Abstract
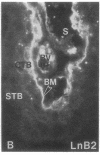
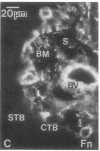
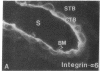
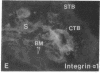
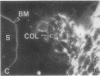
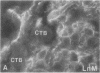
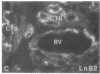
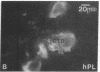
Development of the human embryo depends on the ability of first trimester cytotrophoblastic stem cells to differentiate and invade the uterus. In this process, transient expression of an invasive phenotype is part of normal cytotrophoblast differentiation. Morphologically, this process begins when polarized chorionic villus cytotrophoblasts form multilayered columns of nonpolarized cells, and invade the uterus. Using immunocytochemistry, we compared the presence of adhesion receptors and extracellular matrix ligands on cytotrophoblasts in villi, cell columns, and the uterine wall. Villus cytotrophoblasts, anchored to basement membrane, stained for alpha 6 and beta 4 integrin subunits and both merosin and A-chain-containing laminin. Nonpolarized cytotrophoblasts in columns expressed primarily alpha 5 and beta 1 integrin subunits and a fibronectin-rich matrix. Cytotrophoblast clusters in the uterine wall stained for alpha 1, alpha 5, and beta 1 integrins, but not for most extracellular matrix antigens, suggesting that they interact primarily with maternal cells and matrices. Tenascin staining was restricted to stroma at sites of transition in cytotrophoblast morphology, suggesting that tenascin influences cytotrophoblast differentiation. Our results suggest that regulation of adhesion molecule expression contributes to acquisition of an invasive phenotype by cytotrophoblasts and provide a foundation for studying pathological conditions in which insufficient or excessive trophoblast invasion occurs, such as preeclampsia or choriocarcinoma.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Brosens I., Dixon H. G. The anatomy of the maternal side of the placenta. J Obstet Gynaecol Br Commonw. 1966 Jun;73(3):357–363. doi: 10.1111/j.1471-0528.1966.tb05175.x. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci M., Classen-Linke I., Mühlhauser J., Kaufmann P., Zardi L., Chiquet-Ehrismann R. The human placenta: a model for tenascin expression. Histochemistry. 1991;95(5):449–458. doi: 10.1007/BF00315740. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A., Beck K., Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988 May 6;53(3):383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Aufderheide E. Stimulation of tenascin expression in mesenchyme by epithelial-mesenchymal interactions. Int J Dev Biol. 1989 Mar;33(1):71–79. [PubMed] [Google Scholar]
- Enders A. C. Cytology of human early implantation. Res Reprod. 1976 Sep;8(5):1–2. [PubMed] [Google Scholar]
- Enders A. C. Fine structure of anchoring villi of the human placenta. Am J Anat. 1968 May;122(3):419–451. doi: 10.1002/aja.1001220302. [DOI] [PubMed] [Google Scholar]
- Engvall E., Earwicker D., Haaparanta T., Ruoslahti E., Sanes J. R. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990 Sep;1(10):731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Fisher S. J., Cui T. Y., Zhang L., Hartman L., Grahl K., Zhang G. Y., Tarpey J., Damsky C. H. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989 Aug;109(2):891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. J Biol Chem. 1989 Apr 15;264(11):6529–6535. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Kennel S. J., Foote L. J., Falcioni R., Sonnenberg A., Stringer C. D., Crouse C., Hemler M. E. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem. 1989 Sep 15;264(26):15515–15521. [PubMed] [Google Scholar]
- Klein G., Ekblom M., Fecker L., Timpl R., Ekblom P. Differential expression of laminin A and B chains during development of embryonic mouse organs. Development. 1990 Nov;110(3):823–837. doi: 10.1242/dev.110.3.823. [DOI] [PubMed] [Google Scholar]
- Klein G., Langegger M., Timpl R., Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988 Oct 21;55(2):331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kurman R. J., Main C. S., Chen H. C. Intermediate trophoblast: a distinctive form of trophoblast with specific morphological, biochemical and functional features. Placenta. 1984 Jul-Aug;5(4):349–369. doi: 10.1016/s0143-4004(84)80015-6. [DOI] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librach C. L., Werb Z., Fitzgerald M. L., Chiu K., Corwin N. M., Esteves R. A., Grobelny D., Galardy R., Damsky C. H., Fisher S. J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991 Apr;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. M., Korzelius C. A., Mercurio A. M. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of alpha 6 beta 4 and alpha 2 beta 1 integrins. Cell Regul. 1990 Feb;1(3):249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhenn V. B., Schreiber A. B., Soriero O., McMillan W., Allison A. C. A monoclonal antibody against basal cells of human epidermis. Potential use in the diagnosis of cervical neoplasia. J Clin Invest. 1985 Nov;76(5):1978–1983. doi: 10.1172/JCI112197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Daams H., Van der Valk M. A., Hilkens J., Hilgers J. Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986 Aug;34(8):1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K. J., Hall D. E., Flier L. A., Gehlsen K. R., Turner D. C., Carbonetto S., Reichardt L. F. A neuronal cell line (PC12) expresses two beta 1-class integrins-alpha 1 beta 1 and alpha 3 beta 1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990 Nov;5(5):651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P., Damsky C. H. Regulation of extracellular matrix degradation by cell-extracellular matrix interactions. Cell Differ Dev. 1990 Dec 2;32(3):299–306. doi: 10.1016/0922-3371(90)90043-v. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Faber M., Liotta L. A., Albrechtsen R. Immunochemical and ultrastructural assessment of the nature of the pericellular basement membrane of human decidual cells. Lab Invest. 1985 Dec;53(6):624–633. [PubMed] [Google Scholar]
- Yeh I. T., Kurman R. J. Functional and morphologic expressions of trophoblast. Lab Invest. 1989 Jul;61(1):1–4. [PubMed] [Google Scholar]