Abstract
To ensure long-term consistent neural recordings, next-generation intracortical microelectrodes are being developed with an increased emphasis on reducing the neuro-inflammatory response. The increased emphasis stems from the improved understanding of the multifaceted role that inflammation may play in disrupting both biologic and abiologic components of the overall neural interface circuit. To combat neuro-inflammation and improve recording quality, the field is actively progressing from traditional inorganic materials towards approaches that either minimizes the microelectrode footprint or that incorporate compliant materials, bioactive molecules, conducting polymers or nanomaterials. However, the immune-privileged cortical tissue introduces an added complexity compared to other biomedical applications that remains to be fully understood. This review provides a comprehensive reflection on the current understanding of the key failure modes that may impact intracortical microelectrode performance. In addition, a detailed overview of the current status of various materials-based approaches that have gained interest for neural interfacing applications is presented, and key challenges that remain to be overcome are discussed. Finally, we present our vision on the future directions of materials-based treatments to improve intracortical microelectrodes for neural interfacing.
Keywords: Intracortical Microelectrodes, Brain Machine Interfaces, Neuro-inflammatory Response, Neurodegeneration, Biocompatibility
1. INTRODUCTION
Neural interfaces bridge the central nervous system to the outside world. Originally, neural interfaces were developed as a basic science tool, and as such, have been used extensively to develop our understanding of how the nervous system works. bell (1–4) Additionally, neural interfaces hold great potential for functional restoration in persons with paralysis, other forms of motor dysfunction, or limb loss. Such rehabilitative applications are commonly referred to as brain machine (or brain computer) interfaces. (5) In brain machine interface (BMI) applications, a recording device is used to extract volitional intent in the form of consciously modulated neuronal signals. Using a variety of signal transducing systems and processing algorithms, extracted neural signals can then be used to drive external devices such as limb prostheses or computers. (6–12)
A number of types of recording electrode devices have been developed to access different forms of neural information through varying levels of invasiveness (Figure 1). For example, non-penetrating recording electrodes placed externally on the scalp or on the brain surface can gain functional information. (11, 12) However, many researchers believe that recording devices that penetrate into specific regions of the brain will provide the most useful control signals for complex BMI applications. (13) Despite the potential that penetrating intracortical microelectrodes have shown, widespread implementation is impeded by the inability to consistently record high quality neural signals over clinically relevant time frames. (14–17) As such, this review focuses on intracortical microelectrodes implanted within the cerebral cortex, which record from single or small populations of nearby neurons.
Figure 1.
Examples of recording neural electrodes for brain machine interface devices. (A) EEG activity is recorded non-invasively with electrodes placed on the scalp. (B) ECoG electrodes are placed either outside the dura mater (epidural ECoG) or under the dura mater (subdural ECoG) and can record neural activity on the cortical surface. (C) Intracortical microelectrodes penetrate the cortex and can record action potentials from individual or small populations of neurons within the cortex.
In particular, the evolution of traditional intracortical microelectrode systems is discussed from a materials science perspective (Section 2). Emphasis is given to key developments that have facilitated the longest and highest quality in vivo recordings. In addition, a number of primary failure modes are discussed that must be overcome to achieve the full potential of intracortical microelectrodes for in vivo recording applications (Section 3). Lastly, the impressive progress that has been made in recent years to develop the next generation of intracortical microelectrodes is reviewed (Section 4). By framing recent advancements within the context of current successes, the most promising strategies are highlighted and the most critical challenges for improving intracortical electrode-based neural interfaces are discussed.
2. TRADITIONAL INTRACORTICAL MICROELECTRODES FOR BRAIN MACHINE INTERFACING
A number of intracortical microelectrodes have been designed to interface with cortical neurons, including insulated metal microwires and semiconductor-based devices such as the Michigan and Utah electrode arrays. Regardless of the specific design or manufacturer, a similar compound circuit can be used to describe how microelectrodes extract electrical signals generated from single target neurons (Figure 2). Extensive descriptions of each of the primary portions of the compound circuit are available elsewhere, (18, 19) and therefore only a brief description will be included here.
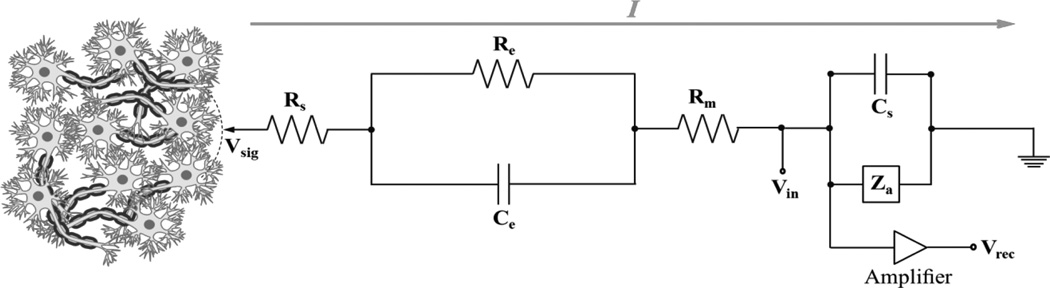
Figure 2.
A commonly used equivalent circuit model (Robinson Model) of metal microelectrode recoding in the brain. signals at the tip of the microelectrode (Vsig) generate currents (I) that flow to ground through the microelectrode and effective amplifier circuit, creating the potential (Vin) at the input of the amplifier before being recorded (Vrec); Rs is the resistance of the electrolyte; Re is the leakage resistance which models the flow of the charge carriers crossing the electric double layer; Ce is the capacitance of the microelectrode-electrolyte interface; Rm is the resistance of the microelectrode; Cs is all the shunt capacitance to ground; and Za is the input impedance of the amplifier. Thus, the effective impedance of the microelectrode is comprised of the resistance of the electrolyte (Rs), the resistance and capacitance of the double layer interface of the electrolyte (Re and Ce) and the (negligible) resistance of the microelectrode (Rm). The impedance of the microelectrode is frequency dependent. At low frequencies, the impedance is dominated by the series combination of Rs and Re, whereas at high frequencies Ce bypasses the effect of Re so that the impedance is now close to Rs. Thus, by measuring the impedance of an electrode at high and low frequencies, it is possible to determine the component values for the equivalent circuit.
The first portion of the circuit involves the cortical column and the complex set of presynaptic inputs that innervate the target neurons being recorded from. These inputs can be both excitatory and inhibitory. If a sufficient excitatory postsynaptic potential is created then a compound action potential is generated through depolarization of the axon hillock. The ion-based signal then travels through the extracellular space to the electrode-recording site. As transport is primarily diffusion based, the distance traveled and the impedance of the extracellular space governs the strength of the signal reaching the recording site. It has been suggested that the maximum effective recording range for classic microelectrode designs is roughly 50–150 µm. (20)
At the recording site, the electric potential produced by the ion-based signal is recorded as a voltage change. Signals can then be amplified and analyzed using various acquisition and processing techniques. (21) Once analyzed, algorithms are applied to translate the signal into device commands/orders that carry out the user’s volitional intent. (21–23) Output devices can vary from application to application and have ranged from moving a cursor on a computer screen, to facilitating a robot to walk on a treadmill, driving a wheelchair, or controlling a robotic arm. (24)
2.1. Microwires: From an Acute Electrophysiology Tool to a Useful Interface Between Man and Machines
Metal wire electrodes have an extensive history as the “go to” tool of neuroscientists for acute electrophysiology experiments. Therefore, it is not surprising that metal wire electrodes have been further developed to extend their use to long-term BMI applications. Prominent developments include reducing wire size, enhancing electrode geometry as well as optimizing both the underlying conducting and insulating materials. A schematic example of a microwire electrodes described in the literature can be found in Figure 3A.
Figure 3.
Schematic representation of the generation design of leading microelectrode array technologies, including A) Microwires, B) Michigan-style Microelectrodes, C) Utah Electrode Arrays (EUA) and D) Cone (glass pipette) Electrodes.
Many of the earliest descriptions of metal wire electrodes used to record from single or small populations of neurons date back to the 1940s. Renshaw performed one of the earliest studies utilizing metal wire electrodes to record electrical signals from single neurons, using Ag/AgCl based electrodes. (3) Other metals that would prove more effective and safer in chronic recording applications, such as stainless steel, tungsten and platinum, also had roots during this early period. (1, 25, 26) For example, in 1942, Grundfest and Campbell conducted one of the first studies utilizing stainless steel electrodes to record electrical impulses from neurons within the feline spinal cord. (1) Improving upon their original design, Grundfest next began utilizing electrolytic pointing to create sharpened stainless steel microwires. (2) Electrolytic pointing reduced the variability between individual electrodes and improved insertion into cortical tissue.
Despite the limited recording duration (hours to days) of early devices, metal recording electrodes facilitated a rapid increase in knowledge concerning neural pathways and volitional movement. For example, in 1966, Evarts described that specific patterns of neuronal activity correlate with set motor responses. (27) Specifically, Evarts found that the electrical activity of pyramidal neurons in the precentral cortex of primates correlated with specific behavior patterns. The results of Evarts’ study contributed greatly to the idea of using volitionally controlled neural signals to manipulate external devices.
Several new electrode designs and materials were introduced in the 1970s that permitted recordings to be performed for longer durations. A collaboration between Salcman, Bak and Schmidt at the National Institute of Health (NIH) led to the development of microwire electrodes from iridium (Ir), platinum (Pt) and platinum-iridium- alloys (Pt/Ir-). (28, 29) Either a glass (28) or poly (monochloro-p-xylene) (Parylene-C) coating (29) was incorporated to insulate the microwire electrodes. Iridium electrodes in particular demonstrated that microwire-based electrodes are capable of chronic recording by detecting single unit activity from primate cortex up to 223 days after implantation. (29, 30) However, in addition to the promise shown by the NIH studies, the authors also observed that average recording performance was inconsistent and decreased over time. (30) Salcman, Bak and Schimdt were among the first to propose that inflammatory-mediated device encapsulation reduced recording performance over time by increasing impedance and electrically isolating the device from the surrounding tissue. (30, 31) Section 3.4 provides further information on inflammatory and encapsulation-mediated electrode failure.
Over the last few decades, a number of groups have shown that a variety of microwire devices are capable of recording the signals needed for brain machine interface applications over extended periods. (16, 30, 32, 33) Despite these successes, a major hurdle for microwire-based microelectrodes is still the challenge of consistently recording high quality units over time. (34, 35)
2.2. Silicon-based Microelectrodes
2.2.1. Michigan-Style Microelectrodes
Beginning in the 1960s, advancements in semiconducting materials and improvements in micromachining capabilities drove the development of silicon-based microelectrodes. Based on foundational work with silicon etching for beam-lead integrated circuits at Bell Telephone Laboratories, Angell, Starr and Wise developed the first intracortical microelectrode with a silicon substrate in 1966. (36) Similar to existing microwire devices, early silicon-based devices consisted of a penetrating tine with an exposed conducting tip capable of electrically interacting with nearby cells. (36, 37)
By the 1980’s, further work by Wise and colleagues at the University of Michigan led to the development of what is commonly referred to as the “Michigan (MI)-style microelectrode.” (38) Applying newly developed microfabrication processes such as diffusion-based etch stops, silicon microelectrodes were fabricated with multiple recording sites placed along a single or multiple planar shanks. (39) The advantage of MI-style electrodes over traditional metal microwire devices is their ability to record from numerous sites at well-controlled tissue depths.
The basic structure of a single-shank MI-style microelectrode is shown in Figure 3B. Several microfabrication processes are used in the creation of MI-style microelectrodes. (39) First a diffused boron etch stop is created using a thermal oxide mask to define the substrate’s dimensions. Etch stops allows for all processes to be performed on the topside of the silicon wafer rather than having to pattern both sides of the wafer, as was done when creating the original MI-style devices. Following definition of the probe dimensions, a dielectric layer is added to insulate the backside of the device. The dielectric usually consists of a silicon oxide/silicon nitride stack or an alternative passivation layer. Building on the dielectrics, a series of conducting traces are applied to the length of the probe to link the recording sites to the bond pads. The recording sites and bond pads are then created from conducting metals such as gold or iridium. A second stack of stress-compensated silicon oxide/silicon nitride is then deposited through chemical vapor deposition to insulate the conducting traces. To further shield the device and protect the dielectrics from dissolution under in vivo conditions (Section 3.3), insulating polymer coatings such as Parylene-C or Epoxylite have been adopted over time. Additionally, improved hermetic protection through anodic silicon-glass bonding for on-chip processors has also been developed.
A number of groups have shown that MI-style microelectrodes are capable of chronic recording in a variety of species. (40–42) As with microwires, despite a number of studies showing that chronic recording is feasible, the major hurdle for MI-style microelectrodes has been the challenge of consistently recording high quality units over time. (43)
Today MI-style microelectrodes are being further developed at a number of universities and laboratories. MI-style microelectrodes are also commercially available for neuroscience and preclinical applications from NeuroNexus®, a subsidiary of GreatBatch Inc®. Advanced MI-style microelectrodes have been developed with on-chip processing as well as wireless telemetry systems. Additionally, microfluidics and optical waveguides have been incorporated to expand the number of ways in which MI-style microelectrodes can interact with the surrounding tissue. However, as discussed in Section 3, several factors still limit the clinical success of MI-style microelectrode technology. For further details on the development and successes of MI-style microelectrodes, readers are referred to the excellent review by Wise. (39)
2.2.2. The Utah Electrode Array
Normann and colleagues developed an alternative, silicon-based microelectrode, which due to its origin at the University of Utah is referred to as the Utah Electrode Array (UEA). (44) Instead of the thin film design of the MI-style arrays, the UEA uses glass reflow, dicing and etching to create an array of well-defined penetrating electrode tines. Figure 3C shows a basic schematic of the UEA along with the slanted UEA design created using slight modifications of the original processing steps (described below). While originally designed for stimulation applications, the UEA has been widely used as a recording tool. In fact, the UEA is the only high-density, penetrating recording electrode approved by the US Food and Drug Administration and that has received the CE mark for use in Europe.
Since the first generation, significant development efforts have been devoted to improve the performance of the UEA. For example, to improve charge transfer, Pt/Ti/W/Pt, and then subsequently sputtered iridium oxide (SIROF), have been used instead of the original gold or platinum contacts on the terminal recording sites. (45) Furthermore, conformal Parylene-C coatings have been applied through chemical vapor deposition to provide additional insulation to the electrode tines and protect the underlying dielectrics from dissolution. (46) Electrical isolation of individual channels has been further enhanced by incorporating a glass dielectric between individual bond pads on the backside of the wafer. (47)
Beyond the initial design of the UEA with a 10 × 10 array of 1.5 mm tines, developers have also shown that a number of alternative structures can be created. For example, the Utah Slanted Electrode Array (USEA) was developed to facilitate stimulation and recording at various tissue depths. (48) Recent studies have also shown that the UEA’s structure can be further manipulated to create devices that better conform to complex anatomical geometries using variable depth dicing and wet isotropic etching. (49) Ultra-high aspect ratio devices made from highly conductive bulk silicon have been created using microwire electrical discharge machining. (50) In addition, high density arrays have also recently been created. (51)
Similar to the MI-style devices, significant recent efforts have focused on creating wireless versions of the UEA, where power and telemetry systems are incorporated on the base of the UEA via flip-chip bonding. (52, 53) Other developments that are being pursued include the addition of optical waveguides for optogentic research. (54)
The ability of the UEA to record the neural signals needed for chronic rehabilitative applications has been demonstrated by a number of groups. For example, the UEA has been utilized for recording in the visual and auditory cortex. (55, 56) Additionally a number of primate studies have shown the usefulness of the UEA in brain machine interface applications, such as cursor and prostheses control. (57, 58)
The commercialization and clinical translation of the UEA began by spinning-off Bionic Technologies, from the University of Utah in 1997. Cyberkinetics Neurotechnology Systems, Inc. later acquired this spin-off. In 2004, the FDA granted Cyberkinetics the first of two Investigational Device Exemptions (IDEs) to begin human clinical trials with a UEA-based system (BrainGate™).
After achieving major milestones in the clinical development of the UEA, portions of Cyberkinetics were acquired in 2008 by two distinct, yet collaborative, entities. Blackrock® Microsystems, LLC, under Dr. Florian Solzbacher, acquired rights to many of the underlying hardware components. Today, Blackrock® Microsystems, LLC operates as the original equipment manufacturer for the UEA. The BrainGate™, co-founded by Jeffery Stibel, acquired rights to the BrainGate Neural Interface System and many of Cyberkinetics’ clinical applications for the technology. Further information can be found on Blackrock® Microsystem’s and the BrainGate™ websites.
As part of the BrainGate™ clinical trials, Hochberg et al. have shown that UEAs implanted into the primary motor cortex can be used to restore volitional control of external devices, including a computer cursor and a simple robotic hand, to patients with tetraplegia. (7) Building on Hochberg’s seminal work, recently another publication from the BrainGate™ group has described the success achieved in an additional pair of patients. In the second study, the authors demonstrated that patients with long-standing tetraplegia were able to produce useful movements of a prosthetic arm with the UEA based system. (6) For example, one patient was able to use her thoughts to control a robotic arm, reach and grasp a bottle of coffee, bring it towards her mouth to drink, and then return the bottle to the table for the first time in 14 years. (6) Excitingly, results were achievable five years after implantation, and the systems were still functional at the time of publication. While the successes of animal studies and the BrainGate™ project in particular are quite promising, improving recording consistency is still a primary focus for the UEA. (59)
2.3. Neurotrophic Cone Electrode
Diverging from many in the field who believed that single units were the key to BMI success, Kennedy et al. took a new multi-unit approach towards BMIs and developed the neurotrophic cone electrode (Figure 3D). (60) Kennedy’s electrode was built around a glass cone with a Teflon®-insulated gold wire. This design is very similar to pipette electrodes used in acute electrophysiology experiments prior to the development of microwire devices. Building on the work of David and Aguayo, who had demonstrated endogenous innervation of peripheral nerve grafts, Kennedy placed a segment of sciatic nerve into the glass cone. (61) Similar to the innervation of the nerve graft, implantation of the cone electrode into rat cortex elicited the ingrowth of neuronal processes into the glass cone. Using his novel approach, Kennedy was able to record neural signals for up to 11 months following implantation in the rat cortex and up to 15 months in the monkey cortex. (60, 62)
Beyond successful animal experiments, Kennedy implemented the neurotrophic cone electrodes as the earliest platform for successful human clinical studies of brain machine interfaces. (8–10) In their seminal study, Kennedy and colleagues implanted neurotrophic cone electrodes into the cortex of three patients and consciously modulated neural signals were used to drive the movement of a computer cursor.
While the data obtained using neurotrophic cone electrodes are quite promising, widespread adoption has been limited, possibly due to the fragility and boutique fabrication scheme of the cone electrode. However, Kennedy’s approach does highlight the potential of bioactive strategies for improving neural interfacing (Discussed further in Section 4.5).
3. CHALLENGES TO OBTAINING CONSISTENT, HIGH-QUALITY NEURAL RECORDINGS
Despite the substantial success that has been demonstrated using intracortical microelectrodes in neural interface applications, many studies have shown chronic cortical recording to be inconsistent in a variety of species and with multiple electrode types. As early as 1974, Burns et al. showed a progressive decline in unit recordings in cat cerebral cortex after implantation, with only 8% of the electrodes functioning after 5 months. (31) Forty years later, recording instability is still a commonly documented problem. For example, Liu et al. reported that implanted electrodes are unstable during the acute phases of tissue remodeling, and thereafter experience a continual decrease in recording ability over the ensuing months. (16, 17) Additionally, recently Ludwig et al. and Freire et al. have both described fluctuations in recording stability that agree well with previous findings. (43, 63)
A number of failure modes likely influence chronic recording stability and quality including: 1) direct mechanical damage of the electrode; 2) corrosion of electrical contacts; 3) degradation of passivation layers and insulating coatings; and 4) the neuro-inflammatory response that the brain mounts against chronically implanted devices. (14, 34) Figure 4 illustrates how each failure mode could impact the compound circuit describing how microelectrodes extract electrical signals from neural tissue. Traditionally, microelectrode failure modes have largely been studied independently from one another. However, there is likely considerable interplay among the various modes making it difficult to attribute failure to a single mechanism.
Figure 4.
(A) Cellular level failure modes. Cellular level failure modes act 1) disrupting the neuronal signal source, 2) impeding charge transport through the extracellular space or 3) disrupting charge transfer at the electrode recording site. (B) Higher, tissue level failure modes that have not been as widely described or considered in the literature. Asterisks denote failure modes with an underlying inflammatory component. As nearly all failure modes have an underlying inflammatory component, strategies that reduce neuro-inflammation will be critical to improving the biocompatibility and function of intracortical microelectrodes.
