Testosterone (T) is the principal sex hormone responsible for growth and development of the reproductive system in male vertebrates.1 It has historically received much attention not only from the medical and scientific communities2 but also from the general public, owing to its psychological and behavioral effects.3 T drives the asymmetry of several biological processes spanning virilization to anabolism, disease prevention,1 and aging.4 In this issue of Molecular Therapy, Aghazadeh et al.5 describe a novel fusion peptide, TVS167, that can induce T formation in rat testes and increase its serum level as well as rescue its synthesis in adult male rats exposed to antagonists of the gonadotropin-releasing hormone, which constitutes the initial step in the hypothalamic–pituitary–gonadal axis governing T production. This peptide acts by exploiting the interaction between the voltage-dependent anion channel 1 (VDAC1) and the 18-kDa translocator protein (TSPO) within the mitochondrial transduceosome, a multicomponent molecular machine that controls lipid import and steroidogenesis. This peptide reveals a regulatory mechanism in the mitochondrial pathway of steroid anabolism that could provide a new target for therapeutic intervention (see model in Figure 1).
Figure 1.
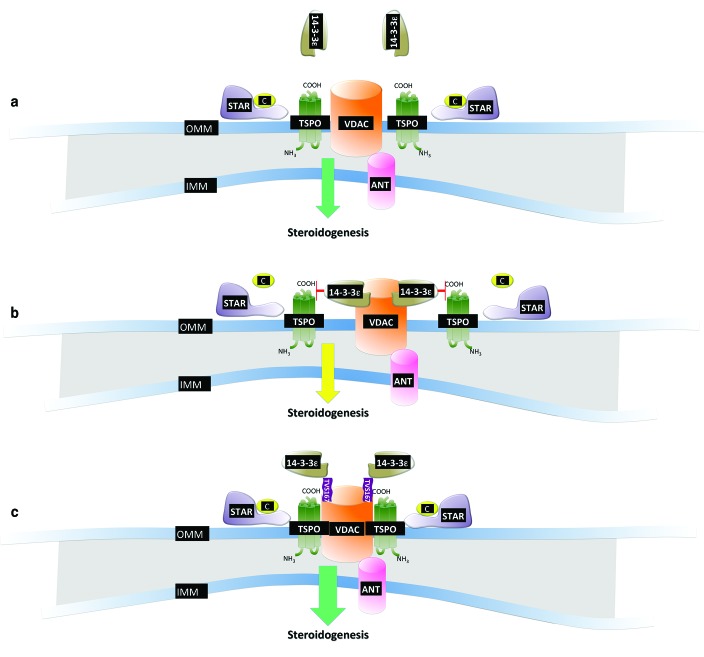
Optimization of TSPO-VDAC1 binding and mitochondrial steroidogenesis via the fusion peptideTVS167. (a) The molecular cooperation among the steroidogenic acute regulatory program (STAR), the voltage-dependent anion channel 1 (VDAC1), and the 18-kDa translocator protein (TSPO) is core to the transduceosome complex and drives steroidogenesis. (b) During hormone stimulation, the mitochondrial recruitment of 14-3-3ε prevents the functional interaction between VDAC1 and TSPO, thereby perturbing steroidogenesis. (c) TAT-VDAC1 Ser167 (TVS167) prevents the interaction between 14-3-3ε and VDAC1, facilitating TSPO-VDAC1 binding and steroidogenesis. ANT, adenine nucleotide translocase; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane.
T is produced in testicular Leydig cells, but the molecular mechanisms underlying this process remain unclear. Our poor understanding of the mechanisms governing T production limits the design of agonistic therapeutics, despite the pressing need to treat patients affected by reduced serum T levels.6 Conditions such as hypogonadism or castration lead to health issues that go beyond infertility and include fatigue, depression, as well as decreased lean body mass and bone mineral density. These clinical manifestations also occur in aged subjects for whom T availability is greatly limited.1,2,7 Currently, interventions to restore T levels are based on testosterone replacement therapy (TRT),6 a direct successor to the organotherapy-based approach that originated almost two centuries ago. In 1889, Charles-Edouard Brown-Séquard announced that he had rejuvenated after injecting himself with testicular extracts from guinea pigs and dogs, which he dubbed a “rejuvenating elixir.”8 However, it was later shown that the self-administered formulation contained little if any androgen, and the effects were therefore prevalently placebo-dependent.
