Abstract
Milk, the secretory product of the lactation genome, promotes growth of the newborn mammal. Milk delivers insulinotropic amino acids, thus maintains a molecular crosstalk with the pancreatic β-cell of the milk recipient. Homeostasis of β-cells and insulin production depend on the appropriate magnitude of mTORC1 signaling. mTORC1 is activated by branched-chain amino acids (BCAAs), glutamine, and palmitic acid, abundant nutrient signals of cow´s milk. Furthermore, milk delivers bioactive exosomal microRNAs. After milk consumption, bovine microRNA-29b, a member of the diabetogenic microRNA-29-family, reaches the systemic circulation and the cells of the milk consumer. MicroRNA-29b downregulates branched-chain α-ketoacid dehydrogenase, a potential explanation for increased BCAA serum levels, the metabolic signature of insulin resistance and type 2 diabetes mellitus (T2DM). In non-obese diabetic mice, microRNA-29b downregulates the anti-apoptotic protein Mcl-1, which leads to early β-cell death. In all mammals except Neolithic humans, milk-driven mTORC1 signaling is physiologically restricted to the postnatal period. In contrast, chronic hyperactivated mTORC1 sig-naling has been associated with the development of age-related diseases of civilization including T2DM. Notably, chronic hyperactivation of mTORC1 enhances endoplasmic reticulum stress that promotes apoptosis. In fact, hyperactivated β-cell mTORC1 signaling induced early β-cell apoptosis in a mouse model. The EPIC-InterAct Study demonstrated an association between milk consumption and T2DM in France, Italy, United Kingdom, Germany, and Sweden. In contrast, fermented milk products and cheese exhibit an inverse correlation. Since the early 1950´s, refrigeration technology allowed widespread consumption of fresh pasteurized milk, which facilitates daily intake of bioactive bovine microRNAs. Persistent uptake of cow´s milk-derived microRNAs apparently transfers an overlooked epigenetic diabetogenic program that should not reach the human food chain.
Keywords: Branched-chain amino acid dysmetabolism, β-cell apoptosis, endoplasmic reticulum stress, diabetogenic milk-derived microRNAs, mTORC1, type 2 diabetes mellitus
INTRODUCTION
The worldwide rising prevalence of type 2 diabetes mellitus (T2DM) has been attributed to rising rates of obesity and poor lifestyles, genetic and developmental susceptibility to disease [1]. According to the US National Diabetes Statisitics Report 2014 [2] 29.1 million people or 9.3% of the U.S. population have diabetes mellitus.
T2DM is characterized by progressive β-cell dysfunctioning, early β-cell apoptosis resulting from low-grade local and systemic inflammation, increased oxidative stress and insulin resistance, recently extensively reviewed elsewhere [3-6].
Notably, since the 1960´s there is a steady increase in the prevalence rate of T2DM in industrialized countries [7]. Shortly prior to the 1960’s, the refrigerator and widespread cooling technology was introduced into most households and food stores of developed countries. Cooling technology allows daily access to fresh pasteurized cow´s milk, whereas in former times and less developed countries primarily fermented milk and milk products have been consumed due to the unavailability of cooling facilities.
The pathogenesis of age-related diseases of civilization such as obesity, T2DM, metabolic syndrome, cancer, neurodegenerative diseases and early aging have all been related to persistently increased activation of the nutrient-sensitive kinase mechanistic target of rapamycin complex 1 (mTORC1) [8-14]. Remarkably, milk is the only and sufficient nutrient system of all mammals that allows appropriate postnatal growth. It has recently been recognized that milk is not “just food” but represents a sophisticated signaling system of mammalian evolution that adequately activates mTORC1 of the cells of the milk recipient to drive controlled species-specific growth [15]. Milk contains abundant essential branched-chain amino acids (BCAAs: leucine, isoleucine, and valine) and glutamine that are important nutrient signals that communicate with pancreatic β-cells of the milk recipient to promote mTORC1-mediated insulin synthesis and secretion. Insulin itself is an important growth hormone that activates mTORC1 of insulin-dependent cells facilitating anabolism and growth of the newborn organism. Notably, it is not the carbohydrate content of milk and skim milk that exerts strong insulinotropic effects, but the insulinotropic amino acids that operate as nutrient signals stimulating insulin secretion. In all mammals, except humans since the Neolithic revolution, this insulinotropic signaling system is restricted to the postnatal growth phase and has not been designed by evolution for lifelong use. Downregulation of intestinal lactase expression resulting in lactose intolerance after the weaning period is thus the original genetic makeup of humans and all other mammals.
It is the intention of this review to provide evidence that persistent abuse of mTORC1-activating and microRNA-transmitting bovine milk is a major overlooked pathogenic and epigenetic factor promoting the epidemic of T2DM.
1. MILK´S HARDWARE DRIVING MTORC1 SIGNALING
mTORC1 of β-cells plays a pivotal role in β-cell homeostasis, insulin synthesis and insulin secretion [16-19]. mTORC1 orchestrates cell growth and proliferation [20]. mTORC1 is the central hub of metabolism that activates nucleotide, protein and lipid synthesis and promotes cell cycle progression under conditions of nutrient and growth factor availability [20-30]. Moreover, mTORC1 plays a fundamental role in lipid accumulation and adipogenesis [31-35]. Thus, persistently overactivated mTORC1 signaling stimulates weight gain, increases body mass, and fat mass [31-36], known risk factors promoting the development of T2DM.
Basically, there are five major pathways that activate mTORC1: 1) growth factors such as insulin and IGF-1 [20, 23, 24,28], 2) sufficient cellular energy (glucose, ATP) [36-38], 3) the availability of amino acids, predominantly essential BCAAs such as leucine [21-29, 39], 4) the presence of glutamine for cellular leucine uptake and glutaminolysis-mediated activation of mTORC1 [40-42], and 5) the availability of saturated fatty acids, especially palmitic acid [43] (Fig.1).
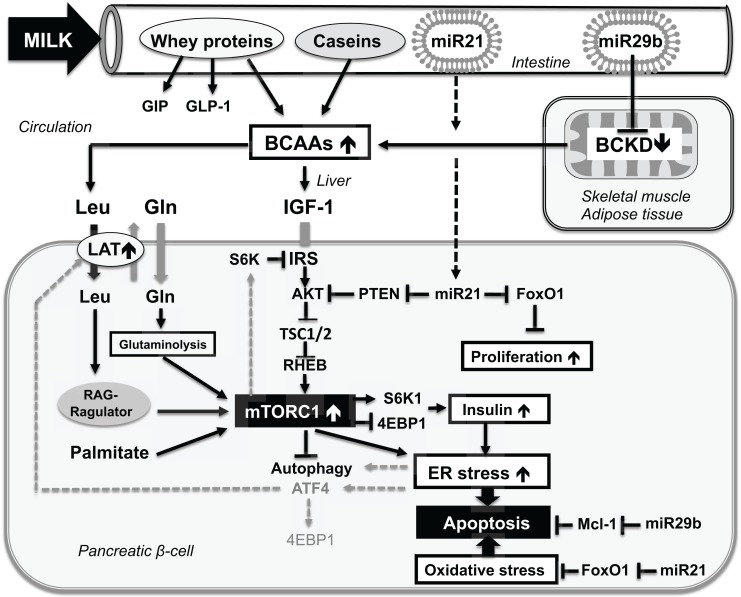
Fig. (1).
Schematic working model representing the potential crosstalk bewteen milk signaling and persistent β-cell mTORC1 hyperactivation promoting endoplasmic reticulum (ER)-stress and early β-cell apoptosis. Milk is a rich source of branched-chain amino acids (BCAAs). Leucine (Leu) and glutamine (Gln) synergistically activate β-cell mTORC1. mTORC1 activation is important for insulin synthesis. Insulin synthesis is further promoted by whey-stimulated secretion of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1(GLP-1). Milk-derived exosomal microRNA-29b (miR29b) may represent a shutoff mechanism of mitochondrial BCAA catabolism that increases BCAA plasma levels enhancing BCAA-driven β-cell mTORC1 activation. Persistent milk-mediated β-cell mTORC1 activation promotes ER-stress leading to early β-cell apoptosis. ER-clearance is impaired by mTORC1-mediated inhibition of autophagy further promoting β-cell apoptosis. ER-stress in a vicious cycle via activating transcription factor 4 (ATF4)-mediated upregulation of L-type amino acid transporter (LAT) aug-ments leucine-mTORC1 signaling. Further enhancement of β-cell apoptosis may result from milk miR29b-mediated inhibition of anti-apoptotic Mcl-1. Milk miR-21-mediated inhibition of FoxO1 may increase β-cell proliferation and may increase oxidative stress further promoting β-cell apoptosis. See list of abbreviations.
It will be demonstrated that persistent and excessive cow´s milk consumption provides and mediates abundant nutrient and growth factor signals that overactivate β-cell mTORC1 signaling of the human milk recipient promoting endoplasmic reticulum (ER)-stress and early β-cell apoptosis.
1.1. Milk Provides BCAAs Activating mTORC1
Milk proteins provide highest amounts of essential BCAAs, especially leucine [44]. Leucine plays a pivotal role for activating mTORC1 [39]. Of all animal proteins, whey proteins contain the highest amount of leucine (14%) [44], and in comparison to meat (8% leucine), whey proteins undergo fast intestinal hydrolysis, thus operate like an i.v.-amino acid infusion [45-49].
1.2. Milk Provides Glutamine Activating mTORC1
Milk protein (8.09 g glutamine/100 g) in comparison to beef protein (4.75 g glutamine/100 g) provides 70% more glutamine [49]. In the β-cell, glutamine is an important activator of mTORC1. Glutamine functions as a gatekeeper for cellular leucine uptake via the L-type amino acid transporter (LAT) and is a precursor of the glutaminolysis pathway that activates mTORC1 [16, 50-52]. Leucine is an allosteric activator of glutamate dehydrogenase (GDH), the key-regulating enzyme of the glutaminolysis pathway [50-52]. This intimate interplay of glutamine and leucine maximizes the flux through GDH in pancreatic β-cells, which is important for mTORC1-S6K1-dependent insulin secretion [52]. Thus, leucine and glutamine should be regarded as milk´s amino acid messengers that activate β-cell mTORC1 signaling during the postnatal period of mammalian life.
1.3. Milk Stimulates Incretin and Insulin Secretion
The insulinemic index of whole cow´s milk (148±14) and skim milk (140±13) is much higher than the glycemic indices of whole milk (42±5) and skim milk (37±9), repectively [53,54]. Fast hydrolysis and immediate intestinal absorption of insulinotropic amino acids of the whey protein fraction of cow´s milk raise insulin levels to much higher magnitudes than intestinal digestion of structural proteins such as beef (insulinemic index: 51) [53,54]. The major insulinotropic protein fraction of cow´s milk is the whey protein fraction [55]. Whey-derived leucine and other whey-derived amino acids stimulate glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP-1) secretion of enteroendocrine K- and L-cells, respectively [56-60]. Dipeptidyl peptidase-IV (DPP-IV) activity is of critical importance for the biological activity and half-life of these incretins. Remarkably, milk protein-derived peptides have been shown to inhibit DPP-IV activity and thus increase incretin activity enhancing incretin-mediated insulin synthesis and secretion [61,62].
Additionally, whey-derived amino acids directly exert inulinotropic effects on pancreatic β-cells [16,18,19]. Milk protein consumption in comparison to meat protein intake thus results in significant hyperinsulinemia [63]. Furthermore, i.v.-infusion of bovine β-casomorphin 4, a bovine milk protein peptide that is generated in the intestinal tract, has been shown to stimulate μ-receptors of β-cells and substantially increases insulin secretion in dogs [64]. Thus, milk intake may also stimulate neuroendocrine regulatory networks of the β-cell that augment insulin secretion.