3.1. Direct Mechanical Damage
Several studies have indicated that mechanical damage during or following insertion can lead to microelectrode failure. For example, Ward et al. experienced mechanical failure in seven of nineteen devices, regardless of the type of electrode. (14) Interestingly, while a number of the electrodes used in Ward’s study were made from materials that are commonly considered brittle, such as silicon or ceramics, only one failure of a penetrating shank was described. (14) Similar occurrences of mechanical failures away from the penetrating wires, shanks and tines of traditional microelectrode recording systems have independently been described in recent reports. (34, 35, 64) Thus, improvements in the mechanical stability of the entire recording system, and not just the intracortical microelectrode should be further pursued.
3.2. Corrosion of Electrical Contacts
While descriptions concerning electrode corrosion have been reported for stimulating electrodes, relatively few have been provided for recording microelectrodes. However, even under non-stimulating conditions (i.e. under conditions in which no electrochemical reactions should occur via an externally applied electric field) some materials used in recording microelectrodes likely experience faradaic charge transfer and corrosion over time. (65) In fact, structural changes at the electrode-recording sites have been observed to progress with time after electrode insertion for tungsten microwires (Figure 5), (34) while little corrosion was reported for Pt/Ir electrodes. (35)
Figure 5.
Pre-Implant (left) and Post-Explant (right) SEM images from acute (top, 1 day), recovery (middle, 16 days), and chronic (bottom, 187 days). The images show the progression of corrosion of the tungsten metal over the increased implantation time. Figure from Prasad et al. 2012.
The rate of corrosion is likely environment and material specific. For example, Patrick et al. have shown that bare tungsten and gold-plated tungsten wires corrode readily in phosphate-buffered saline even under non-stimulating conditions. (66) Patrick and colleagues also found that tungsten corrosion was increased in the presence of oxidative species in vitro. The critical role of oxidative species in electrode corrosion provides an important link to the brain’s inflammatory response, since reactive oxygen species are actively produced surrounding implanted microelectrodes (Section 3.4.4). In vivo corrosion rates were reported to be as high as 100 µm/year, indicating that corrosion is a likely contributor to at least tungsten-based electrode failure.
By contrast, titanium forms a natural passivation layer, and is more resistant to oxidative corrosion. McCarthy et al. have begun to develop titanium-based MI-style microelectrodes that may perform better in the oxidative environment that develops surrounding implanted microelectrodes. (67) Furthermore, platinum wires are not only stable in saline/H2OS environments, but actively convert hydrogen peroxide species to water, (66) mirroring the catalytic activity of natural anti-oxidative enzymes. (68) Potter et al. have previously demonstrated that reactive oxygen species accumulation may facilitate neurodegeneration at the microelectrode surface. (69, 70) Therefore, the ability for platinum materials to reduce the concentration of oxidative species could explain the improved performance of platinum-based microelectrodes in neural interface applications.
Beyond impacting recording site stability, electrode corrosion can also generate toxic species. For example, the generation of toxic species from Ag/AgCl electrodes has been well documented. (71) In addition, Patrick et al. reported that the primary species generated by tungsten corrosion were tungstic ions, which are known to be moderately toxic. (66) Production of toxic species could be another important connection linking biotic and abiotic failure modes.
Information regarding the corrosion rates of many common microelectrode materials under non-stimulating conditions is not readily available. In view of the above-mentioned findings further analysis of the corrosion of common electrode materials would be valuable. Furthermore, the impact of corrosive species generated from the breakdown of many electrode materials is not well understood and deserves additional study. When conducting corrosion analysis it is important to mimic the in vivo environment, including the presence of oxidative species and acidic pH. Additionally, the impact of corrosion products should be considered when examining and comparing the biocompatibility of chronically implanted microelectrodes made from different materials.
3.3. Degradation of Passivation Layers and Insulating Coatings
Similar to electrode recording sites, the passivation layers and insulating coatings commonly incorporated into microelectrodes may degrade over time. Significant degradation of an electrode’s insulating or passivation layers could reduce an electrodes ability to detect local ionic signals (Figure 4). (32)
The susceptibility for silicon or glass passivation layers to degrade in vivo has been shown in a number of studies. For example, Wang et al. observed that the corrosion of silicon begins in as little as ten days from implantation into the rat brain. (72) Furthermore, Hämmerle et al. have shown that while silicon oxide is stable for over 21 months in saline solution, significant degradation occurs after implantation in a subretinal model. (73) Beyond the removal of the as-fabricated silicon oxide surface layer within 12 months of implantation, Hämmerle and colleagues also observed progressive corrosion of their underlying silicon substrate. (73) Degradation is not exclusive to silicon and silicon oxide layers, as Maloney and colleagues have shown similar degradation rates (~1 µm/year) in triple layered silicon oxide/nitride stacks. (74)
The degradation of traditional passivation layers is not surprising as they were originally designed to serve as dielectrics in dry, noncorrosive environments that are shielded from mechanical stresses. A number of mechanisms may influence the degradation of traditional passivation materials. These mechanisms include mechanical stress, film defects, as well as chemical or electrochemical reactions. Further information regarding these mechanisms is presented by Scmitt et al. (75)
To overcome the limitations of traditional passivation materials, Cogan et al. developed an amorphous silicon carbide (a-SiC) dielectric film for microelectrodes. (76) Degradation testing showed that the a-SiC had a dissolution rate of 0.1 nm/h at 90°C (1/20th that of silicon nitride) and no measurable dissolution at 37 °C.
Due to the chemical vulnerability of common dielectric passivation layers, further encapsulation of microelectrodes with insulating polymers has become common practice. (32, 46, 77) While no direct comparison has been made, historically there has been a significant trend towards improved recording longevity when silicon microelectrodes were coated with polymeric insulators. However, in vivo rodent studies have shown no difference in the neuro-inflammatory response of Parylene-coated Michigan-style microelectrodes compared to uncoated devices. (78) Therefore, it is likely that any increased recording longevity is not due to a significant reduction in the neuro-inflammatory response on account of reduced degradation of the Si-based devices. Nevertheless, as many descriptions of the loss of recording quality come from electrodes with polymer-based insulating coatings, it is clear that improving insulation alone is not a silver bullet and that other sources of instability, such as the neuro-inflammatory response, are still at play.
While insulating polymer coatings have significantly improved recording systems, a limited number of studies have described degradation of common insulators used on microelectrodes. For example, Prasad et al. showed evidence that polyimide insulation on tungsten microwires was peeled away from the recording site, and had signs of cracking as early as 42 days after implantation (Figure 6). (34) Insulation damage was particularly common in chronic implants, where seven of twelve electrodes implanted showed damage. However, it is unclear if changes to the insulation were a result of direct damage of the polyimide or a result of corrosion of the underlying tungsten.
Figure 6. Insulation Deterioration Post-Explant SEM.
Post-explant SEM of individual microwire to indicate deterioration in electrode insulation for parylene-C coated Pt/Ir microwires. The deterioration occurs in the form of delamination and cracks. While the insulation deterioration varies among microwires even with the same array, Prasad et al. observed it to be present in all the wires across animals for all implant durations (7 days – 6 months). Figure and caption from Prasad et al. 2014.
As with recording site corrosion, further analysis of the degradation of common passivation layers and insulting coatings is needed. While in vitro experiments can facilitate higher throughput analysis, it is critical to also investigate degradation using in vivo models to more accurately understand the contribution of the neuro-inflammatory response (Section 3.4).
3.4. The Neuro-Inflammatory Response
There is increasing consensus that the neuro-inflammatory response to intracortical microelectrodes is a primary hurdle preventing microelectrode-driven BMIs from reaching their full potential. Therefore, improving the understanding of the neuro-inflammatory response that develops following microelectrode implantation in the brain, and developing strategies to reduce its impact are critical to achieving the promise of BMIs and to enable longer recording durations for basic science experiments.
Over 100 studies have described stereotypic features of the brain’s response to microelectrodes that occur irrespective of the type of implant, method of sterilization, species studied, or implantation method. From this rich body of literature, it has become increasing clear that the brain’s response consists of an interconnected web of molecular and cellular components. The ultimate result of which is the continuous perpetuation of the response, and the prevention of microelectrode integration into the surrounding tissue.
With respect to the molecular and cellular components, several theories have been presented to explain how individual components of the response might adversely impact recording quality. However, it is highly likely that multiple aspects of the response are at play simultaneously. Thus, further study into the details of the neuro-inflammatory response and the development of more comprehensive mitigation strategies are indicated.
3.4.1. Initial Injury and Early Wound Healing Events
Due to the dense and, in many cases highly vascularized nature of nervous tissue, microelectrode implantation inevitably causes vascular and cellular injury. (79, 80) Following the initial iatrogenic injury, several acute cascades and processes are initiated to induce wound closure and promote tissue remodeling. Directly after injury, the coagulation cascade is initiated and forms a provisional matrix to restore vascular integrity. (81, 82) Simultaneously the complement system is also initiated. The complement cascade may directly induce apoptosis in nearby cells or invading pathogens through the membrane attack complex. Additionally, complement assists in recruiting inflammatory cells to the site of injury through the alternative arm of the cascade. (83)
Much is known about early wound healing events and their roles in injury and other device implantation models. (84, 85) However, comparably few studies have explored the early wound healing events after implantation of intracortical microelectrodes. (79, 86) The majority of what is known about the brain’s response to implanted microelectrodes comes from end-point histological studies focused on later time points that range from ~1–24 weeks post-implantation. Figure 7 provides images and an illustration of the stereotypic response of the brain to chronically implanted microelectrodes. (87)
Figure 7.
Electrode implantation results in localized pro-inflammatory cellular and biochemical events. Early after implantation, activated microglia begin to attach to the surface of the electrode and locally release pro-inflammatory factors. Glia cell adhesion is followed by astrocytic encapsulation along the entire shaft of the electrode (formation of the glial scar). These events, as well as localized hemorrhaging, have been shown to be correlated with neurodegeneration at the interface. Representative IHC images of the dominant cell types are shown left. Scale = 100 µm. Figure and caption from Potter et al. 2012.
3.4.2. Motion Induced Injury at Later Time Points
Microelectrode-induced injury events are likely not limited to the initial iatrogenic trauma. It is widely accepted that propagation of the neuro-inflammatory response may be due to perpetual motion-induced damage at the interface of traditional microelectrodes. The base materials used in traditional microelectrodes are significantly stiffer than cortical tissue. Therefore, starting with Goldstein and Salcman’s work in 1973, a number of groups have suggested that motion of the brain with respect to the microelectrode may induce damage to the surrounding tissue. (88–95)
In silico studies support the hypothesis that even micromotion of the brain relative to a stiff microelectrode could induce strain on the surrounding tissue. (89) However, to date, limited work has been performed to quantify microelectrode-induced strain on the surrounding tissue. Recently, the Muthsuwamy lab developed a method to measure the mechanical properties of the biotic component of the brain-electrode interface, surrounding non-compliant stainless steel microelectrode implants. (96) Specifically, they have found that the estimated shear and elastic modulus in the surrounding brain tissue fluctuates and evolves over time. Ongoing studies are investigating the effects of implant stiffness on the strain placed on the cortical tissue adjacent the implant. Determining whether implanted microelectrodes induce sufficient strain to affect neural and inflammatory cells is a critical gap in the field. Additionally, quantifying in vivo or ex vivo strain data would be extremely useful in the creation of improved predictive models for driving future microelectrode designs (Discussed further in Sections 4.1).
Despite the infancy of strain quantification, recent in vivo studies have shown that microelectrodes made from materials that more closely match the brain’s mechanical properties may elicit a reduced neuro-inflammatory response. (94, 97–99) However, the precise mechanism underlying how mechanical mismatch facilitates the neuro-inflammatory response is still being debated. Nevertheless, the hypothesis that mechanical mismatch between the microelectrode and brain tissue contributes to the neuro-inflammatory response has resulted in the development and use of compliant materials (Section 4.1) to replace the stiffer silicon, ceramic and metal substrates used in traditional microelectrodes. (70, 99–105) It is, however, important to note that the influence, which the compliant behavior exerts on the quality of neural recordings of microelectrodes fabricated from such materials, has yet to be described.
3.4.3. Microglia/Macrophage Response to Intracortical Microelectrodes
Similar to the response in the rest of the body, (84, 85) a key feature of the brain’s response to chronically implanted devices is persistent inflammation at the biotic-abiotic interface. (99, 106) Persistent inflammation involves activation of both resident microglia and the perpetual recruitment of blood-born macrophages. (15, 106–109)
Both microglia and macrophages play a primary role in responding to invading pathogens, recognizing extravasated serum/plasma proteins, phagocytizing damaged or dead cells, (110) and in clearing residual cell debris. (111) Following phagocytosis, microglia and macrophages are known to enter the lymphatic system and act as antigen presenting cells in a variety of diseases and pathological states. (112–116) As suggested by Skousen et al., cell trafficking to and from the implant interface provides a potentially persistent stimulus for the neuro-inflammatory response via extravasated fibrinogen, fibronectin, complement factors and other blood products. (117) Following extravasation, blood products adsorb to the microelectrode surface and perpetuate inflammatory cell activation through receptor-mediated pathways such as Toll-like receptor (TLR)-mediated pathways (Figure 8). (69, 118, 119)
Figure 8. Self-perpetuating neuroinflammatory pathways.
After intracortical microelectrode implantation, the damage of localized vasculature can result in two mechanistic paradigms at the interface of the implanted device. The order of events in either cycle is unknown, as any one event can perpetuate the subsequent step. Left: Extravasated blood-derived proteins from damaged vasculature become adsorbed onto the surface of the implanted device and dispersed throughout the local tissue environment. Blood-derived proteins then activate inflammatory cells and stimulate the release of pro-inflammatory and cytotoxic soluble factors. Release of pro-inflammatory molecules facilitates selfperpetuation of both blood-brain barrier (BBB) breakdown and persistent neuroinflammation around the implant. Right: Release of pro-inflammatory and cytotoxic soluble factors can directly and indirectly lead to neuronal apoptosis. Cellular debris from apoptotic cells can further stimulate microglia activation and initiate further BBB instability. Therefore, the neuro-inflammatory response to intracortical microelectrodes will last as long as the implant is implanted in the tissue, and interacting with cells or proteins. Figure adapted from Potter et al. 2013.
3.4.4. The Critical Role of Pro-Inflammatory and Cytotoxic Soluble Factors
Multiple studies have shown that activated microglia and macrophages release a plethora of pro-inflammatory/cytotoxic soluble factors that can damage healthy bystander cells and the surrounding tissue. (120–124) Furthermore, as described in Sections 3.2 and 3.3, a number of soluble factors may also be involved in recording site corrosion and degradation of insulating coatings.
It should be noted that astrocytes and other cells are also known to secrete pro-inflammatory and cytotoxic soluble factors. However in general these cells are believed to produce significantly less pro-inflammatory and cytotoxic soluble factors than activated macrophages and microglia. (120) Furthermore, comparative studies have indicated that macrophages/microglia, and not astrocytes, are the key source of pro-inflammatory and cytotoxic soluble factors that mediate neurodegeneration in a number of disease states. (125–127)
Of the plethora of soluble factors within a macrophage’s available palette, previous work from Biran et al. has shown that adherent cells retrieved from explanted devices secrete both tumor necrosis factor-alpha (TNF-α) and monocyte chemotactic protein-1 (MCP-1). (107) TNF-α can have direct toxic effects on neurons and oligodendrocytes, while MCP-1 is a chemokine involved in opening the blood-brain barrier (BBB) and recruiting new macrophages to sites of injury and inflammation. (120, 121, 124, 128–134)
Several recent studies have also provided further support for the predominant role of macrophage-released soluble factors on recording function and the neuro-inflammatory response. For example, Karumbaiah et al. have shown that gene expression for various pro-inflammatory soluble factors, specifically IL-1,6 and 17 as well as TNF-α, is up-regulated in tissue surrounding poorly performing microelectrodes. (135, 136) In addition, Potter et al. have shown that accumulation of reactive oxygen species surrounding implanted microelectrodes may impact neuronal viability. (69)
Skousen et al. have suggested that macrophage-secreted soluble factors may be critical in both propagating as well as shaping the response to traditional microelectrode designs. (117, 137) Specifically, it was shown that predicted distributions for macrophage-released soluble factors correlate well with the shape and structure of the neuro-inflammatory response to traditional microelectrode designs regardless of device compliance. These observations reveal that presented architecture is a major contributing factor to the overall neuro-inflammatory impact on surrounding neural tissue. Taken together, the studies referred to above indicate the utility of strategies that reduce the concentration of pro-inflammatory and cytotoxic soluble factors to improve recording function. To have maximal impact, as suggested by Skousen et al., strategies should focus on 1) limiting the local number of activated macrophages at the device interface, 2) reducing the degree of inflammatory cellular activation, and 3) directly antagonizing the accumulation of pro-inflammatory and cytotoxic soluble factors themselves. (117, 137)
3.4.5. Astrogliosis and Fibrotic Encapsulation
Surrounding the inflammatory core, a region consisting of hypertrophic astrocytes as well as infiltrating fibroblasts and meningeal cells has also been observed. (69, 70, 107, 109, 117, 138–146) In healthy brain tissue, astrocytes regulate the local microenvironment. Astrocytes sequester a number of neurotransmitters and ions, while also maintaining the BBB that isolates the cellular and ionic milieu of the brain from that of the supporting vasculature. (147–150)
Following injury, astrocytes increase the number and size of their cellular processes, and are primarily identified by increased staining for glia fibrillary acid protein (GFAP), an astrocyte-specific intermediate filament. Hypertrophic astrocytes are believed to play a similar role to that of reactive fibroblasts in the foreign body response in other tissue compartments. (151) Specifically, astrocytes create a dense scar-like layer that limits volume transmission. (152)
Many neuro-inflammatory studies have hypothesized that the astrocytic diffusion barrier may play a beneficial role in restricting the impact of macrophage-secreted factors on the surrounding tissue, as well as mechanically shielding the surrounding tissue from micromotion induced strains surrounding the historically stiff microelectrodes. (94, 98, 153–157) However, astrogliosis or other forms of fibrotic encapsulation, may also increase the tissue’s impedance to small ion transport, potentially limiting recording function as suggested by Porter et al. and later by Schmidt and colleagues. (30, 31, 158)
3.4.6. Neuronal Loss at the Electrode-Tissue Interface
Associated with the regions of inflammation and reactive gliosis, studies have described a decrease in the local nerve fiber and neuronal cell body densities surrounding implanted devices. (107, 109, 143, 145, 159, 160) While a significant number of neurons remain within the recording range, the overall decrease in neuronal density (approximately 40–60% in most studies) indicates that the environment may no longer be ideal for promoting neuronal health and function. Clearly any compromise of the target neuronal population may influence device function.
It has become well established that chronic inflammation and neuronal loss are associated with the persistent presence of the implant, and are not solely the result of iatrogenic injury. In their seminal paper, Biran et al. compared various markers of neuro-inflammation in chronically implanted animals to animals that received only a stab wound injury. (107) The authors found that chronic neuro-inflammation and neuronal loss does not accompany stab wound injuries made with microelectrodes identical to those left in place.
Biran’s findings have been confirmed and expanded upon by several groups, including McConnell et al., and Potter et al. (109, 161) Both of these studies also observed that microelectrode implantation within the cerebral cortex may trigger a multiphasic neuro-inflammatory and neurodegenerative response. However, it should be noted that the time-course of the neuro-inflammatory response is still being debated due to discrepancies between, and even within, different laboratories. (78, 87, 109, 161, 162) Furthermore, several studies have failed to establish a direct correlation between neuro-inflammation and recording quality. This disconnect may be due to the complex interconnectedness of microelectrode failure modes or, as discussed in Section 3.4.10, non-linear relationships between electrode function and the neuro-inflammatory response. (160)
3.4.7. Local Extracellular Matrix Changes
Associated with the region of astrocyte hypertrophy and reduced neuronal density, a number of studies have described changes in local extracellular matrix (ECM). Injury-induced changes in ECM have been widely reported following traumatic brain injuries and in many neurological diseases. (163–165) Following microelectrode implantation, Zhong et al. have described an up-regulation of chondroitin sulfate proteoglycans (CSPGs) at the biotic/abiotic interface. (166) CSPGs are generally considered neuro-inhibitory. (167–173) Therefore, as with successful repair and regeneration following spinal cord injury, it is likely that the altered ECM impedes successful neuronal regeneration in tissue adjacent to the implanted microelectrode. Furthermore, changes in the ECM density could also further limit volume transmission surrounding implanted microelectrodes.