The interest in T encouraged chemists to develop protocols to synthesize it in the laboratory, for which the Nobel Prize was awarded to Butenandt and Ruzicka in 1939.2 The availability of synthetic T enabled TRT without the need to isolate it from animals or tissues. Despite advancements in delivery formulations, TRT is associated with many side effects, warranting the development of alternative therapeutic strategies.
Mitochondria are central to the health of cells and tissues and act as decisional “hubs” for cellular responses by integrating different physiological and pathological input signals.9 They are tightly linked to basic energy-dependent functions as well as to more specialized cellular activities, such as maintenance of ion homeostasis, reactive oxygen and nitrogen species signaling, and apoptotic/necrotic cell death. They possess the ability to fuse or divide, move along microtubules and microfilaments, and undergo active turnover as a consequence of autophagy-related organelle quality control. Furthermore, they determine the quality and pace of androgen formation.10
Surprisingly, steroidogenesis has received relatively little attention compared to the many other symbiotic, tissue-specific functions performed by mitochondria. The principal regulatory mechanism of steroid hormone biosynthesis is the control of transfer of cholesterol from the outer to inner mitochondrial membrane. Hormonal stimulation of steroidogenic cells promotes this lipid import through a multiprotein complex, termed the transduceosome, spanning the two membranes. Cholesterol is thereby trafficked from cytosolic sources to be cleaved into pregnenolone by the product of the CYP11A1 gene.10 This gene encodes a member of the cytochrome P450 superfamily, which catalyzes many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids. The CYP11A1 protein localizes to the mitochondrial inner membrane and catalyzes the first and rate-limiting step in the synthesis of the steroid hormones.
The rate of cholesterol import itself depends on the transduceosome, which comprises cytosolic and outer mitochondrial membrane–based proteins: the hormone-induced steroidogenic acute regulatory protein (STAR), the high-affinity cholesterol-binding protein TSPO that contains a cytosolic cholesterol 64 recognition/interaction domain, and VDAC1.11 A precise physical interaction between TSPO, VDAC1, and STAR is essential to the function of the transduceosome, and interference in their homeostasis compromises the synthesis of steroids and overall mitochondrial physiology.12 Aghazadeh et al. demonstrate that the isoform ε of the 14-3-3 family of adapter proteins, located in the cytosol at resting conditions, is recruited to mitochondria following cell stimulation with human chorionic gonadotropin. This leads to reduced cholesterol import due to competition between 14-3-3 and TSPO for binding to VDAC1, which is dependent on amino acid Ser167 of the latter. By fusing part of HIV transcription factor 1 (TAT) to the in silico–predicted 14-3-3ε-binding motif of VDAC1, the authors created a fluorescence-labeled TAT-VDAC1 Ser167 (TVS167) peptide that easily penetrates cells and membranes and gives rise to effects on de novo synthesis or recovery of T formation in vitro and in vivo. TVS167 acts by limiting the interaction of the ε isoform of the 14-3-3 adapter protein with VDAC1, which increases the interaction between VDAC1 and TSPO, which in turn results in increased uptake of cholesterol and stimulation of steroidogenesis.