1.4. Milk Stimulates IGF-1 Secretion Activating mTORC1
A meta-analysis confirmed that continued milk consumption increases serum levels of insulin-like growth factor-1 (IGF-1) [65]. The European Prospective Investigation into Cancer and Nutrition (EPIC) confirmed a relationship between milk intake in 2,109 European women with increased IGF-1 serum levels [66]. A 20% increase in serum IGF-1 levels has been observed in prepubertal children previously not used to milk consumption after a daily intake of 710 mL of ultra-heat treated (UHT) milk for 4 weeks [67]. A recent study including 193 overweight adolescents aged 12-15 years drank either 1L/day of skim milk, whey, casein or water for 12 weeks. All milk-based-drinks contained 35 g milk protein/L. IGF-1 significantly increased with skim milk and tended to increase with casein compared to the pre-test control group [68].Casein in comparison to whey protein has been shown to differentially enhance hepatic IGF-1 synthesis [55]. Notably, per capita cheese comsumption, the major dairy source of casein, increased in Germany from 5 kg in 1950 to 24.4 kg in 2013 [69].
1.5. Milk Provides Palmitic Acid Activating mTORC1
Bovine milk contains about 3.5 to 5% total lipid. About 98% of the lipid is composed of triacylglycerols, transported in milk fat globules [70]. The major fatty acid of total fatty acids of milk lipids is palmitate (C16:0) with 32.3 wt% [70,71]. Palmitate like BCAAs activates mTORC1 at the lysosomal compartment [43].
Thus milk, the promoter of postnatal growth of mammals, activates mTORC1 of the milk recipient either by transfer or induction of pivotal mTORC1 activating signaling pathways.
2. MILK´S SOFTWARE: EXOSOMAL MICRORNAS
In 2013, Melnik and colleagues [15] hypothesized that milk functions as a genetic transfection system of the milk recipient by transfer of exosomal bioactive bovine milk microRNAs to modulate early metabolic programming. The microRNA regulatory network appears to represent milk´s epigenetic software that augments mTORC1 signaling and cell cycle progression to optimize growth conditions of the newborn mammal. Valadi et al. [72] were the first who demonstrated that exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Thus, secreted exosomal microRNAs have been appreciated as important players of gene regulation and intercellular communication [73-75]. MicroRNAs bind through partial sequence homology to the 3´-untranslated region (UTR) of target mRNAs and cause either translational block or mRNA degradation [76].
Recently, Witwer and Hirschi [77] challenged the hypothesis that diet-derived foreign microRNAs, especially plant-derived xenomirs, might be absorbed and could regulate vertebrate metabolism and function. The caveat of Witwer and Hirschi [77] is primarily based on repeated negative results evaluating the intestinal uptake of plant-derived microRNAs [78-80], which could not confirm the reported intestinal absorption of rice-derived MIR168a [81].
In mammals however, microRNAs enclosed by membranous microvesicles (exosomes) play a pivotal role for horizontal microRNA transfer [82]. Milk is apparently mammal´s longest distance interindividual exosomal signaling system that allows maternal-neonatal communication for metabolic regulation of the newborn. In fact, breast milk in comparison to all other human body fluids contains the highest amounts of total RNAs [83]. It has been suggested that bovine and human milk exosomal microRNAs may be transferred to the infant to promote immune regulatory functions [84-86]. MicroRNA-containing exosomes of 30-100 nm diameter have been identified in human breast milk, cow´s milk, bovine whey and colostrum [87-89]. Exosomes from bovine colostrum and mature milk are able to deliver microRNAs into cultured cells thereby increasing cytoplasmic microRNA levels [89]. Recently, Baier et al. [90] provided evidence that microRNAs of commercial pasteurized cow´s milk are absorbed by adult human subjects in biologically meaningful amounts from nutritionally relevant doses of cow´s milk and affected gene expression of human peripheral blood mononuclear cells, HEK-293 kidney cell cultures and mouse liver cells. The authors estimated that the 245 microRNAs of bovine milk may modulate the transcription of more than 11,000 human genes [90]. The lipid bilayer of milk exosomes protects their microRNA cargo against harsh degrading conditions like low acidic pH of 1-2 and RNase-mediated degradation [86, 91]. Boiling of milk, however, results in complete microRNA degradation [92]. Recently, Howard et al. [93] studied the influence of homogenization, pasteurization, microwave treatment and storage at 4°C of commercial whole milk, fat reduced (2%) milk and skim cow´s milk on microRNA recovery. Notably, more than 50% of microRNA-29b was still detectable after pasteurization and homogenization of whole and 2% fat milk in comparioson to raw milk [93]. Nearly one third of microRNA-29b was recovered in skim milk after these procedures [93]. Storage of 2% fat milk for 15 days did not affect microRNA-29b concentrations [93]. Thus, raw cow´s milk contains the highest amounts of bioactive microRNAs, whereas pasteurized refrigerated commercial milk still contains substantial amounts of bioactive microRNAs [90, 93].
2.1. MicroRNA-21 Activates mTORC1 and Inactivates FOXO1
A major microRNA type in cow´s milk is bovine microRNA-21 [85], which is identical with the humun microRNA-21 (www.mirbase.org). Notably, microRNA-21 is involved in phosphoinositol-3-kinase (PI3K)-AKT and mTORC1 signaling pathways [94]. Critical targets of microRNA-21 are mRNAs of important tumor suppressor proteins involved in upstream and downstream suppression of mTORC1 signaling such as phosphatase and tensin homolog (PTEN) [95-97], Sprouty1 and Sprouty2 [98-100], and programmed cell death 4 (PDCD4) [101-103]. Moreover, microRNA-21 has been shown to induce the cell cycle promoter cyclin D1 in an mTORC1-dependent manner [104]. MicroRNA-21-mediated inhibition of Sprouty1 and Sprouty2 amplifies RAS-RAF-MEK-ERK signaling, which suppresses TSC2 (tuberin) and thus raises mTORC1 activity. Furthermore, microRNA-21 stimulates the initiation of translation by repression of PDCD4, which is a suppressor of translation initiation that inhibits the RNA helicase eIF4A [105]. Both, 4E-binding protein-1 (4E-BP-1) and PDCD4 are thus crucial regulatory inhibitors of translation initiation that control protein synthesis. Activation of the mTORC1 pathway and its substrate kinase S6K1 results in subsequent phosphorylation of 4E-BP-1 and PDCD4 that promotes eIF4E-eIF4G complex assembly stimulating mRNA translation [104].
Furthermore, microRNA-21 induces adipogenic differentiation and proliferation of human adipose tissue-derived mesenchymal stem cells [106, 107], thus promotes fat mass accretion. MicroRNA-21 orchestrates high glucose-induced signals to TORC1, resulting in renal cell pathology in T2DM [108]. Moreover, microRNA-21 has been demonstrated to promote renal injury in a mouse model of T2DM [109]. Thus, excess intake of bovine milk microRNA-21 may overstimulate β-cell mTORC1 activation and may contribute to renal mTORC1-driven pathology in T2DM.
Recently, the mRNA of forkhead box class O1A transcription factor (FoxO1) has been indentified as a direct target of microRNA-21 [110]. FoxO1 is a key transcription factor that plays a central role in the regulation of glucose metabolism, insulin signaling, and β-cell homeostasis [6, 110-115]. FoxO1 controls β-cell replication via the insulin/IGF-1 signaling pathway [6]. Moreover, FoxO1 regulates the expression of antioxidant enzymes such as catalase and superoxide dismutase 2 [116]. FoxO1 apparently protects β-cells against oxidative stress [6, 117] and oxidative stress-induced apoptosis [6, 117, 118] (Fig. 1). Milk is physiologically provided during the postnatal growth phase, a period with enhanced insulin demands. From a mechanistical point of view, it is conceivable that milk promotes β-cell replication and β-cell mass expansion by epigenetic transfer of exosomal microRNA-21 that downregulates FoxO1.
2.2. microRNA-29b: Milk´s Inhibitor of BCAA Catabolism?
Serum levels of circulating BCAAs are increased in obese individuals and are associated with future insulin resistance and T2DM [119-140]. Thus, increased serum BCAA levels are believed to represent a critical metabolic signature of insulin resistance and T2DM [128,132, 134-142].
The rate-controlling step of BCAA catabolism is catalyzed by the multienzyme mitochondrial branched-chain α-ketoacid dehydrogenase (BCKD) [143]. About half of the BCKD catalytic activity resides in skeletal muscle, whereas a considerable portion of activity also resides in adipose tissue [143]. The BCKD complex is composed of the branched-chain keto acid dehydrogenase (BCKDHA), the dihydrolipoamide branched-chain transacylase (DBT), and the dihydrolipoamide dehydrogenase (DLD) [143, 144]. Notably, the DBT forms the core of the BCKD complex [145]. Mersey and coworkers [146] provided evidence that human microRNA-29b controls the expression of the BCKD complex at the level of mRNA translation. Human microRNA-29b recognizes the mRNA of DBT, which forms the core of the BCKD complex and provides the binding site for all other proteins in the BCKD complex including the branched-chain α-keto acid dehydrogenase kinase (BCKDK) [146, 147]. Thus, microRNA-29b, a major microRNA of bovine milk [86, 92], suppresses BCAA catabolism. Intriguingly, Baier and coworkers [90] demonstrated that bovine microRNA-29b, which is identical to their human ortholog (www.mirbase.org), increased in substantial amounts and in a dose-dependent manner in healthy milk consumers. Furthermore, microRNA-29b increased in peripheral blood mononuclear cells (PBMCs) associated with a doubling of cellular microRNA-29b levels six hours after oral exposure to commercial cow´s milk [90]. Remarkably, milk consumption evoked 30% changes in microRNA-29b target gene expression in human PBMCs [90]. From these data, it is conceivable to suggest that bovine microRNA-29b may modify mRNA levels of its target DBT mRNA, which translates the functionally most important core component of the BCKD complex (Fig.1).
Why should milk inhibit BCAA catabolism? There is good reason to believe that milk provides a regulatory epigenetic microRNA program that rescues valuable essential BCAAs from mitochondrial oxidation in order to favor their incorporation into important functional and structural proteins required for growth and development. Leucine, isoleucine and valine are among the most hydrophobic amino acids, which are crucial determinants for hydrophobic clefts of enzymes and are important for the insertion of proteins such as receptors into cell membranes [143]. Hydrophobic residues are crucial for oxygen binding in hemoglobin and myoglobin and substrate binding of various enzymes [148]. Remarkably, surfactant protein B contains 37% of BCAAs (17.7% leucine) [149]. Leucine-rich transcription factors such as leucine zippers are most important for adequate regulation of transcription and cell signaling [150]. It is thus conceivable, that BCAA catabolism should be attenuated during the process of postnatal growth and development. From this perspective, it makes sense that milk provides a signal to switch off BCAA catabolism in order to increase BCAA levels for the formation of vitally important BCAA-dependent proteins. Notably, in stored and refrigerated whole milk and 2% fat milk more than 50% of microRNA-29b is preserved compared to unprocessed raw milk [93].
There is accumulating evidence that commercial milk consumption is associated with increased body mass index and obesity in humans and in a recently reported mouse model, which demonstrated increased mTORC1-S6K1 activation during bovine milk consumption [33,151-155]. In addition, milk-mediated transfer of microRNA-21, which induces adipogenesis [106,107], may function as a further mechanism that promotes the development of obesity, the most common comorbidity of T2DM. It has recently been demonstrated in db/db mice that long-term inhibition of microRNA-21 reduced obesity [156].
