Abstract
Background: Previous studies relating smoking and alcohol drinking with the incidence of dementia have been inconsistent. Objectives: We assessed whether smoking and alcohol drinking was associated with the risk of dementia, including Alzheimer disease (AD) and vascular dementia (VaD) after seven years of follow-up. Design: We prospectively analysed the incidence of dementia from 2004 to 2011 among 2959 elderly men, according to their smoking and alcohol drinking status. Setting: six neighbourhoods from three districts mentioned in Chongqing city. Participants: A total of 3170 men were followed up annually for 7 years. Measurements: Cox proportional hazards models were established to evaluate the association between smoking, alcohol drinking and the risk of dementia. Results: The incidences of AD and VaD were higher respectively in current smoking than never smoking, daily drinking than never drinking over 7 years of follow-up (p<0.01). After adjusting for age and other potential confounders, current smoking was associated with increased risk of AD (HR= 2.14, 95% CI 1.20-4.46) and VaD (HR= 3.28, 95% CI 1.14-4.52), meanwhile, daily drinking was related to increased risk of AD (HR= 2.25, 95% CI 1.43-3.97) and VaD (HR= 3.42, 95% CI 1.18-4.51). In addition, co-smoking and drinking were related to with a significantly higher risk of AD and VaD than non-smoking and drinking (HR= 3.03, 95% CI 1.65-4.19) and VaD (HR= 3.96, 95% CI 1.64-4.71). Moreover, co-smoking and drinking had higher risk of AD and VaD compared with current smoking and daily drinking. Conclusions: Current smoking and daily drinking were found to be significantly associated with dementia in elderly men.
Keywords: Alcohol drinking, Alzheimer’s disease, smoking, vascular dementia
INTRODUCTION
Addiction to smoking and alcohol drinking are serious problems of public health in worldwide, especially in China [1, 2]. Smoking and alcohol drinking are closely related to atherosclerosis, cardiovascular disease, stroke, hypertension, diabetes mellitus, dementia, etc. [3, 4]. In the past 50 years, an estimated 17.7 million deaths might be related to smoking all over the world [5].
The effects of cigarette consumption and alcohol drinking on dementia among elderly people have been paid attention [6, 7]. The association of smoking and alcohol drinking with dementia risk has shown various results in past study. Some studies finding suggested that smoking was a risk factor of cognitive impairment for AD and VaD [8]. Another three researches hoped that nicotinic stimulation may have promise for improving cognitive [9]. In addition, several results have recently suggested that alcohol drinking in elderly people might impair cognitive function [10, 11], whereas others did not indicate that alcohol consumption would be harmful to cognition function, and it was not possible to define a specific beneficial level of alcohol drinking [12].
The study of dementia among elderly people has been taken in some large cities in China [13, 14]. China is a country with a large production and consumption of cigarette and alcohol. It is different from western countries in China that the cigarette and alcohol users are mainly in adult men, rather than in women. In past study, association of smoking and alcohol drinking with risk of dementia in both sexes was discussed together in China. The association was not specially analysed in men, so previous researches could not accurately reflect the effect of smoking and alcohol drinking on dementia. We examined the relationship of smoking, alcohol consumption with dementia, AD and VaD in a large cohort of elderly men.
MATERIALS AND METHODS
Study Population
Chongqing City is the largest city of the southwest region. Chongqing City has a population of 35 million people, and is the most population city in China. We randomly selected six neighbourhoods from three districts mentioned in Chongqing city. The following inclusion criteria were applied: (1) 60 years of age and older. (2) long-term residents of local communities. The exclusion criteria included (1) serious illness, severe hearing or visual impairment precluding a reliable assessment of cognitive function, (2) persistent impairment of consciousness, (3) previous long-lasting mental retardation, (4) no reliable information, (5) a history of severe head trauma or neurosurgery. The present study was approved by the Institutional Review Board of Daping Hospital and all men provided informed consent.
Baseline Screening
In this study, baseline screening was performed between January 1 and June 30, 2004. Formal questionnaire and neuropsychological tests for all men were completed at the community medical centers. For elderly men who were absent at these center, or who were not able to attend due to physical disabilities, our staff went to their home to perform the questionnaire and tests. Among 4057 examined men, 3728 men were eligible (response rate 92%), while 221 men were not available at the time of screening (n = 106) or declined to participate (n = 115). An additional 337 men were diagnosed with dementia and excluded. The remaining 3170 men without dementia were enrolled at baseline (Fig. 1).
Fig. (1).
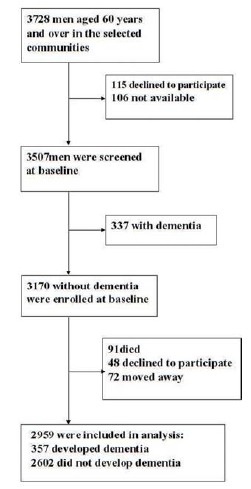
Flowchart of analysis sample selection.
The following data on formal questionnaire and neuropsychological tests were collected. These procedures were administered by experienced neurologists, psychiatrists and senior nurses. The agreement on data collection was excellent based on the κ values of 0.92 for baseline screening questionnaire and 0.91 for neuropsychological tests.