Such a discovery primes mitochondria as a therapeutic target site to treat T deficiencies, as well as providing further insight into the role of 14-3-3ε, as a scaffold protein that regulates the interplay between core components of the transduceosome during steroidogenesis. Notably, TSPO, which is an established biomarker in clinical diagnostics,13 emerges as the downstream effector of this cascade, because 19-Atriol—a chemical blocker of its cholesterol-binding capacity—abrogates the effects of TVS167. Moreover, the effects of TVS167 are independent from the luteinizing hormone, which is a major effector of the side effects of TRT, thus implying a greater safety profile for systemic use. TVS167 therefore represents a promising lead agent for the development of medical treatments of hypogonadism or conditions currently treated with the exogenous administration of T. The high degree of cross-species homology of both 14-3-3ε and VDAC1, which are the molecular targets for TVS167, provides hope for its possible efficacy in humans.
TVS167 applications could also be extended to aging subjects whose health and quality of life is jeopardized by T deficiency. The age-related decline of the endocrine system, known as endocrinosenescence, notably affects the production of sex steroids with an increased production of inflammatory cytokines that contribute to chronic inflammation underlining cellular and tissue senescence,14 in which malfunctioning mitochondria play an important role. The mitochondrial theory of aging entails progressive oxidative damage to mitochondrial DNA that results in dysregulation of cell and organ function leading to overall system decline.15 The mitochondrial DNA mutations that have been reported are likely to underlie loss of function in the testis, but this as yet remains poorly defined. TVS167 could therefore represent a therapeutic aid for this as well as a much-needed tool to study this aspect of senescence.
In summary, the identification of the lead peptide TVS167 brings to light a critical step in androgen biosynthesis focused on a functional interplay between TSPO and VDAC1. It further established a possible curative strategy to advance an organelle-targeted therapy for androgen equilibrium.
Acknowledgments
A thanks goes to Jemma Gatlliff for assisting with the model depicted in Figure 1. The research activity on TSPO led by the author is supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Medical Research Council (MRC), Petplan Charitable Trust (PPCT), MarieCurie Actions, LAM-BIGHI Research Grant and Umberto Veronesi Foundation.
References
- Huhtaniemi I., and, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. 2011;8:335–344. doi: 10.1038/nrurol.2011.47. [DOI] [PubMed] [Google Scholar]
- Freeman ER, Bloom DA., and, McGuire EJ. A brief history of testosterone. J Urol. 2001;165:371–373. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Naef M, Snozzi R, Heinrichs M., and, Fehr E. Prejudice and truth about the effect of testosterone on human bargaining behavior. Nature. 2010;463:356–359. doi: 10.1038/nature08711. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser F, Raum WJ, Perry HM, Flood JF, Jensen J.et al. (1997Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone Proc Natl Acad Sci USA 947537–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M., and, Papadopoulos V. Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3ε protein adaptor and mitochondrial VDAC1 interactions. Mol Ther. 2014;22:1779–1791. doi: 10.1038/mt.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay AT, Spark RF, Bansal S, Cunningham GR, Goodman NF, Nankin HR.et al. (2003American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: a couple's problem Endocr Pract 977–95. [DOI] [PubMed] [Google Scholar]
- Carruthers M. Time for international action on treating testosterone deficiency syndrome. Aging Male. 2009;12:21–28. doi: 10.1080/13685530802699067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Séquard CE. Note on the effects produced on man by subcutaneous injections of liquid obtained from the testicles of animals. Lancet. 1889;134:105–107. [Google Scholar]
- McBride HM, Neuspiel M., and, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J., and, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import. Mol Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P.et al. (2006Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function Trends Pharmacol Sci 27402–409. [DOI] [PubMed] [Google Scholar]
- Gatliff J, East D, Crosby J, Abeti R, Craigen W, Harvey J.et al. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control Autophagyin press). [DOI] [PMC free article] [PubMed]
- Gatliff J., and, Campanella M. The 18 kDa translocator protein (TSPO): a new perspective in mitochondrial biology. Curr Mol Med. 2012;12:356–368. doi: 10.2174/1566524011207040356. [DOI] [PubMed] [Google Scholar]
- Scarf AM., and, Kassiou M. The translocator protein. J Nucl Med. 2011;52:677–680. doi: 10.2967/jnumed.110.086629. [DOI] [PubMed] [Google Scholar]
- Bratic A., and, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951-957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]