Remarkably, BCKD component transcripts were significantly lower in subcutaneous adipocytes from obese versus lean Pima Indians [139]. Moreover, mRNA abundances for BCAA catabolic enzymes were markedly reduced in omental white adipose tissue of obese persons with metabolic syndrome compared with weight-matched healthy obese subjects [139]. Lynch and Adams [157] recently concluded that attenuated mitochondrial BCAA metabolism, which appears to be a critical metabolic deviation in T2DM, is either caused by altered gene expression, mutations, or epigenetic factors that affect gene expression. Notbably, BCKDHA (branched-chain α-keto acid dehydrogenase), the gene encoding the regulated unit of BCKDC is one of the primary susceptibility genes identified that affected the risk of both T2DM and obesity [158].
Nevertheless, genetic mutations cannot explain the worldwide and steady rise in diabetes prevalence. Thus, an epigenetic modifier such as a very common dietary factor may promote BCAA dysregulation of human beings. Bovine milk microRNA-29b-attenuated BCAA catabolism may be the missing overlooked epigenetic factor that is responsible for the epidemic of T2DM.
2.3. Milk-microRNA-Mediated Insulin Resistance
Milk contains substantial amounts of the microRNA let-7 family (let-7a, let-7b, let-7c and let-7f) [85, 89]. There is accumulating evidence that the Lin28/let-7 axis regulates glucose metabolism [159]. Muscle specific loss of Lin28a, a developmentally regulated RNA-binding protein, and overexpression of let-7 resulted in insulin resistance and impaired glucose tolerance in mice [159]. Intriguingly, let-7 targets are enriched for genes that contain SNPs associated with T2DM and fasting glucose in human genome-wide association studies [159]. Lin28 selectively blocks the processing of pri-let-7 micoRNAs [160]. Notably, the restoration of the Lin28 protein blocked let-7 expression and restored glucose metabolism in adipose-derived stem cells derived from obese tissues [161]. The most interesting microRNAs in inflammatory microvesicles in association with metabolic and cardiovascular diseases recently reported are the let-7 family, microRNA17/92 family, microRNA-21, microRNA-29, microRNA-126, microRNA-133, microRNA-146 and microRNA-155 [162], which exhibit a substantial overlap with the spectrum of bovine milk microRNAs [84-89].
Palmitate, the major fatty acid of milk fat, significantly increases the expression of microRNA-29a in myocytes [163]. MicroRNA-29a targets and suppresses mRNA of insulin receptor substrate-1 (IRS-1) and attenuates IRS-1 expression. Ectopic expression of microRNA-29a thus impairs insulin signaling and glucose uptake in myocytes through a substantial decrease in IRS-1. Intriguingly, bovine milk contains microRNA-29a [89], thus presents a putative exogeneous source of microRNA-29a-mediated suppression of insulin signaling. The suppression of skeletal muscle insulin signaling via milk-derived microRNA-29a transfer may spare insulin for more important anabolic purposes as skeletal muscle activity is rather low during the nursing period. Increased circulating blood levels of palmitate in obesity may induce palmitate-mediated microRNA-29a expression in skeletal muscle, which may be fortified by milk-derived palmitate as well as direct transfer of bovine milk microRNA-29a into the systemic circulation of the milk consumer. MicroRNA-29a and let-7-family of bovine milk may thus promote microRNA-driven insulin resistance [159,164].
3. BCAA-MTORC1-S6K1-MEDIATED INSULIN RESISTANCE
Fatty acids and their metabolites have been implicated in the development of insulin resistance and T2DM. However, metabolomics technologies revealed that BCAAs and related metabolites are more strongly associated with insulin resistance than many common lipid species. Nevertheless, in animal feeding studies, BCAA supplementation required the background of a high-fat diet to promote insulin resistance [136].
Palmitate-driven microRNA-29a-mediated suppression of IRS-1 in combination with increased BCAA levels may synergize in the induction of insulin resistance. Excessive amounts of circulating BCAAs activate mTORC1 and its downstream substrate, the kinase S6K1 [23,33,165,166],which reduces insulin signaling by inhibitory phosphorylation of IRS-1 [165-174]. BCAA-mediated insulin resistance is explained by enhanced mTORC1/S6K1-mediated inhibitory phosphorylation of IRS-1 [142].
4. MILK ACCOMPANIES THE LIFELONG MARCH TO DIABETES
Accelerated fetal growth and increased birth weight are well-known risk constellations increasing the risk of T2DM and obesity later in life [175-177]. As medicine and nutritional sciences still regard milk as a source of valuable proteins, vitamins, and calcium, an increase (4 servings per day) of either milk or dairy products is recommended by most societies of gynecology and obstetrics during pregnancy [178]. Data from 50,117 mother-infant pairs of the Danish National Birth Cohort collected from 1996-2002 showed an increase of placental weight and birth weight across the whole range of milk intake [179]. The Generation R Study, a population-based prospective cohort study from fetal life until young adulthood in Rotterdam, investigated 3,405 mothers during pregnancy [180]. Maternal milk consumption of >3 glasses (450 mL of milk) per day was associated with greater fetal weight gain in the third trimester of pregnancy, which led to an 88 g higher birth weight than that with milk consumption of 0 to 1 glass per day [180]. Notably, this association was limited only to milk, whereas protein intake from nondairy food or cheese was not associated with increased birth weight. A possible explanation for this finding is the presence of biologically active microRNAs in milk and their potential absence in processed and fermented milk products such as cheese [93]. Jiang et al. [94] recently detected increased levels of microRNA-21 in placental tissue of mothers with macrosomal infants. Bovine microRNA-21 transfer to the pregnant mother by milk consumption may overstimulate trophoblast mTORC1, which controls nutrient and BCAA transfer to the fetus [181-186]. A recent literature review provided translational evidence that milk consumption during pregnancy but not fermented dairy products increased placental and birth weight [187].
After birth, high protein infant formula feeding maintains exaggerated BCAA-mTORC1 signaling associated with rapid weight gain [188-191]. Infant formula with higher protein versus formula with lower protein content has been linked to increased mTORC1 signaling [188,189], which is associated with higher weight gain velocity [190,191]. Importantly, rapid weight gain in infancy has been related to an increased risk of T2DM [192]. Data derived from the National Health and Nutrition Examination Survey (NHANES) 1999-2004 confirmed that milk consumption in children increased body mass index (BMI) [151] and induced earlier onset of menarche [193]. Increased BMI and early onset of menarche are both explained by milk-mTORC1-mediated anabolism, which obviously accelerates human growth trajectories. Remarkably, early onset of menarche is a well-known risk factor for the development of T2DM recently confirmed in a systematic review and meta-analysis [194]. Thus, there is good reason to assume that persistent bovine milk signaling deviates the physiological human axis of mTORC1 signaling during intrauterine and extrauterine life.
5. MTORC1 INDUCES ENDOPLASMIC RETICULUM-STRESS-MEDIATED Β-CELL APOPTOSIS
To maintain appropriate β-cell mass and β-cell homeostasis, a physiological level of mTORC1 signaling is required [195]. In order to adapt to the increased metabolic burden of obesity and insulin resistance, β-cells increase mass by proliferation, neogenesis and hypertrophy to enhance β-cell function [195]. There is substantial evidence that ER-stress of chronically overacticated β-cells results in early β-cell apoptosis, the hallmark of T2DM [196-198]. Studies in humans indicate that glucose intolerance appears after 20% reduction in β-cell mass, while overt diabetes develops with 65% reduction [199]. Pancreatic β-cells have a heavy engagement in insulin synthesis (> 50% of total protein synthesis) and express high levels of the ER-stress transducers eukaryotic translation initiation factor 2-alpha kinase 3 (EIF2AK3) and endoplasmic reticulum-to-nucleus signaling 1 (ERN1), which induce the unfolded protein response (UPR) [197]. Recent evidence supports the concept that hyperactivation of the UPR is closely related to β-cell dysfunction and apoptosis [196,197].
ER-stress and β-cell dysfunction have been related to increased lipotoxicity [198]. The saturated fatty acid palmitate, the most abundant fatty acid of milk lipids, has been implicated to play a major role in β-cell apoptosis [199]. Glucose amplifies fatty acid-induced ER-stress in pancreatic β-cells via activation of mTORC1 [200]. Notworthy, palmitate and BCAAs are both able to activate mTORC1 on lysosomal compartments [43].
Importantly, increased mTORC1 singaling upregulates the ER-stress response [201]. mTORC1-driven ER-stress has been associated with the induction of apoptosis [202-204]. Ozcan et al. [203] convincingly demonstrated that increased mTORC1 activity (due to loss of TSC suppressors) triggered UPR-driven apoptosis. Upon prolonged ER-stress, mTORC1 contributes to apoptotic signaling by suppressing the survival kinase AKT through feedback inhibition [205].
Apparently, in a vicious cycle, ER-stress via induction of eIF2α-P/activating transcription factor-4 (ATF4) signaling stimulates the expression of LAT thereby enhancing intra-β-cell leucine levels that further stimulate mTORC1 signaling during ER-stress [204,206] (Fig.1). Han et al. [207] demonstrated that ER-stress via ATF4 and DNA damage-inducible transcript 3 (DDIT3) upregulation increased protein synthesis leading to cell death.
ER-stress stimulates autophagy as an adaptive response to clean up terminally misfolded proteins from the ER [198] (Fig.1). It is well established that mTORC1 is a negative regulator of autophagy [208,209]. Remarkablly, it has been shown that stimulation of autophagy improved ER-stress-induced diabetes in a mouse model [210]. Furthermore, inhibition of mTORC1 during ER-stress increased autophagy and attenuated apoptosis [211].
Thus, persistent milk-mediated overstimulation of β-cell mTORC1 may promote chronic ER-stress promoting β-cell apoptosis, the hallmark of T2DM. In fact, persistent mTORC1 activation of β-cells in β-cell TSC2-/- mice resulted in a biphasic response with hyperinsulinemia and hypoglycemia at young ages (4-28 weeks) and hypoinsulinemia and hyperglycemia due to enhanced β-cell apoptosis in adult mice [212]. These findings are in accordance with the observations of Ozcan et al. [203], who found enhanced UPR signaling in cells lacking TSC2. This experimental constellation thus resembles chronic milk-mediated mTORC1 hyperactivation, which apparently provides a most critical mechanism promoting early death of β-cells in milk consuming societies (Fig.1).
6. ARE MILK-DERIVED MICRORNAS DIABETOGENIC?
It is predicted that more than 30% of human genes are regulated by microRNAs. There is most recent scientific interest in the influence of microRNAs in the regulation of insulin signaling pathways and insulin resistance in type 1 and type 2 diabetes [213-217]. As milk promotes insulin secretion and mTORC1-driven growth, it is most conceivable that milk-derived exosomal microRNAs that reach the systemic circulation [90] may be taken up by the pancreatic β-cell to modify translation during the period of lactation and milk feeding.
The β-cell must respond immediately to changes of blood glucose levels and thus has an intimate connection to the systemic blood circulation. To facilitate this connection, the β-cells of the islets of Langerhans are embedded in a dense capillary network. Notably, islet capillaries show about ten times more fenestration than those within the exocrine tissue and are highly permeable [218-220]. Endothelial cell fenestrae produce a pore of about 100 nm in diameter and allow rapid passage of macromolecules [220].
There is recent evidence that β-cells release microRNA-containing exosomes [221, 222]. It is thus conceivable, that milk exosomes, which have a size of 50-100 nm, may reach the β-cells from the systemic circulation. The purpose of milk-mediated exosomal microRNA signaling may be the stimulation of β-cell growth and insulin secretion during the postnatal growth period, which requires an adaptation of the infant´s β-cell mass to fulfill increased insulin demands. However, persistent milk-driven microRNA signaling may deteriorate β-cell homeostasis.
Notably, the microRNA-29 family, which consists of microRNA-29a, b, and c, has been identified as diabetic microRNA markers [223]. MicroRNA-29a has been identified as one of the microRNAs that was upregulated in the serum of children with type 1 diabetes [224]. microRNA-29 has pro-apoptotic functions and is involved in renal and cardiovascular injury [225], common complications of T2DM.