The following data were collected at baseline, including age, weight, body mass index (BMI) and educational level and available medical records. Medical records were collected to clinical assessment. The assessment included presence of ischemic heart diseases, stroke, hypertension (systolic/diastolic blood pressure > 140/90 mmHg or being on antihypertensive treatment), diabetes mellitus (fasting blood glucose > 110 mg/dl or being on antidiabetic treatment), hypercholesterolemia (total cholesterol > 200 mg/dl). Measurements of blood pressure and electrocardiogram were performed on-site. In addition, fasting serum samples were collected at baseline and stored at -80°C for measurement of glucose, cholesterol and ApoE. Men with potential diseases that had not previously been diagnosed, were admitted to the Daping Hospital for further investigation and a diagnosis diseases state. The diagnosis of diseases, including ischemic heart diseases, stroke, hypertension, diabetes mellitus and hypercholesterolemia, were based on the International Classification of Diseases, 9th Revision (ICD-9).
Smoking and Alcohol Drinking
Tobacco smokers were classified as past smokers who had ceased smoking for at least 6 months, current smokers, or those who never smoked. Alcohol drinking status was classified as daily drinking, weekly drinking, monthly drinking, and occasional drinking. Non-smoking and drinking was neither smoking nor drinking, only smoking included past smoking and current smoking except drinking, only drinking included weekly drinking and monthly drinking except smoking. Co-smoking and drinking was defined as current smokers and daily drinking. In drinking men of our study, 86.3% men drank distilled spirit, 13.1% men drank beer, and only 0.6% men wine. In fact, 96.5% men drank distilled spirit and beer at the same time, so types of alcohol consumption were not analyzed in this study.
Diagnosis of Dementia
The diagnosis of dementia was described in our previous studies [15]. In brief, diagnosis of dementia was based on criteria modified from the DSM-IV [16]. The subjects with dementia were further subjected to brain CT or MRI. Diagnosis of AD dementia was made according to the criteria of National Institute of Neurological and Communicative Diseases and Stroke/AD and Related Disorders Association [17]. Diagnosis of vascular dementia (VaD) was based on the criteria of the National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) [18]. The differentiation of AD from VaD is based on the NINDS-AIREN criteria and HIS (HIS≤4: AD, 4≤HIS≤7: mixed dementia (AD plus VaD), HIS ≥7: VaD [19]. The diagnosis of other dementias, including Parkinson's disease dementia, frontotemporal dementia, Lewy body dementia and Huntington’s disease, were based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [16].
Follow-up
A total of 3170 men were followed up annually for 7 years from July 2004 to June 2011. These men were interviewed by questionnaire and neuropsychological tests at the community medical centers or our staff went to their home to perform the interview when needed. Cognitive status was re-assessed using the same neuropsychological tests as baseline screening. There was no difference in characteristics between dropped out and remained men in the study.
Statistical Analysis
Using univariate analyses, baseline variables between men with and without dementia were compared using the Pearson Chi square test, the Fisher exact test, the t test or the Mann-Whitney U test as deemed appropriate. We assessed the associations (hazard ratio) between smoking or drinking status and the risk of dementia development in the Cox proportional hazards models. The associations were firstly analyzed without adjustment for potential confounders. Then, the associations were further analyzed after adjustment for age BMI, education, ApoE and vascular risk factors. Finally, the statistical analyses were performed using SPSS 18.0 for Windows.
RESULTS
Baseline Characteristics in Men Who Did and Did not Develop Dementia
In the seven years course of the study, 91 (2.9%) men died, 48 (1.5%) declined to further participate, and 72 (2.3%) moved away from the area, leaving 2959 men who completed the follow-up (Fig. 1). During follow-up, 357 cases of dementia were detected, of which 172 (48.2%) was AD, 156 (43.7%) were VaD and 29 (8.1%) were other dementias. For the men who completed the follow-up, the average age was 67.4±4.7 years. Compared with the men who did not develop dementia, those who did were significantly older, hypertension, diabetes mellitus, ischemic heart disease and stroke were more frequent in men with dementia, AD and VaD (p<0.01). Hypercholesterolemia was more prevalent in men with dementia, AD and VaD (p<0.05). ApoE was significantly more in men with dementia and AD (p<0.01), and VaD (p<0.05) (Table 1).
Table 1.