3.4.8. Blood-Brain Barrier Dysfunction
New observations from Tresco’s group have opened other potential explanations as to how the neuro-inflammatory response to an implanted microelectrode could influence recording. As observed in many neurodegenerative disorders, it was found that local BBB integrity is compromised in the tissue immediately surrounding implanted microwires and Michigan-style microelectrodes. (78, 117) These findings suggest that an altered local ionic milieu could influence recording instability. (174–176) Recently Potter et al. studied the progression of BBB integrity over time and found that similar to neuro-inflammatory diseases such as multiple sclerosis, BBB dysfunction is highly dynamic. (87, 109, 177, 178)
Additional recent data further highlights the potential role of BBB dysfunction in connection with poor recording performance. Findings from the Bellamkonda group with Michigan-style and microwire electrodes (162) have shown that recording performance correlates with markers of BBB dysfunction such as extravasated immunoglobulin G (IgG) or labeled albumin.
Beyond directly impacting neurons and recording function, it is important to emphasize that infiltrating blood products also serve as persistent stimuli for perpetuating neuro-inflammation and vice-versa. For example, extravasated fibrinogen, plasma soluble fibronectin, complement factors, and other blood products have been shown to be potent mediators of macrophage and microglial activation. (118) Following extravasation, blood-products are involved in inflammatory cell activation through TLR, CD14 (i.e. glycosylphosphatidylinositol-anchored membrane glycoprotein), and other receptor-mediated pathways. (119, 120) A variety of blood components are likely present at the microelectrode/tissue interface throughout the lifetime of the implant as a combination of phenomena. This combination includes 1) the initial damage of microelectrode implantation into the cortex, 2) motion-induced damage at later timepoints, 3) macrophage/microglia trafficking at both early and chronic time points and 4) persistent pro-inflammatory signaling. Thus, developing a combination of methods to break the self-perpetuating cycle of inflammation and BBB dysfunction should be of key focus in the field to improve microelectrode biocompatibility.
3.4.9. Connecting the Neuro-Inflammatory Response and Recording Quality
While a number of potential mechanisms have been presented to describe how the brain’s response may impact recording function, the direct connection remains unclear. However, there is increasing evidence indicating that the neuro-inflammatory response may be a primary hurdle to consistently obtaining high quality recordings. For example, in 2007, Rennekar et al. examined whether systemic anti-inflammatory administration could improve recording performance. (179) The drug used in Rennekar’s study, Minocycline, is a tetracycline antibiotic known to shift macrophages and microglia away from a pro-inflammatory (M1) phenotype. (180, 181) Electrodes in rats that received oral minocycline treatment showed a significant improvement in both signal to noise ratio (SNR), and the number of channels that recorded stimulus-driven neural activity. Unfortunately, while likely implicating inflammation, little histological examination was performed to link particular cells types (such as macrophages) or reactive species to recording function.
Delivery of another anti-inflammatory drug, dexamethasone, has been shown to reduce the inflammatory response to inserted microelectrodes. (182–185) However, studies describing the impact of dexamethasone administration on recording performance have, to our knowledge, not been performed. Interestingly, studies that delivered dexamethasone locally around an implanted microelectrode showed no significant impact on the reactivity at later time points. This apparent discrepancy is likely due to exhaustion of the drug source. Therefore a chronic anti-inflammatory regimen or more permanent solution will be needed to regulate the neuro-inflammatory response through the lifetime of the implanted microelectrode.
Unfortunately, even if it was possible to continually deliver dexamethasone or minocycline, this would not be an adequate long-term solution, as chronic use of either drug can result in immune system impairment, decreased renal function, vertigo, bone discoloration/loss, fatal colitis, and intracranial hypertension. (186–189) Therefore, while the use of Minocycline and dexamethasone provides a mechanistic understanding regarding how inflammation may impact electrode performance, better-tolerated pharmaceutical and materials-based approaches need to be developed.
To further elucidate the role of inflammation on recording function, Tyler and colleagues examined whether exacerbation of the inflammatory response would reduce recording performance. (190) To answer this question, Tyler’s group compared the recording quality of Michigan-style devices in control animals to that from animals that were administered the bacterial endotoxin lipopolysaccharide (LPS). LPS is a known stimulus for driving macrophages and microglia to a pro-inflammatory state through Toll-like receptor pathways. (191–193) Microelectrodes in rats that received LPS had significantly lower signal to noise ratios and number of recorded units compared to saline-only control animals. (98) Thus, Tyler and colleagues’ results further implicate neuro-inflammation as a primary biological mediator of recording performance.
While Tyler’s work demonstrated that large-scale exacerbation of neuroinflammation impacts recording quality, more recent evidence from Ravikumar et al. suggests that even small-scale shifts in neuroinflammation can dramatically impact the local tissue. (160) Specifically, Ravikumar et al. examined the brain tissue response to sterilized silicon microelectrodes with varied amounts of low-level endotoxin contamination. Histological evaluation at two weeks showed a direct correlation between microglia/macrophage activation and residual endotoxin levels. By contrast, astrogliosis, neuronal loss, and blood brain barrier dysfunction demonstrated a threshold-dependent response to bacterial endotoxins and macrophage/microglia activation. A threshold-dependent response demonstrates that even subtle changes in the neuro-inflammatory environment over time could underlie observations of recording inconsistency as the environment shifts back and forth beyond a critical inflammatory threshold.
In their study, Ravikumar et al. also indicated that in the 108 published microelectrode studies that they reviewed, a wide range of sterilization methods were used. Quite strikingly, different distributions of sterilization methods are seen in studies that utilize functional and non-functional electrodes, respectively. It appears to be rather concerning that in as many as 20% of the studies, no details on how the implants were cleaned and sterilized were reported. This raises the question, to what extent our understanding of electrode performance is confounded by lack of attention to potentially critical details, which should be noticed and reported.
3.5. Summary of the Challenges to Achieving Consistent, High-Quality Neural Recordings
The above section summarizes the many mechanisms that can spatially and temporally mediate microelectrode failure. These failure modes include, but are not limited to, 1) direct mechanical damage; 2) corrosion of electrical contacts; 3) degradation of passivation layers and insulating coatings; and 4) the neuro-inflammatory response that the brain mounts against chronically implanted devices. Figure 4 highlights how each of these various failure modes may impact the overall neural interface circuit.
Due to the variety of failure modes and the high level of interplay involved, it is increasingly evident that combinatorial strategies may be needed to obtain consistent, high quality neural recordings. While a number of anti-inflammatory drugs have been investigated and have provided information on whether/how neuro-inflammation may impact electrode performance, better-tolerated, longer-lasting approaches need to be further developed.
Furthermore, one could argue that all four of the described failure modes could be mitigated through the appropriate choice and/or development of more appropriate materials. Therefore, it is not surprising that material-based approaches to mitigating microelectrode failure and/or poor tissue integration have received considerable attention.
4. MATERIAL STRATEGIES FOR IMPROVING MICROELECTRODE BIOCOMPATIBILITY AND RECORDING PERFORMANCE
In the last decade, various materials-based strategies have been investigated with the objective of minimizing the neuro-inflammatory response and enabling high-fidelity neural interfacing over clinically relevant timeframes. In all cases, developers have sought to address one or a set of the limitations discussed in Sections 2 and 3. Throughout the remainder of the paper we will review the primary approaches to develop the next generation of intracortical microelectrodes including:
Minimizing motion-induced injury using compliant microelectrode substrates
Limiting surgical trauma and/or inflammatory cell accumulation by manipulating microelectrode architecture
Preventing protein and inflammatory cell adhesion through non-fouling surface coatings
Manipulating inflammatory cell phenotype through use of surface topography
Directing tissue integration at the microelectrode-tissue interface using bioactive materials
Reducing the concentration or impact of inflammatory soluble factors through the use of passive and active antagonists
Improving the electrical performance of intracortical microelectrodes using conducting polymers and nanomaterials
Each subsection is concluded with our interpretation on the strengths/limitations and questions that must be addressed to enable consistent, high-quality long-term neural recordings.
There are a number of important facts to consider when comparing and analyzing the impact of material-based approaches for improving microelectrode function. First, isolating the impact of a given strategy to one specific variable that could influence the neuro-inflammatory response is difficult at best. For example, as will be discussed in Section 4.1, a major strategy in the field for reducing the neuro-inflammatory response is the creation of compliant, polymer-based microelectrodes that better match the mechanical properties of the surrounding tissue. However, many of the polymers used to create compliant microelectrodes absorb a significant degree of water and are likely permeable to small molecules. Thus the innate permeability of these complaint materials adds the possibility that findings from these studies have been influenced by improved clearance of pro-inflammatory and cytotoxic soluble factors (Section 4.6.1). Therefore, to elucidate the overall design space available for microelectrode designers, further studies should be conducted to isolate the impact of individual design variables as well as to identify possible interactions or emergent phenomena.
Equally as important when analyzing findings from studies that have examined new strategies for reducing the neuro-inflammatory response, one must critically assess the role that tissue processing and other techniques may have on reported results. For example, in almost all cases the implanted microelectrodes are removed from tissue prior to analysis. Microelectrode removal may disrupt the tissue interface and influence data interpretation, especially for coatings that impact cell attachment. (78, 107, 108, 194) Different groups also use a variety of diverse markers to describe related cellular and molecular features of the neuro-inflammatory response. An example of this is the use of pan-macrophage markers such as OX-42 and IBA-1 versus markers for activated macrophages such as CD-68. There are also large to subtle differences in the methods used to image, quantify, and statistically compare histological results that can lead to differences in interpretation. Common differences include the use of confocal versus traditional microscopy, the use of boutique quantification packages, as well as discrepancies in defining what makes for an independent measurement/observation. Therefore, efforts to improve the quality and consistency of methods across and even within groups would be useful for improving intra-study comparisons.
4.1. Mechanically Compliant Intracortical Microelectrodes
As discussed above, traditional microelectrodes have been composed of extremely stiff materials such as metals or silicon. The high stiffness has facilitated microelectrode implantation into the cortical tissue. (79) Unfortunately, a number of groups have hypothesized that increased stiffness may adversely impact neuronal tissue through a number of mechanisms. (88–95) First, in vitro evidence indicates that substrate stiffness, even in a static culture environment, may adversely impact neuronal and glial cell types. However, a number of in vivo studies looking at either stiff materials or those coated with compliant polymers have indicated that haptic-mediated mechanotransduction may not play as significant a role as initially thought (see Sections 4.1.3, 4.2.1, 4.3 and 4.6.1). (117, 195) The second, and perhaps more predominant hypothesis, is that mechanical differences between the brain and microelectrodes induce adverse strains in the surrounding tissue during regular brain micromotion. (95, 138, 196) Therefore, compliant materials that have mechanical properties closer to that of brain tissue have received extensive attention towards improving microelectrode integration within the surrounding tissue.
4.1.1. Mechanical Factors Impacting Intracortical Microelectrode Biocompatibility
When manipulating microelectrode compliance it is important to further discuss a number of mechanical factors that may impact microelectrodes or the surrounding tissue during insertion and throughout the indwelling period. During insertion, three primary forces act on the microelectrode, namely: an axial tip force, frictional forces excreted on any presented surface, and a compressive clamping force (Figure 9). The summation of these three forces is commonly referred to as the total insertion force (IF). The IF for traditional microelectrodes ranges from 500–1000 µN depending on the shape of electrode’s tip and dimensions of the electrode’s shank. (196, 197)
Figure 9.
Forces acting on the intracortical microelectrodes upon penetration.
To avoid buckling during insertion, the IF must be lower than the critical loading force (CLF) for a given design. Therefore, both the IF and CLF should be considered when creating any new microelectrode design. Given similar dimensions to traditional microwire or planar silicon microelectrodes, compliant devices should have an IF > 1000 µN to avoid buckling. (198–201) To satisfy this design criterion many compliant microelectrodes were designed with larger cross sectional areas than traditional microwires or silicon microelectrodes. As will be discussed in Sections 4.1.3 and 4.1.4, a number of groups have also moved to using insertion aides or in situ softening materials in order to insert smaller or more compliant devices.
As stated, there is substantial belief in the field that mechanical differences between the brain and traditional microelectrodes induce adverse strain in the surrounding tissue during normal respiratory and circulatory pulsations. Unfortunately, while in vivo studies have described insertion and extraction mechanics, (96, 157) only one study has been performed to directly quantify microelectrode-induced strain over the indwelling period. (96) Determining whether implanted microelectrodes induce sufficient strain to adversely impact neural and inflammatory cells is a critical gap in the field.
Aside from the one in vivo study, several computational models have been developed to estimate this elusive parameter. (89, 202–204) Such modeling studies support the hypothesis that mechanical mismatch between the implanted microelectrode and surrounding brain tissue could lead to adverse strains and stresses being generated during normal brain micromotion. (95) Furthermore, while the majority of the field has focused on electrode stiffness, the models suggest that tethering scheme and the degree of tissue adherence are additional variables that can be manipulated to reduce microelectrode-induced strains.
Of these additional variables, perhaps the best characterized in vivo is the impact of device tethering. Starting with Biran et al., multiple studies have indicated that tethering devices to the skull exacerbates the neuroinflammatory response. (108, 145, 205) While these findings support the hypothesis that mechanical mismatch plays a role in propagating the neuroinflammatory response, there are alternative explanations and contradictory evidence in the field. For example, one alternative explanation is that anchoring exacerbates inflammation by facilitating meningeal fibroblast migration into the brain. This is supported by recent findings indicating that submeningeal implantation reduces the neuro-inflammatory response. (206) Furthermore, the only in vivo tethering study driven directly by a computational model failed to confirm the predicted impact of various tethering schemes. (207)
Therefore, while mechanical models have expanded our understanding of the impact of a number of biotic and abiotic parameters, there is still further work that should be pursued. Direct validation of the in silico models with in vivo data is necessary. Mechanical models also could be further improved by incorporating more accurate mechanical properties of the surrounding glial scar. Work has only recently been completed by the Muthuswamy group to quantify microelectrode-induced changes in the mechanical properties of the surrounding tissue. (96)
4.1.2. “Off-the-Shelf” Compliant Polymeric Materials for Intracortical Microelectrodes
Several groups have developed compliant microelectrode substrates and coatings from “off-the-shelf” polymeric materials. These materials include polyimide, benzocyclobutene (BCB), polydimethylsiloxane (PDMS), Parylene-C and SU-8. (201, 207–213)
To date, only a limited examination of the tissue response to microelectrodes made from “off-the-shelf” complaint materials has been performed. In vitro culture has been the predominant characterization tool. The results of these studies have indicated that a number of traditional compliant polymers are non-toxic and support the attachment of neuronal and glial cells. Interestingly, there is little evidence that any of these “off-the-shelf” materials significantly reduce the in vivo neuro-inflammatory response.
Characterization of recording performance from electrodes made from “off-the-shelf” compliant materials is also quite limited. Again the majority of microelectrodes made from “off-the-shelf” compliant materials have proven successful during in vitro recording studies. A limited number of materials have undergone acute in vivo testing. For example, using BCB-based microelectrodes, Clement et al. succeeded in recording neural signals from rat cortex. (214) Additionally, Altuna et al. were able to record multi-unit activity as well as local field potentials using an SU-8 based microelectrode. (215) Longer-term studies are now needed to examine if these complaint microelectrodes reduce the neuro-inflammatory response or improve chronic recording performance.
There are still a number of inherent limitations to these “off-the-shelf” materials. For example, in nearly all cases their stiffness is still at least 3 orders of magnitude higher than brain tissue. While modelling studies have indicated that reducing microelectrode stiffness to the MPa range will limit tissue strain, it is unclear exactly how soft a material must be to achieve reductions in the neuroinflammatory response or improvements in recording function.
As with many of the early metals used for microelectrodes, toxicity has been a concern with a number of “off-the-shelf” compliant polymers. Toxicity may be caused from the polymer itself or from leaching of residual solvents, plasticizers, or degradation products. For example, Vernekar showed that thick untreated SU-8 substrates are not compatible with primary neuronal culture as less than 10% of primary neurons survived when cultured on SU-8 substrates. (216) The authors suggested that the poor cytocompatibility of SU-8 was due to leachables as neuronal survival increased when substrates underwent heated vacuum treatment and sonication in isopropanol. It is important to note that leachable-mediated toxicity is not isolated to SU-8 alone, as other groups have described toxicity with PDMS and Poly Vinyl Alcohol (PVA) as well. Therefore toxicity testing should be performed on any new polymer system before time consuming and costly animal trials. However, as a word of encouragement, it is likely that more careful preparation of these materials will overcome issues related to toxicity, as many are routinely used in biomedical applications without incident.
Moisture uptake is another factor that may impact the performance of polyimide, as well as a number of the in situ softening materials that will be discussed in Section 4.14. Polyimide, for example, swells by approximately 4–6% (w/w) upon implantation. (208, 217, 218) Swelling of polyimide has been linked to a rapid decrease in electrode performance after implantation. However swelling may not be altogether negative. As will be described in Section 4.6.1, Tresco and colleagues have proposed that polymer swelling may also provide an additional clearance mechanism for pro-inflammatory soluble factors. (137) Therefore, swelling may provide an alternative/complimentary explanation for improvements in the neuroinflammatory response to compliant polymer substrates, provided that the increased water uptake does not interfere with the electrical circuit or electrode insulation.
4.1.3. Strategies to Prevent Buckling During Insertion of Compliant Microelectrodes
While the stiffness of many “off-the-shelf” compliant materials is still significantly higher than brain tissue, it is low enough to cause buckling in devices made on the same scale as traditional microwires or planar MI-style microelectrodes. To prevent buckling a number of larger device designs and insertion aides have been developed.
Perhaps the simplest method to prevent buckling of more compliant microelectrodes is to increase the size of the device beyond the traditional architectures used in microwire or siliconbased implants. LaPlaca took this approach to facilitate insertion of Parylene-C based microelectrodes. (201) Similar to MI-style microelectrodes LaPlaca’s probes were designed to be 100 µm wide; however, the thickness was roughly doubled to 25 µm. LaPlaca’s findings indicate that only slightly larger designs may be needed to prevent buckling. Obviously increasing device size may have consequences. For example, increasing device size will reduce device compliance and increase strain induced on the surrounding tissue. In addition, increasing device size will also exacerbate the initial iatrogenic injury. Interestingly, Skousen et al. has indicated that the neuroinflammatory response to single penetrating devices may not be greatly impacted by increasing device size beyond that found in traditional designs. (117) However, findings from Skousen and others also indicate that the neuroinflammatory response can be greatly reduced by transitioning to architectures that are smaller than traditional designs (see Section 2.1). (117, 145, 159)
Other efforts to prevent buckling of compliant microelectrodes have focused on reinforcing compliant polymers with stiffer materials. For example, Lee et al. reported on a new design for polyimide-based intracortical microelectrodes, which provides adequate stiffness for insertion into neural tissue. (217, 218) In Lee’s design, a 5–10 µm thick silicon layer was applied to the polymer to prevent buckling during insertion. Similar designs have been reported for BCB. (219, 220) Penetration tests into rat brains showed that reinforced polyimide based microelectrodes of similar size to a standard MI array could penetrate the rat pia without buckling. However it should be noted, that the composite Young’s modulus of these electrodes increased significantly from 2.8 GPa (neat polyimide without silicon backbone) to 31GPa and 58GPa with a 5 or 10 µm thick silicon layer, respectively. (217, 218) Therefore, the use of permanent reinforcement may be counterproductive towards minimizing chronic tissue strain as the overall compliance is reduced little, if at all, compared to traditional devices.
As an alternative to the silicon-reinforced systems discussed above, Takeuchi et al. incorporated a microfluidic channel into a compliant Parylene-C based microelectrode. (209) Takeuchi filled the channel with a dissolvable poly(ethylene glycol) (PEG) reinforcement. Using this approach, the authors were able to successfully insert their compliant device and record neural signals directly after implantation. Despite the promise of these early reports, longer-term studies have not been reported.
More recently, and perhaps inspired by Takeuchi’s dissolvable system, several groups have investigated the use of biodegradable polymers as shuttles for compliant devices.243, 251, 252 One of the most promising examples of a degradable shuttle was explored by Shain and Kohn. In a series of investigations, Shain and Kohn studied several tyrosine-based polycarbonates as biodegradable carriers for intracortical microelectrodes. (221–223) Tyrosine-based polymers have many attractive properties including the neutral pH of products created during hydrolysis of the polymer. Other degradable shuttles that have also been investigated include poly(lactic acid), glucose and gelatin. (212, 222, 224–228)
In summary, a number of strategies have been developed to facilitate insertion of compliant microelectrodes including increasing device size and the use of permanent and temporary reinforcement schemes. It is important to note that the use many of these methods will increase the initial iatrogenic injury. Furthermore increasing device size or the use of a permanent insertion aide may negate any mechanical benefit achieved by using a compliant polymer, thus providing no increase to the quality and stability of neural recordings.
