Abstract
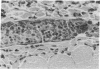
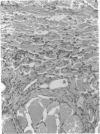
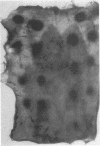
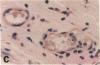
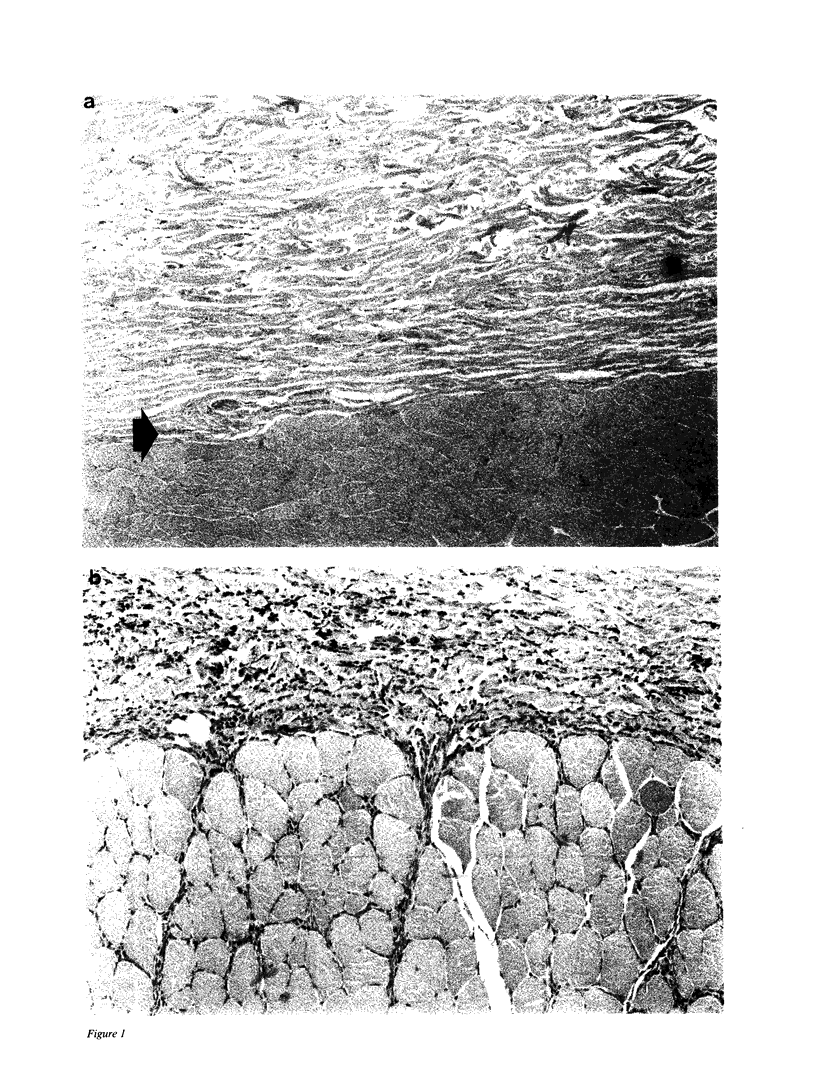
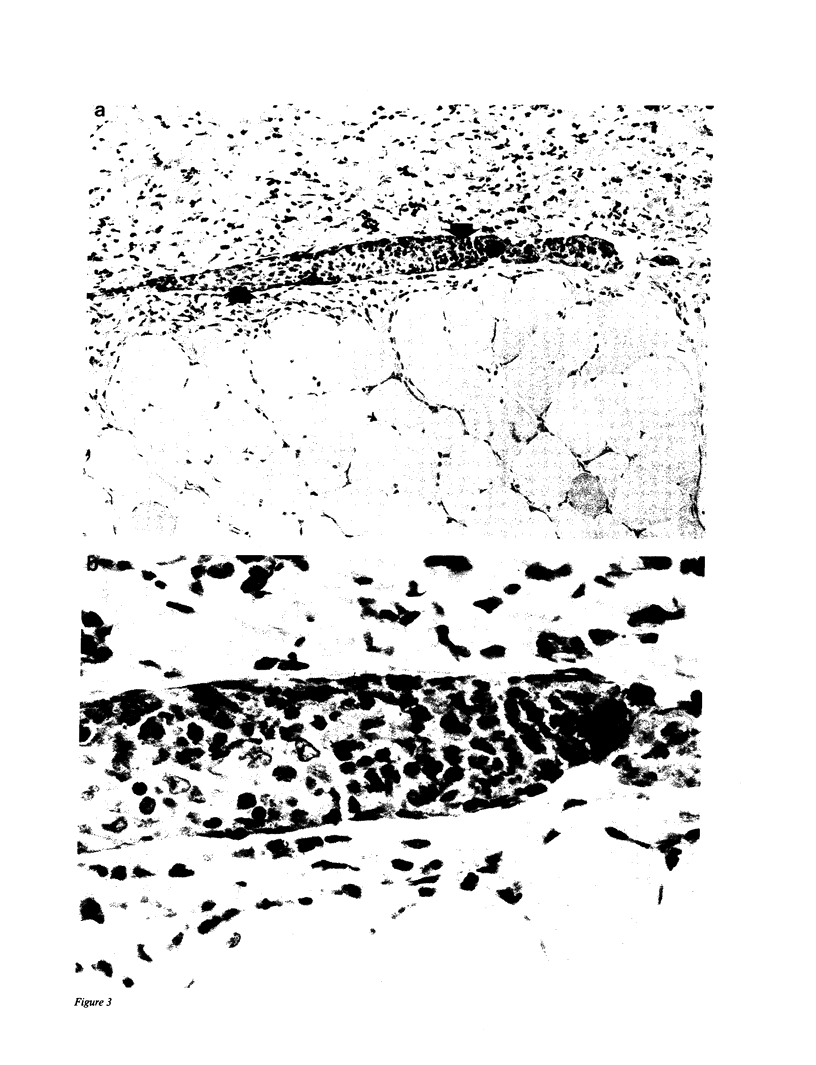
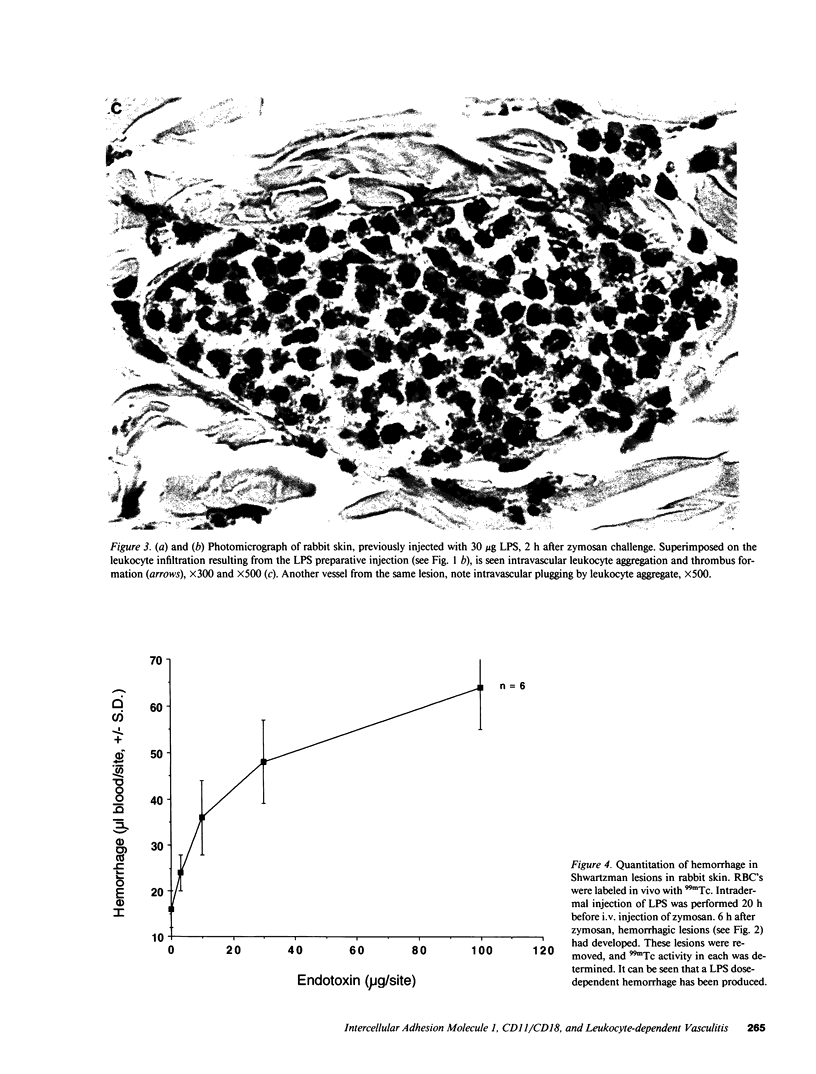
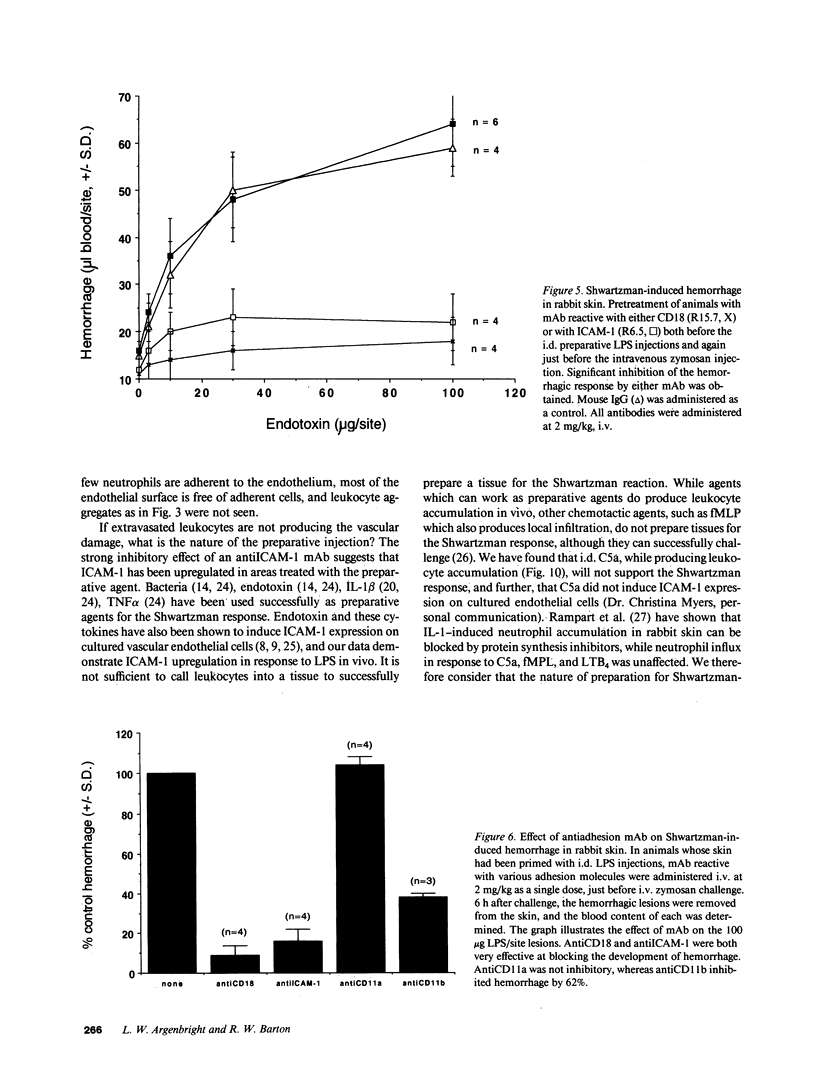
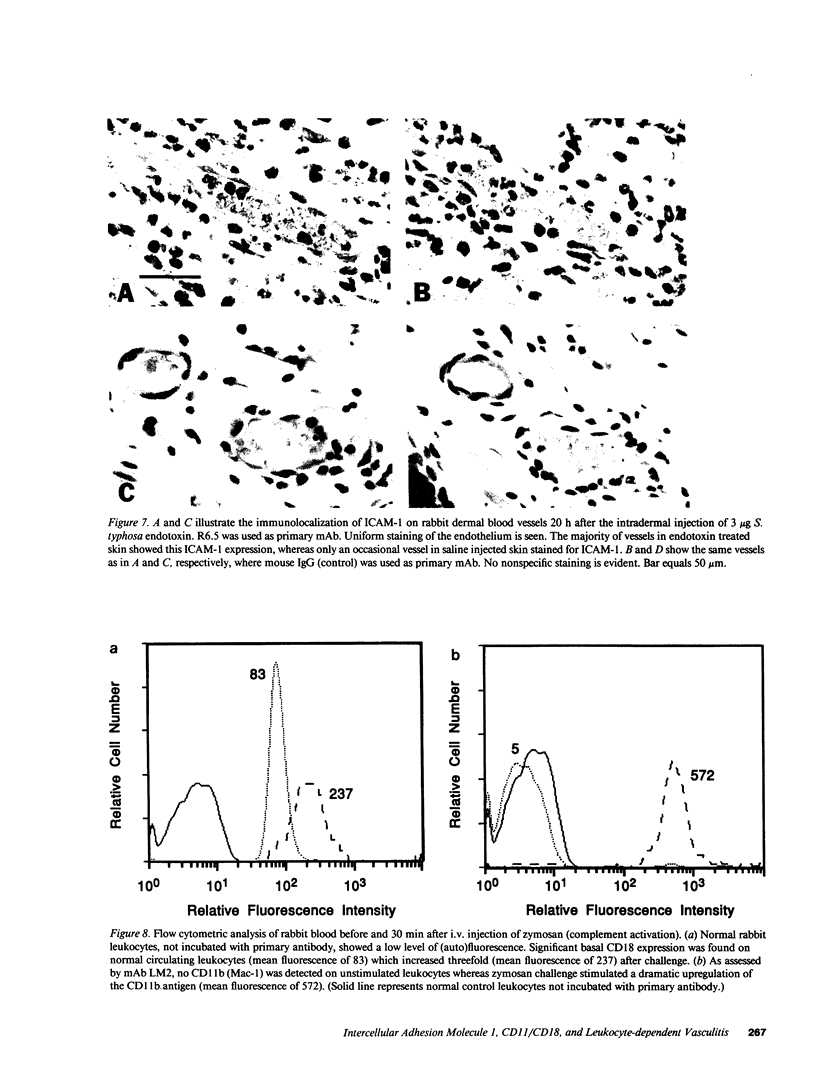
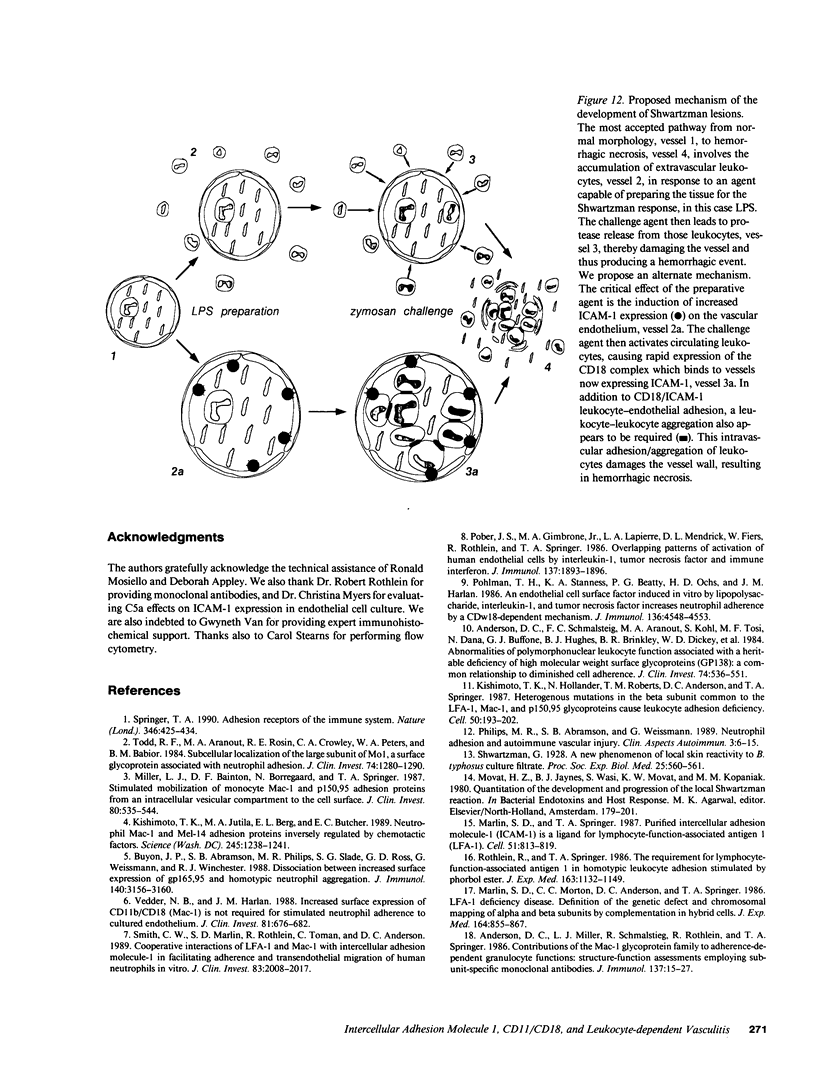
We have investigated the role of leukocyte-endothelial cell interactions in a rabbit model of hemorrhagic vasculitis. Microvascular injury was produced in the skin by intradermal injection of Salmonella typhosa endotoxin followed 20 h later by intravenous zymosan, which activates complement. Hemorrhagic necrosis develops in the "prepared" skin sites which is characterized by microthrombi, neutrophil aggregation, platelet and fibrin deposition, and massive extravasation of erythrocytes. Hemorrhage in these Shwartzman-like lesions was quantitated by 99mTc-labeled autologous erythrocytes. Inhibition of the hemorrhagic response was obtained with mAb reactive with ICAM-1 as well as mAb against the leukocyte CD18 when either was administered intravenously just before intravenous zymosan