MicroRNA-29b downregulates the expression of the anti-apoptotic protein myeloid cell leukemia 1 (Mcl-1) [226]. In non-obese diabetic (NOD) mice, upregulation of microRNA-29a, b, and c caused pancreatic β-cell death via suppression of Mcl-1, an essential member of the pro-survival Bcl-2 family genes [227]. Thus, the microRNA-29-Mcl-1 axis may thus play a role in the pathogenesis of diabetes and may contribute to β-cell dysfunction in prediabetic NOD mice [227]. Furthermore, studies on microRNA expression in skeletal muscles of Goto-Kakizaki (GK) rats, a non-obese model of T2DM, identified upregulated microRNA-29a and microRNA-29b in these diabetes animals compared to control animals [228]. Intriguingly, increased urinary levels of microRNA-29a, b, and c have been detected in patients with T2DM [229]. Thus, upregulation of the microRNA-29 family may be involved in the pathogenesis of type 1 and type 2 diabetes.
The systemic uptake of microRNA-29 by consumption of “fresh” commercial milk may augment diabetogenic microRNA-29 signaling. Bovine milk microRNA-29a by targeting the mRNA of insulin receptor substrate-1 (IRS-1) enhances insulin resistance [163]. Milk microRNA-29b by targeting the mRNA of DBT, the critical core component that assembles the BCKD complex [146], may explain increased BCAA-mTORC1-S6K1-mediated insulin resistance. Furthermore, by targeting Mcl-1 of β-cells, milk-derived microRNA-29b may promote early β-cell apoptosis (Fig. 1). Persistent activation of mTORC1 may impair β-cell autophagy, augmenting ER-stress-induced β-cell apoptosis. However, as a feedback mechanism, mTORC1 has been shown to promote cell survival through translation of Mcl-1 [230].
7. EPIDEMIOLOGY OVERLOOKS THE IMPACT OF MILK´S MICRORNAS
The majority of epidemiological studies show that “dairy consumption“ in general is inversely related to the risk of T2DM [231-235]. Rice and coworkers recommend the intake of more than three servings of dairy per day to reduce the risk of T2DM [236]. It is of most critical concern that most cohort studies do not accurately differentiate milk from other fermented dairy products. There are only few studies that selectively analyse the association between milk consumption and T2DM [237-242]. Surprisingly, milk, the starting material of all other processed dairy products does not convincingly exhit diabetes-protective effects as observed with fermented or processed milk products such as yoghurt. Liu et al. [237] prospectively examined the incidence of T2DM in 37,183 women in relation to the number of skim milk and whole milk servings and found no significant decrease in diabetes risk. Elwood et al. [238] studied 2,375 men of the Caerphilly cohort. Milk intake showed no significant trend with incident diabetes. Villegas et al. [239] investigated 64,191 women of the Shanghai women cohort and reported a significant decrease in diabetes risk in women consuming > 200 g milk/day versus none. Kirii et al. [240] analysed 59,796 men and women of a Japanese cohort and found no significant risk for diabetes for milk consumption in men, whereas women exposed to > 200 g milk/day showed a trend to lower diabetes risk, although not reaching statisitical significance. The Physicians´ Health Study [241] (21,660 men) exhibited a significant increase in diabetes risk. Less than 1 serving of whole milk/week was associated with a diabetes prevalence of 1.7% and > 2 servings/week with 2.6% (p<0.001), respectively. For skim/low fat milk the diabetes percentages were 1.6 and 2.3 (p<0.001) respectively [241].
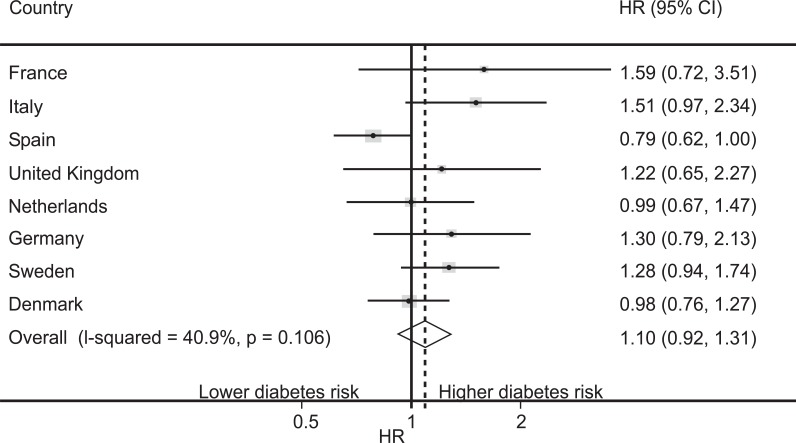
The worldwide largest prospective study investigating the type of dairy product intake and incident T2DM is the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct study [242], a nested case cohort within 8 European countries (n=340,234). Although, the pooled hazard ratios (HRs) demonstrated only a slight but not significiant increase of diabetes risk in relation to increased milk intake, HRs of individual countries showed substantial variations. Higher diabetes risks (HR > 1) were observed in the French, Italian, UK, German and Swedish cohorts, whereas the Netherlands and Denmark exhibited HR=1 (Fig. 2). Only Spain (UHT milk consumption > 96% of total milk consumption [243]) exhibitied a HR < 1 [242]. Sluijs et al. [242] convincingly concluded that the findings for milk and diabetes risk remain inconclusive and require further research of the associations with various types of milk.
Fig. (2).
Hazard ratios (HRs) (and 95% CIs) for the association of milk consumption with diabetes risk (highest compared with lowest quintile) per Eu-ropean country of the EPIC-InterAct Study (n=340,234) according to the Sluijs et al. [242] with permission the American Society of Nutrition. Notably, 80.3% of the EU population (France, Italy, UK, Germany, Sweden) exhibited an increased diabetes risk in relation to milk consump-tion, whereas 6.4% (Netherlands, Denmark) showed no correlation and 13.3% (Spain) exhibited an inverse association
Differences in heat exposure and time during milk processing may modify the biological activity of bovine microRNAs. High temperature short time (HTST) pasteurization (72°C, 15 sec), and UHT processing (140°C, 14 sec) may have different effects on the bioactivity of milk´s exosomal microRNAs. Furthermore, milk processing by the consumer prior to use (boiling or microwave treatment) is also of critical importance to evaluate milk microRNA-mediated effects on human health [93].
DISCUSSION
“Milk and sugar” are the inseparable and most common food items on everyman´s table in Western societies. It is known for a long time, that the addition of milk to a low glycemic carbohydrate meal exaggerates the insulinemic response comparable to that of a hyperglycemic carbohydrate meal [244]. Thus, milk intake is an overlooked metabolic burden of the β-cell that increases ER-stress. However, in comparison to glucose, milk´s origin and function differ. Milk is the secretory product of the lactation genome of a mammalian species that executes a sophisticated growth program designed to upregulate anabolic mTORC1 signaling of the milk recipient, the species-specific newborn mammal. Milk maintains an intimate molecular crosstalk with the pancreatic β-cell via stimulation of incretin signaling and transfer of abundant insulinotropic BCAAs that increase insulin synthesis and secretion by upregulation of β-cell mTORC1 activity. Furthermore, milk provides a microRNA signaling software. By transfer of the bovine exosomal microRNA-29 family, milk apparently attenuates BCAA catabolism, a meaningful metabolic deviation that favors BCAA incorporation into functionally important proteins during postnatal development. However, this metabolic deviation increases BCAA-mTORC1-driven β-cell ER-stress as well as BCAA-mTORC1-S6K1-driven insulin resistance of peripheral tissues.
In mammals, milk signaling generally is limited to the physiological nursing period, except the Neolithic Homo sapiens, who introduced milk consumption 8000-10,000 years ago into his food chain [245,246]. Noteworthy, humans of the early Neolithic period consumed preferentially fermented milk and milk prodcuts [245,246]. Whereas lactase persistence in most Europeans resulted from LCT mutations during the early Neolithic period [247], most Asian populations are still lactose intolerant. However, the dairy industry is able to bypass physiological lactose intolerance in humans by treating milk with exogeneous microbial lactase (β-galactosidase) [248].
The Chinese transition to the Western dietary lifestyle is reflected by the Hong Kong Dietary Survey [249]. In this cohort, a dietary pattern with “more meat and milk products” was associated with a 39 % greater risk of diabetes [249]. In Europe as well, higher animal protein (milk protein plus meat protein) consumption has been associated with a higher prevalence of T2DM compared to plant-derived protein intake [250].
Most recent evidence has been provided that fermentation such as in yoghurt or cheese production destroys or attenuates the exosomal microRNA signaling of milk [93]. Populations that gained mutations of the lactase gene resulting in lactase persistance have lifelong access to fresh milk containing both the amino acid hardware and microRNA software of milk. Modern populations experienced a further boost of “milk doping” and growth acceleration by the widespread distribution of refrigeration technology since the early 1950´s. With the intention to preserve valuable bioactive ingredients of milk such as vitamins, pasteurized fresh milk prodcuts reached the daily food chain of the modern consumer. This unnoticed change exposed people of technologically developed societies to daily microRNA signaling of milk [15,90,93]. Indeed, since the introduction of the refrigerator into our households the prevalence of diseases of civilization increased progressively. Persistent “abuse” of an mTORC1 sigaling system designed by mammalian evolution to operate only during the early postnatal growth phase of life may be a key mTORC1-dependent mechanism promoting age-related diseases of cilvilization such as obesity, T2DM, cancer, and neurodegenerative diseases [8-15]. The presented concern about the association of milk consumption and diseases of civilization is in accordance with recent results of Michaëlsson et al. [251], who reported a higher mortality in two large Swedish cohorts of men and women with high intake of milk but not fermented or processed dairy products. Furthermore, the authors observed a correlation between milk intake and serum levels of the inflammatory cytokine interleukin 6 (IL-6) [251], which is also increased in patients with T2DM [252]. Experimental evidence underlines that exposure of β-cells to IL-6 induces early β-cell death [253]. Milk-mediated IL-6 signaling may be the connecting piece that links milk consumption with low grade inflammation that promotes β-cell failure and early β-cell apotosis [3-5]. Remarkably, β-cells express IL-6 receptors, which after ligand binding induce phosphorylation of signal transducer and activator of transcription-3 (STAT3) [254]. Notably, activated STAT3 promotes the expression of microRNA-21 [255-257]. Furthermore, IL-6-mediated activation of mTORC1 [258] may further increase ER-stress-mediated β-cell apoptosis.
Milk´s biological function as a BCAA- and microRNA-donating system for mTORC1-driven growth may explain accelerated ageing and increased mortality with persistent milk consumption [251]. As early as 1934, McCay and Crowell [259] provided translational evidence that slower growth favours longevity of various animal species.
Remarkably, comorbidities of T2DM such as ischemic heart disease have been associated with milk consumption [260], whereas populations with a high prevalence of lactose malabsorption, whose milk intake is low, have a reduced risk of ischemic heart disease than populations with low (<30%) lactose malabsorption [261]. In another large recent Swedish cohort study, subjects with lactose intolerance had decreased risks of lung, breast, and ovarian cancer [262]. Increased whole milk intake has also been associated with prostate cancer-specific mortality among U.S. male physicians [241]. Activated mTORC1 signaling plays a pivotal role in the initiation and progression of prostate cancer [263,264].Thus, persistently over-activated mTORC1, milk´s crucial biological function, accelerates aging and the onset of age-related diseases of civilization [8-5].