Baseline characteristics of men who developed dementia and who did not.
| Did not Develop Dementia | All Developed Dementia | Developed Dementia | |||
|---|---|---|---|---|---|
| AD | VaD | Other Dementia | |||
| n =2602 | n = 357 | n = 172 | n = 156 | n = 29 | |
| Physical characteristics | |||||
| Age (years), ±SD | 67.1±4.7 | 69.6±4.6* | 69.3±4.7* | 69.8±4.6* | 70.9±4.5* |
| Weight (Kg), ±SD | 64.7±10.5 | 64.4±10.3 | 64.2±10.2 | 64.5±10.1 | 64.8±9.9 |
| BMI (kg/m2), ±SD | 22.1±3.8 | 22.4± 3.6 | 22.3±3.6 | 22.4±3.6 | 22.5±3.5 |
| Education (years), ±SD | 11.2±5.1 | 10.9±4.9 | 10.6±4.8 | 11.3±5.0 | 10.9±4.8 |
| ApoE ε4 n (%) | 343(13.2) | 65 (18.2) * | 32(18.6) * * | 30(19.2) * * | 3 (10.3) |
| Vascular risk factors | |||||
| Hypertension, n (%) | 759(29.2) | 128 (35.9) * | 63(36.6) * * | 61(39.1) * | 4(13.8) |
| Diabetes mellitus, n (%) | 629(24.2) | 109 (30.5) * | 54(31.4) * * | 52(33.3) * | 3 (10.3) |
| Hypercholesterolemia, n (%) | 617(23.7) | 105 (29.4) * * | 53(30.8) * * | 48(30.8) * * | 11(37.9) |
| Ischemic heart disease, n (%) | 380(14.6) | 72(20.2) * | 35(20.3) * * | 32(20.5) * * | 5(17.2) |
| Stroke, n (%) | 487(18.7) | 88 (24.6) * | 43(25.0) * * | 43(27.6) * | 2(6.9) |
BMI: body mass index, AD: Alzheimer disease, VaD: vascular dementia
Two sided p values for χ2 test and t test, * p<0.01; * * p<0.05 vs did not develop dementia
Association of Smoking and Drinking with Dementia
Table 2 showed the association of smoking and alcohol drinking with dementia incidence. Among the three groups of smoking, it was found that the men with current smoking had significantly higher incidence of AD and VaD than those with never smoking (p < 0.01). When the four groups of drinking was analysed, the men with weekly drinking had significantly higher incidence of dementia and VaD than those with occasional drinking (p < 0.05). The association was even stronger when daily drinking was considered, showing a similar trend in dementia, AD and VaD (p < 0.01). While the men with Co-smoking and drinking had significantly higher incidence of dementia, AD and VaD than those with non-smoking and drinking (p < 0.01). Other dementias in our dementia classification included Parkinson's disease dementia 10 cases, frontotemporal dementia 9 cases, Lewy body dementia 7 cases and Huntington’s disease 3 cases. The number of other dementia cases was smaller, so the correlation of smoking and alcohol drinking with other dementias was not further researched.
Table 2. Dementia incidence in smoking, alcohol drinking status for men.
| Total | Did not Develop Dementia | All Developed Dementia | Developed Dementia | |||
|---|---|---|---|---|---|---|
| AD | VaD |
Other
Dementia |
||||
| n =2959 | n =2602 | n =357 | n =172 | n =156 | n =29 | |
| Smoking status | ||||||
| Never smoking n (%) | 829(28.0) | 734(28.2) | 95(26.6) | 44(25.6) | 41 (26.3) | 10(34.5) |
| Past smoking n (%) | 879(29.7) | 778(29.9) | 101(28.3) | 46(26.7) | 43(27.6) | 12(41.4) |
| Current smoking n (%) | 1251(42.3) | 1090 (41.9) | 161(45.1)* | 82(47.7)* | 72(46.2)* | 7(24.1) |
| Drinking status | ||||||
| Occasional drinking n (%) | 765(25.8) | 674 (42.5) | 91(25.5) | 38(22.1) | 42 (26.9) | 11(38.0) |
| Monthly drinking n (%) | 491(16.6) | 440 (16.9) | 51(14.3) | 25(14.5) | 21(13.5) | 5(17.2) |
| Weekly drinking n (%) | 402(13.6) | 361(13.9) | 41(11.5)* * | 18(10.5) | 16(10.3)* * | 7(24.1) |
| Daily drinking n (%) | 1301(44.0) | 1127(43.3) | 174 (48.7)* | 91(52.9)* | 77(49.4)* | 6 (20.7) |
| Co-smoking and drinking status | 1134(38.3) | 1325(50.9) | 203 (56.9)* | 105 (61.0)* | 89 (57.1)* | 9 (31.0) |
Co-smoking and drinking: co-existence of smoking and drinking
Two sided p values for χ2 test
* p<0.01 vs never smoking or occasional drinking or Non- smoking and drinking
* * p<0.05 vs never smoking or occasional drinking or Non- smoking and drinking
Dementia Incidence by Age and Smoking and Drinking Status
Fig. (2) showed dementia incidence over 7 years of follow-up by age and smoking, drinking status. These men were divided into three groups according to age. As follows: 60- to 69-year-old group, the 70- to 79-year-old group and the ≧ 80-year-old group. Interestingly, in each age-group, the incidence of Alzheimer’s disease per 100 person-years was significantly higher in co-smoking, drinking status than in current smoking and daily drinking (p < 0.01). In each age-group, the incidence of vascular dementia per 100 person-years was significantly higher in co-smoking, drinking status than in current smoking and daily drinking (p < 0.01).
Fig. (2).

