2, through reactions with other compounds, through their own chemical transformation influencing atmospheric lifetime of other GHGs, and by affecting the absorptive characteristics of the atmosphere such as cloud formation.18,19 In an indirect radiative reaction, a chemical breaks down in the atmosphere producing a GHG, or interacts with other chemicals in the atmosphere changing atmospheric concentrations of GHGs. The size of the indirect effect is dependent upon when and where the gas is emitted.20,21 CO2 is a GHG that is transparent to incoming solar radiation but with the capacity to easily absorb and trap infrared radiation. This allows heat to be retained at the Earth’s surface, contributing to ground-level ozone and global climate change.22 Ground-level ozone can damage crops and human health, and can lead to reduced crop production.23,24
This study is the first to identify five direct and/or indirect mechanisms by which CS2 contributes CO2 to the atmosphere and exhibits its capacity as a GHG and contributor to global climate change. This study is also one of the first to identify the presence of CS2 and other sulfide compounds in emissions from unconventional shale gas E&P operations.
Materials and Methods
A literature review was performed examining previous work related to occupational exposure to CS2 and any mechanism by which CS2 contributes to CO2 atmospheric concentrations and to CS2 use or presence in emissions from natural gas extraction or processing operations.
Key words searched included CS2, CS2 and CO2, CS2 occupational exposure, natural gas E&P emissions, and CS2 mechanism of CO2 formation. Databases searched included MEDLINE, TOXLINE, Scopus, Science Direct, TOXNET, and PubMed. Although many articles were found identifying CS2 exposure in occupational settings, they were mostly published prior to 1980. Recent publications on ambient air monitoring in regions experiencing energy extraction analyzed air samples collected for CS2, but failed to identify the potential CS2 has as a GHG and contributor to global climate change. No article to date was found identifying all five potential mechanisms by which CS2 might form CO2 in the atmosphere or the potential for COS and SO2 to be contributors to CO2 formation.
Results
The five potential mechanisms by which CS2 may form CO2 either directly or indirectly and contribute to GHG atmospheric concentrations are described in this paper (Fig. 1).
Figure 1.
Five mechanisms of CO2 formation from CS2.
Mechanism 1 – Chemical interaction
The interaction of CS2 and natural gas with hydrogen sulfide (H2S) at high temperatures can form CO2.25
In the document Report to Congress on Hydrogen Sulfide Air Emissions Associated with the Extraction of Oil and Gas, gas processing operations were identified as potential sources of H2S releases. Impurities present in natural gas (produced water, H2S, and CO2) are removed prior to the gas being compressed and shipped in pipelines. This removal process is often performed at pad sites located in residential areas where extraction occurs and may be a source of H2S.26
Mechanism 2 – Combustion
Combustion of CS2 in the presence of oxygen produces CO2 and SO2.
CS2 is highly flammable and can ignite easily. When exposed to spark or friction, it is known to easily combust. Contact with steam pipes or even light bulbs have been known to initiate the combustion of CS2.27–31
Mechanism 3 – Photolysis
Photolysis of CS2 leads to the formation of carbonyl sulfide (COS), CO, and SO2.
CS2 may have an indirect effect on global climate change through the main transformation product COS. CO is only a weak direct GHG but has important indirect effects on global climate change. CO reacts with hydroxyl (OH) radicals in the atmosphere, reducing their abundance. As OH radicals help the atmospheric lifetimes of strong GHGs such as methane, CO may indirectly increase the global climate change potential of these gases from OH radical scourging.32–34
The half-life of CS2 in the atmosphere from photolysis is estimated to be 1 week, whereas that of COS is estimated at 2 years, which provides the opportunity for long-range chemical transport and atmospheric conversions.35,36
Chin reported an additional indirect source of CS2 present in sulfur recovery operations in the oil industry, which is oxidized COS in the atmosphere.6
Mechanism 4 – One-step hydrolysis
Shangguan identified two mechanisms of hydrolysis of CS2. In the one-step hydrolysis mechanism, CS2 directly reacts with the hydrogen atoms in two molecules of water, producing CO2 and H2S.37–39
Mechanism 5 – Two-step hydrolysis
In a two-step hydrolysis mechanism, CS2 reacts with a hydrogen atom in a water molecule, leading to reactive intermediate COS. COS then reacts with an additional water molecule to form CO2 and H2S.