4.1.4. In Situ Softening Materials
An alternative approach to the strategies described above has been the development of in situ softening materials as substrates for intracortical microelectrodes. Such “smart” materials are being considered for a broad range of biomedical applications, including use as delivery vehicles for therapeutic molecules, use as mechanical actuators, and as scaffolds for regenerative medicine applications. (229–234) In situ softening materials have received attention as microelectrode substrates as they are sufficiently stiff to facilitate implantation into the brain, but then soften in vivo to better match the mechanical properties of cortical tissue.
The first realization of an in situ softening microelectrode was reported by Capadona et al. in 2008. (100) Taking a biomimetic approach, the team utilized the microstructure of the sea cucumber dermis as the blueprint for a new class of stimuli-responsive, mechanically adaptive polymer nanocomposites. Specifically, the current generation of mechanically-adaptive nanocomposites is based on a poly(vinyl acetate) (PVAc) matrix reinforced with rigid cellulose nanocrystals (CNCs). (103) When dry, these nanocomposites are in a rigid state (E' = 5.1 GPa), due to the glassy matrix and the rigid percolating network of the CNCs. Upon exposure to physiological conditions, the nanocomposite absorbs fluid and swells considerably (30–90% w/w depending on the type of CNCs). Subsequently the nanocomposite undergoes phase transition and softens (E' = 12 MPa) as water plasticizes the matrix and disassembles the CNC network (Figure 10). It was shown that dry implants of this nanocomposite can readily be inserted through the pia mater into the cerebral cortex of a rat without the need for assistive devices. The insertion of the chronically compliant materials was a significant feat as reference implants consisting of the neat matrix polymer (PVAc) buckled under lower loads before they could be inserted into the cortical tissue. (98) Ex vivo studies confirmed that the initially stiff microscale nanocomposites rapidly softened when implanted into the rodent brain. Figure 11 shows the stiffness of a 12.2% v/v PVAc/CNC nanocomposite upon introduction into artificial cerebrospinal fluid (ACSF) at 22 °C (Young’s modulus ~3400 MPa), and reveals that the softening occurs over the period of 15 min to reach a Young’s modulus of ~33 MPa. A comparison between the Young’s modulus of microprobes that had either been implanted in a living rat cortex showed no statistical difference immersed for 15 min in ACSF (Figure 11). (97, 98)
Figure 10.
Top: Pictures of a sea cucumber, in the threatened (stiff) and relaxed (soft) and state. Bottom: Simplified schematic representation of the switching mechanism found in the sea cucumber dermis and used in physiologically responsive mechanically-adaptive nanocomposites. A soft matrix is reinforced with rigid particles, whose interactions in are moderated by a chemical agent.
Figure 11.
Plot showing the log of the Young’s modulus of mechanically adaptive PVAc/CNC nanocomposites as function of exposure time to ACSF or implantation time in the rat cortex. Data were acquired by either a dynamic mechanical analysis (DMA Data, open squares; bulk materials) using a submersion clamp and exposing the sample to ACSF preheated to 37 °C or mechanical tests of microprobes that had been implanted into the rat cortex for the time indicated and which were explanted for microtensile testing (Ex vivo Data, open circles). The x-axis indicates the time of exposure to either ACSF or implanted in the rat cortex, respectively. Reprinted with permission from Harris et al. Copyright © 2011 IOP Publishing.
In preliminary investigations, Hess et al. (91, 92) have shown that functional microelectrodes can be made using laser micromachining followed by deposition of a Parylene-C insulating layer, sputtering of Ti/Au electrodes, and deposition of an overlaying Parylene-C coating. Initial histological evaluations of in situ softening materials in vivo demonstrated that compliant implants more rapidly stabilized neural cell populations than rigid microwires at four weeks post-implantation. (94) However, no significant difference was observed at 8 weeks post-implantation. Thus, the results of Harris’s initial studies could be interpreted that, despite acute benefits, the mechanical mismatch between microelectrodes and cortical tissue appears to have little effect on the chronic neuro-inflammatory response. However, this oversimplified interpretation deserves further consideration. Previously Tresco and colleagues have observed that microwires, on a similar size scale as those used by Harris et al., have a reduced response compared to larger MIstyle microelectrodes. (194) Therefore the lack of significant difference at 8 weeks may actually quite promising.
To verify this interpretation, Nguyen et al. recently completed a more comprehensive evaluation of the neuroinflammatory response to PVAc/CNC nanocomposite implants through a 16 week implantation period. At this later time point, they observed nearly complete attenuation of inflammatory cell activation, and the absence of any appreciable neuron loss surrounding PVAc/CNC nanocomposites compared to chemically-matched PVAc-coated MI-style microelectrodes (Figure 12). (235) Interestingly, unlike Harris’ initial study, few statistically significant differences were observed between compliant and stiff PVAc implants at early time points. This discrepancy could be due to differences in either the controls, surgical technique, or a number of other factors, but it does emphasize the difficulty of intra-study comparisons and the need for further standardization of techniques throughout the field.
Figure 12.
(A) Immunohistochemical analysis of CD68, a cellular marker for activated microglia/macrophages. Representative fluorescence microscopy images of stained tissue show a distinct benefit of mechanically compliant implants, compared to the chemically matched non-compliant implants (16 weeks post-implantation; p < 0.05). Scale bar = 100 µm. Error bars represent standard error. (B) Immunohistochemical analysis of neuronal nuclei (NeuN) around the implant site. Representative fluorescence microscopy images of stained tissue show that neuronal dieback around the non-compliant implant was significantly higher than in case of the compliant nanocomposite implant at 16 weeks postimplantation. The bar graphs show quantification of neuron densities. * Denotes significance between non-compliant and compliant samples; # Denotes significance between noted implant and age-matched sham control (p < 0.05). The horizontal dashed line represents the 100% neuron level as determined by quantification of age-matched sham animals. Error bars represent standard error. Figure adapted from Nguyen et al. 2014.
In situ softening intracortical implants have also been made from shape memory polymers (SMPs). (236, 237) (104, 236) For example, Sharp et al. developed SMP-based microelectrodes from an epoxy-based polymer using a micro-casting technique. (237) More recently, Ware et al. developed SMP based-microelectrodes from acrylate and thiol-ene/acrylate polymers. (93, 236) (104, 236) Due to acrylate’s sensitivity to a number of environmental factors necessary for photolithographic processing, a transfer-by-polymerization process was used instead. In vivo studies demonstrated that both acrylate and thiolene/acrylate-based microelectrodes were capable of recording neuronal signals in a rat cortex. However, improved histological studies to further verify these findings, and a head-to-head comparison of recording performance to that of traditional microelectrodes have not been reported to date. (104)
Recently Tien et al. used silk fibroin to fabricate a third type of mechanically adaptive microelectrode. (238) The elastic modulus of silk is reduced upon hydration from 1.8 GPa in the dry state to 20 MPa in the wet state. Beyond being a compliant, Tien et al. have also engineered their system to release scar-inhibiting agents such as the enzyme chondroitinase ABC (chABC) as a potential avenue to ameliorate axonal growth inhibition by CSs within the glial scar. While promising, to date, the effect of silk fibroin coatings has only been examined using in vitro models and no recording studies have been performed.
4.1.5. Summary of Important Considerations Regarding Compliant Microelectrodes
Mechanical mismatch has been hypothesized to promote and perpetuate the neuro-inflammatory response. If this hypothesis proves true, an obvious solution is to increase the compliance of implanted microelectrodes. However, microelectrode design also must be concerned with the conflicting mechanical requirements necessary to prevent microelectrode buckling during insertion. Fortunately, a number of strategies have been developed to overcome this hurdle including insertion aides and in situ softening materials.
Although, some of the strategies described above have facilitated insertion of single-shank electrodes, challenges still exist if they are to be applied to multi-shank designs. Parallel to the idea that it is improbable that one absolute mechanism mediates microelectrode failure, it is also unlikely that one device will serve the needs of the entire recording community. Therefore, even single shank implants may provide promise to individual laboratories and in specific applications.
As stated, perhaps the two largest gaps in the field of compliant microelectrodes is that no studies have been performed to directly quantify: 1) the effects of device stiffness/compliance on electrode recording quality, and 2) if microelectrode-induced strain over the indwelling period can be reduced by material choice or electrode design. Therefore, due to the well-established strategies for overcoming mechanical mismatch, particular emphasis should be directed at validating this widely pursued hypothesis. If in vivo studies confirm that compliant materials are indeed better than traditional rigid microelectrodes, one must ask how compliant do they need to be? While answering this question it will be critical to utilize properly designed experiments and appropriate controls to avoid the insertion of confounding explanations and interpretations.
Finally, and most importantly, one of the larger problems with softening implants is the requirement for some degree of water to ‘switch’ the mechanical properties. In our unpublished experience, the accumulation of water into early devices created delamination between material components, and created problems which limits evaluation of the probes to histological studies, preventing long-term electrophysiology experiments. Recent advances in fabrication and materials processing has overcome this limitation, (91) and electrophysiology studies are underway.
4.2. Manipulating Microelectrode Architecture to Reduce Neuro-Inflammation
A second major strategy to reduce the neuro-inflammatory response to intracortical microelectrodes has focused on altering device geometry and architecture. A number of theories have been proposed for how altering geometry and architecture could be used to reduce the neuro-inflammatory response. Theories include 1) altering tip geometry or the penetrating profile of the microelectrode to reduce surgical trauma, 2) reducing feature size to improve mechanical compliance, and 3) minimizing presented surface area to reduce the local number of inflammatory cells in a given volume.
4.2.1. Theories for how Microelectrode Architecture Impacts Neuro-Inflammation
Szarwoski et al. conducted the first study investigating the potential of changing device architecture to alter the neuro-inflammatory response. (239) The authors studied the neuro-inflammatory response to a variety of devices with different cross sectional areas, tip geometries, and surface roughness and concluded that the tissue response was independent of these electrode properties. However, it is possible that their results and conclusions stem from the fact that the range of given parameters were too narrow to induce significant changes in the neuro-inflammatory response. Indeed, the broad conclusions of Szarwoski et al. have since been contradicted by several recent studies. (145, 159, 240)
Of the contradicting studies, both Stice et al. and Thelin et al. provided evidence that changing device geometry and reducing presented surface area impact the neuro-inflammatory response (145, 240). The two studies independently revealed significant differences in classic hallmarks of the neuro-inflammatory response between microwires of different diameters. Both groups hypothesized that reducing the initial iatrogenic injury by presenting a smaller cross-sectional area drove their results. However, while plausible, this interpretation is confounded due to differences between the presented surface area and curvature of the disparate sized microwires as well as possible differences in mechanical compliance.
Additionally, work presented by Seymour and Kipke found significant differences in both the neuronal and non-neuronal cell responses between a SU-8/Parylene-C based electrode’s larger shank and an adjoining lateral platform designed with a variety of different sized lattice architectures. (159) Using devices with identical penetrating profiles Seymour and Kipke removed the impact of the initial iatrogenic injury from their findings/interpretations. Thus, while the extent of iatrogenic injury may play a role in the severity of the neuro-inflammatory response, Seymour and Kipke clearly demonstrated that other architecture-governed properties are at play as well. The authors concluded that mechanical differences between the thin adjoining lattice structures and the larger primary solid shanks were the underlying cause of their results. While studies have suggested that mechanics may play a role in the neuro-inflammatory response (Sections 3.4.2 and 4.1), further mechanisms could also be at play.
For example, Skousen et al. have suggested that architecture (specifically the local surface area) may be manipulated to control the number of inflammatory cells and the concentration of pro-inflammatory and cytotoxic soluble factors at the device interface. (117) To test their hypothesis, Skousen et al. compared the tissue response of planar silicon microelectrodes with either a solid shank or thin lattice architecture. Despite being less compliant than the polymer-based lattice devices used by Seymor and Kipke or the compliant materials discussed in Section 4.1, (159) the silicon-based lattice microelectrodes still significantly reduced the neuroinflammatory response after an 8-week indwelling period. Specifically, Skousen et al. observed a reduction in inflammatory cell activation, blood brain barrier dysfunction and neuronal loss surrounding the lattice microelectrodes. Skousen’s findings confirm that other architecture-governed properties beyond iatrogenic injury or microelectrode compliance influence the severity of neuro-inflammatory response. Current studies are investigating the use of novel architectures to improve microelectrode function and biocompatibility.
4.2.2. Driving Next-Generation Microelectrode Designs Using Predictive Modelling
To facilitate further investigation of the role that microelectrode architecture and other constitutive properties play in the neuro-inflammatory response, several computational models have been developed. The first series of these models estimated the mechanical strains induced in the surrounding tissue due to microelectrode architecture and stiffness. (89, 202–204) Such modeling studies support the hypothesis that mechanical mismatch between the implanted microelectrode and surrounding brain tissue could lead to adverse strains and stresses being generated. (95)
While mechanical models have expanded our understanding of the impact of a number of biotic and abiotic parameters, there is still further work that should be pursued. For instance, mechanical models could be further improved by incorporating more accurate mechanical properties of the surrounding glial scar. Work has only recently been completed by the Muthuswamy group to quantify microelectrode-induced strain on the surrounding tissue. (96) This newly described quantified strain data will be extremely useful to understand whether modeled in vivo strain fields around microelectrodes compare to strain ranges shown to affect various neural and inflammatory cells in vitro.
In addition to models describing the mechanical impact of microelectrode design on the surrounding tissue, Skousen et al. have introduced models to estimate soluble factor distribution surrounding various electrode designs. (117) Results indicate that the spatial distribution of proinflammatory factors surrounding an implanted device is governed by the local number of adherent inflammatory cells, and the spatial summation of their released soluble factor gradients. Thus, reducing feature size and isolating architectural components spatially from one another can be used to minimize the concentration of negative soluble factors. This specific architectural approach reduces soluble factor concentration by limiting the quantity of source cells at the microelectrode/tissue interface. Other means of reducing the concentration of pro-inflammatory and cytotoxic molecules by incorporating passive permeability sinks or using active antagonists will be discussed further in Section 4.6.
Similar to the mechanical models described by above, Skousen et al.’s models to describe soluble factor distribution are still relatively simple and should be further improved to fully establish their validity while also increasing their accuracy and usefulness. For example, to date soluble factor distribution models have focused primarily on estimating the distribution of the potent cytokines TNFα and MCP-1. (117) However, as mentioned above in Section 3.4.4, various interleukins, reactive oxygen species, and matrix metallo proteinases (MMP) 2 and 9 are also believed to mediate the neuro-inflammatory response. (69, 107, 135) Therefore expanding these models to include other soluble factors that have different effective concentration levels, half-lives, clearance rates or diffusivity is needed. Additionally, the models could be further improved by incorporating a graded diffusivity that better reflects the impact of the glial scar on volume transmission. To best understand the impact of the glial scar, as well as to ultimately validate such models, techniques need to be developed or adapted to accurately measure soluble factor distribution in tissue surrounding implanted microelectrodes.
Despite the considerable amount of progress based, at least in part, on modeling studies, predictive models are still under-utilized as a research and design tool. For example, to date mechanical models have only been used to predict the potential strains surrounding classic single tine microelectrode designs. Expanding such models to understand how microelectrode architecture can be manipulated to reduce persistent mechanical damage would be extremely useful. Ultimately, once properly validated, both mechanical and soluble factor modeling will be pivotal tools in the toolboxes of microelectrode designers.
4.2.3. Important Considerations when Manipulating Microelectrode Architecture
While considering architectural modifications for improving microelectrode function and biocompatibility, it is important to consider the limitations and hurdles inherent to this approach. For example, while already in existence, newer methods of placing conducting traces along the substrate may need to be employed. Use of such fabrication techniques could increase production costs, at least in the near future until such techniques become more commonplace.
In addition, as microelectrode dimensions are becoming smaller the devices become increasingly fragile and prone to mechanical failure. Use of structural elements such as lattice designs could be employed to improve the durability of small features. However, as noted by Seymor and Kipke as well as Skousen et al., lattice structures permit increased tissue growth through the device. (117, 159) Such ingrowth could potentially exacerbate the amount of tissue removed upon device extraction as a result of failure or infection. Therefore, a higher-level view must be taken to ensure that any architectural changes used to reduce the neuro-inflammatory response do not limit microelectrode performance or biocompatibility by eliciting new complications.
4.3. Non-Fouling Surface Modifications to Prevent Cell Adhesion
An alternative strategy to reduce the neuro-inflammatory response to implanted microelectrodes is the use of non-adhesive coatings to prevent inflammatory cell attachment and activation at the microelectrode surface. Several surface coatings or materials have been described to reduce or prevent cell adhesion.
Silicon carbide (SiC) has been long studies as an alternative substrate for neural electrodes due to its documented biocompatibility in other medical device applications. SiC has gained attention because it is chemically ‘inert’, and lends itself readily to MEMs fabrication and chemical surface modifications to improve in vitro biocompatibility (or biologically ‘inert’ / non-fouling). SiC was recently reviewed by Saddow. (241)
The most broadly applied approach in biomaterial science for preventing cell adhesion is through the presentation of biologically ‘inert’ chemical moieties on the surface of the implant. For the purposes of this section, the term ‘inert’ refers to the ability to resist protein adsorption and cell adhesion, but is not meant to reflect the material’s ability to resist corrosion. For review of a variety of surface treatment approaches to create non-fouling substrates, see Raynor et al. (242)
Tresco and colleagues first investigated whether reducing cell adhesion could alter the neuro-inflammatory response to microelectrode arrays. (194, 243) Specifically, Leung et al. characterized microglial adhesion to a variety of surfaces in vitro.273 Subsequently, Winslow et al. compared the neuro-inflammatory response of planar silicon microelectrodes that had a uniform coating of the hydrophobic insulator Parylene-C to that of identical uncoated devices.191 In vitro, Parylene-C reduced microglial adhesion by ~95%. A similar reduction of cell adhesion was observed following device removal after two, four or 12 weeks of implantation in rat cortical tissue. Interestingly, no significant difference was observed in the neuro-inflammatory response or the level of neuronal loss surrounding the Parylene-C coated devices compared to uncoated microelectrodes.
Garcia and colleagues recently built upon the work by Winslow et al. using conformal microgel coatings of poly(N-isopropylacrylamide) (pNIPAm) cross-linked with poly(ethylene glycol diacrylate) (PEG-DA) on silicon microelectrodes. (195) Long polyethylene glycol (PEG) chains take advantage of entropic and osmotic repulsion to prevent cell adhesion through the inhibition of protein adsorption. In vitro analyses demonstrated significantly reduced astrocyte and microglia adhesion to microgel-coated microelectrodes, compared to uncoated controls. However, persistent inflammation was observed surrounding both uncoated and coated microelectrodes following one, two and 24 weeks of implantation in rat cortex. Furthermore, neuronal density around the implanted electrodes was also lower for both implant groups compared to the uninjured controls.
As no cells were found adhered to either the Parylene-C or pNIPAm/PEG-DA coated microelectrodes upon removal, it would appear that both coatings were still functioning at the end-points studied. Therefore, the combined findings indicate that cell adhesion is not necessary to drive the neuro-inflammatory response. In that respect, Otto at colleagues recently investigated the effects of PEG coatings on electrode impedance in acute in vitro and in vivo models. (244) Otto demonstrated that exposure of the unmodified electrode to bovine serum albumin in vitro, as well as expose to in vivo protein solutions (the brain), resulted in both resistive and capacitive changes to the electrode impedance. Further, by applying a high molecular weight PEG to the electrode, the increase in impedance both in vitro and in vivo was reduced. Otto’s study demonstrated that non-cellular components likely influence the performance of microelectrodes as well. Unfortunately, to the best of our knowledge, no type of inert coating has been shown to effectively reduce chronic device-associated inflammation in any other tissue or implant model over the extended periods of time that may be clinically relevant for BMI applications.