Incidence of dementia per 100 person-years at 7 years end of follow-up by age and smoking, drinking status in men. These men were divided into three groups according to age. A. each age-group, the incidence of Alzheimer’s disease per 100 person-years was significantly higher in co-smoking, drinking status than in current smoking and daily drinking (p < 0.01). B. In each age-group, the incidence of vascular dementia per 100 person-years was significantly higher in co-smoking, drinking status than in current smoking and daily drinking (p < 0.01).
Relationship Between Smoking, Alcohol Drinking and Dementia in Cox Proportional Hazard Regression Model
The relationship between smoking or drinking status and incidence of dementia was shown in (Table 3). After adjusting for age, BMI, education, ApoE, vascular risk factors, it was noteworthy that current smoking was associated with a significantly higher risk of AD and VaD than never smoking, and daily drinking was associated with a significantly higher risk of AD and VaD than occasional drinking. In addition, co-smoking and drinking were related to with a significantly higher risk of AD and VaD than non-smoking and drinking. Moreover, co-smoking and drinking had higher risk of AD and VaD compared with current smoking and daily drinking.
Table 3. Relationship between smoking, alcohol drinking and dementia risk in Cox proportional hazard regression model in men.
| Alzheimer’s Disease | Vascular Dementia | Other Dementia | ||||
|---|---|---|---|---|---|---|
| Crude HR for AD (95% CI) | Adjusted HR for AD (95% CI) * | Crude HR for VaD (95% CI) | Adjusted HR for VaD (95% CI) * | Crude HR for Other Dementia (95% CI) | Adjusted HR for Other Dementia (95% CI) * | |
| Smoking status | ||||||
| Never smoking | 1 | 1 | 1 | 1 | 1 | 1 |
| Past smoking | 1.21(0.87-2.28) | 1.20(0.89-2.31) | 1.27(0.92-2.18) | 1.25(0.93-2.21) | 1.14(0.72-1.93) | 1.15(0.76-1.96) |
| Current smoking | 2.15(1.21-4.06) | 2.14(1.20-4.46) | 3.25(1.29-4.27) | 3.28(1.14-4.52) | 1.21(0.79-1.36) | 1.18(0.72-1.39) |
| Drinking status | ||||||
| Occasional drinking | 1 | 1 | 1 | 1 | 1 | 1 |
| Monthly drinking | 1.06(0.76-1.32) | 1.03(0.83-1.35) | 1.12(0.67-1.29) | 1.18(0.86-1.24) | 1.13(0.58-1.36) | 1.01(0.69-1.28) |
| Weekly drinking | 1.28(0.75-1.31) | 1.31(0.69-1.43) | 1.37(0.87-1.29) | 1.35(0.82-1.25) | 1.07(0.70-1.29) | 1.09(0.76-1.34) |
| Daily drinking | 2.29(1.45-3.92) | 2.25(1.43-3.97) | 3.34(1.29-4.28) | 3.42(1.18-4.51) | 1.08(0.81-1.36) | 1.06(0.82-1.39) |
| Co-smoking and drinking status | ||||||
| Non-smoking and drinking | 1 | 1 | 1 | 1 | 1 | 1 |
| Only smoking | 1.01(0.82-1.12) | 1.01(0.86-1.13) | 1.02(0.76-1.06) | 1.06(0.77-1.08) | 1.01(0.71-1.02) | 1.01(0.74-1.03) |
| Only drinking | 1.03(0.96-1.14) | 1.03(0.97-1.14) | 1.03(0.82-1.07) | 1.05(0.85-1.09) | 1.01(0.72-1.03) | 1.01(0.74-1.04) |
| Co-smoking and drinking | 3.02(1.61-4.16) | 3.03(1.65-4.19) | 3.94(1.63-4.68) | 3.96(1.64-4.71) | 1.12(1.13-1.27) | 1.12(1.14-1.28) |
Co-smoking and drinking: co-existence of smoking and drinking
HR: hazard ratio, CI: confidence interval
* adjusted for age, BMI, education, ApoE, vascular risk factors
DISCUSSION
Our study firstly showed that current smoking was associated with a 2.1-fold greater risk of AD and a 3.3-fold greater risk of VaD, and daily drinking was associated with a 2.3-fold greater risk of AD and a 3.4-fold greater risk of VaD, and co-smoking and drinking was also associated with a 3-fold greater risk of AD and a 3.9-fold greater risk of VaD in Chinese elderly men. Moreover, co-smoking and drinking had higher risk of AD and VaD compared with current smoking and daily drinking. Our study indicated the incidence of VaD in China is higher than that in other counties [20]. It could be related to high development of stroke and vascular risk factors in China.
In 2011, a study summarized the potentially modifiable risk factors for AD: smoking, diabetes, midlife hypertension, and so on [21]. Together, up to half of AD cases worldwide and in the USA are potentially attributable to these factors. A 10-25% reduction in all seven risk factors could potentially prevent as many as 1·1-3·0 million AD cases worldwide and 184,000-492,000 cases in the USA.