Research has shown that hydrolysis of CS2 can occur at room temperature at a slow rate.40
A previous study by the authors identified CS2 and 12 other sulfide compounds present in ambient air in residential communities in the Barnett Shale geologic formation where natural gas E&P operations were being carried out.41 CS2 concentrations and the detection frequency were found to vary with distance from the natural gas emission source. Values ranged from 0.3 to 200 parts per billion by volume (ppbv) based on a 24-hour air monitoring period, and from 1.5 to 980 ppbv based on a 1-hour monitoring period. The results presented in Table 1.
Table 1.
CS2 and associated sulfide compounds in natural gas emissions 24- and 1-hour minimum and maximum concentrations in parts per billion by volume (ppbv).
| CHEMICAL (PPBV) | MIN. 24 HOURS |
MAX. 24 HOURS |
MIN. 1 HOUR |
MAX. 1 HOUR |
|---|---|---|---|---|
| Carbon disulfide | 0.7 | 103.0 | 3.4 | 504.6 |
| Carbonyl sulfide | 0.3 | 36.7 | 1.5 | 180.0 |
| Dimethyl disulfide | 0.3 | 200.0 | 1.5 | 980.0 |
| Methyl ethyl disulfide | 0.3 | 145.0 | 1.5 | 710.0 |
| Methyl propyl disulfide | 0.3 | 41.6 | 1.5 | 204.0 |
| Diethyl disulfide | 0.3 | 32.7 | 1.5 | 160.2 |
| Ethyl, methylethyl disulfide | 0.3 | 46.7 | 1.5 | 228.8 |
| Dimethyl trisulfide | 1.2 | 46.3 | 5.9 | 226.8 |
| Ethyl n-propyl disulfide | 0.3 | 25.2 | 1.5 | 123.5 |
| Diethyl trisulfide | 0.3 | 8.2 | 1.5 | 40.3 |
| Methyl n-butyl disulfide | 0.3 | 15.5 | 1.5 | 76.0 |
| Propyl n-butyl disulfide | 0.3 | 14.6 | 1.5 | 71.5 |
| Dipropyl disulfide | 0.3 | 23.1 | 1.5 | 113.2 |
The U.S. Environmental Protection Agency’s Urban Air Toxcs Monitoring Program (NMP) comprising 52 sites around the US reported only CS2 as a monitored sulfide compound comparable to the values in this study. CS2 atmospheric concentrations based on a 24-hour air monitoring period showed a minimum level for CS2 of 0.005 ppbv and a maximum of 0.193 ppbv. Sampling period was for up to 12 consecutive months.42 CS2 is denser than air (2.62 vapor density when compared to air = 1) and can settle close to the ground in ground-level breathing zones.43
Discussion
The potential contribution of CS2 to atmospheric CO2 and GHG levels warrant further examination due to its unique qualities as a NMVOC and HAP. COS, CO, SO2, breakdown products of CS2, and indirect greenhouse gases additionally contribute to CO2 formation in the atmosphere. The transparency of CO2 to incoming solar radiation allows it to trap and absorb infrared radiation at the Earth’s surface. This, in turn, gives rise to ground-level ozone formation, which is a major factor in climate change.
Many urban cities experiencing natural gas E&P are designated as nonattainment areas with air quality below National Ambient Air Quality Standards (NAAQS). Identification of airborne chemicals contributing to CO2 levels as well as the sources of those emissions is critical in order to manage air quality and achieve NAAQS compliance. The quantification of emissions from chemical manufacturing facilities producing CS2 in the US may be easy due to the major source reporting requirements. Quantification of CS2 emissions from natural gas E&P operations is more difficult due to the lack of required emission reporting and the vast number of well sites. Additionally, each pad site may have multiple sources of CS2 emission. As CS2 escapes into the atmosphere, to quantify secondary atmospheric conversion becomes a challenge. While CS2 is a direct contributor to atmospheric CO2 levels, it is also an indirect contributor and is responsible for intermediates that may also produce CO2 in the atmosphere.