4.4. Topographical Control of Cell Phenotype
In contrast to the general failure of non-adhesive surfaces to improve the long-term biocompatibility of microelectrodes, active approaches that permit adhesion while controlling cell phenotype have shown considerable promise. One active approach that has received extensive study is the use of controlled surface topography. Specifically, topographical cues have been used to control the adhesion, migration, orientation and gene expression of a variety of cell types. (245, 246) Despite studies showing that nanostructured surfaces can have a positive influence towards controlling cell functions, the underlying mechanism(s) are not well understood. (247)
Building in part upon this research, Moxon and coworkers conducted studies to investigate the potential of controlled topography to improve microelectrode biocompatibility. (248–250) Specifically, Moxon et al. examined the impact of presenting roughened, porous silicon or ceramic surfaces, which were designed to better mimic the nanostructured and fibrous nature of the extracellular matrix. Implantation of nano-porous surfaces was found to induce less glial activation and to improve neuronal density at the microelectrode/tissue interface. However, Moxon and coworkers only examined the response out to one week post-implantation, and the lasting impact on the neuro-inflammatory response or recording is still unclear and deserves further study.
More recently, the VandeVord laboratory has also begun investigating the effects that nanopatterned substrates may have on astrocyte reactivity. (251) Utilizing nanofabrication techniques, Ereifej et al. created poly(methyl methacrylate) (PMMA) surfaces with various degrees of groove width (no grooves, 555 nm, or 277 nm). Cultured astrocytes were less responsive to the narrower 277 nm grooves, compared to other surfaces. This preliminary study further demonstrates the role of surface topography in manipulating glia cell reactivity. However, future studies will require characterization with microglia and macrophages, which are more likely to experience topographical cues presented by implanted microelectrodes.
While surface topography is very important for cell-material interactions, cells cannot adhere directly to synthetic materials. The natural extracellular matrix is composed of many fibular proteins. Surface topography (or architecture) alone does not facilitate all cell function. Typically, the presentation of biological motifs are equally as important, (252) and should be considered in combination with the above approaches.
Additionally, the above studies, which investigated surface topography, did not differentiate between the insulating substrate of the electrode and the conductive metal / polymer portions of functional microelectrodes. However, it appears to be important to probe how changes to the conducting electrical contact would affect the surface area of the contact, and thus the electrical impedance and recording performance. For example, studies with increased surface area of the recording site must critically evaluate if the source of improved impedance measurements are a result of reduced neuroinflammation or the increased surface area.
4.5. Incorporating Bioactive Materials
Over the last decade, another promising approach to control cell phenotype at the biotic/abiotic interface has been developed, which involves the decoration of microelectrodes with bioactive surface coatings. (226, 253–263) A broad-spectrum of bioactive materials has been immobilized on the implant surface to control the neuro-inflammatory response (Table 1). Bioactive materials have been shown to be at least temporarily successful in attenuating the neuro-inflammatory response to intracortical microelectrodes within the brain tissue. However, it is not clear if the temporary effect of most bioactive strategies is a result of biomolecule consumption (degradation or exhaustion of the coating), or an evolution of redundant biology overcoming the initial effect of the surface modification.
Table 1.
Non-Exhaustive List of Bioactive Surface Treatments for Intracortical Microelectrodes.
| Bioactive Agent | Coating Material | Electrode Type | Reference |
|---|---|---|---|
| Dexamethasone (DEX) | Poly(pyrrole) (PPy) | Gold-coated coverslip | (185) |
| PLGA nanoparticles embedded in alginate hydrogel | Silicon | (262) | |
| Nitrocellulose | Silicon | (166) | |
| PLGA nanofibers | Silicon | (264) | |
| Poly(propylene sulfide) nanoparticles embedded in poly(ethylene oxide) | Platinum | (265) | |
| Laminin (LN) | Dextran | Silicon, gold and polyimide | (266) |
| Poly(ethyleneimine) (PEI) or Chitosan (CH) | Silicon | (260) | |
| Poly(pyrrole) (PPy) | Gold-coated coverslip | (267) | |
| Poly(ethyleneimine) (PEI) | Silicon | (268) | |
| L1 | Poly(ethylene glycol) (PEG) | Silicon | (257) |
| Silicon dioxide modified using silane chemistry | Silicon | (256) | |
| Parylene-C | Tungsten | (269) | |
| α-MSH | Nitrocellulose | Silicon | (263) |
| Silicon modified using silane chemistry | Silicon | (255) | |
| Nerve Growth Factor (NGF) | Parylene-C | Silicon | (224) |
| Poly(ethylene dioxythiophene) (PEDOT) | Platinum | (270) | |
| Poly(pyrrole) (PPy) | Polystyrene | (271) | |
| Superoxide Dismutase (SOD) | Surface functionalization | Silicon | (272) |
Perhaps one of the simplest and most common biomaterials approaches is the passive adsorption or covalent immobilization of ECM components to promote “directed” cell attachment. As biomaterialists became interested in the device-mediated neuro-inflammatory limitations to intracortical microelectrodes, the attachment of ECM proteins and peptides onto the microelectrode surface were among the first methods reported. Extracellular matrix-based materials for neural interfacing applications have been recently reviewed by Chen and Allen. (273) Therefore, only select representative examples or new considerations will be discussed here.
Among the most important ECM proteins for neural applications is laminin (LN), an adhesive protein that plays crucial roles in cell migration, differentiation, and axonal pathfinding. (274) The two main peptide sequences from LN that are often targeted for biomaterial applications include Ile-Lys-Val-Ala-Val (IKVAV) and Tyr-Ile-Gly-Ser-Arg (YIGSR). In 1993, Massia and Hubble were among the first to report on receptor-specific cell spreading on surfaces covalently immobilized with YIGSR. (275) Twenty years later, Massia developed a surface grafting method that allows for the covalent immobilization of IKVAV on the surface of silicon, silicon oxide, gold and insulating polymers such as polyimide, all common components of intracortical microelectrodes. (266) This work highlights the specificity of LN peptides for supporting neuronal attachment, and reinvigorated the use of LN-derived strategies for intracortical microelectrode applications.
Likely based on Massia’s work, the Bellamkona group reported a series of publications utilizing LN-based coatings as surface modifications for intracortical microelectrodes applications. (260, 268) First, a layer-by-layer (LBL) assembly was used to build up a deposition of poly(ethyleneimine) (PEI) and LN on silicon wafers with an oxide layer. It was found that the PEI-LN layer was stable for at least 7 days under simulated physiological conditions, and significantly improved neuron adhesion and differentiation in vitro. The subsequent in vivo study revealed that PEI-LN coatings are able to reduce the counts of reactive microglia and astrocytic tissue response to Si-based electrodes after 4 weeks post-implantation. (268) However, LN coating elicited a more robust pro-inflammatory response at one day post-implantation than uncoated devices, as indicated by increased CD-68 positive microglia, GFAP astrocytes, and proinflammatory cytokine expression. Interestingly, neuron densities were statistically similar at all of the time points investigated, suggesting no advantage to the LN coating for recording applications.
In parallel to the LN work in the Bellamkonda lab, several laboratories have investigated the ability of doping conductive polymers (discussed further in Section 4.7), with LN-based peptides in order to increase neuronal attachment. For example, Stauffer and Cui investigated two different LN fragments, YIGSR and RNIAEIIKDI, as dopants in an electropolymerized poly(pyrrole) (PPy). The goal of the initial in vitro study was to combine the critical electrical properties of conducting polymers with the ability to promote specific-cell attachment (YIGSR) and neurite outgrowth (RNIAEIIKDI). (267) Stuaffer and Cui’s results confirm the cell-specific attachment and growth seen over the previous decades in many laboratories. The novelty of their work was that the combination of the two peptides on a conducting polymer scaffolding synergistically increased both neuronal attachment, and neurite outgrowth, while also demonstrating low impedance and increased charge capacity. Despite the promising in vitro results suggesting that LN-containing coatings may enhance the long-term recording stability of neural interfaces, no studies to date have described the in vivo recording performance of these materials.
In a second example of LN-derived peptide incorporation into conducting polymers, Green et al. doped PEDOT with DEDEDYFQRYLI and DCDPGYIGSR. (276) Interestingly, Green et al. demonstrated that large peptide dopants produced softer PEDOT films with a minimal decrease in electrochemical stability. However, despite the retained bioactivity of dopant peptides, the effects were largely dependent on initial cell attachment, and neither of the peptides investigated provided the bioactivity of the native LN protein. In a later study, Green et al. also examined the effect of entrapping nerve growth factor (NGF) within the PEDOT during electrodeposition. (277) The incorporation of NGF was shown to remain biologically active within the PEDOT. However, Green et al. also found that the use of both a LN peptide dopant and NGF in the PEDOT resulted in polymers with decreased mechanical and electrical properties compared with controls containing only NGF. (277)
Despite retained biological activity of the incorporated peptides, the combination of biological molecules within conducting polymers has thus far failed to provide the synergistic benefits that the field has anticipated. In fact, the Poole-Warren group recently provided a report of the performance of conducting polymer electrodes, without the incorporation of biological molecules. (278) Since isolated peptide sequences typically demonstrate enhanced activity of targeted functions compared to full protein controls, (279) it is likely that the immobilization methods employed within conducting polymer requires further optimization, perhaps insulating spacer groups to isolate the biomolecules from the polymers.
While the use of LN surface modification alone has not successfully improved the biocompatibility or recording quality of microelectrodes, there is increasing evidence that the presentation of other specific ECM molecules or networks of ECM components that mimic the complexity of natural brain tissue may be useful. For example, the Cui group has demonstrated encouraging work with the neural adhesion molecule L1. Azemi et al. demonstrated that neural electrode arrays coated with immobilized L1 showed enhanced levels of attachment of mouse cerebellum neurons in vitro. (257) Azemi et al. directly compared the efficacy of L1 with LN. The study showed that while the LN-functionalized surfaces greatly promoted the growth of astrocytes, the L1-functionalized surfaces showed significantly reduced astrocyte attachment compared to both LN-coated and uncoated control surfaces. (257) In a subsequent paper, (256) Azemi et al. investigated the neuro-inflammatory response to L1-functionalized Michigan-type microelectrodes implanted in a rat cortex for up to 8 weeks. The study revealed that L1-functionalized microelectrodes show significant reduction in reactive tissue gliosis when compared with uncoated electrodes. The most promising aspect of the L1 approach is the ability to maintain normal neuronal populations while also significantly increasing the density of neuronal filament at the interface (Figure 13). The Cui laboratory has more recently begun to explore the utility of L1-functionalized electrodes for peripheral nervous system applications. Unfortunately, to date, no description of the impact of L1 immobilization on recording quality for intracortical microelectrode applications has been reported, but deserves further attention due to the success of foundational studies.
Figure 13.
(a, b) Representative images of NeuN+ cells (green) around the NM (unmodified) and L1 (L1-peptide grafted) probes after 8 weeks of implantation in rat cortex. Below the set off images, the corresponding normalized cell count differences between L1 and NM probes for the 0–100 µm region away from the interface (*p < 0.05). (c, d) Representative images of neuronal filament (green) stained tissue after 8 weeks of implantation in rat cortex. Below the set off images, the corresponding normalized neuronal filament intensity level differences between L1 and NM probes for the 0–100 µm region away from the interface (*p < 0.05). Scale bar = 100 µm. Reprinted with permission form Azemi et al. Copyright © 2008 Elsevier.
While Azemi et al. showed the supremacy of L1 over LN, (256) other studies have also shown that presentation of single ECM components such as LN or L1 alone may not be sufficient to mitigate the neuro-inflammatory response. For example, Tanaka et. al. showed that microglia cultured on fixed (dead) astrocyte monolayers, even in the presence of serum, display a resting phenotype. (280, 281) Tanaka’s results indicate that cues presented by the astrocyte ECM are sufficient to regulate microglia activation. Interestingly, the impact of fixed astrocyte ECM was significantly more effective at reducing microglial activation than individual ECM components such as LN or fibronectin (FN).
Building, in part on the findings of Tanaka et al., Tresco and colleagues have developed approaches to harvest the extracellular matrix (ECM) produced by CNS cells, including astrocytes, cultured on sacrificial open-celled foam substrates. (282) Immunohistochemical and proteomic analysis with tandem mass spectroscopy revealed that the harvested material consisted of a complex network of ECM components including collagen, fibronectin, laminin and various glycosaminoglycans. In vitro cytocompatibility studies of the decellularized material have shown the material to be non-toxic and adhesive to various cell types. Tresco and colleagues are currently investigating the impact of astrocyte-derived ECM coatings on the neuro-inflammatory response in rodent models.
Utilizing complete, tissue-specific ECM may provide additional benefits to single protein approaches. Although ECM throughout the body shares common protein and glycosoaminoglycan building blocks, subtle differences indicate that the precise make-up of a tissues-specific ECM is vital in regenerative applications. (283) Several studies have shown that culturing cells on tissue-specific ECM improves infiltrating cell proliferation rates and increases the expression of desired phenotypic cell and tissue characteristics. (284–288) In contrast, implantation of non-tissue specific ECM induces the formation of undesired, phenotypically irregular tissue at the implantation site. (289, 290)
While bioactive approaches, based primarily on ECM proteins and peptides, have shown promise in improving the neuro-inflammatory response to intracortical microelectrodes, one limitation of these strategies is their short-lived nature. For example, inflammatory cells that unavoidably become activated in response to the initial iatrogenic trauma are known to phagocytize and remove adherent and even covalently immobilized proteins over time. Therefore, bioactive coatings should primarily be thought of and used as one component of a combinatorial strategy for improving microelectrode function and biocompatibility. Specifically, bioactive materials may serve as a key component to direct initial wound healing events and tissue integration following implantation, but are not as likely to be used to improve the long-term recording performance.
A further, and often overlooked, concern with protein-based coatings is their potential for immunogenicity. While the majority of proteins found in the ECM are well conserved between animals and humans, interspecies differences do exist. As a result the implantation of even decellularized, xenogenic ECM has been shown to elicit an adaptive immune response. (291–294) Therefore the use of autologous or allogenic materials may prove key to maximizing the clinical success of ECM-based coatings.
4.6. Antagonizing Pro-inflammatory and Cytotoxic Soluble Factors
4.6.1. Passive Permeability Sinks
Several strategies have been developed to antagonize the pro-inflammatory and cytotoxic effector molecules secreted by inflammatory cells at the microelectrode/tissue interface. The simplest form of antagonism is the use of passive diffusion to reduce the concentration of pro-inflammatory and cytotoxic factors in the adjacent tissue. For example, it has long been hypothesized that permeability of the adjacent tissue may influence the overall integration of biomedical devices by allowing better clearance of pro-inflammatory and cytotoxic molecules away from the device/tissue interface. (152) However new evidence indicates that the permeability of implants themselves may also be manipulated to reduce soluble factor concentration and facilitate improved wound healing. (295–299)
While not a microelectrode, one of the earliest descriptions using a passive permeability sink is work with semi-permeable hollow fiber membranes (HFMs) for cell delivery and encapsulation. Despite having a larger penetrating profile than any single tined microelectrode, HFMs elicit a very minimal neuro-inflammatory response. (152, 205, 299) While the roughened surface of the HFM wall may play a role in reducing the response. (248, 249) Tresco and colleagues also hypothesize that HFMs act as a permeability sink for pro-inflammatory and cytotoxic soluble factors. Specifically, the membrane’s semi-permeable wall structure and fluid filled lumen permit diffusion of reactive soluble factors into the device, and thus away from the surrounding brain tissue. Due to the short half-life of many pro-inflammatory and cytotoxic molecules, a large portion of the molecules are believed to be degraded within the HFM, without ever impacting the surrounding tissue.
To investigate whether a permeable sink strategy could be used to improve microelectrode biocompatibility, Tresco and colleagues have investigated the use of thick hydrogel coatings to reduce the neuro-inflammatory response. (137) For this strategy to be effective the “coating" or “sink volume" must be thick enough to passively entrap the soluble factors until they breakdown by hydrolysis and other passive degradation mechanisms. Finite element modeling showed that it is possible to retain pro-inflammatory cytokines in the diffusion sink to passively reduce their concentration and their subsequent impact on the biology of the surrounding tissue as long as their residence time in the sink exceeded their biological half-life. Given the appropriate size of the sink, cytokines diffusing into the semipermeable surface of the device would be unlikely to diffuse out of this region in their active state, and thus would be effectively silenced.
Subsequent in vivo studies showed that the incorporation of the thick permeable hydrogel significantly reduced a number of classic hallmarks of the neuro-inflammatory response compared to uncoated microelectrodes or those that received a thin hydrogel coating (surface chemistry control). Reduced hallmarks included macrophage recruitment and activation, astrogliosis, blood brain barrier dysfunction and neuronal cell loss.
It should be noted that better mechanical matching between the soft hydrogel and the surrounding brain tissue may contribute to the reduced neuro-inflammatory response seen around hydrogel coated microelectrodes described by Tresco and colleagues. However, a reduced inflammatory response to other stiff semipermeable devices suggests that mechanical matching is not the only factor involved. For example, work from Desai and colleagues have shown a similar reduction in the inflammatory response, as well as complement activation, to stiff silicon and metal membranes with a semipermeable membrane/lumen structure. (300, 301) Furthermore, the similarities in the inflammatory response to passive permeability sinks across a broad range of stiffness combined with the high moisture uptake of many of the polymers described in Section 4.1 could suggest a complimentary/alternative mode of action for many compliant materials.
Further studies aimed at better characterizing the response to a variety of design variables are needed due to the potential that well-established and controllable strategies for reducing the neuro-inflammatory response could have on intracortical microelectrode technology. Specifically, more in depth studies to isolate the individual and composite roles of mechanical mismatch, microelectrode architecture and device permeability are needed. To facilitate these types of advanced design studies, a number of new or improved methods are needed to quantify response variables, including both in vivo strain profiles and cytokine distributions surrounding implanted devices. In addition, novel test devices that better isolate or control individual design elements need to be created.
4.6.2. Active Antagonism
In contrast to the passive strategies explored by Tresco and colleagues, a number of groups have investigated active antagonism of pro-inflammatory and cytotoxic soluble factors to improve microelectrode technology. For example, the Bellamkonda group investigated alpha melanocyte-stimulating hormone (α-MSH) as an anti-inflammatory target molecule. α-MSH is an endogenous tridecapeptide with potent anti-inflammatory properties. Specifically, α-MSH acts through the inhibition of pro-inflammatory cytokines and neurotoxic nitric oxide (NO) production. (302) Zhong and Bellamkonda were the first to investigate the anti-inflammatory properties of the neuropeptide in the context of intracortical microelectrodes. They initially developed nitrocellulose-based coatings that were capable of locally delivering α-MSH from Michigan-type microelectrodes. (263) Zhong et al. found that α-MSH released over 21 days remained bioactive and successfully inhibited NO production by LPS-stimulated microglia, in vitro.
Subsequent work by He and Bellamkonda further demonstrated that the immobilization of α-MSH retained anti-inflammatory properties both in vitro and in vivo. (255) Specifically, when immobilized on the microelectrode surface, α-MSH again successfully inhibited NO and pro-inflammatory cytokine production by LPS-stimulated microglia in vitro. More importantly, immobilization of α-MSH on the surface of Michigan-type microelectrodes qualitatively reduced the detection of TNF-α mRNA one week post implantation in rat cortex, and quantitatively reduced the density of both CD-68 and GFAP positive microglia/macrophages and astrocytes, respectively. Unfortunately, He et al. did not report on the effects of α-MSH functionalized microelectrodes on the local neuron density or viability. (255) Furthermore, despite the promise of their short-term results, He and Bellamkonda have not pursued longer histological or functional studies with α-MSH.
As an alternative means of antagonizing the impact of pro-inflammatory soluble factors, Taub et al. recently reported on the use of interleukin receptor antagonist (IL-1Ra)-coated microelectrodes. (259) While IL-1Ra-coated microelectrodes demonstrated significantly reduced astrogliosis compared to non-coated microelectrodes, no other histology was provided, and it remains unclear how these materials affect neuronal viability or inflammatory cell activation.
Another active strategy for antagonizing pro-inflammatory and cytotoxic soluble factors has been the use of the anti-oxidant resveratrol. (69) While the use of resveratrol to date has been limited to systemic delivery, the overall approach and findings fit well with other active antagonist strategies and show exciting promise for improving microelectrode function and biocompatibility. Specifically, at two weeks post-implantation, Potter et al. found that animals receiving resveratrol therapy at the time of implantation demonstrated reduced blood-brain barrier instability, accompanied with increased neuronal density at the microelectrode-tissue interface (Figure 14). At four weeks post implantation, no difference was observed in neuronal density between resveratrol-receiving and control cohorts. The authors have suggested that the loss of impact on neuronal density is likely due to clearance or inactivation of resveratrol over time.
Figure 14.
Rats treated with Resveratrol showed increased neuronal densities at the electrode interface, no appreciable neurodegeneration, and a significant improvement in the stability of the blood-brain barrier 2 weeks post-implantation of Michigan-type silicon microelectrodes. Scale=100 µm. Modified from Potter et al. 2013.