Some studies revealed the association of smoking and alcohol drinking status with incidence of dementia [22, 23]. Park et al. [24] evaluated cognitive function impairment, smoking and drinking status in 3,174 inhabitants aged 60–64 years in Korea, with a follow-up assessment of cognitive function 7 years later. Current smokers showed a higher risk for developing cognitive function impairment than did never smokers. Nevertheless, for AD, the results examined the association between smoking, alcohol drinking and AD, and suggested that heavy drinking and smoking and ApoE can lower the age of onset for AD in an additive fashion [25]. García et al. [26] performed a case-control study. Risk of AD was unaffected by tobacco smoking, alcohol drinkers also showed a lower risk of AD than never consumers.
Furthermore, excessive smoking and alcohol consumption has a great effect on VaD in our study. For VaD, there is substantial evidence from observational studies that conventional risk factors such as smoking, hypertension, diabetes and dyslipidemia play a role in the development of vascular cognitive impairment [27]. Smoking was associated with an increased rate of progression of vascular brain injury and decline in executive function a decade later [28]. Panza et al. [29] reported that light to moderate alcohol drinking might be associated with a reduced risk of unspecified incident dementia and AD, while for VaD, the current result is only suggestive of a protective effect.
It has been argued that joint effects of alcohol and tobacco use on ADs [30, 31]. In recent study, interaction between tobacco and alcohol consumption with AD was investigated. Unadjusted logistic regression model showed statistical significance for the interaction term between tobacco and alcohol, and this interaction term was also statistically significant in the model including our potentially confounding variables [26]. We assessed the interactions between tobacco and alcohol in large prospective studies, and found the joint effects of alcohol and tobacco use on AD and VaD.
There have been different results about the type of alcohol and risk of dementia. In the previous study, we suggested that a significantly lower risk of dementia existed for light-to-moderate drinkers of wine. A light-to-moderate intake of beer was associated with a significantly higher risk of dementia. Copenhagen study revealed that monthly and weekly intake of wine was significantly associated with a lower risk of dementia [32]. A monthly intake of beer was associated with increased risk of dementia. However, Rotterdam Study did not find the relationship between alcohol and dementia depended on the type of alcoholic beverage [33].
Gender, drinking patterns, interactions with other lifestyle-related and genetic factors genotyping may be sources of great variability. Previous studies on smoking, drinking and dementia stratified by gender showed contradictory results. A study suggested that frequent alcohol drinking increased the risk of developing cognitive impairment among male subjects (P =0.044) [24]. Another report from a Japanese rural community revealed that smoking was associated with an increased risk of disabling dementia. A twofold excess risk was found for smoking duration of ≥45 years. For men, we also found that excessive smoking and drinking were associated with increased risk of dementia [34].
Smoking and alcohol drinking were found to be significantly related to the development of dementia [35, 36]. However, the mechanism by which smoking and alcohol drinking contributes to dementia is unclear. Evidence from neuropathological and clinical studies suggested considerable overlap in risk factors of AD and VaD, and two diseases shared common pathogenic mechanisms. Zhu and others [37] demonstrated that oxidative stress is the earliest pathological changes of the brain in AD patients. Ho et al. [8] investigated the cigarette smoke-induced pathological changes and significantly increased levels of oxidative stress in AD rats. Cigarette impacted amyloid precursor protein (APP) processing by increasing the production of APPβ and accumulation of β–amyloid peptide.
Ritchie et al. [38] suggested the hypothesis that a genetic score for alcohol processing capacity moderates the association between alcohol consumption and lifetime change in cognitive ability. A significant gene × alcohol consumption interaction on lifetime cognitive change was found (p = 0.007). The effect of alcohol drinking on cognitive change may thus depend on genetic differences in the ability to metabolize alcohol.
The associated of apoE with risk of AD have been defined through in the study, APOE plays a critical role in transporting cholesterol in AD [39]. The effects of apoE isoforms on AD have been confirmed in humans, animal models and cellular studies. This evidence revealed the ε4 allele of the APOE gene is a stronger genetic risk factor for AD than the more common ε3 allele, whereas the presence of the ε2 allele is protective [40, 41]. The ε4 allele of the APOE gene not only dose dependently increases the risk for AD but also lowers the age of onset. ApoE4 contributes to the pathogenesis of AD by both loss-of-function in neuroprotection and gain-of-function in neurotoxicity compared to apoE3 [40, 42]. Whether apoE4 confers a risk for the development of VaD has been intensive investigation with conflicting conclusions. The results revealed a positive association between harboring the APOE4 allele and increased risk for VaD [43-45]. However, another studies found that APOE4 allele does not confer risk for VaD [46, 47].
In recent reports, alleles for alcohol dehydrogenase (ADH) and aldehyde dehydrogenases (ALDH) had been implicated in the risk of AD. Lewis et al. [48] used a set of four SNPs in ADH three genes (ADH1A, ADH1B, and ADH7) to examine the effect of maternal consumption of alcohol on offspring cognition. The total number of rare alleles on this SNP set interacted significantly with alcohol drinking. Moreover, ALDH not only transform aldehydes to acids but also act as antioxidant enzymes. This study found that the mitochondrial ALDH activity was significantly increased in the putamen of patients with AD compared to controls [49]. In addition, a Mendelian randomization study carried out in southern Chinese men, in which alcohol drinking is low to moderate and is influenced by genotype, offers an alternative and superior approach for clarifying the causal effect of moderate alcohol drinking on cognitive function [50]. ALHD2 genotype was strongly associated with alcohol consumption.