Currently, there is a profound lack of information and quantification of CS2 emissions from natural gas E&P operations. At facilities where combustion takes place, CS2 may be counted overall in VOC emissions. Quantification of CS2 or speciation of sulfide compounds is not currently required. Additionally, these data are self-reported and may be more qualitative rather than quantitative. Although CS2 and COS are HAPs, capable of adversely impacting health, this chemical has been considered to be primarily an occupational exposure agent. Current peer-reviewed nonoccupational health studies are lacking and outside the scope of this paper.
This study provides additional knowledge to the atmospheric puzzle, but more questions remain. Future studies are needed to examine and quantify the direct and indirect contribution of CS2 and intermediates of CS2 to atmospheric CO2 formation and GHG levels. Quantification of direct source CS2 emissions from natural gas E&P operations is also required to better understand how CS2 originating from energy extraction may contribute to local and regional CO2, GHG, and HAP levels. With additional knowledge, we can prepare proper mitigation strategies for this previously unidentified and underreported chemical that contributes to CO2 concentrations in the atmospheric and contributes to global climate change.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: ALR, JTP. Analyzed the data: ALR, JTP. Wrote the first draft of the manuscript: ALR, JTP. Contributed to the writing of the manuscript: ALR, JTP. Agree with manuscript results and conclusions: ALR, JTP. Jointly developed the structure and arguments for the paper: ALR, JTP. Made critical revisions and approved final version: ALR, JTP. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.World Health Organization . WHO Air Quality Guide for Europe. 2nd ed. Denmark: 2000. Carbon disulfide; pp. 1–8. [Google Scholar]
- 2.U.S. Environmental Protection Agency . Integrated Risk Information System (IRIS) on Carbon Disulfide. Washington, DC: National Center for Environmental Assessment, Office of Research and Development; 1999. [Google Scholar]
- 3.U.S. Environmental Protection Agency . Health and Environmental Effects Profile for Carbon Disulfide. Cincinnati, OH: Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development; 1986. EPA/600/x-86/155. [Google Scholar]
- 4.U.S. Environmental Protection Agency Chemical summary for carbon disulfide. 1994. [Accessed July 19, 2010]. Available at: http://www.epa.gov/chemfact/s_carbds.txt.
- 5.Greenberg MI, Hamilton RJ, Phillips SD. Occupational, Industrial and Environmental Toxicology. St Louis Missouri: Mosby-Year Books Inc; 1997. [Google Scholar]
- 6.World Health Organization . Concise international chemical assessment document 46: carbon disulfide. 2002. [Accessed October 11, 2010]. Available at: www.inchem.org/documents.cicads/cicads/cicad46.htm. [Google Scholar]
- 7.West Virginia University Statler College of Engineering and Mineral Resources Production of specialty chemicals from a coal gasification acid gas waste stream. 2014. [Accessed October 10, 2014]. Available at: http:www.che.cemr.wvu.edu/publications/projects/large_proj/acidgas.PDF.