While the findings from the resveratrol studies have not provided a long-term solution, they do support the use of active antagonism of pro-inflammatory and cytotoxic factors to improve microelectrode technology. Therefore, ongoing studies are currently investigating the impact of repeated dosing and local delivery of resveratrol, as well as other natural and synthetic antioxidants, that may prove safer and more effective than resveratrol itself. Additionally, in order to develop a system to provide sustained neuroprotection, Potter et al. also investigated modifying the microelectrode surface with an anti-oxidative coating. (272) For initial proof of concept, they chose the superoxide dismutase (SOD) mimetic Mn(III)tetrakis(4-benzoic acid)porphyrin (MnTBAP). Their system utilizes a composite coating of adsorbed and immobilized MnTBAP designed to provide an initial burst-release followed by sustained presentation of an immobilized layer of the antioxidant. Potter’s results indicate that the hybrid modified surfaces provide sustained anti-oxidative activity, and reduced the accumulation of reactive oxygen species both intra- and extracellularly.
4.6.3. Further Considerations Concerning Soluble Factor Antagonization
While comparing passive and active approaches to antagonize pro-inflammatory and cytotoxic soluble factors, it is important to consider the benefits, limitations and hurdles inherent to the two approaches. For example, passive approaches are inherently indiscriminant and will reduce the concentration of likely any soluble factor. This indiscriminate nature could make passive approaches desirable as they can impact the entire range of pro-inflammatory and cytotoxic soluble factors. Unfortunately, the indiscriminant nature of passive approaches also necessitates that beneficial, pro-healing factors will be impacted along with any negative factors. In contrast, active approaches can be tailored to specific targets. However, due to the overlapping impact of many pro-inflammatory soluble factors, singling out one or even a limited number of factors may not be effective.
Another limitation of many active approaches is their limited effective duration. There are currently few if any viable options for locally delivering soluble antagonists for the extended periods of time that may be clinically relevant for BMI applications. Systemic delivery over time may be effective at reducing neuro-inflammation to a tolerable level. However, the unwanted side effects associated with the drugs and supplements studied to date make continual systemic dosing unattractive. Passive approaches involving the use of permeability sinks, on the other hand, should persist for much greater periods of time, if not indefinitely, depending how they are implemented.
Due to the benefits and limitations of passive and active approaches, we believe that ultimately a composite approach will be most effective at enabling effective recording over clinically relevant time frames for BMI applications. Such an approach will utilize active strategies over the initial acute time frames to promote effective wound healing, while longeracting passive approaches such as increased substrate compliance, reduced surface area or incorporation of a permeability sink will be used to maintain a homeostasic environment throughout the implant duration. For other applications where microelectrodes will be implanted for shorter durations (weeks-months), composite strategies may not be needed and even systemic dosing of soluble antagonists or anti-inflammatory drugs may be sufficient.
4.7. Conducting Polymers
The inherent conductive properties of intrinsically conductive polymers make them a useful class of materials for a wide range of biomedical applications, such as biosensors, tissue engineering, neuroprosthetic electrodes, drug delivery, and actuators. (303, 304) Conducting polymers are particularly attractive for intracortical microelectrode applications because they have mechanical properties that lie between those of conventional metallic microelectrodes and the brain tissue, can provide high surface area and therewith facilitate an efficient ion exchange between recoding sites and the brain tissue, and can at least in principle, be processed into a broad range of geometries/structures/architectures. Charge transfer is improved through reduced impedance and greater selectivity for both recording and stimulating neural interfacing applications; although their intrinsic conductivity is lower than that of gold, platinum, or stainless steel electrodes.
The key feature of conducting polymers is conjugated double bonds along the backbone with a high degree of π-orbital overlap, results in electrically conductive materials. Conducting polymers with various morphologies can be directly deposited onto intracortical microelectrode surfaces. As a result, the conducting polymer coatings lower the impedance of the electrodes and can provide a mechanical buffer between the stiff intracortical microelectrode and the compliant brain tissue. Additionally, bioactive agents such as anti-inflammatory drugs and neurotrophic factors can be incorporated and delivered from these conducting polymer coatings. Several studies have shown that intracortical microelectrode functionality can be improved to some extent by coating the microelectrode surface with low-impedance conductive polymer with nanoscale roughness or porosity, (43, 305) or through addition of cell adhesion peptides, (305) proteins, (260, 261, 306) or anti-inflammatory drugs. (262, 263) Overall, in vivo studies have shown that these conducting coatings may enhance the chronic recording performance of intracortical microelectrodes. (43)
Among the currently available conducting polymers, poly(pyrrole) (PPy) and poly-(3,4-ethylene dioxythiophene) (PEDOT) (Figure 15) are the most studied conducting polymers for intracortical microelectrode applications. Such conducting polymers have been doped with various dopants such as poly(styrene sulfonate) (PSS), (305) perchlorate (ClO4−), (307) para-toluene sulfonate (pTS), (278) or sulphate (SO4) (308) to modify the surface of metallic intracortical microelectrodes. (309) In this section we discuss the development of conducting polymers used to modify the intracortical microelectrode surfaces with particular attention to the use of PPy and PEDOT.
Figure 15.
Chemical structures of poly(pyrrole) (PPy) and poly-(3,4-ethylene dioxythiophene) (PEDOT), examples of conducting polymers explored in neural interfaces.
Much of the initial research on conducting polymers for neural interfacing focused on PPy due to the ease of preparation, high conductivity, controllable surface properties, and the possibility to electropolymerize this polymer from water. In 2001, Martin and co-workers investigated the use of PPy as a surface coating for neural electrodes. (226) In his report, PPy was combined with a genetically engineered protein, designed to incorporate GAGAGS sequences of silk alternated with the cell-binding sequence RGD. The polymers were deposited electrochemically onto the silicon microelectrodes. The study showed that the PPy-coated Michigan-style microelectrodes had a higher surface area and charge density compared with uncoated electrodes, which facilitates charge transport, and more efficient neural communication. (305) In addition, it has been reported that a higher surface area significantly lowers the overall impedance of the intracortical microelectrodes. (310)
PSS has been used commonly as a dopant material for PPy due to its stability and in vitro compatibility with mammalian neuronal cells. (305, 311–313) Cui and Martin electrochemically deposited PPy doped with PSS on the neural electrodes, and found that the coated electrodes had an increased surfaces area, which resulted in a 30-fold decrease in impedance. (305) In 2005, George et al. reported on the biocompatibility of PPy-based cortical implants that had been doped with PSS or sodium dodecylbenzenesulfonate (NaDBS). (312) Immunohistochemical studies showed that PPy-based intracortical implants-doped either with PSS or NaDBS after 3 and 6 weeks implanted in a rat cerebral cortex had less gliosis than Teflon-coated microwire controls. However, the differences in gliosis at the 6-week time point had lessened compared to 3 weeks. George’s study also showed that incorporating neurotrophic molecules such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) into the PPy matrix promoted the ingrowth of neural tissue into the lumen of the PPy-based implants, compared to implants without growth factors. As noted in earlier sections, analysis at limited intermediate time points can lead to incomplete conclusions. Therefore, further works need to be done to demonstrate whether the bioactive molecules can enhance neuronal adhesion and interaction with conducting polymerbased intracortical implants.
The incorporation of conducting polymers within hydrogels that are used to coat conventional microelectrodes is another intriguing approach to better integrate intracortical microelectrodes with the neural tissue. Hydrogels are attractive due to their use in many biomedical device applications, their high water content which causes the mechanical properties to be similar to those of the brain tissue, and their porous network structure which can facilitate charge transport especially if conducting polymers are insulated. In one example, Michigan-style microelectrodes were first coated with cross-linked alginate and then PPy/PSS was subsequently electrochemically polymerized on the device surface. (90) PPy was observed to grow vertically form the electrode surface, and at the recording site recording site. It was found that the impedances of the porous hydrogel modified with conducting polymer films are around three orders of magnitude less than the impedance of the metal microelectrodes. The authors also found that the PPy/PSS-alginate-coated recording sites were capable to transporting charges as efficiently as conventional electrodes. Despite the growing number of studies being conducted with PPy for intracortical microelectrode applications, electrochemically made PPy has a poorly defined chemical structure in which there are a significant amount of α-β couplings. The presence of defective α-β couplings along the polymer backbone induce structural disorder, limits the electrochemical response, and is contributing significantly to polymer breakdown due to overoxidation. (314) With these limitations in mind, new and highly stable conducting materials must be found that can endure the long-term implantation lifetime as well as attack from biological agents present in the brain tissue.
To overcome the drawbacks of PPy, poly(3,4-ethylene dioxythiophene) (PEDOT) has recently been explored as an alternative to PPy for neural interfacing electrodes. Specifically, PEDOT is more stable to oxidation and more conductive than PPy. Unlike PPy, undesired α-β couplings and structural disorder are eliminated in PEDOT by “blocking” the 3- and 4-positions of the monomer by the attachment of ethylenedioxy groups (Figure 16). Early studies by Cui and Martin (315) explored the benefits of PEDOT as coating for neural microelectrodes. Cyclic voltammetry experiments demonstrated that PEDOT-coated electrodes were more stable than those coated with PPy. In addition, high quality acute neural signals were recorded with the PEDOT-coated Michigan-style microelectrodes in the cerebellum of guinea pig with higher signal amplitude than in reference experiments with un-coated microelectrodes with gold contacts. This is likely due to the deposition of conducting polymer (i.e. PEDOT), which decreases the impedance of the electrode (increase sensitivity). Cui and Martin’s findings are highly desirable for potential use of PEDOT as alternative conducting material for neural electrodes.
Figure 16.
Chemical structures of monomers used to fabricate PEDOT and PEDOT-MeOH.
After Cui and Martin initial report, several studies followed, which further explored the use of PEDOT as electrically conductive material in intracortical microelectrode applications. For example, Xiao et al. modified the surface of intracortical microelectrodes using PEDOT-MeOH that was electrochemically doped with poly(styrene sulfonate) (PSS). (316) In this study, Xiao et al. improved the limited processability (aqueous solubility) of PEDOT through the addition of an appropriate pendant side group onto the backbone. To this end, polar derivatives of EDOT, specifically EDOT-MeOH (the chemical structure of which has been shown in Figure 16) was used. Xiao et al. found that the PEDOT-MeOH coating decreased the impedance by almost two orders of magnitude in comparison to the uncoated Michigan-style microelectrode. Decrease in electrode impedance leads to improved charge transfer from the surrounding brain tissue to the intracortical microelectrode, which is argued to lead to more effective recording and stimulating. In another investigation, Yang et al. (317) electrochemically deposited surfactant-induced ordered PEDOT onto gold-coated Michigan-style microelectrodes. Although, this ordered PEDOT polymer coated-electrodes exhibited a lower impedance and a higher charge capacity than uncoated electrodes, it was found that the surfactant used in the preparation leached from the device when placed in a cell culture medium and killed all nearby cells in the culture.
More recent studies by Martin and co-workers have shown that neural cells can be incorporated into PEDOT, while still maintaining cell viability and signal transduction capabilities. As a result, functional hybrid PEDOT-neural cell electrode coatings were created that can be used as highly biomimetic conductive substrates for intracortical microelectrodes. Polymerization of PEDOT around living cells has been reported in vitro (318) as well as in vivo (319) through living tissue. In one study, Richardson-Burns et al. (318) reported the electrochemical polymerization of PEDOT in the presence of live neural cells that had been cultured on in-house fabricated Au/Pd sputter-coated electrodes and/or Applied Biophysics (Troy, NY) electrodes, as shown in Figure 17, resulting in the formation of PEDOT film around and onto adhered neural cells. Additionally, PEDOT, PEDOT/live neurons, and neuron-templated PEDOT coatings on the electrodes significantly enhanced the electrical properties and increased charge transfer capacity. While in vitro experiments show successful electropolymerization of conducting polymers around neural cells, it is critical to apply the concept in animal models to fully characterize the performance of the materials to more accurately understand the contribution of this strategy to the field.
Figure 17.
(a) Schematic of the electrochemical deposition cell and the neural cell monolayer cultured on the surface of the metal electrode prior to polymerization. (b) PEDOT polymerized around living cells. Reprinted with permission from Richardson-Burns. Copyright © 2007 Elsevier.
The first chronic, long-term neural recording studies of PEDOT-coated electrodes were conducted by Kipke and co-workers in 2006. (43, 320) It was found that chronically implanted control microelectrodes were unable to record well-isolated unit activity due to a dramatically increased noise floor, while PEDOT-coated Michigan-style microelectrodes consistently recorded neural activity, and showed a much lower noise floor than un-coated gold-based controls over a six-week period following implantation in three male rats. (320)
More recently, Kozai et al. took a further step towards long-lasting conjugated-polymerbased neural interfaces that elicit little tissue response in the brain, fabricating an integrated composite microelectrode consisting of electrically conductive carbon fiber core, a coating-based layer, and a PEDOT:PSS-based recording pad. (321) Specifically, electrodes were fabricated by mounting carbon fiber, which serves as the conducting core and provides the mechanical backbone of the device, with a diameter of 7 µm onto a NeuroNexus microelectrode. The carbon fibers were then coated with a 800 nm thin poly(p-xylylene) layer (Figure 18a), and subsequently a 50 nm thick layer of poly((p-xylylene-4-methyl-2-bromoisobutyrate)-co-(p-xylylene)), both with a chemical vapor deposition (CVD) process (Figure 18b). The later polymer provides initiator groups for a subsequent atom transfer radical polymerization (ATRP). This was used to apply a ~200 nm thin layer of poly(ethylene glycol) methacrylate (PEGMA) (Figure 18c). Finally, a carbon recording site was exposed at the tip of the neural stainless-steel wire by cutting away the insulation, and the recording site was coated with a layer of PEDOT:PSS that was applied by electrochemical deposition (Figure 18d). Chronic neural recordings from the inserted implants into the motor cortex of rats showed that the resulting microelectrodes provided stable single-neuron recording over five weeks in the brain. Interestingly, the electrodes also showed reduced neuro-inflammation response compared with traditional silicon electrodes. Thus far, these electrodes with mechanically-compliant coatings are the smallest implantable neural electrodes that were able to record neuronal activity in animals.
Figure 18.
(a–d) Schematic representation of the fabrication of microtherad electrodes, and (e) SEM images of a fully assembled, functional electrode. Reprinted with permission from Kozia et al.. Copyright © 2012 Nature Publishing Group.
Conducting polymers aim to enhance the chronic performance of intracortical microelectrodes through providing a high surface area, and more conductive materials. Also, the charge transfer is likely improved through reduced impedance, thereby providing greater selectivity for both recording and stimulating neural applications. Despite the vast amount of research being produced in recent years on the conducting polymers for neural interfacing applications, the field is still growing and many challenges, limitations and questions remain to be answered. One key challenge of conducting polymers in neural interfacing applications is the preservation of the conductivity or electrochemical stability over long periods of time. For example, PPy and PEDOT films have been shown to lose up to 95% and 30% of their conductivity when subjected to 16 h of polarisation, respectively. (322) Also, conducting polymers are often brittle and the addition of dopants to the system in many cases exacerbates this effect. (309) Therefore, developing conducting polymers that are less brittle and more malleable while maintaining the conductivity and avoid delamination over time is required. Moreover, limited studies on the mechanical properties of conducting polymers for the duration of implant lifetime suggest that further research is needed to assess the durability of a coated intracortical microelectrode in animal models. This will provide an insight into possible delamination and/or mechanical erosion of conducting polymers. Another major drawback of conducting polymer systems is the diffusion of the employed dopants, unreacted monomers and typical process contaminations (e.g. solvents) into the medium, which all are slightly to moderately toxic. Unfortunately, most of the studies that have revealed a positive biological performance have been conducted in vitro. We believe that the cytotoxicity of released dopants is likely a limiting factor to the use of in-situ polymerization in vivo, despite the success in vitro – at least with the chemistries commonly explored. Thus, toxicity testing needs to be assessed using in vivo implantation studies to answer whether conducting polymers are useful for long-term neural implants. Today, most/all of the studies have relied on electropolymerization of conducting polymers on conductive electrodes (e.g. Au). In fact, the substrate to be coated needs to be conductive. So, the conducting polymer is really a mediator between the brain tissue and the conducting intracortical microelectrodes. Therefore, it is also of particular interest to look at printed polymer-based conductive microelectrodes for future neural interfacing applications. Few studies have investigated how conducting polymers tolerate sterilization. Given the correlation between sterilization method and the inflammatory response to microelectrodes discussed above, (160) and the fact that not all formulations of PEDOT have the same durability, (323) the translation of any conducting polymer technology to long-term stable in vivo applications remains unclear. Finally, it is clear that long-term in vivo studies in this area of research is required for a better understanding of the impact of conducting polymers in improving long-term performance of intracortical microelectrodes include electrochemical stability, delamination, mechanical integrity, the maintenance of an intimate contact between the electrode and surrounding neural tissue, and the ability to remove the implants without causing significant damage to the surrounding tissue.
4.8. Nanomaterials
Nanomaterials have been explored in a variety of biomedical applications due to their unique properties arising from their nanoscale dimensions. (324–326) Nanomaterials can be useful in cortical interfacing applications for several reasons. (327, 328) Perhaps most importantly, nanomaterials can interact with biological systems with a high degree of specificity, are able to stimulate and interact with target cells with minimizing undesirable effects, and the electronic properties of nanostructures can be tailored to match the needs associated with charge transport required for electrical/ionic level cellular interfacing.
Charge transfer reactions involving the exchange of charge between various carriers occur at the electrode–polymer and polymer–tissue interfaces. (329) In particular, it has been established that increasing the effective surface area of the interface will increase the ability for charge transfer to occur. Also, the electrical impedance of the microelectrode is inversely proportional to the surface area of the recording site. (310) As a result, the intrinsically large surface area of nanomaterials results in high charge transfer as well as lower overall impedance of the intracortical microelectrode.
Additionally, nanoscience approaches can present subcellular stimuli that can vary from one part of the neuron to another. (330) For example, a combination of photolithography and layer-bylayer (LbL) self-assembly have been used to pattern secreted phospholipase A2 (sPLA2), which promotes neuronal adhesion, on a non-fouling background of poly(diallyldimethylammoniumchloride) (PDDA). (331) The approach used by Mohammed et al. facilitates nanoscale patterning with complex functional architectures that are tailored to the needs of a particular experiment. LbL self-assembly has also been used by Ai et al. on silicon rubber to pattern alternating laminin and poly-D-lysine or fibronectin/poly-D-lysine ultrathin layers which supported neurite outgrowth of cerebellar neurons. (332) Taken together, the studies by Mohammad et al. and Ai et al. suggest that bioactive ultrathin coatings could be used to promote cell adhesion and limit immune responses, and may facilitate improved performance of intracortical microelectrodes.
Additionally, several types of nanomaterials have been utilized in neural interface devices, including carbon nanotubes, carbon nanofibers, and graphene. The use of nanomaterials and nanotools for neuroscience has been recently reviewed. (327, 333) In this Section, we therefore limit the discussion to the current state of the most widely investigated nanomaterials in the content of neural interfacing applications.