Lahiri DK et al. [51] proposed the LEARn (Latent Early-life Associated Regulation) model for AD. LEARn-AD might be a “two-hit” disorder, wherein the first hit might occur due to environmental stress within the regulatory sequences of AD-associated genes. This hit might come in early childhood. The second hit could consist of further stress, such as poor mid-life diet, or changes in expression of genes that occur later in life independent of any pathogenesis. Further, the LEARn model operates through the regulatory region of the gene, specifically through changes in methylation and oxidation within the promoter of specific genes [52]. The LEARn model combines genetic and environmental risk factors in an epigenetic pathway to explain the etiology of the most common forms of neurobiological disorders. Additionally, this study suggested that the LEARn model might explain the etiology of AD and other neuropsychiatric disorders [53]. The model provides us with a novel direction for identifying potentially harmful agents that may induce neurodegeneration and provides hope that we may be able to prevent age-related neurodegenerative disease by “detoxifying” our environment [54].
There were several limitations in the present study. First, the association between different types of alcohol beverage and dementia was not observed in this study. Some results pointed to a protective effect of alcohol drinking, mostly associated to wine consumption [55, 56]. Second, vascular contributions to cognitive impairment and dementia are important. Carotid artery stenosis, carotid intimal-medial thick and bilateral present carotid plaques were not assessed in this study, which might be associated with AD and VaD [57]. Third, another limitation is that measurements of confounding factors, including smoking and alcohol consumption status, were based on self-report.
In conclusion, the present study revealed that heavy smoking and drinking were related to the incidence of both AD and VaD, and a higher risk of dementia in men. Thus, these findings might suggest that to control smoking and drinking could be of importance for decreasing dementia incidence.
Sponsor’s Role
All sponsors have funded the underlying research scope of all respective authors where related aims are applicable to this study. However, no specific sponsor was explicitly involved in the design, methods, subject recruitment, data collection, analysis, and/or preparation of this particular manuscript.
Author Contributions
Shiming Zhou designed the study concept, Rui Zhou, Tingting Zhong and Rui Li aquired the subjects and clinical data. Shiming Zhou analyzed the data, and with Huadong Zhou, Rui Li and Rui Zhou performed further data analyses and interpretation. Shiming Zhou and Huadong Zhou drafted the manuscript and all authors revised and finally approved the initial manuscript and its revision.
TABLE.
| Elements of Financial/Personal Conflicts | Huadong Zhou | Shiming Zhou | Rui Zhou |
Tingting Zhong
Rui Li Jun Tan |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | No | No | No | No | ||||
| Grants/Funds | No | No | No | No | ||||
| Honoraria | No | No | No | No | ||||
| Speaker Forum | No | No | No | No | ||||
| Consultant | No | No | No | No | ||||
| Stocks | No | No | No | No | ||||
| Royalties | No | No | No | No | ||||
| Expert Testimony | No | No | No | No | ||||
| Board Member | No | No | No | No | ||||
| Patents | No | No | No | No | ||||
| Personal Relationship | No | No | No | No | ||||
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Schroeder S. Smoking-related mortality in the United States. N. Engl. J. Med. 2013;368(18):1753–1754. doi: 10.1056/NEJMc1302783. [DOI] [PubMed] [Google Scholar]
- 2.Zaridze D., Brennan P., Boreham J., Boroda A., Karpov R., Lazarev A., Konobeevskaya I., Igitov V., Terechova T., Boffetta P., Peto R. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373(9682):2201–2214. doi: 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clair C., Rigotti N.A., Porneala B., Fox C.S., D’Agostino R.B., Pencina M.J., Meigs J.B. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014–1021. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman N.D., Leitzmann M.F., Hollenbeck A.R., Schatzkin A., Abnet C.C. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9(7):649–656. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holford T.R., Meza R., Warner K.E., Meernik C., Jeon J., Moolgavkar S.H., Levy D.T. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA. 2014;311(2):164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahtiluoto S., Polvikoski T., Peltonen M., Solomon A., Tuomilehto J., Winblad B., Sulkava R., Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75(13):1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Wang Y.J., Zhang M., Xu Z.Q., Gao C.Y., Fang C.Q., Yan J.C., Zhou H.D., Chongqing Ageing Study Group Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76(17):1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 8.Ho Y.S., Yang X., Yeung S.C., Chiu K., Lau C.F., Tsang A.W., Mak J.C., Chang R.C. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One. 2012;7(5):e36752. doi: 10.1371/journal.pone.0036752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelton M.C., Kahn H.J., Conrath C.L., Newhouse P.A. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43(1-3):274–282. [PubMed] [Google Scholar]