challenge. This observation suggests that an intravascular event occurring in response to complement activation is required for the development of hemorrhagic vasculitis. We hypothesize that agents which successfully prepare the skin for the Shwartzman response after their intradermal injection do so by promoting increased intercellular adhesion molecule 1 (ICAM-1) expression on the vascular endothelium. Activation of complement then induces CD11/CD18 expression on circulating leukocytes thus producing an intravascular CD11/CD18-ICAM-1 (leukocyte-endothelium) adhesion event. Inhibition of intravascular leukocyte-leukocyte aggregation with mAb against CD11b (Mac-1) showed partial inhibition of hemorrhage, while mAb against CD11a (LFA-1) showed no inhibitory activity. This type of cytokine-primed, neutrophil-dependent vascular damage may be a model of human vasculitic processes where microvascular damage is produced in the absence of immune-complex deposition.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenbright L. W., Letts L. G., Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J Leukoc Biol. 1991 Mar;49(3):253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Buyon J. P., Abramson S. B., Philips M. R., Slade S. G., Ross G. D., Weissmann G., Winchester R. J. Dissociation between increased surface expression of gp165/95 and homotypic neutrophil aggregation. J Immunol. 1988 May 1;140(9):3156–3160. [PubMed] [Google Scholar]
- Buyon J. P., Shadick N., Berkman R., Hopkins P., Dalton J., Weissmann G., Winchester R., Abramson S. B. Surface expression of Gp 165/95, the complement receptor CR3, as a marker of disease activity in systemic Lupus erythematosus. Clin Immunol Immunopathol. 1988 Jan;46(1):141–149. doi: 10.1016/0090-1229(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Fehr J., Dahinden C., Russi R. Formylated chemotactic peptides can mimic the secondary, provoking endotoxin injection in the generalized Shwartzman reaction. J Infect Dis. 1984 Jul;150(1):160–161. doi: 10.1093/infdis/150.1.160. [DOI] [PubMed] [Google Scholar]
- Gu J., McGrath L. B. Localized endocrine conversion of ventricular cardiocytes in ventricular aneurysm. J Histochem Cytochem. 1990 Nov;38(11):1659–1668. doi: 10.1177/38.11.2145358. [DOI] [PubMed] [Google Scholar]