Evidence accumulates that the widely prescribed anti-diabetic drug metformin functions as an inhibitor of mTORC1 signaling [265]. Intriguingly, the mTORC1 inhibitor metformin not only controls T2DM, but also reduces the risk of cancer [266], and apparently prolongs life span in humans [267]. Metformin thus counteracts milk-mediated activation of mTORC1.
From the perspective of evolutionary biology, milk consumption represents a novel human behavior that could exert adverse long-term health effects [151]. Continued overstimulation of mTORC1 signaling in humans due to persistent consumption of milk may accelerate aging of the β-cell associated with β-cell apoptosis. Half a century ago, the antagonistic pleiotropy theory solved the mystery of aging by postulating genes beneficial early in life at the cost of aging [268]. Early in life, milk via mTORC1 activation drives a developmental program, which persists later in life as an aimless quasi-program of aging and age-related diseases [268]. In this regard mTORC1 signaling works as pacemaker of aging [269]. In order to prevent age-related diseases such as T2DM Kapahi et al. [269] suggested to reduce mTORC1 signaling: “with TOR less is more” (Fig.3).
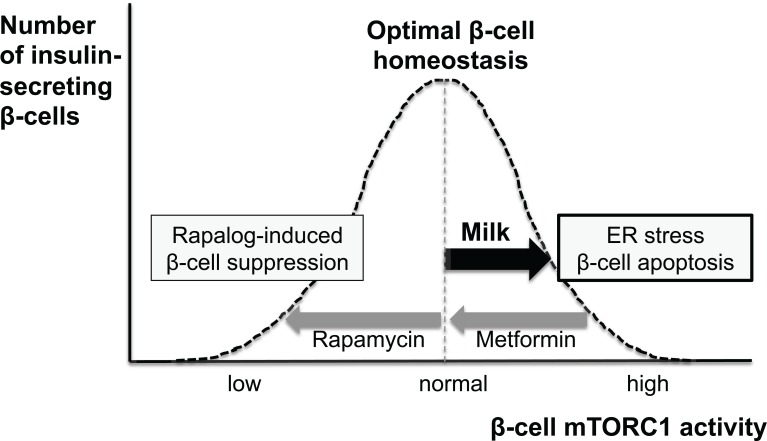
Fig. (3).
Schematic representation of milk-mediated disturbances of β-cell homeostasis. Persistent milk signaling overactivates β-cell mTORC1 promot-ing endoplasmic reticulum (ER)-stress resulting in early β-cell apoptosis leading to type 2 diabetes. Metformin treatment attenuates nutrient (milk)-driven overstimulation of mTORC1. Rapamycin treatment inhibits mTORC1 resulting in β-cell suppression and diabetes. Long-term β-cell survial and optimized β-cell homeostasis requires an appropriate well balanced magnitude of β-cell mTORC1 signaling
Refrigeration technology has been implemented into Western households since the 1950´s. This technological achievement allows daily and widespread access to bioactive bovine microRNAs that may affect more than 11,000 human genes [90]. This unnoticed change in human nutrition apparently plays a major role in the pathogenesis of mTORC1-driven T2DM and its mTORC1-driven comorbidities. It is of critical concern that in Western societies milk-driven mTORC1 signaling starts already during fetal life (maternal milk consumption during pregnancy), is further promoted by high protein infant formula feeding, maintained by school milk intake during adolescence, and continued into adulthood and senescence.
Despite the lack of sound evidence powered by randomized controlled trials [270], industry-associated authors still proclaim milk as a “source of healthy nutrition” preventing diseases of civilization such as T2DM [271,272]. Future randomized controlled trials have to differentiate the health effects of pasteurized, HTST versus UHT milk and fermented dairy products in relation to their microRNA bioactivity, especially of the diabetogenic microRNA-29 family.
At present, no epidemiological study has considered the impact of biologically active milk microRNAs in relation to obesity and T2DM. The adipogenic and diabetogenic risk of fresh pasteurized milk has to be differentiated from UHT milk and fermented milk products such as yoghurt. Notably, Howard et al. [93] detected only trace amounts of microRNA-29b in yoghurt. The inactivation of diabetogenic microRNAs during the fermentation process may explain the reduced risk of T2DM in association with yoghurt intake [273, 274]. Hyperactivated β-cell mTORC1 signaling in combination with milk-derived diabetogenic microRNAs superimposed by high glucose and palmitate-driven mTORC1 signaling may in a synergistic fashion accelerate ER-stress and early β-cell apoptosis resulting in its final clinical outcome: type 2 diabetes mellitus (Table 1).
Table 1.
Potential diabetogenic mediators of cow´s milk consumption
| Component of Milk | Potential Diabetogenic Mechanism | References |
|---|---|---|
| Leucine, other BCAAs, and whey peptides | β-cell mTORC1 activation, whey-driven incretin secretion, whey peptide-mediated inhibition of dipeptidyl peptidase IV increasing incretion activity leading to exaggerated insulin production, ER-stress, early β-cell apoptosis | [16-19, 39, 52, 58, 59, 61, 62, 195, 203] |
| Glutamine | Activation of β-cell mTORC1 via the glutaminolysis pathway, glutamine-mediated leucine uptake, leucine-driven mTORC1 activation | [16, 41, 42] |
| Palmitate | β-cell mTORC1 activation, ER-stress driven by lipotoxicity and hyperactivated mTORC1, ER-stress | [43, 200] |
| MicroRNA-21 | Inhibition of translation of tumor suppressor proteins (PTEN, Sprouty, PDCD4), increased mTORC1 signaling; inhibition of FOXO1 promoting β-cell proliferation and enhanced oxidative stress leading to β-cell apoptosis | [15, 108, 110, 116, 117] |
| MicroRNA-29a | Suppression of IRS-1 translation enhancing insulin resistance | [163] |
| MicroRNA-29b | Suppression of BCAA catabolism enhanching BCAA-driven mTORC1 signaling; suppression of anti-apototic Mcl-1 translation, early β-cell apoptosis | [90, 93, 146, 226, 227] |
| Let-7b | Impairment of glucose homeostasis | [159] |
| Casomorphin 4 | Neurogenic stimulation of insulin secretion enhancing ER-stress | [64] |
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
ABBREVIATIONS
- ATF4 =
Activating Transcription Factor 4
- BCAA =
Branched-Chain Amino Acid
- BCAA =
Branched-Chain Amino Acid
- BCKDHA =
Branched-chain Keto Acid Dehydrogenase
- HCR =
Hypoxic cytotoxicity ratio
- BCKDK =
Branched-chain α-Keto Acid Dehydrogenase Kinase
- Bcl2 =
B-cell CLL/lymphoma 2
- Bcl2 =
B-cell CLL/lymphoma 2
- DBT =
Dihydrolipoamide Branched-Chain Transacylase
- DDIT3 =
DNA Damage-Inducible Transcript 3
- DLD =
Dihydrolipoamide Dehydrogenase
- DPP-IV =
Dipeptidyl Peptidase IV
- EIF2AK3 =
Eukaryotic Translation Initiation Factor 2-alpha Kinase 3
- EPIC =
European Prospective Investigation into Cancer and Nutrition
- ER =
Endoplasmic Reticulum
- ERN1 =
Endoplasmic Reticulum-to-Nucleus Signaling 1
- FOXO1 =
Forkhead Box Class O1
- GDH =
Glutamate Dehydrogenase
- GIP =
Glucose-Dependent Insulinotropic Polypeptide
- GLP-1 =
Glucagon-like Peptide-1
- HTST =
High Temperature Short Time
- IGF1R =
Insulin-Like Growth Factor-1 Receptor
- IL-6 =
Interleukin 6
- IR =
Insulin Receptor
- IRS =
Insulin Receptor Substrate
- PI3K =
Phosphoinositol-3 Kinase
- LCT =
Lactase Gene
- LAT =
L-type Amino Acid Transporter
- Let-7 =
microRNA let-7
- Leu =
Leucine
- MicroRNA =
Micro-ribonucleic Acid
- Mcl-1 =
Myeloid Cell Leukemia Sequence 1
- mTORC1 =
Mechanistic Target Of Rapamycin Complex 1
- NHANES =
National Health and Nutrition Examination Survey
- NOD =
Non-obese Diabetic
- PDCD4 =
Programmed Cell Death 4
- PTEN =
Phosphatase And Tensin Homolog
- S6K1 =
Ribosomal Protein S6 Kinase, 70-kD Kinase 1
- STAT3 =
Signal Transducer and Activator of Transcription 3
- T2DM =
Type 2 Diabetes Mellitus
- PTEN =
Phosphatase And Tensin Homolog
- TSC2 =
Tuberin
- UHT =
Ultra-heat Treated
- UPR =
Unfolded Protein Response
- UTR =
3´-Untranslated Region
REFERENCES
- 1.Forouhi NG, Wareham NJ. The EPIC-InterAct Study A study of the interplay between genetic and lifestyle behavioral factors on the risk of type 2 diabetes in European populations. Curr Nutr Rep. 2014;3:355–63. doi: 10.1007/s13668-014-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reference: Available from http://www.cdc.gov/ diabe-tes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-states.pdf&ie=UTF-8&oe=UTF-8&gfe_rd=cr&ei= mHliVIynHNKq8weeiIHADQ .
- 3.Akash MS, Shen Q, Rehman K, Chen S. Interleukin-1 receptor antagonist a new therapy for type 2 diabetes mellitus. J Pharm Sci. 2012;101:1647–58. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 4.Akash MS, Rehman K, Chen S. An overview of valuable scientific models for diabetes mellitus. Curr Diabetes Rev. 2013;9:286–93. doi: 10.2174/15733998113099990062. [DOI] [PubMed] [Google Scholar]
- 5.Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525–31. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T. The role of FOXO1 in ß-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–23. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 7.CDC´s Division of Diabetes Translation Long-term trends in diagnosed diabetes. http://www.cdc.gov/diabetes/statistics. October . 2011.