- 10.Anttila T., Helkala E.L., Viitanen M., Kåreholt I., Fratiglioni L., Winblad B., Soininen H., Tuomilehto J., Nissinen A., Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329(7465):539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J., Zhou D.H., Li J., Wang Y.J., Gao C., Chen M. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin. Neurol. Neurosurg. 2006;108(4):378–383. doi: 10.1016/j.clineuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Mukamal K.J., Kuller L.H., Fitzpatrick A.L., Longstreth W.T., Jr, Mittleman M.A., Siscovick D.S. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289(11):1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z.X., Zahner G.E., Román G.C., Liu J., Hong Z., Qu Q.M., Liu X.H., Zhang X.J., Zhou B., Wu C.B., Tang M.N., Hong X., Li H. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch. Neurol. 2005;62(3):447–453. doi: 10.1001/archneur.62.3.447. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H., Deng J., Li J., Wang Y., Zhang M., He H. Study of the relationship between cigarette smoking, alcohol drinking and cognitive impairment among elderly people in China. Age Ageing. 2003;32(2):205–210. doi: 10.1093/ageing/32.2.205. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D.H., Wang J.Y., Li J., Deng J., Gao C., Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J. Neurol. 2004;251(4):421–427. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association Committee on Nomenclature and Statistics . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV TR). 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 17.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Román G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., Amaducci L., Orgogozo J.M., Brun A., Hofman A., et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 19.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E., Powers W.J., DeCarli C., Merino J.G., Kalaria R.N., Vinters H.V., Holtzman D.M., Rosenberg G.A., Wallin A., Dichgans M., Marler J.R., Leblanc G.G. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa M., Ohara T., Ninomiya T., Hata J., Yoshida D., Mukai N., Nagata M., Uchida K., Shirota T., Kitazono T., Kiyohara Y. Milk and dairy consumption and risk of dementia in an elderly Japanese population: the Hisayama Study. J. Am. Geriatr. Soc. 2014;62(7):1224–1230. doi: 10.1111/jgs.12887. [DOI] [PubMed] [Google Scholar]
- 21.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalapatapu R.K., Delucchi K.L. APOE e4 genotype and cigarette smoking in adults with normal cognition and mild cognitive impairment: a retrospective baseline analysis of a national dataset. Am. J. Drug Alcohol Abuse. 2013;39(4):219–226. doi: 10.3109/00952990.2013.800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piazza-Gardner A.K., Gaffud T.J., Barry A.E. The impact of alcohol on Alzheimer’s disease: a systematic review. Aging Ment. Health. 2013;17(2):133–146. doi: 10.1080/13607863.2012.742488. [DOI] [PubMed] [Google Scholar]
- 24.Boyoung Park, Jonghan Park, Jae Kwan Jun, Kui Son Choi, Mina Suh Gender Differences in the Association of Smoking and Drinking with the Development of Cognitive Impairment PLoS One. 2013;8:e75095. doi: 10.1371/journal.pone.0075095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwood D.G., Kalechstein A., Barker W.W., Strauman S., St George-Hyslop P., Iglesias C., Loewenstein D., Duara R. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2010;25(5):511–518. doi: 10.1002/gps.2372. [DOI] [PubMed] [Google Scholar]
- 26.García A.M., Ramón-Bou N., Porta M. Isolated and joint effects of tobacco and alcohol consumption on risk of Alzheimer’s disease. J. Alzheimers Dis. 2010;20(2):577–586. doi: 10.3233/JAD-2010-1399. [DOI] [PubMed] [Google Scholar]
- 27.Rincon F., Wright C.B. Vascular cognitive impairment. Curr. Opin. Neurol. 2013;26(1):29–36. doi: 10.1097/WCO.0b013e32835c4f04. [DOI] [PubMed] [Google Scholar]
- 28.Debette S., Seshadri S., Beiser A., Au R., Himali J.J., Palumbo C., Wolf P.A., DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panza F., Capurso C., D’Introno A., Colacicco A.M., Frisardi V., Lorusso M., Santamato A., Seripa D., Pilotto A., Scafato E., Vendemiale G., Capurso A., Solfrizzi V. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. J. Alzheimers Dis. 2009;17(1):7–31. doi: 10.3233/JAD-2009-1009. [DOI] [PubMed] [Google Scholar]
- 30.Tyas S.L. Alcohol use and the risk of developing Alzheimer’s disease. Alcohol Res. Health. 2001;25(4):299–306. [PMC free article] [PubMed] [Google Scholar]
- 31.Doll R., Peto R., Boreham J., Sutherland I. Smoking and dementia in male British doctors: prospective study. BMJ. 2000;320(7242):1097–1102. doi: 10.1136/bmj.320.7242.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truelsen T., Thudium D., Grønbaek M., Copenhagen City Heart Study Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59(9):1313–1319. doi: 10.1212/01.WNL.0000031421.50369.E7. [DOI] [PubMed] [Google Scholar]