- 8.Susan B. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals. New Jersey, USA: Rahway Publishers, Inc; 1989. [Google Scholar]
- 9.U.S. EPA . Carbon Disulfide Emission Control Options. Research Triangle Park, NC: U.S. EPA; 1992. [Google Scholar]
- 10.Manning WP. Talk Presented at 86th Gas Producers Association (GPA) Annual Convention. San Antonio, TX: 2007. Removing Organic Sulfur Compounds from Natural Gas. Available at: https://www.gpaglobal.org/publications/view/id/1036/ [Google Scholar]
- 11.Andre RC. In: Cooper’s Toxic Exposure Desk Reference. Andre RC, editor. Boca Raton, FL: CRC Press Inc; 1997. [Google Scholar]
- 12.Hultman N, Rebois D, Scholten M, et al. The greenhouse impact of unconventional gas for electricity generation. Environ Res Lett. 2011;6:044008. [Google Scholar]
- 13.O’Sullivan F, Paltsev S. Shale gas production: potential versus actual greenhouse gas emissions. Environ Res Lett. 2012;7:044030. [Google Scholar]
- 14.Rich AL, Grover JP, Sattler ML. An exploratory study of air emissions associated with shale gas development and production in the Barnett Shale. J Air Waste Manage Assoc. 2014;64(1):61–72. doi: 10.1080/10962247.2013.832713. [DOI] [PubMed] [Google Scholar]
- 15.Zielinska B, Fujita E, Campbell D. Monitoring of emissions from barnett shale natural gas production facilities for population exposure assessment. NUATRC Research Report. 2011. [Accessed October 21, 2010]. Available at: https://sph.uth.edu/mleland/Webpages/publications.htmhttps://sph.uth.edu/mleland/attachments/DRI-BarnettReport19Final.pdf.
- 16.ERG City of fort worth natural gas air quality study. 2011. [Accessed December 11, 2011]. Available at: http://fortworthtexas.gov/uploadedFiles/Gas_Wells/AirQualityStudy_final.pdf.
- 17.Texas Commission on Environmental Quality Barnett Shale Air Monitoring Results 2009–2011. 2011. [Accessed December 15, 2011]. Available at: http://www.tceq.state.tx.us/airquality/barnettshale/fw_sampling.
- 18.Gillenwater M. Forgotten carbon: indirect CO2 in greenhouse gas emission inventories. Env Sci Policy. 2008;11:195–203. [Google Scholar]
- 19.U.S. Environmental Protection Agency Greenhouse gas emissions reductions. 2013. [Accessed April 28, 2013]. Available at: http://www.epa.gov/greeningepa/ghg/
- 20.U.S. Environmental Protection Agency Climate change: Inventory of U.S. Greenhouse Gas Emissions and Sinks 1990–2011. 2011. [Accessed April 28, 2013]. Available at: http://www.epa.gov/climatechange/Downloads/ghgemissions/US-GHG-Inventory-2013-ES.pdf.
- 21.Satein H. Chemical relationship between greenhouse gases and air pollutants in biomass energy production. oregon toxic alliance. 2009. [Accessed July 31, 2014]. Available at: http://www.beyondtoxics.org/wp-content/uploads/2011/11/OTA_Report-BiomassContributors_to_ClimateChange7-28-09.pdf.
- 22.Forster P, Ramaswamy V, Artaxo P. Changes in atmospheric constituents and radiative forcing. In: Solomon S, Qin M, Manning M, et al., editors. Climate Change2007: The Physical Science Basis. Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel of Climate Change. Cambridge: Cambridge University Press; 2007. pp. 129–235. http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg1_report_the_physical_science_basis.htm. [Google Scholar]
- 23.U.S. Environmental Protection Agency Natural gas-fired reciprocating engines. AP42 Compilation of Air Pollutant Emission Factor Vol. 1: Stationary Point and Area Sources. 1995. [Accessed October 11, 2010]. Available at: http://www.epa.gov/ttnchie1/ap42/ch03/final/c03s02.pdf.
- 24.U.S. Environmental Protection Agency Climate change: agriculture and food supply. 2013. [Accessed July 30, 2014]. Available at: http://www.epa.gov/climatechange/impacts-adaptation/agriculture.html.