- HALPERN B. N. INHIBITION OF THE LOCAL HEMORRHAGIC SHWARTZMAN REACTION BY A POLYPEPTIDE POSSESSING POTENT ANTIPROTEASE ACTIVITY. Proc Soc Exp Biol Med. 1964 Feb;115:273–276. doi: 10.3181/00379727-115-28889. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Weaver L. J., Hudson L. D., Craddock P. R., Jacob H. S. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980 May 3;1(8175):947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Hopkins P., Belmont H. M., Buyon J., Philips M., Weissmann G., Abramson S. B. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum. 1988 May;31(5):632–641. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Hollander N., Roberts T. M., Anderson D. C., Springer T. A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987 Jul 17;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Morton C. C., Anderson D. C., Springer T. A. LFA-1 immunodeficiency disease. Definition of the genetic defect and chromosomal mapping of alpha and beta subunits of the lymphocyte function-associated antigen 1 (LFA-1) by complementation in hybrid cells. J Exp Med. 1986 Sep 1;164(3):855–867. doi: 10.1084/jem.164.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori W. Intravascular coagulation as a clinical manifestation of the Shwartzman reaction. Bibl Haematol. 1983;(49):41–48. doi: 10.1159/000408445. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Wasi S. Severe microvascular injury induced by lysosomal releasates of human polymorphonuclear leukocytes. Increase in vasopermeability, hemorrhage, and microthrombosis due to degradation of subendothelial and perivascular matrices. Am J Pathol. 1985 Dec;121(3):404–417. [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Horn J. K., Ong R., Goldstein I. M. Complement (C5)-derived chemotactic activity in serum from patients with pancreatitis. J Lab Clin Med. 1983 Jan;101(1):123–129. [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., Giclas P. C., Terry R., Boyer J. T., O'Rourke R. A. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975 Sep;56(3):740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., GOOD R. A. Studies on the mechanism of the Shwartzman-phenomenon; evidence for the participation of polymorphonuclear leucocytes in the phenomenon. J Exp Med. 1951 Jan;93(1):49–63. doi: 10.1084/jem.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich R. P., Jevnikar A. M., Takei F., Glimcher L. H., Kelley V. E. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990 Feb;136(2):441–450. [PMC free article] [PubMed] [Google Scholar]