Carbon nanotubes (CNTs) are perhaps the most widely studied class of nanomaterials for intracortical microelectrodes, (327, 334, 335) in view of their extraordinary strength, toughness, electrical conductivity, and surface area. As with the exploration of any new application for potential biomaterials, early work with CNTs towards intracortical microelectrodes began with in vitro applications. For example, Mattson et al. reported that multi-walled carbon nanotubes (MWCNTs) could be used as platform for neuronal growth. (336) Since then, several studies have been devoted to evaluate neuronal growth on CNTs. (337–341) Wang et al. showed the first in vitro stimulation of primary neurons with CNT-based electrodes. (342) The authors found that neurons can grow and differentiate on the microelectrode, and more importantly, that the neurons can be repeatedly stimulated with CNT electrodes. In parallel, several other groups confirmed the possibility to stimulate the neural cells via single-walled and/or multi-walled carbon nanotubes in cultured brain circuits. (337, 343, 344) In 2008, Keefer et al. reported that CNT-coated metal microelectrodes improved both the recording and electrical stimulation of neurons in culture, and in vivo in rats and monkeys. (345) In vivo recording studies of CNT-coated microelectrodes were conducted in the rat motor cortex and monkey visual cortex. It was found that CNTs-coated microelectrodes had increased the neuronal recording sensitivity as well as decreased neuronal noise compared to un-coated tungsten microelectrode controls. It was possible to record local field potential (LFPs), multiple unit activity, as well as neuronal spiking simultaneously with one CNT-coated microelectrode in vivo. Additionally, Keefer et al. found that the combination of CNTs and the conducting polymer PPy increased the charge transfer beyond that seen with CNTs alone. Further, CNT/PPy-coated microelectrodes had a significantly lower impedance (higher electrode neuronal sensitivity) than bare conventional tungsten and stainless steel microelectrodes. In a similar study, Luo et al. also reported that PEDOT/CNT-coated Pt microelectrodes showed a much lower impedance than the bare Pt electrodes as characterized by electrochemical impedance spectrum (EIS) in PBS. (346) Electrochemical impedance spectroscopy was used to show that the PEDOT/CNTs decreased the impedance of Pt microelectrodes with increasing coating thickness due to the increase of the electroactive surface area, which results in a bigger capacitance. The PEDOT/CNT-coated electrodes exhibited higher charge injection than traditional electrodes and in vitro tests with neurons showed that the neurons attached tightly to the PEDOT/CNT surface and exhibited long neurite extensions, suggesting that PEDOT/CNT-coatings are non-neurotoxic and support the growth of neurons. Similar conducting polymer/CNT composite coatings were also studied by several other groups. (347, 348) Lu et al. reported that co-deposited PPy/SWCNT coatings significantly reduce the impedance of platinum/tungsten microelectrodes. Additionally, the brain tissue response of PPy/SWCNT coated microelectrodes and un-coated Pt microelectrodes were studied with immunochemistry after a 6-week implantation in the cortex of rats. Quantitative analysis of glial fibrillary acidic protein (GFAP) expression and neuronal nuclei (NeuN) showed significantly lower GFAP and higher NeuN counts for the PPy/SWCNT coated microelectrodes within the first 100 µm from the implant/tissue interface. (348)
Despite the perspective advantages of CNTs for intracortical microelectrode applications, the studied devices are rigid (non-compliant) and may not be optimally suited for chronic neural in vivo applications. As a result, there is an increased attention towards the development of flexible CNTs-based intracortical microelectrodes by combination of flexible polymeric substrates and CNTs to overcome both the mechanical failure of more brittle CNTs-based devices, and devicemediated tissue strain. Flexible CNT-based neural electrodes were created by the combination of flexible polymeric substrates and CNT-based electrodes. Lin et al. were the first to fabricate a flexible CNT-based electrode array for neural recording applications. (349) In this work, the CNT electrodes were partially embedded into a flexible Parylene-C film using a microfabrication process based on four steps: CNT growth, polymer binding, flexible film transfer, and partial isolation. The flexible CNT electrodes produced were used to successfully record the spontaneous spikes from a crayfish nerve cord.
Alternatively, the direct growth of CNTs on flexible polyimide substrates has been reported by Hsu et al. (350) Hsu et al. utilized UV-ozone exposure as a simple and low-cost route to improve the interfacial properties between the CNT electrodes. In culture, UV-ozone treated CNTs electrodes promoted neuron and neurite growth in close contact with CNTs, suggesting the biocompatibility of modified CNTs for neuronal growth. (350) In a subsequent study, spontaneous spikes were recorded from a crayfish with a signal-to-noise (SNR) ratio of 6.2. The flexible CNT electrodes were also used to record the electrocorticography (i.e. placing the electrode in the subdural region) of a rat motor cortex with a SNR of 8.7. (351) More examples of CNT-based flexible electrodes for neural recording and simulating applications have been developed by Hanein et al, (335, 352, 353) who transferred single-walled carbon nanotubes (SWCNTs) onto a flexible PDMS substrate. Recent evoked electrical activity recording studies with chick retinas demonstrated the device capability for high efficacy neuronal recording applications. (335)
Carbon nanofibers (CNFs), which consist of multi-walled graphene structures stacked on top of each other have also been explored as substrates for neural interfacing applications. Researchers at NASA Ames Research Center developed forest-like vertically aligned CNFs on a Si wafer by plasma enhanced chemical vapour deposition (PECVD). (354) After the CNF film was submerged in a liquid and dried, the CNFs irreversibly stuck together to form microbundles. Stable 3D “fuzzy” films were created by electrochemically coating the CNFs with a thin layer of electrically conducting polypyrrole (PPy). It was found that the impedance of this kind of electrode decreased significantly compared to common metal electrodes due to the large surface area of 3D nanostructured CNFs, which results in high ion mobility. Furthermore, the PPy coating was shown to improve the biocompatibility of CNF-based electrodes compared to uncoated, and further reduces the electrode impedance by more than 20 times due to the redox potentials of the polymer. A subsequent study by the same group showed that an intimate neural-electrical interface can be formed between the vertically aligned CNFs and a neuronal network of PC12 cells. (355) The addition of neuronal growth factor (NGF) on the vertically aligned CNFs facilitated the formation of well-differentiated cells with mature neurites. Although, the freestanding CNFs coated with PPy and NGF were mechanically rigid to maintain their vertical alignment, they were found to be flexible enough to bend toward the cell body when driven by traction forces of the cells, thereby facilitating cell adhesion. Thus, it was suggested that the soft PPy coating not only improved the mechanical stability by forming a core-shell structure with the CNFs, but also promotes a better mechanical contact with neuronal cells due to a reduction of the local mechanical stresses. (355)
Several studies suggest that graphene exhibits excellent biocompatibility, low cytotoxicity and supports neuronal growth. (356–358) Therefore, graphene is another carbon nanomaterial that has been recently utilized in neural interface application. Chen et al. (359) reported the fabrication of graphene-based neural microelectrodes, while Luo et al. (360) reported graphene oxide-based conducting polymer nanocomposites for potential neural interfacing applications. Chen’s study showed that graphene-based microelectrodes are capable of recording neural signals in a crayfish. These graphene electrodes showed biocompatibility and non-toxicity throughout the 16 day cell culture experiment with neuronal cells. In the second investigation, Luo et al. electrochemically doped PEDOT with graphene oxide (GO) and demonstrated that conducting PEDOT/GO nanocomposites supported the growth of neural cells with minimal toxicity along with low electrochemical impedance. (360) To clearly demonstrate the potential of this class of grapheme-based materials for neural microelectrodes, future studies need to investigate the chronic in vivo performance defined by both the ability to maintain a clinical viable signal quality and stimulation capabilities, as well as the biotic and abiotic failure modes (defined above). Alternatively, the Martin group designed multifunctional nanobiomaterials that can be used for coating neural microelectrodes. (264, 361) These materials significantly decrease the electrode impedance and increase the charge density. While in vivo data are in process, and to our knowledge yet to be reported, the approach nicely demonstrates how several attractive concepts can be merged to create “smart nanobiomaterials” that are soft, have a low impedance, high charge density, and also can deliver anti-inflammatory drugs to alleviate the brain immune response to neural interfaces.
Together with the Kipke group, Martin’s group also reported the use of conducting polymer nanotubes for chronic neural interfaces. (362) This work showed that PEDOT nanotube-coated electrodes have a markedly lower impedance over a 7-week period than conventional Michigan-style microelectrodes. Figure 19 shows the fabrication process of such PEDOT nanotubes on the surface of Michigan-style microelectrodes. Martin and Kipke later investigated the effect of nanotube morphology on the properties of the electrodes in vitro. (363) Their work demonstrated that the PEDOT nanotubes decreased the impedance of the electrode site by about two orders of magnitude, and increased the capacity of charge density by about three orders of magnitude compared to bare iridium microelectrodes. (363) The team further showed that the mechanical properties of the conducting polymer can be tuned by their surface morphology. For instance, it was demonstrated that PPy and PEDOT nanotubes can adhere better to the surface of the neural electrodes than films of these polymers. Despite the many incredibly attractive materials properties that nanomaterials have demonstrated towards long-term neural interfaces, several of the devices created from these materials in their current form still suffer from limitation that have chronically plagued neural electrodes (degradation and delamination). Therefore, the combination approaches, such as thus being developed by Martin and colleagues, represent the starting point towards integrating nanomaterials into intracortical microelectrodes for neural interfacing applications.
Figure 19.
Schematic illustration of conducting polymer (PEDOT) nanotube fabrication on neural microelectrodes: (a, b) Electrospining of polylactic acid (PLLA) nanofibers. (c) Electrochemical deposition of PEDOT. (d) Dissolution of the PLLA core. (e, f) Optical microscopy images of the entire microelectrode (e) and single electrode site (f) before surface modification. (g, h) Optical microscopy images of the entire microelectrode (g) and single electrode site (h) after electrospining of PLLA nanofibers. (i, j) Optical microscopy images of the entire microelectrode (i) and single electrode site (j) after electrochemical deposition of PEDOT and remove of the PLLA core. Reprinted with permission from Abidian et al. Copyright © 2009 Wiley-VCH.
Recent research activities focused on use of nanomaterials in the domain of neural interfacing applications has contributed significantly to our understanding of the development of better biocompatible intracortical microelectrodes. The application of nanomaterials in chronic neural interfaces is still in its infancy despite an impressive body of research that is emerging, partly because of the complexities associated with interacting with neural cells and the mammalian nervous system. Moreover, there are still fundamental gaps of knowledge regarding the potential toxicity of nanomaterials within the brain that need to be addressed. Additionally, like other devices discussed above, it remains unclear how functional devices of this nature can be removed from patients without damaging surrounding tissue, or breaking the device off in the cortical tissue, due to their size and brittleness. We believe that there are still tremendous opportunities for nanomaterials to contribute to neural interfacing devices to generate intracortical microelectrodes that can enhance, and improve the current technologies. Thus, herein, we present a brief outline of the most important future areas of nanomaterials in the chronic neural interfacing applications that need to be investigated:
Integration of traditional approaches and nanomaterials to design new types of intracortical microelectrodes.
Development of new types of nanostructured coatings offered by nanomaterials that could also increase the charge injection capacity and reduction of impedance.
Incorporation of anti-inflammatory agents in the coatings using layer-by-layer self-assembly technique.
Miniaturization of the traditional intracortical microelectrodes using smaller and more compliant flexible nanomaterials, while maintaining efficient electrochemical function.
Improvement of electrical and biological properties of the neural tissue-electrode interface.
Design of easily manufacturable, highly conductive, and mechanically strong and flexible intracortical electrodes using nanomaterials, and conductive and adaptive polymers.
Investigation the chronic long-term potential toxicity profiles as well as delamination and degradation of nanomaterials over the period of implantation.
Comprehensive long-term recording and stimulation studies of nanomaterials in animal models.
Finally, these exciting avenues must be tempered with the realization that the toxicity of nanomaterials is still a developing field, and that a better understanding of how nanoscale materials interact with the central nervous system is required before one can use nanomaterials widely in neural interfacing applications.
5. CONCLUSIONS
Undoubtedly, the recordings of consciously modulated neuronal signals using intracortical microelectrodes have advanced our fundamental understanding of brain function in both normal and diseased states. In addition, chronic microelectrode recordings provide a way for paralyzed or “locked in” individuals to directly control various assistive devices through brain machine interfacing. Unfortunately, the implementation of brain machine interfacing applications has been severely hindered due to inconsistent recording quality and premature electrode failure.
Poor recording quality and microelectrode failure are likely a function of an array of variables resulting from both biotic and abiotic factors. The high degree of complexity makes improving microelectrode performance a challenging problem. While a number of failure modes have been identified, a more in-depth mechanistic understanding is needed.
A number of strategies have been studied to improve microelectrode performance. Such strategies include those to improve the stability of conducting and insulating materials as well as those to improve microelectrode integration with the surrounding tissue. As neuro-inflammation has been shown to play a significant role in each of the major microelectrode failure modes, specific emphasis should be given to reduce the reactive environment that results following microelectrode implantation.
Due to the variety of failure modes and the large degree of interplay involved, it is increasingly evident that a combination of strategies may be necessary to enable consistent, high quality recording over a clinically relevant time frame. To optimally implement and integrate such strategies it will be necessary to further identify the most potent strategies for improving microelectrode performance. It will also be useful to isolate possible interactions or emergent phenomena to elucidate the overall design space available to engineers. Most importantly, we must all consider that differences in microelectrode design may be a major contributing source for discrepancies between the dominant failure mechanisms reported for traditional designs. Each class of microelectrode presents with a different primary failure mode, and time course for failure. Therefore, one approach will most likely not provide a global solution to all electrode types or to all recording applications.
It is also important to note that despite the vast amount of research being developed in the last decade on polymeric materials for neural interfacing applications, in most cases, researchers have simply used polymers developed for different purposes, with the aim to re-use them for neural microelectrodes. Although the development of such materials-based intracortical microelectrodes has contributed significantly to our understanding of how improve device integration and increase recording consistency, designing novel materials, strategies and concepts for the specific purposes of this field must be accelerated.
Despite the challenges and questions that remain, we should be encouraged by the exciting possibilities that will be opened by further developing our understanding of the mechanisms that influence microelectrode performance. Furthermore, due to the exciting advances in the fields of material science, neural engineering and bioengineering, we should foster dynamic multidisciplinary teams, in order to accumulate the skills and knowledge to design, test, and integrate the next generation intracortical microelectrodes, capable of long-term clinical deployment for neuro-rehabilitative applications, and beyond.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from Adolphe Merkle Foundation, the Swiss National Science Foundation (Weder, NRP 62: Smart Materials, Nr. 406240_126046), the Department of Veterans Affairs (Capadona, Rehabilitation Research and Development: B7122R and B1495-R), Presidential Early Career Award for Scientist and Engineers (Capadona, Rehabilitation Research and Development: PECASE), and the Nation Institute of Health (Capadona, National Institute of Neurological Disorders and Stroke, 1R01NS082404-01A1). We also thank M. Ravikumar, K. Potter-Baker, and J. Nguyen for assistance in figure generation. None of the funding sources aided in the collection, analysis and interpretation of data, in the writing of the review, or in the decision to submit the paper for publication.
Footnotes
The authors have no conflicts of interest related to this work to disclose.
REFERENCES
- 1.Grundfest HCB. Origin, conduction and termination of impulses in the dorsal spinocerebellar tract of cats. J Neurophysiol. 1942;5(4):275–294. [Google Scholar]
- 2.Grundfest HSR, Oettinger WH, Gurry RW. stainless steel micro-needle electrodes mae by electrolytic pointing. Rev Sci Instrum. 1950;21(4):360–362. [Google Scholar]
- 3.Renshaw BFA, Morison BR. Activity of isocortex and hippocampus: electrical studies with micro-electrodes. J Neurophysiol. 1940 Jan 1;3(1):74–105. 1940. [Google Scholar]
- 4.Pancrazio JJ, Peckham PH. Neuroprosthetic devices: how far are we from recovering movement in paralyzed patients? Expert Review of Neurotherapeutics. 2009;9(4):427–430. doi: 10.1586/ern.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolelis MAL. Brain–machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012 May 17;485(7398):372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul 13;442(7099):164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998 Jun 1;9(8):1707–1711. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy PR, Bakay RA, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000;8(2):198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy PR, Kirby MT, Moore MM, King B, Mallory A. Computer control using human intracortical local field potentials. Ieee T Neur Sys Reh. 2004 Sep;12(3):339–344. doi: 10.1109/TNSRE.2004.834629. [DOI] [PubMed] [Google Scholar]
- 11.Kellis S, Miller K, Thomson K, Brown R, House P, Greger B. Classification of spoken words using surface local field potentials. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:3827–3830. doi: 10.1109/IEMBS.2010.5627682. [DOI] [PubMed] [Google Scholar]
- 12.Kellis S, Miller K, Thomson K, Brown R, House P, Greger B. Decoding spoken words using local field potentials recorded from the cortical surface. J Neural Eng. 2010 Oct;7(5):056007. doi: 10.1088/1741-2560/7/5/056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006 Sep;29(9):536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Ward MP, Rajdev P, Ellison C, Irazoqui PP. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 2009;1282:183–200. doi: 10.1016/j.brainres.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Tresco PA, Winslow BD. The challenge of integrating devices into the central nervous system. Crit Rev Biomed Eng. 2011;39(1):29–44. doi: 10.1615/critrevbiomedeng.v39.i1.30. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, McCreery DB, Bullara LA, Agnew WF. Evaluation of the stability of intracortical microelectrode arrays. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2006 Mar;14(1):91–100. doi: 10.1109/TNSRE.2006.870495. [Research Support, N.I.H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, McCreery DB, Carter RR, Bullara LA, Yuen TG, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999 Sep;7(3):315–326. doi: 10.1109/86.788468. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods. 2008 Mar 30;169(1):141–157. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson DA. The electrical properties of metal microelectrodes. Proceedings of the IEEE. 1968;56(6):1065–1071. [Google Scholar]
- 20.Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 21.Tillery SIH, Taylor DM. Signal acquisition and analysis for cortical control of neuroprosthetics. Curr Opin Neurobiol. 2004 Dec;14(6):758–762. doi: 10.1016/j.conb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002 Jun 7;296(5574):1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 23.Gilja V, Chestek CA, Diester I, Henderson JM, Deisseroth K, Shenoy KV. Challenges and opportunities for next-generation intracortically based neural prostheses. IEEE transactions on bio-medical engineering. 2011 Jul;58(7):1891–1899. doi: 10.1109/TBME.2011.2107553. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donoghue J. Bridging the Brain to the World: A Perspective on Neural Interface Systems. Neuron. 2008;60(3):511–521. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Hubel DH. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- 26.Wolbarsht ML, Macnichol EF, Jr, Wagner HG. Glass Insulated Platinum Microelectrode. Science. 1960 Nov 4;132(3436):1309–1310. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]
- 27.Evarts EV. Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol. 1966 Nov;29(6):1011–1027. doi: 10.1152/jn.1966.29.6.1011. [DOI] [PubMed] [Google Scholar]
- 28.Salcman M, Bak MJ. Design, fabrication, and in vivo behavior of chronic recording intracortical microelectrodes. IEEE Trans Biomed Eng. 1973 Jul;20(4):253–260. doi: 10.1109/TBME.1973.324189. [DOI] [PubMed] [Google Scholar]
- 29.Salcman M, Bak MJ. A new chronic recording intracortical microelectrode. Med Biol Eng. 1976 Jan;14(1):42–50. doi: 10.1007/BF02477088. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt EM, Bak MJ, McIntosh JS. Long-term chronic recording from cortical neurons. Exp Neurol. 1976 Sep;52(3):496–506. doi: 10.1016/0014-4886(76)90220-x. [DOI] [PubMed] [Google Scholar]