- 33.Ruitenberg A., van Swieten J.C., Witteman J.C., Mehta K.M., van Duijn C.M., Hofman A., Breteler M.M. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359(9303):281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda A., Yamagishi K., Tanigawa T., Cui R., Yao M., Noda H., Umesawa M., Chei C., Yokota K., Shiina Y., Harada M., Murata K., Asada T., Shimamoto T., Iso H. Cigarette smoking and risk of disabling dementia in a Japanese rural community: a nested case-control study. Cerebrovasc. Dis. 2008;25(4):324–331. doi: 10.1159/000118377. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K., Izumi M. Alcohol is a risk factor not for thalamic but for putaminal hemorrhage: the Akita Stroke Registry. J. Stroke Cerebrovasc. Dis. 2013;22(7):1064–1069. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Peters R. Blood pressure, smoking and alcohol use, association with vascular dementia. Exp. Gerontol. 2012;47(11):865–872. doi: 10.1016/j.exger.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Smith M.A., Honda K., Aliev G., Moreira P.I., Nunomura A., Casadesus G., Harris P.L., Siedlak S.L., Perry G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007;257(1-2):240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie S.J., Bates T.C., Corley J., McNeill G., Davies G., Liewald D.C., Starr J.M., Deary I.J. Alcohol consumption and lifetime change in cognitive ability: a gene × environment interaction study. Age (Dordr.) 2014;36(3):9638. doi: 10.1007/s11357-014-9638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanekiyo T., Xu H., Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohn T.T. Is apolipoprotein E4 an important risk factor for vascular dementia? Int. J. Clin. Exp. Pathol. 2014;7(7):3504–3511. [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Li L., Liu F., Deng S., Zhu R., Li Q., He Z. ApoE gene polymorphism and vascular dementia in Chinese population: a meta-analysis. J Neural Transm (Vienna) 2012;119(3):387–394. doi: 10.1007/s00702-011-0714-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu B., Shen Y., Cen L., Tang Y. Apolipoprotein E gene polymorphism in a Chinese population with vascular dementia: a meta-analysis. Dement. Geriatr. Cogn. Disord. 2012;33(2-3):96–103. doi: 10.1159/000337025. [DOI] [PubMed] [Google Scholar]
- 46.Kim K.W., Youn J.C., Han M.K., Paik N.J., Lee T.J., Park J.H., Lee S.B., Choo I.H., Lee D.Y., Jhoo J.H., Woo J.I. Lack of association between apolipoprotein E polymorphism and vascular dementia in Koreans. J. Geriatr. Psychiatry Neurol. 2008;21(1):12–17. doi: 10.1177/0891988707311028. [DOI] [PubMed] [Google Scholar]
- 47.Orsitto G., Seripa D., Panza F., Franceschi M., Cascavilla L., Placentino G., Matera M.G., Paris F., Capurso C., Solfrizzi V., Dallapiccola B., Pilotto A. Apolipoprotein E genotypes in hospitalized elderly patients with vascular dementia. Dement. Geriatr. Cogn. Disord. 2007;23(5):327–333. doi: 10.1159/000100972. [DOI] [PubMed] [Google Scholar]
- 48.Lewis S.J., Zuccolo L., Davey Smith G., Macleod J., Rodriguez S., Draper E.S., Barrow M., Alati R., Sayal K., Ring S., Golding J., Gray R. Fetal alcohol exposure and IQ at age 8: evidence from a population-based birth-cohort study. PLoS One. 2012;7(11):e49407. doi: 10.1371/journal.pone.0049407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michel T.M., Gsell W., Käsbauer L., Tatschner T., Sheldrick A.J., Neuner I., Schneider F., Grünblatt E., Riederer P. Increased activity of mitochondrial aldehyde dehydrogenase (ALDH) in the putamen of individuals with Alzheimer’s disease: a human postmortem study. J. Alzheimers Dis. 2010;19(4):1295–1301. [Google Scholar]
- 50.Au Yeung S.L., Jiang C.Q., Cheng K.K., Liu B., Zhang W.S., Lam T.H., Leung G.M., Schooling C.M. Evaluation of moderate alcohol use and cognitive function among men using a Mendelian randomization design in the Guangzhou biobank cohort study. Am. J. Epidemiol. 2012;175(10):1021–1028. doi: 10.1093/aje/kwr462. [DOI] [PubMed] [Google Scholar]
- 51.Lahiri D.K., Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp. Gerontol. 2010;45(4):291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahiri D.K., Maloney B., Zawia N.H. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry. 2009;14(11):992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lahiri D.K., Maloney B., Basha M.R., Ge Y.W., Zawia N.H. How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr. Alzheimer Res. 2007;4(2):219–228. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- 54.Maloney B., Sambamurti K., Zawia N., Lahiri D.K. Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr. Alzheimer Res. 2012;9(5):589–599. doi: 10.2174/156720512800617955. [DOI] [PubMed] [Google Scholar]
- 55.Lindsay J., Laurin D., Verreault R., Hébert R., Helliwell B., Hill G.B., McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 56.Luchsinger J.A., Tang M.X., Siddiqui M., Shea S., Mayeux R. Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 2004;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 57.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C., Launer L.J., Laurent S., Lopez O.L., Nyenhuis D., Petersen R.C., Schneider J.A., Tzourio C., Arnett D.K., Bennett D.A., Chui H.C., Higashida R.T., Lindquist R., Nilsson P.M., Roman G.C., Sellke F.W., Seshadri S., American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]