- 8.Zoncu R, Efeyan A, Sabatini DM. mTOR from growth signal integration to cancer, diabetes and ageing. Nature Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–45. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci. 2012;S4:941–52. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, Bereczki E, Zhang H , et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis implication for Alzheimer disease. J Biol Chem. 2013;288:15556–70. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelsohn AR, Larrick JW. Dissecting mammalian target of rapamycin to promote longevity. Rejuvenation Res. 2012;15:334–7. doi: 10.1089/rej.2012.1347. [DOI] [PubMed] [Google Scholar]
- 14.Xu S, Cai Y, Wei Y. mTOR signaling from cellular senescence to organismal aging. Aging Dis. 2014;5:263–73. doi: 10.14336/AD.2014.0500263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J. 2013;12:103. doi: 10.1186/1475-2891-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel ML, Marshall CA, Pappan KL, Kwon G. Metabolic and autocrineregulation of the mammalian target of rapamycin by pancreatic ß-cells. Diabetes. 2002;51:2877–85. doi: 10.2337/diabetes.51.10.2877. [DOI] [PubMed] [Google Scholar]
- 17.Leibowitz G, Cerasi E, Ketzinel-Gilad M. The role of mTOR in the adaptation and failure of ß-cells in type 2 diabetes. Diabetes Obes Metab. 2008;10:157–69. doi: 10.1111/j.1463-1326.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–9. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bacquer O, Queniat G, Gmyr V, Kerr-Conte J, Lefebvre B, Pattou F. mTORC1 and mTORC2 regulate insulin secretion through Akt in INS-1 cells. J Endocrinol. 2013;216:21–9. doi: 10.1530/JOE-12-0351. [DOI] [PubMed] [Google Scholar]
- 20.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–7. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, Peterson T, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling. Cold Spring Harb Perspect Biol. 2012;4:pii a011593. doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Guan KL. Amino acid signaling in TOR activation. Ann Rev Biochem. 2011;80:1001–32. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. 2013;35:463–73. doi: 10.1007/s10059-013-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci. 2013;38:233–42. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efeyan A, Sabatini DM. Nutrients and growth factors in mTORC1 activation. Biochem Soc Trans. 2013;41:902–5. doi: 10.1042/BST20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–9. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Proud CG. Nutrient control of mTORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–7. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–81. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricoult SJ, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013;14:242–51. doi: 10.1038/embor.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamin HB, Barnea M, Genzer Y, Chapnik N, Froy O. Long-term commercial cow's milk consumption and its effects on metabolic parameters associated with obesity in young mice. Mol Nutr Food Res. 2014;58:1061–8. doi: 10.1002/mnfr.201300650. [DOI] [PubMed] [Google Scholar]
- 34.Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans. 2013;41:906–12. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- 35.Yoon MS, Zhang C, Sun Y, Schoenherr CJ, Chen J. Mechanistic target of rapamycin controls homeostasis of adipogenesis. J Lipid Res. 2013;54:2166–73. doi: 10.1194/jlr.M037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Ji J, Yan XH. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52:373–81. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- 37.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol. 2014;397:67–77. doi: 10.1016/j.mce.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab. 2012;302:E1329–42. doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]
- 40.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen A, Hall MN. An amino acid shuffle activates mTORC1. Cell. 2009;136:399–400. doi: 10.1016/j.cell.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Duran RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–58. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda M, Tanaka Y, Kume S, et al. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta. 2014;1842:1097–108. doi: 10.1016/j.bbadis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87:1576S–81S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 45.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He T, Giuseppin ML. Slow and fast dietary proteins differentially modulate postprandial metabolism. Int J Food Sci Nutr. 2014;65:386–90. doi: 10.3109/09637486.2013.866639. [DOI] [PubMed] [Google Scholar]
- 47.Boutrou R, Gaudichon C, Dupont D, et al. Sequential releases of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr. 2013;97:1414–23. doi: 10.3945/ajcn.112.055202. [DOI] [PubMed] [Google Scholar]
- 48.Mahé S, Roos N, Benamouzig R , et al. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans the influence of the nature and quantity of the protein. Am J Clin Nutr. 1996;63:546–52. doi: 10.1093/ajcn/63.4.546. [DOI] [PubMed] [Google Scholar]
- 49.Lenders CM, Liu S, Wilmore DW , et al. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur J Clin Nutr. 2009;63:1433–9. doi: 10.1038/ejcn.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Li C, Allen A, Stanley CA, Smith TJ. The structure and allosteric regulation of mammalian glutamate dehydrogenase. Arch Biochem Biophys. 2012;519:69–80. doi: 10.1016/j.abb.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorin S, Tol MJ, Bauvy C, et al. Glutamate dehydrogenase contributes to leucine sensing in the regulation of autophagy. Autophagy. 2013;9:850–60. doi: 10.4161/auto.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes. 2001;50:353–60. doi: 10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]
- 53.Holt S, Brand Miller J, Petocz P. An insulin index of foods The insulin demand generated by 1000-kK portions of common foods. Am J Clin Nutr. 1997;66:1264–76. doi: 10.1093/ajcn/66.5.1264. [DOI] [PubMed] [Google Scholar]
- 54.Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93:175–7. doi: 10.1079/bjn20041304. [DOI] [PubMed] [Google Scholar]
- 55.Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/ IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63:1076–83. doi: 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
- 56.Thomas FB, Sinar D, Mazzaferri EL, et al. Selective release of gastric inhibitory polypeptide by intraduodenal amino acid perfusion in man. Gastroenterology. 1978;74:1261–5. [PubMed] [Google Scholar]
- 57.Chen Q, Reimer RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition. 2009;25:340–9. doi: 10.1016/j.nut.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 59.Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in helathy subjects studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85:996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 60.Salehi A, Gunnerud U, Muhammed SJ, et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on ß-cells. Nutr Metab (Lond) 2012;9:48. doi: 10.1186/1743-7075-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacroix IM, Li-Chan EC. Inhibition of dipetidyl peptidase (DPP)-IV and a-glucosidase actitvities by pepsin-treated whey proteins. J Agric Food Chem. 2013;61:7500–6. doi: 10.1021/jf401000s. [DOI] [PubMed] [Google Scholar]
- 62.Power O, Nongonierma AB, Jakeman P, FitzGerald RJ. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc Nutr Soc. 2014;73:34–46. doi: 10.1017/S0029665113003601. [DOI] [PubMed] [Google Scholar]
- 63.Hoppe C, Mølgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increases s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr. 2005;59:393–8. doi: 10.1038/sj.ejcn.1602086. [DOI] [PubMed] [Google Scholar]
- 64.Schusdziarra V, Schick A, de la Fuente A , et al. Effect of ß-casomorphins and analogs on insulin release in dogs. Endocrinology. 1983;112:855–9. doi: 10.1210/endo-112-3-885. [DOI] [PubMed] [Google Scholar]
- 65.Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level a systematic literature review. Int J Food Sci Nutr. 2009;60:330–40. doi: 10.1080/09637480903150114. [DOI] [PubMed] [Google Scholar]
- 66.Norat T, Dossus L, Rinaldi S , et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61:91–8. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- 67.Rich-Edwards JW, Ganmaa D, Pollak MN , et al. Milk consumption and the prepubertal somatotropic axis. Nutr J. 2007;6:28–0. doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larnkjær A, Arnberg K, Michaelsen KF, Jensen SM, Mølgaard C. Effect of milk proteins on linear growth and IGF variables in overweight adolescents. Growth Horm IGF Res. 2014;24:54–9. doi: 10.1016/j.ghir.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes. 2012;3:38–53. doi: 10.4239/wjd.v3.i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen RG, Ferris AM, Lammi-Keefe CJ. The composition of milk fat. J Dairy Sci. 1991;74:3228–43. doi: 10.3168/jds.S0022-0302(91)78509-3. [DOI] [PubMed] [Google Scholar]
- 71.Bitman J, Wood DL. Changes in milk fat phospholipids during lactation. J Dairy Sci. 1990;73:1208–16. doi: 10.3168/jds.S0022-0302(90)78784-X. [DOI] [PubMed] [Google Scholar]
- 72.Valadi H, Ekstöm K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNA and microRNAs is novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 73.Liang H, Huang L, Cao J, Zen K, Chen X, Zhang CY. Regulation of mammalian gene expression by exogeneous microRNAs. Wiley Interdiscip Rev RNA. 2012;3:733–42. doi: 10.1002/wrna.1127. [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Liang H, Zhang J, Zen K, Thang CY. Secreted mi-croRNAs a new form of intercellular communication. Trends Cell Biol. 2012;22:125–32. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 76.Ludwig AK, Giebel B. Exosomes small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–5. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Witwer KW, Hirschi KD. Transfer and functional consequences of dietary microRNAs in vertebrates Concepts in search of corroboration. Bioessays. 2014;36:394–406. doi: 10.1002/bies.201300150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickinson B, Zhang Y, Petrick JS, Heck G, Ivashuta S, Marshall WS. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat Biotechnol. 2013;31:965–7. doi: 10.1038/nbt.2737. [DOI] [PubMed] [Google Scholar]
- 79.Snow JW, Hale AE, Isaacs SK, Baggish AL, Chan SY. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA Biol. 2013;10:1107–16. doi: 10.4161/rna.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs limited evidence for general uptake of dietary plant xenomiRs. RNA Biol. 2013;10:1080–6. doi: 10.4161/rna.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Hou D, Chen X , et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–26. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs molecular mechansism and clinical applications. Protein Cell. 2012;3:28–37. doi: 10.1007/s13238-012-2003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396:528–33. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Gao C, Li H, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010;20:1128–37. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- 86.Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci. 2012;95:4831–41. doi: 10.3168/jds.2012-5489. [DOI] [PubMed] [Google Scholar]
- 87.Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics. 2012;75:1486–92. doi: 10.1016/j.jprot.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Purification of RNA from milk whey. Methods Mol Biol. 2013;1024:191–201. doi: 10.1007/978-1-62703-453-1_15. [DOI] [PubMed] [Google Scholar]
- 89.Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell. 2013;4:197–210. doi: 10.1007/s13238-013-2119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baier SR, Nguyen C, Xie F, Wood Jr, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr. 2014;144:1495–1500. doi: 10.3945/jn.114.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Q, Li M, Wang X, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8:118–23. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howard KM, Kusuma RJ, Baier SR, et al. Loss of MiRNAs during processing and storage of cow´s (Bos taurus) milk. J Agric Food Chem. 2015 doi: 10.1021/jf505526w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang H, Wu W, Zhang M, et al. Aberrant upregulation of miR-21 in placental tissues of macrosomia. J Perinatol. 2014;34:658–63. doi: 10.1038/jp.2014.58. [DOI] [PubMed] [Google Scholar]