- 25.Harbison RD. Hamilton & Hardys Industrial Toxicology. 5 ed. St Louis, MO: Mosby-Year Book, Inc; 1998. Carbon disulfide; pp. 364–68. [Google Scholar]
- 26.Liang Y-X, Qu DZ. Cost-benefit analysis of the recovery of carbon disulfide in the manufacturing of viscose rayon. Scan J Work Environ Health. 1985;11(suppl4):60–3. [PubMed] [Google Scholar]
- 27.Chin M, Davis DD. Global sources and Sinks of OCS and CS2 and their distributions. Global Biogeochem Cycles. 1993;7:321–37. [Google Scholar]
- 28.U.S. Environmental Protection Agency Report to congress on hydrogen sulfide air emissions associated with the extraction of oil and natural gas. 1993. [Accessed July 15, 2014]. Available at: http://www.fossil.energy.gov/programs/gasregulation/authorizations/2012_applications/sierra_ex12_97/Ex._21_-_EPA_Hydrogen_Sulfide_Report_-_p.pdf.
- 29.Lindeburgh MR. Mechanical Engineering Reference Manual for the PE Exam. 13th ed. Belmont, CA: Professional Publications; 2006. [Google Scholar]
- 30.U. S. Environmental Protection Agency Method 15 A-determination of total reduced sulfur emissions from sulfur recovery plants in petroleum refineries. 2014. [Accessed July 15, 2014]. Available at: http://www.epa.gov/ttn/emc/promgate/m-15a.pdf.
- 31.NJ Dept of Health Hazardous substance fact sheet: carbon disulfide. 2010. [Accessed August 15, 2014]. Available at: http://www.nj.gov/health/eoh/rtkweb/documents/fs/0344.pdf.
- 32.Sevas Educational Society Physical properties of carbon disulfide. 2014. [Accessed July 31, 2014]. Available at: http://www.sbioinformatics.com/design_thesis/Carbon_Disulfide/Carbon-2520Disulfide_Properties&uses.pdf.
- 33.West Virginia University Statler College of Engineering and Mineral Resources Production of specialty chemicals from a coal gasification acid gas waste stream. 2013. [Accessed October 10, 2013]. Available at: http:www.che.cemr.wvu.edu/publications/projects/large_proj/acidgas.PDF.
- 34.Erve JC, Amarnath V, Sills RC, Morgan DL, Valentine WM. Disulfide or N,N,-diethyldithiocarbamate in Vivo. Chem Res Toxicol. 1998;11:1128–36. doi: 10.1021/tx980077p. [DOI] [PubMed] [Google Scholar]
- 35.Wood WP, Heicklen J. The photooxidation of carbon disulfide. J Phys Chem. 1971;75:854–60. [Google Scholar]
- 36.Wine PH, Chameides WL, Ravishankara AR. Potential role of CS photo-oxidation in tropospheric sulfur chemistry. Geophys Res Lett. 1981;8(5):543–6. [Google Scholar]
- 37.Peyton TO, Steele RV, Mabey WR. Carbon Disulfide, Carbonyl Sulfide: Literature Review and Environmental Assessment. Washington, DC: US Environmental Protection Agency; 1976. EPA-600/9-78-009. [Google Scholar]
- 38.Chin M, David DD. A reanalysis of OCS (COS) as a source of stratospheric background sulfur aerosol. J Geophys Res. 1993;100(D5):8993–9005. [Google Scholar]
- 39.Shangguan J, Guo HX. The surface basicity and catalysis over the alumina based catalysts for COS and CS2 hydrolysis. J Mol Cat. 1997;11:337–42. [Google Scholar]
- 40.Zhao S, Yi H, Tang X, et al. The hydrolysis of carbonyl sulfide at low temperature: a review. Sci World Journal. 2013;2013:8. doi: 10.1155/2013/739501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watts SF. The mass budgets of carbonyl sulfide, diethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos Environ. 2000;34:761–79. [Google Scholar]
- 42.Ling L, Zhang R, Han P, et al. A theoretical study on hydrolysis mechanism of carbon disulfide. J Mol Model. 2010;18(4):1625–32. doi: 10.1007/s00894-011-1183-4. [DOI] [PubMed] [Google Scholar]
- 43.Wallace KL, Kunkel DB. Carbon disulfide. In: Burke-Sullivan J, Krieger G, editors. Clinical Environmental Health and Toxic Exposure. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1206–10. [Google Scholar]