- 95.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han M, Liu M, Wang Y, et al. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 incactivation by targeting PTEN. PLoS One. 2012;7:e39520–0. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFß-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One. 2012;7:e42316. doi: 10.1371/journal.pone.0042316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sayed D, Rane S, Lypowy J, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrouths. Mol Biol Cell. 2008;19:3272–82. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dariminpourain M, Wang S, Ittmann M, Kwabi-Addo B. Transcriptional and post-transcriptional regulation of Sprouty1, a receptor tyrosine kinase inhibitor in prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:279–85. doi: 10.1038/pcan.2011.33. [DOI] [PubMed] [Google Scholar]
- 100.Frey MR, Carraro G, Batra RK, Polk DB, Warburton D. Sprouty keeps bowel kinases regular in colon cancer, while miR-21 targets Sprouty. Cancer Biol Ther. 2011;11:122–4. doi: 10.4161/cbt.11.1.14176. [DOI] [PubMed] [Google Scholar]
- 101.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21):post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 102.Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–9. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, Platanias LC. Suppression of programmed cell death 4 (PDCD4):protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem. 2008;28:8601–10. doi: 10.1074/jbc.M707934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng R, Song G, Roll GR, Frandsen NM, Willenbring H. A mi-croRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J Clin Invest. 2012;122:1097–108. doi: 10.1172/JCI46039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dennis MD, Jefferson LS, Kimball SR. Role of p70S6K1-mediated phosphorylation of eIF4B and PDCD4 proteins in the regulation of protein synthesis. J Biol Chem. 2012;287:42890–9. doi: 10.1074/jbc.M112.404822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27:3093–102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 107.Kim YJ, Hwang SH, Cho HH, Shin KK, Bae YC, Jung JS. MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21 expression in white adipose tissues. J Cell Physiol. 2012;227:183–93. doi: 10.1002/jcp.22716. [DOI] [PubMed] [Google Scholar]
- 108.Dey N, Das F, Mariappan MM, et al. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J Biol Chem. 2011;286:25586–603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong X, Chung AC, Chen HY, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–74. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 110.Lei BX, Liu ZH, Li ZJ, Li C, Deng YF. miR-21 induces cell proliferation and suppresses the chemosensitivity in glioblastoma cells via downregulation of FOXO1. Int J Clin Exp Med. 2014;7:2060–6. [PMC free article] [PubMed] [Google Scholar]
- 111.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–36. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 112.Kitamura T, Ido Kitamura Y. Role of FoxO proteins in pancreatic beta cells. Endocr J. 2007;54:507–15. doi: 10.1507/endocrj.kr-109. [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi M, Kikuchi O, Sasaki T, et al. FoxO1 as a double-edged sword in the pancreas analysis of pancreas- and ß-cell-specific FoxO1 knockout mice. Am J Physiol Endocrinol Metab. 2012;302:E603–13. doi: 10.1152/ajpendo.00469.2011. [DOI] [PubMed] [Google Scholar]
- 114.Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes. 2006;55:1581–91. doi: 10.2337/db05-0678. [DOI] [PubMed] [Google Scholar]
- 115.Guo S, Dunn SL, White MF. The reciprocal stability of FOXO1 and IRS2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol. 2006;20:3389–99. doi: 10.1210/me.2006-0092. [DOI] [PubMed] [Google Scholar]
- 116.Dansen TB. Forkhead Box O transcription factors key players in redox signaling. Antioxid Redox Signal. 2011;14:559–61. doi: 10.1089/ars.2010.3778. [DOI] [PubMed] [Google Scholar]
- 117. Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–63. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 118.Anuradha R, Saraswati M, Kumar KG, Rani SH. Apoptosis of beta cells in diabetes mellitus. DNA Cell Biol. 2014;33:743–8. doi: 10.1089/dna.2014.2352. [DOI] [PubMed] [Google Scholar]
- 119.Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968;25:52–7. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- 120.Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–6. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 121.Belfiore F, Iannello S, Rabuazzo AM. Insulin resistance in obesity a critical analysis at enzyme level. A review. Int J Obes. 1979;3:301–23. [PubMed] [Google Scholar]
- 122.Pennetti V, Galante A, Zonta-Sgaramella L, Jayakar SD. Relation between obesity, insulinemia, and serum amino acid concentrations in a sample of Italian adults. Clin Chem. 1982;28:2219–24. [PubMed] [Google Scholar]
- 123.Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983;25:400–3. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- 124.Forlani G, Vannini P, Marchesini G, Zoli M, Ciavarella A, Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism. 1984;33:147–50. doi: 10.1016/0026-0495(84)90127-6. [DOI] [PubMed] [Google Scholar]
- 125.Schauder P, Zavelberg D, Langer K, Herbertz L. Sex-specific differences in plasma branched-chain keto acid levels in obesity. Am J Clin Nutr. 1987;46:58–60. doi: 10.1093/ajcn/46.1.58. [DOI] [PubMed] [Google Scholar]
- 126.Caballero B, Finer N, Wurtman RJ. Plasma amino acids and insulin levels in obesity response to carbohydrate intake and tryptophan supplements. Metabolism. 1988;37:672–6. doi: 10.1016/0026-0495(88)90089-3. [DOI] [PubMed] [Google Scholar]
- 127.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–63. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Newgard CB, An J, Bain Jr, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeng M, Che Z, Liang Y, et al. GC-MS based plasma metabolic profiling of type 2 diabetes. Chromatographia. 2009;69:941–8. [Google Scholar]
- 130.Zeng YJ, Zeng FQ, Dai L, et al. Characteristics and risk factors for hyperglycemia in Chinese female patients with systemic lupus erythematosus. Lupus. 2010;19:1344–50. doi: 10.1177/0961203310375439. [DOI] [PubMed] [Google Scholar]
- 131.Tai ES, Tan ML, Stevens RD. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953–0. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5:e15234–0. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Würtz P, Mäkinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes. 2012;61:1372–80. doi: 10.2337/db11-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–14. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–31. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–87. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim MJ, Yang HJ, Kim JH, et al. Obesity-related metabolomic analysis of human subjects in black soybean peptide intervention study by ultraperformance liquid chromatography and quadrupole-time-of-flight mass spectrometry. J Obes. 2013;2013:874981–0. doi: 10.1155/2013/874981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morris C, O'Grada C, Ryan M, Roche HM, Gibney MJ, Gibney ER, Brennan L. The relationship between BMI and metabolomic profiles a focus on amino acids. Proc Nutr Soc. 2012;71:634–8. doi: 10.1017/S0029665112000699. [DOI] [PubMed] [Google Scholar]
- 142.Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7:53–9. doi: 10.1007/s11684-013-0255-5. [DOI] [PubMed] [Google Scholar]
- 143.Brosnan JT, Brosnan ME. Branched-chain amino acids enzyme and substrate regulation. J Nutr. 2006;136:207S–11S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 144.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT, Hutson SM. Branched-chain amino acid metabolon interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase (BCATm) J Biol Chem. 2010;285:265–76. doi: 10.1074/jbc.M109.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.AEvarsson A, Chuang JL, Wynn RM, Turley S, Chuang DT, Hol WG. Crystal structure of human branched-chain alpha-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure. 2000;8:277–91. doi: 10.1016/s0969-2126(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 146.Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum Mol Genet. 2005;14:3371–7. doi: 10.1093/hmg/ddi368. [DOI] [PubMed] [Google Scholar]
- 147.Davie Jr, Wynn RM, Meng M, et al. Expression and characterization of branched-chain alpha-ketoacid dehydrogenase kinase from the rat. Is it a histidine-protein kinase?. J Biol Chem. 1995;270:1986 1–7. doi: 10.1074/jbc.270.34.19861. [DOI] [PubMed] [Google Scholar]
- 148.Chou PY, Fasman GD. Structural and functional role of leucine residues in proteins. J Mol Biol. 1973;74:263–81. doi: 10.1016/0022-2836(73)90372-0. [DOI] [PubMed] [Google Scholar]
- 149.Hawgood S, Derrick M, Poulain F. Structure and properties of surfactant protein, B. Biochim Biophys Acta. 1998;1408:150–60. doi: 10.1016/s0925-4439(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 150.Glover JN, Harrison SC. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature. 1995;373:257–61. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- 151.Wiley AS. Dairy and milk consumption and child growth Is BMI involved? An analysis of NHANES 1999-2004. Am J Hum Biol. 2010;22:517–25. doi: 10.1002/ajhb.21042. [DOI] [PubMed] [Google Scholar]
- 152.Berkey CS, Rocket HR, Willet WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain. Arch Pediatr Adolesc Med. 2005;159:543–50. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- 153.Matthews VL, Wien M, Sabaté J. The risk of child and adolescent overweight is related to types of food consumed. Nutr J. 2011;10:71–0. doi: 10.1186/1475-2891-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arnberg K, Mølgaard C, Michaelsen KF, Jensen SM, Trolle E, Larnkjær A. Skim milk, whey, and casein increase body weight and whey and casein increase plasma C-peptide concentration in overweight adolescents. J Nutr. 2012;142:2083–90. doi: 10.3945/jn.112.161208. [DOI] [PubMed] [Google Scholar]
- 155.Barr SI, McCarron DA, Heaney RP, et al. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. Am J Diet Assoc. 2000;100:810–7. doi: 10.1016/S0002-8223(00)00236-4. [DOI] [PubMed] [Google Scholar]
- 156.Seeger T, Fischer A, Muhly-Reinholz M, Zeiher AM, Dimmeler S. Long-term inhibition of miR-21 leads to reduction of obesity in db/db mice. Obesity (Silver Spring) 2014;22:2352–60. doi: 10.1002/oby.20852. [DOI] [PubMed] [Google Scholar]
- 157.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tiffin N, Adie E, Turner F, et al. Computational disease gene identification a concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic Acids Res. 2006;34:3067–81. doi: 10.1093/nar/gkl381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu H, Shyh-Chang N, Segrè AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Viswanathan SR, Dalex GQ, Gregory RI. Selective blockade of microRNA processing by Lin-28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pérez LM, Bernal A, San Martin N, Lorenzo M, Fernández-Veledo S, Gávez BG. Metabolic rescue of obese adipose-derived stem cells by Lin28/Let7 pathway. Diabetes. 2013;62:2368–79. doi: 10.2337/db12-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic diesease. Cardiovasc Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 163.Yang WM, Jeong HJ, Park SY, Lee W. Induction of miR-29a by saturated fatty acids impairs insulin signaling and glucose uptake through translational repression of IRS-1 in myocytes. FEBS Lett. 2014;588:2170–6. doi: 10.1016/j.febslet.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 164.Chakraborty C, Doss CG, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014;5:697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 165.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 166.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–2. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 167.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–9. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 169.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–6. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 170.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 171.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–84. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 172.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Tremblay F, Brulé S, Hee Um S, et al. Identification of Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–61. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gulati P, Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans. 2007;35:236–8. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 175.Lawlor DA, Smith GD, O'Callaghan M, et al. Epidemiologic evidence for the fetal overnutrition hypothesis findings from the Mater-University Study of Pregnancy and its Outcomes. Am J Epidemiol. 2007;165:418–24. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 176.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92:876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.The American College of Obstetricians and Gynecologists FAQ1 Nutrition during pregnancy. Sep http://www.acog.org/~/media/For%20Patients/faq001.Pdf?dmc=1&ts=20140823T1014147121 . 2013.
- 179.Olsen SF, Halldorsson TI, Willett WC, et al. Milk consumption during pregnancy is associated with increased infant size at birth prospective cohort study. Am J Clin Nutr. 2007;86:1104–10. doi: 10.1093/ajcn/86.4.1104. [DOI] [PubMed] [Google Scholar]
- 180.Heppe DH, van Dam RM, Willemsen SP, et al. Maternal milk consumption, fetal growth, and the risks of neonatal complications the Generation R Study. Am J Clin Nutr. 2011;94:501–9. doi: 10.3945/ajcn.111.013854. [DOI] [PubMed] [Google Scholar]
- 181.Lager S, Oowell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Larqué E, Riuz-Palacios M, Koletzko B. Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care. 2013;16:292–7. doi: 10.1097/MCO.0b013e32835e3674. [DOI] [PubMed] [Google Scholar]
- 183.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted growth. J Physiol. 2007;582:449–59. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33:e23–9. doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297:C723–31. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 186.Jansson N, Rosario FJ, Gaccioli F, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98:105–13. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med. 2015 doi: 10.1186/s12967-014-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Preedy VR, Watson RR, Zibadi S, Melnik BC, editors. Human Health Handbooks no. 8 Wageningen Academic Publishers. 2014. Formula feeding promtes adipogenic, diabetogenic, hypertonic and allergic mTORC1-programming Handbook of dietary and nutritinal aspects of bottle feeding; pp. 545–568. [Google Scholar]
- 189.Melnik BC. Excessive leucine-mTORC1-signalling of cow milk-based infant formula the missing link to understand early childhood obesity. J Obes. 2012;2012:197653. doi: 10.1155/2012/197653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Escribano J, Luque V, Ferre N, et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age the EU Childhood Obesity Programme. Int J Obes. 2012;36:548–53. doi: 10.1038/ijo.2011.276. [DOI] [PubMed] [Google Scholar]
- 191.Koletzko B, Beyer J, Brands B, et al. Early influences of nutrition on postnatal growth. Nestle Nutr Inst Workshop Ser. 2013;71:11–27. doi: 10.1159/000342533. [DOI] [PubMed] [Google Scholar]
- 192.Dunger DB, Salgin B, Ong KK. Session 7: Early nutrition and later health Early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66:451–7. doi: 10.1017/S0029665107005721. [DOI] [PubMed] [Google Scholar]
- 193.Wiley AS. Milk intake and total dairy consumption associations with early menarche in NHANES 1999,-2004. PLoS One. 2011;6:e14685. doi: 10.1371/journal.pone.0014685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Janghorbani M, Mansourian M, Hosseini E. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol. 2014;51:519–28. doi: 10.1007/s00592-014-0579-x. [DOI] [PubMed] [Google Scholar]
- 195.Bartolome A, Guillén C. Role pf the mammalian target of rapamycin (mTOR) complexes in pancreatic ß-cell mass regulation. Vitam Horm. 2014;95:425–69. doi: 10.1016/B978-0-12-800174-5.00017-X. [DOI] [PubMed] [Google Scholar]
- 196.Back SH, Kang SW, Han J, Chung HAT. Endoplasmic reticulum stress in the ß-cell cell pathogenesis of type 2 diabetes. Exp Diabetes Res. 2012:618396. doi: 10.1155/2012/618396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–93. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic ß-cell dysfunction. Diabetes Obes Metab. 2010;12:76–82. doi: 10.1111/j.1463-1326.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 199.Meier JJ, Breuer TG, Bonadonna RC, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55:1346–54. doi: 10.1007/s00125-012-2466-8. [DOI] [PubMed] [Google Scholar]
- 200.Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic ß-cells via activation of mTORC1. PLoS One. 2009;4:e4954. doi: 10.1371/journal.pone.0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Reiling JH, Sabatini DM. Increased mTORC1 singaling UPRegulates stress. Mol Cell. 2008;29:533–5. doi: 10.1016/j.molcel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 202.Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation oft he IRE-JNK pathway. Cell Death Differ. 2012;19:310–20. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ozcan U, Ozcan L, Yilmaz E, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Krokowski D, Han J, Saikia M, et al. A self-defeating anabolic program leads to ß-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J Biol Chem. 2013;288:17202–13. doi: 10.1074/jbc.M113.466920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–82. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 206.Guan BJ, Krokowski D, Majumder M. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2a. J Biol Chem. 2014;289:12593–611. doi: 10.1074/jbc.M113.543215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dunlop EA, Tee AR. mTOR and autophagy A dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014;36C:121–129. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 209.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bachar-Wikstrom E, Wikstrom JD, Kaiser N, Cerasi E, Leibowitz G. Improvement of ER stress-induced diabetes by stimulating autophagy. Autophagy. 2013;9:626–8. doi: 10.4161/auto.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–47. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 212.Shigeyama Y, Kobayashi T, Kido Y, et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008;28:2971–9. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tang X, Tang G, Ozcan S. Role of microRNAs in diabetes. Biochim Biophys Acta. 2008;1779:697–701. doi: 10.1016/j.bbagrm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ozcan S. Minireview MicroRNA function in pancreatic ß cells. Mol Endocrinol. 2014;28:1922–1933. doi: 10.1210/me.2014-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Plaisance V, Waeber G, Regazzi R, Abderrahmani A. Role of microRNAs in islet beta-cell compensation and failure during diabetes. J Diabetes Res. 2014;2014:618652–0. doi: 10.1155/2014/618652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Roggli E, Britan A, Gattesco S, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59:978–86. doi: 10.2337/db09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Novotny GW, Lundh M, Backe MB, et al. Transcriptional and translational regulation of cytokine signaling in inflammatory ß-cell dysfunction and apoptosis. Arch Biochem Biophys. 2012;528:171–84. doi: 10.1016/j.abb.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 218.Dai C, Brissova M, Reinert RB, et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62:4144–53. doi: 10.2337/db12-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Eberhard D, Kragl M, Lammert E. 'Giving and taking': endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab. 2010;21:457–63. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Zanone MM, Favaro E, Camussi G. From endothelial to beta cells insights into pancreatic islet microendothelium. Curr Diabetes Rev. 2008;4:1–9. doi: 10.2174/157339908783502415. [DOI] [PubMed] [Google Scholar]
- 221.Salama A, Fichou N, Allard M, et al. MicroRNA-29b modulates innate and antigen-specific immune responses in mouse models of autoimmunity. PLoS One. 2014;9:e106153–0. doi: 10.1371/journal.pone.0106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Figliolini F, Cantaluppi V, De Lena M, et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS One. 2014;9:e102521–0. doi: 10.1371/journal.pone.0102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arnold N, Koppula PR, Gul R, Luck C, Pulakat L. Regulation of cardiac expression oft he diabetic marker microRNA miR-29. PloS One. 2014;9:e103284–0. doi: 10.1371/journal.pone.0103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nielsen LB, Wang C, Sørensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362. doi: 10.1155/2012/896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44:237–44. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mott JL, Kobayashi S, Bronk SF, Gores GJ. Mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–40. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Roggli E, Gattesco S, Caille D, et al. Changes in microRNA expression contribute to pancreatic ß-cell dysfunction in prediabetic NOD mice. Diabetes. 2012;61:1742–5. doi: 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly upregulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21:2785–94. doi: 10.1210/me.2007-0167. [DOI] [PubMed] [Google Scholar]
- 229.Peng H, Zhong M, Zhao W, et al. Urinary miR-29 correlates with albuminuria and carotid intima-media thickness in type 2 diabetes patients. PLoS One. 2013;8:e82607–0. doi: 10.1371/journal.pone.0082607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mills Jr, Hippo Y, Robert F, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–8. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults the CARDIA Study. JAMA. 2002;287:2081–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 232.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incididence of vascular disease and diabetes an overview of the evidence. Lipids. 2010;45:925–39. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fumeron F, Lamri A, Abi Khalil C, et al. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome results from a French prospective study, Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2011;34:813–7. doi: 10.2337/dc10-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:1027–31. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 235.Hirahatake KM, Slavin JL, Maki KC, Adams SH. Associations between dairy foods, diabetes, and metabolic health Potential mechanisms and future directions. Metabolism. 2014;63:618–27. doi: 10.1016/j.metabol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rice BH, Quann EE, Miller GD. Meeting and exceeding dairy recommendations effects of dairy consumption on nutrient intakes and risk of chronic disease. Nutr Rev. 2013;71:209–23. doi: 10.1111/nure.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu S, Choi HK, Ford E. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–84. doi: 10.2337/dc06-0256. [DOI] [PubMed] [Google Scholar]
- 238.Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome the Caerphilly prospective study. J Epidemiol Community Health. 2007;61:695–8. doi: 10.1136/jech.2006.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Villegas R, Gao YT, Dai Q, et al. Dietary calcium and magnesium intakes and the risk of type 2 diabetes the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1059–67. doi: 10.3945/ajcn.2008.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kirii K, Mizoue T, Iso H, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–50. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- 241.Song Y, Chavarro JE, Cao Y , et al. Whole milk intake is associated with prostate cancer-specific mortality among, U .; male physicians. J Nutr. 2013;143:189–96. doi: 10.3945/jn.112.168484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sluijs I, Forouhi NG, Beulens JW, et al. The amount and type of dairy product intake and incident type 2 diabetes results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96:382–90. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 243.Gobierno de España. Ministerio de Agricultura Alimentación y Medio Ambiente. El Consumo Alimentario en España. 2011 [Google Scholar]
- 244.Liljeberg Elmståhl H, Björck I. Milk as a supplement to mixed meals may elevate postprandial insulinaemia. Eur J Clin Nutr. 2001;55:994–9. doi: 10.1038/sj.ejcn.1601259. [DOI] [PubMed] [Google Scholar]
- 245.Dunne J, Evershed RP, Salque M, et al. First dairying in green Saharan Africa in the fifth millennium, BC. Nature. 2012;486:390–4. doi: 10.1038/nature11186. [DOI] [PubMed] [Google Scholar]
- 246.Curry A. Archaeology The milk revolution. Nature. 2013;500:20–2. doi: 10.1038/500020a. [DOI] [PubMed] [Google Scholar]
- 247.Beja-Pereira A, Luikart G, England PR, et al. Gene-culture coevolution between cattle milk protein genes and human lactase genes. Nat Genet. 2003;35:311–3. doi: 10.1038/ng1263. [DOI] [PubMed] [Google Scholar]
- 248.Panesar PS, Kumari S, Panesar R. Potential applications of immobilized ß-galactosidase in food processing industries. Enzyme Res. 2010;2010:473137. doi: 10.4061/2010/473137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yu R, Woo J, Chan R , et al. Relationship between dietary intake and the development of type 2 diabetes in a Chinese population the Hong Kong Dietary Survey. Public Health Nutr. 2011;14:1133–41. doi: 10.1017/S136898001100053X. [DOI] [PubMed] [Google Scholar]
- 250.van Nielen M, Feskens EJ, Mensink M , et al. Dietary protein intake and incidence of type 2 diabetes in Europe the EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014;37:1854–62. doi: 10.2337/dc13-2627. [DOI] [PubMed] [Google Scholar]
- 251.Michaëlsson K, Wolk A, Langenskiöld S , et al. Milk intake and risk of mortality and fractures in women and men cohort studies. BMJ. 2014;349:g6015–0. doi: 10.1136/bmj.g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Upadhyaya S, Kadamkode V, Mahammed R, Doraiswami C, Banerjee G. Adiponectin and IL-6: Mediators of inflammation in progression of healthy to type 2 diabetes in Indian population. Adipocyte. 2014;3:39–45. doi: 10.4161/adip.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Oh YS, Lee YJ, Park EY, Jun HS. Interleukin-6 treatment induces beta-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes Metab Res Rev. 2011;27:813–9. doi: 10.1002/dmrr.1233. [DOI] [PubMed] [Google Scholar]
- 254.Russell MA, Cooper AC, Dhayal S, Morgan NG. Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic ß-cells. Islets. 2013;5:95–105. doi: 10.4161/isl.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yang CH, Yue J, Fan M, Pfeffer LM. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res. 2010;70:8108–16. doi: 10.1158/0008-5472.CAN-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ou H, Li Y, Kang M. Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene. PLoS One. 2014;9:e109929–0. doi: 10.1371/journal.pone.0109929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Song S, Abdelmohsen K, Zhang Y, Becker KG, Gorospe M, Bernier M. Impact of pyrrolidine dithiocarbamate and interleukin-6 on mammalian target of rapamycin complex 1 regulation and global protein translation. J Pharmacol Exp Ther. 2011;339:905–13. doi: 10.1124/jpet.111.185678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McCay CM, Crowell MF. Prolonging the life span. Scientific Monthly. 1934;39:405–14. [Google Scholar]
- 260.Moss M. Does milk cause coronary heart diesease? J Nutr Environment Med. 2002;12:207–16. [Google Scholar]
- 261.Segall JJ. Hypothesis Is lactose a dietary risk factor for ischaemic heart disease? Int J Epidemiol. 2008;37:1204–8. doi: 10.1093/ije/dyn169. [DOI] [PubMed] [Google Scholar]
- 262.Ji J, Sundquist J, Sundquist K. Lactose intolerance and risk of lung, breast and ovarian cancers aetiological clues from a population-based study in Sweden. Br J Cancer. 2015;112:149–152. doi: 10.1038/bjc.2014.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow's milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab (Lond) 2012;9:74–0. doi: 10.1186/1743-7075-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16:378–86. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Melnik BC, Schmitz G. Metformin an inhibitor of mTORC1 signaling. J Endocrinol Diabetes Obes. 2014;2:1029–0. [Google Scholar]
- 266.Malek M, Aghili R, Emami Z, Khamseh ME. Risk of cancer in diabetes the effect of metformin. Endocrinology. 2013;2013:636927–0. doi: 10.1155/2013/636927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bannister CA, Holden SE, Jenkins-Jones S , et al. Can people with type 2 diabetes life longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16:1165–73. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- 268.Blagosklonny MV. Revisiting the antagonistic peiotropy theory of aging TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–6. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 269.Kapahi P, Chen D, Rogers AN , et al. With TOR less is more a key role for the conserved nutrient sensing TOR pathway in aging. Cell Metab. 2010;11:453–65. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Weaver CM. How sound is the science behind the dietary recommendations for dairy? Am J Clin Nutr. 2014;99:1217S–22S. doi: 10.3945/ajcn.113.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.McGregor RA, Poppitt SD. Milk protein for improved metabolic health a review of the evidence. Nutr Metab (Lond) 2013;10:46–0. doi: 10.1186/1743-7075-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tunick MH, Van Hekken DL. Dairy products and health recent insights. J Agric Food Chem. 2014 doi: 10.1021/jf5042454. [DOI] [PubMed] [Google Scholar]
- 273.Chen M, Sun Q, Giovannucci E , et al. Dairy consumption and risk of type 2 diabetes 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12:215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wise J. Eating a yoghurt a day is linked to lower risk of type 2 diabetes. BMJ. 2014;349:g7081. doi: 10.1136/bmj.g7081. [DOI] [PubMed] [Google Scholar]