- 31.Burns BD, Stean JP, Webb AC. Recording for several days from single cortical neurons in completely unrestrained cats. Electroencephalogr Clin Neurophysiol. 1974 Mar;36(3):314–318. doi: 10.1016/0013-4694(74)90175-8. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt EM, McIntosh JS, Bak MJ. Long-term implants of Parylene-C coated microelectrodes. Med Biol Eng Comput. 1988 Jan;26(1):96–101. doi: 10.1007/BF02441836. [DOI] [PubMed] [Google Scholar]
- 33.Kruger J, Caruana F, Volta RD, Rizzolatti G. Seven years of recording from monkey cortex with a chronically implanted multiple microelectrode. Front Neuroengineering. 2010;3:6. doi: 10.3389/fneng.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9(5):056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 35.Prasad A, Xue QS, Dieme R, Sankar V, Mayrand RC, Nishida T, Streit WJ, Sanchez JC. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front Neuroeng. 2014;7:2. doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise KD, Angell JB, Starr A. An integrated-circuit approach to extracellular microelectrodes. IEEE Trans Biomed Eng. 1970 Jul;17(3):238–247. doi: 10.1109/tbme.1970.4502738. [DOI] [PubMed] [Google Scholar]
- 37.Wise KD, Angell JB. A low-capacitance multielectrode probe for use in extracellular neurophysiology. IEEE Trans Biomed Eng. 1975 May;22(3):212–219. doi: 10.1109/tbme.1975.324562. [DOI] [PubMed] [Google Scholar]
- 38.BeMent SL, Wise KD, Anderson DJ, Najafi K, Drake KL. Solid-state electrodes for multichannel multiplexed intracortical neuronal recording. IEEE Trans Biomed Eng. 1986 Feb;33(2):230–241. doi: 10.1109/TBME.1986.325895. [DOI] [PubMed] [Google Scholar]
- 39.Wise KD. Silicon microsystems for neuroscience and neural prostheses. IEEE Eng Med Biol Mag. 2005 Sep-Oct;24(5):22–29. doi: 10.1109/memb.2005.1511497. [DOI] [PubMed] [Google Scholar]
- 40.Hetke JF, Lund JL, Najafi K, Wise KD, Anderson DJ. Silicon ribbon cables for chronically implantable microelectrode arrays. IEEE Trans Biomed Eng. 1994 Apr;41(4):314–321. doi: 10.1109/10.284959. [DOI] [PubMed] [Google Scholar]
- 41.Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003 Jun;11(2):151–155. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 42.Vetter RJ, Williams JC, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng. 2004;51(6):896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3(1):59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 44.Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans Biomed Eng. 1991 Aug;38(8):758–768. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- 45.Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Mater. 2010 Feb;5(1):15007. doi: 10.1088/1748-6041/5/1/015007. [DOI] [PubMed] [Google Scholar]
- 46.Hsu JM, Rieth L, Normann RA, Tathireddy P, Solzbacher F. Encapsulation of an integrated neural interface device with Parylene C. IEEE Trans Biomed Eng. 2009 Jan;56(1):23–29. doi: 10.1109/TBME.2008.2002155. [DOI] [PubMed] [Google Scholar]
- 47.Bhandari R, Negi S, Solzbacher F. Wafer-scale fabrication of penetrating neural microelectrode arrays. Biomed Microdevices. 2010 Oct;12(5):797–807. doi: 10.1007/s10544-010-9434-1. [DOI] [PubMed] [Google Scholar]
- 48.Branner ASR, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol. 2001;85(4):1585–1594. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- 49.Bhandari R, Negi S, Rieth L, Normann RA, Solzbacher F. A Novel Method of Fabricating Convoluted Shaped Electrode Arrays for Neural and Retinal Prostheses. Sens Actuators A Phys. 2008;145–146(1–2):123–130. doi: 10.1016/j.sna.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhandari R, Negi S, Rieth L, Solzbacher F. A Wafer-Scale Etching Technique for High Aspect Ratio Implantable MEMS Structures. Sens Actuators A Phys. 2010 Jul 1;162(1):130–136. doi: 10.1016/j.sna.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wark HAC, Sharma R, Mathews KS, Fernandez E, Yoo J, Christensen B, Tresco P, Rieth L, Solzbacher F, Normann RA, Tathireddy P. A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J Neural Eng. 2013;10(4) doi: 10.1088/1741-2560/10/4/045003. [DOI] [PubMed] [Google Scholar]
- 52.Harrison RR, Kier RJ, Chestek CA, Gilja V, Nuyujukian P, Ryu S, Greger B, Solzbacher F, Shenoy KV. Wireless neural recording with single low-power integrated circuit. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2009 Aug;17(4):322–329. doi: 10.1109/TNSRE.2009.2023298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Bhandari R, Klein M, Negi S, Rieth L, Tathireddy P, Toepper M, Oppermann H, Solzbacher F. Integrated wireless neural interface based on the Utah electrode array. Biomed Microdevices. 2009 Apr;11(2):453–466. doi: 10.1007/s10544-008-9251-y. [DOI] [PubMed] [Google Scholar]
- 54.Abaya TV, Diwekar M, Blair S, Tathireddy P, Rieth L, Clark GA, Solzbacher F. Characterization of a 3D optrode array for infrared neural stimulation. Biomed Opt Express. 2012 Sep 1;3(9):2200–2219. doi: 10.1364/BOE.3.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warren DJ, Fernandez E, Normann RA. High-resolution two-dimensional spatial mapping of cat striate cortex using a 100-microelectrode array. Neuroscience. 2001;105(1):19–31. doi: 10.1016/s0306-4522(01)00174-9. [DOI] [PubMed] [Google Scholar]
- 56.Kim SJ, Manyam SC, Warren DJ, Normann RA. Electrophysiological mapping of cat primary auditory cortex with multielectrode arrays. Ann Biomed Eng. 2006 Feb;34(2):300–309. doi: 10.1007/s10439-005-9037-9. [DOI] [PubMed] [Google Scholar]
- 57.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans on Rehabilitation Engineering. 2005;13(4):524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AB, Kettner RE, Georgopoulos AP. Primate motor cortex and free arm movements to visual targets in three-dimensional space. I. Relations between single cell discharge and direction of movement. J Neurosci. 1988;8(8):2913–2927. doi: 10.1523/JNEUROSCI.08-08-02913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82(1):1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy PR. The cone electrode: a long-term electrode that records from neurites grown onto its recording surface. J Neurosci Methods. 1989 Sep;29(3):181–193. doi: 10.1016/0165-0270(89)90142-8. [DOI] [PubMed] [Google Scholar]
- 61.David S, Aguayo AJ. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy PR, Bakay RA. Activity of single action potentials in monkey motor cortex during long-term task learning. Brain Res. 1997 Jun 20;760(1–2):251–254. doi: 10.1016/s0006-8993(97)00051-6. [DOI] [PubMed] [Google Scholar]
- 63.Freire MA, Morya E, Faber J, Santos JR, Guimaraes JS, Lemos NA, Sameshima K, Pereira A, Ribeiro S, Nicolelis MA. Comprehensive analysis of tissue preservation and recording quality from chronic multielectrode implants. PLoS One. 2011;6(11):e27554. doi: 10.1371/journal.pone.0027554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merrill DR. The Electrochemistry of Charge Injection at the Electrode/Tissue Interface. 2010:85–138. [Google Scholar]
- 66.Patrick E, Orazem ME, Sanchez JC, Nishida T. Corrosion of tungsten microelectrodes used in neural recording applications. J Neurosci Methods. 2011;198(2):158–171. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy PT, Otto KJ, Rao MP. Robust penetrating microelectrodes for neural interfaces realized by titanium micromachining. Biomedical Microdevices. 2011;13(3):503–515. doi: 10.1007/s10544-011-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDord JM, Fridovich I. Superoxide Dismutase. The Journal of Biological Chemistry. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 69.Potter KA, Buck AC, Self WK, Callanan ME, Sunil S, Capadona JR. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials. 2013;34:7001–7015. doi: 10.1016/j.biomaterials.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 70.Potter KA, Jorfi M, Householder KT, Foster EJ, Weder C, Capadona JR. Curcumin-releasing mechanically-adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomater. 2014;10(5):2209–2222. doi: 10.1016/j.actbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 71.Dymond AM, Kaechele LE, Jurist JM, Crandall PH. Brain tissue reaction to some chronically implanted metals. J Neurosurg. 1970 Nov;33(5):574–580. doi: 10.3171/jns.1970.33.5.0574. [DOI] [PubMed] [Google Scholar]
- 72.Wang A, Liang X, McAllister JP, 2nd, Li J, Brabant K, Black C, Finlayson P, Cao T, Tang H, Salley SO, Auner GW, Simon Ng KY. Stability of and inflammatory response to silicon coated with a fluoroalkyl self-assembled monolayer in the central nervous system. J Biomed Mater Res A. 2007 May;81(2):363–372. doi: 10.1002/jbm.a.31034. [DOI] [PubMed] [Google Scholar]
- 73.Hammerle H, Kobuch K, Kohler K, Nisch W, Sachs H, Stelzle M. Biostability of micro-photodiode arrays for subretinal implantation. Biomaterials. 2002 Feb;23(3):797–804. doi: 10.1016/s0142-9612(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 74.Maloney JMLS, Baldwin SP, editors. Mater Res Soc Symp Proc. 2005. In Vivo Biostability of CVD Silicon Oxide and Silicon Nitride Films. [Google Scholar]
- 75.G Schmitt J-WS, Faßbender F, Buß G, Lüth H, Schöning MJ. Passivation and corrosion of microelectrode arrays. Electrochimica Acta. 1999;44(21–22):3865–3883. [Google Scholar]
- 76.Cogan SFED, Guzelian AA, Ping Liu Y, Edell R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J Biomed Mater Res A. 2003;67(3):856–867. doi: 10.1002/jbm.a.10152. [DOI] [PubMed] [Google Scholar]
- 77.Loeb GE, Bak MJ, Salcman M, Schmidt EM. Parylene as a chronically stable, reproducible microelectrode insulator. IEEE Trans Biomed Eng. 1977 Mar;24(2):121–128. doi: 10.1109/TBME.1977.326115. [DOI] [PubMed] [Google Scholar]
- 78.Winslow BD, Christensen MB, Yang WK, Solzbacher F, Tresco PA. A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials. 2010;31(35):9163–9172. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006 Sep;3(3):196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- 80.House PA, Macdonald JD, Tresco PA, Normann RA. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurg Focus. 2006;20(5):E4. doi: 10.3171/foc.2006.20.5.5. [DOI] [PubMed] [Google Scholar]
- 81.Hanson SR, Harker LA, Ratner BD, Hoffman AS. In vivo evaluation of artificial surfaces with a nonhuman primate model of arterial thrombosis. J Lab Clin Med. 1980 Feb;95(2):289–304. [PubMed] [Google Scholar]
- 82.Tang L. Mechanisms of fibrinogen domains: biomaterial interactions. J Biomater Sci Polym Ed. 1998;9(12):1257–1266. doi: 10.1163/156856298x00370. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007 Jan;44(1–3):82–94. doi: 10.1016/j.molimm.2006.06.020. [Research Support, N.I.H., Extramural Review]. [DOI] [PubMed] [Google Scholar]
- 84.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 85.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pollock AL. Early Brain Tissue Reaction Surrounding the Michigan-style Microelectrode Arrays Indicates that Extensive Remodeling of Glial and Neuronal Populations Occurs During the Foreign Body Response. Salt Lake City: Department of Bioengineering, University of Utah; 2007. [Google Scholar]
- 87.Potter KA, Simon JS, Velagapudi B, Capadona JR. Reduction of autofluorescence at the microelectrode-cortical tissue interface improves antibody detection. J Neurosci Methods. 2012;203(1):96–105. doi: 10.1016/j.jneumeth.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 88.Mahi H, Rodrigue D. Linear and non-linear viscoelastic properties of ethylene vinyl acetate/nano-crystalline cellulose composites. Rheologica Acta. 2012;51(2):127–142. [Google Scholar]
- 89.Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J Neural Eng. 2005;2(4):103–113. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 90.Kim DH, Abidian M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res A. 2004;71(4):577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 91.Hess A, Capadona J, Shanmuganathan K, Hsu L, Rowan S, Weder C, Tyler D, Zorman C. Development of a stimuli-responsive polymer nanocomposite toward biologically-optimized, MEMS-based neural probes. J Micromech Microeng. 2011;21:54009–54017. [Google Scholar]
- 92.Hess A, Dunning J, Harris J, Capadona JR, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ, Zorman CA. A bio-inspired, chemo-responsive polymer nanocomposite for mechanically dynamic microsystems. Solid-State Sensors, Actuators and Microsystems Conference, 2009 TRANSDUCERS 2009 International; 21–25 June 2009; Denver, CO. 2009. pp. 224–227. [Google Scholar]
- 93.Ware T, Simon D, Arreaga-Salas DE, Reeder J, Rennaker R, Keefer EW, Voit W. Fabrication of responsive, softening neural interfaces. Adv Funct Mater. 2012;22(16):3470–3479. [Google Scholar]
- 94.Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. J Neural Eng. 2011;8(4):046010. doi: 10.1088/1741-2560/8/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldstein SR, Salcman M. Mechanical factors in the design of chronic recording intracortical microelectrodes. IEEE Trans Biomed Eng. 1973;20(4):260–269. doi: 10.1109/TBME.1973.324190. [DOI] [PubMed] [Google Scholar]
- 96.Sridharan A, Rajan SD, Muthuswamy J. Long-term changes in the material properties of brain tissue at the implant–tissue interface. J Neural Eng. 2013;10(6):066001. doi: 10.1088/1741-2560/10/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hess AE, Potter KA, Tyler DJ, Zorman CA, Capadona JR. Environmentally-controlled Microtensile Testing of Mechanically-adaptive Polymer Nanocomposites for ex vivo Characterization. [2013/08/20];JoVE. 2013 78:e50078. doi: 10.3791/50078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harris JP, Capadona JR, Miller RH, Healy BC, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ. Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. J Neural Eng. 2011;8(6):066011. doi: 10.1088/1741-2560/8/6/066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Capadona JR, Tyler DJ, Zorman CA, Rowan SJ, Weder C. Mechanically adaptive nanocomposites for neural interfacing. MRS Bull. 2012;37(06):581–589. [Google Scholar]
- 100.Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science. 2008;319(5868):1370. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]
- 101.Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl Mater Inter. 2010;2(1):165–174. doi: 10.1021/am9006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Bio-inspired mechanically-adaptive nanocomposites derived from cotton cellulose whiskers. J Mater Chem. 2010;20(1):180. [Google Scholar]
- 103.Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Biomimetic mechanically adaptive nanocomposites. Prog Polym Sci. 2010;35(1–2):212–222. doi: 10.1021/am9006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan A, Keefer EW, Rennaker RL, Voit W. Thiol-ene/acrylate substrates for softening intracortical electrodes. Journal of Biomedical Materials Research Part B: Applied Biomaterialss. 2013 doi: 10.1002/jbmb.32946. 10.1002/jbmb.32946. [DOI] [PubMed] [Google Scholar]
- 105.Jorfi M, Roberts MN, Foster EJ, Weder C. Physiologically Responsive, Mechanically Adaptive Bio-Nanocomposites for Biomedical Applications. ACS Appl Mater Interfaces. 2013;5(4):1517–1526. doi: 10.1021/am303160j. [DOI] [PubMed] [Google Scholar]
- 106.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 107.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 108.Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A. 2007;82(1):169–178. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- 109.Potter KA, Buck AC, Self WK, Capadona JR. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J Neural Eng. 2012;9(4):046020. doi: 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
- 110.Ravikumar M, Jain S, Miller RH, Capadona JR, Selkirk SM. An organotypic spinal cord slice culture model to quantify neurodegeneration. J Neurosci Methods. 2012;211(2):280–288. doi: 10.1016/j.jneumeth.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia - Intrinsic Immuneffector Cell of the Brain. Brain Res Rev. 1995 Mar;20(3):269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 112.Betjes MG, Tuk CW, Struijk DG, Krediet RT, Arisz L, Beelen RH. Antigen-presenting capacity of macrophages and dendritic cells in the peritoneal cavity of patients treated with peritoneal dialysis. Clin Exp Immunol. 1993 Nov;94(2):377–384. doi: 10.1111/j.1365-2249.1993.tb03460.x. [In Vitro Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004 Jun 1;172(11):6587–6597. doi: 10.4049/jimmunol.172.11.6587. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 114.Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003 Nov;144(1–2):68–79. doi: 10.1016/j.jneuroim.2003.08.032. [Comparative Study Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 115.Poltorak M, Freed WJ. Immunological reactions induced by intracerebral transplantation: evidence that host microglia but not astroglia are the antigen-presenting cells. Exp Neurol. 1989 Mar;103(3):222–233. doi: 10.1016/0014-4886(89)90046-0. [Comparative Study]. [DOI] [PubMed] [Google Scholar]
- 116.Shaked I, Porat Z, Gersner R, Kipnis J, Schwartz M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J Neuroimmunol. 2004 Jan;146(1–2):84–93. doi: 10.1016/j.jneuroim.2003.10.049. [Comparative Study]. [DOI] [PubMed] [Google Scholar]
- 117.Skousen J, Merriam S, Srivannavit O, Perlin G, Wise K, Tresco P. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194C:167–180. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 118.Goligorsky MS. TLR4 and HMGB1: partners in crime? Kidney Int. 2011 Sep;80(5):450–452. doi: 10.1038/ki.2011.170. [Comment Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 119.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in Inflammation and Cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 120.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007 Jan;8(1):57–69. doi: 10.1038/nrn2038. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 121.Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol. 1995;9(2–3):189–205. [PubMed] [Google Scholar]
- 122.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 123.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 124.Quagliarello VJ, Wispelwey B, Long WJ, Jr, Scheld WM. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991;87(4):1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qin LLY, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 126.Qin LLY, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83(4):973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 127.Gao HMJJ, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81(6):1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 128.Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010 Dec;128(3):519–548. doi: 10.1016/j.pharmthera.2010.08.007. [Review]. [DOI] [PubMed] [Google Scholar]
- 129.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994 Winter;6(4):341–360. [Review]. [PubMed] [Google Scholar]
- 130.Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain Behav Immun. 2007 Mar;21(3):281–289. doi: 10.1016/j.bbi.2006.12.004. [Historical Article Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 131.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction 'opening': signaling via Rho and Rho kinase. J Cell Sci. 2003 Nov 15;116(Pt 22):4615–4628. doi: 10.1242/jcs.00755. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 132.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005 May;25(5):593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 133.Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009 Sep;8(4):277–284. doi: 10.2174/187152809789352249. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 134.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clinica chimica acta international journal of clinical chemistry. 2010 Nov 11;411(21–22):1570–1579. doi: 10.1016/j.cca.2010.07.006. [Review]. [DOI] [PubMed] [Google Scholar]
- 135.Karumbaiah L, Norman SE, Rajan NB, Anand S, Saxena T, Betancur M, Patkar R, Bellamkonda RV. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials. 2012;33(26):5983–5996. doi: 10.1016/j.biomaterials.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Karumbaiah L, Saxena T, Carlson D, Patil K, Patkar R, Gaupp EA, Betancur M, Stanley GB, Carin L, Bellamkonda RV. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34(33):8061–8074. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 137.JL Skousen MB, Tresco PA. A Strategy to Passively Reduce Neuroinflammation Surrounding Devices Implanted Chronically in Brain Tissue by Manipulating Device Surface Permeability. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.08.039. In Press. [DOI] [PubMed] [Google Scholar]
- 138.Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng. 1992;39(6):635–643. doi: 10.1109/10.141202. [DOI] [PubMed] [Google Scholar]
- 139.Schmidt S, Horch K, Normann R. Biocompatibility of silicon-based electrode arrays implanted in feline cortical tissue. J Biomed Mater Res. 1993;27:1393–1399. doi: 10.1002/jbm.820271106. [DOI] [PubMed] [Google Scholar]
- 140.Schultz RL, Willey TJ. The ultrastructure of the sheath around chronically implanted electrodes in brain. J Neurocytol. 1976 Dec;5(6):621–642. doi: 10.1007/BF01181577. [Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 141.Stensaas SS, Stensaas LJ. The reaction of the cerebral cortex to chronically implanted plastic needles. Acta Neuropathol (Berl) 1976;35(3):187–203. [PubMed] [Google Scholar]
- 142.Stensaas SS, Stensaas LJ. Histopathological evaluation of materials implanted in the cerebral cortex. Acta Neuropathol (Berl) 1978 Feb 20;41(2):145–155. doi: 10.1007/BF00689766. [DOI] [PubMed] [Google Scholar]
- 143.Collias JC, Manuelidis EE. Histopathological changes produced by implanted electrodes in cat brains; comparison with histopathological changes in human and experimental puncture wounds. J Neurosurg. 1957 May;14(3):302–328. doi: 10.3171/jns.1957.14.3.0302. [DOI] [PubMed] [Google Scholar]
- 144.Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156(1):33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- 145.Thelin J, Jorntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N, Linsmeier CE. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS One. 2011;6(1):e16267. doi: 10.1371/journal.pone.0016267. [Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Potter-Baker KA, Ravikumar M, Burke AA, Meador WD, Householder KT, Buck AC, Sunil S, Stewart WG, Anna JP, Tomaszewski WH, Capadona JR. A comparison of neuroinflammation to implanted microelectrodes in rat and mouse models. Biomaterials. 2014;34:5637–5646. doi: 10.1016/j.biomaterials.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002 Jun;200(6):629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [Research Support, Non-U.S. Gov't Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andjelkovic AV, Kerkovich D, Pachter JS. Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types. J Leukoc Biol. 2000 Oct;68(4):545–552. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [PubMed] [Google Scholar]
- 149.Goldstein GW. Endothelial cell-astrocyte interactions. A cellular model of the blood-brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [Review]. [DOI] [PubMed] [Google Scholar]
- 150.Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 May 12;24(19):4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004. [In Vitro Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmid-Brunclik N, Burgi-Taboada C, Antoniou X, Gassmann M, Ogunshola OO. Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. American journal of physiology Regulatory, integrative and comparative physiology. 2008 Sep;295(3):R864–R873. doi: 10.1152/ajpregu.00536.2007. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 152.Kim YT, Bridge MJ, Tresco PA. The influence of the foreign body response evoked by fibroblast transplantation on soluble factor diffusion in surrounding brain tissue. J Control Release. 2007 Apr 23;118(3):340–347. doi: 10.1016/j.jconrel.2007.01.002. [Comparative Study Research Support, N.I.H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 153.Aschner M, Sonnewald U, Tan KH. Astrocyte modulation of neurotoxic injury. Brain Pathol. 2002 Oct;12(4):475–481. doi: 10.1111/j.1750-3639.2002.tb00465.x. [Research Support, U.S. Gov't, P.H.S. Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guenard V, Frisch G, Wood PM. Effects of axonal injury on astrocyte proliferation and morphology in vitro: implications for astrogliosis. Exp Neurol. 1996 Feb;137(2):175–190. doi: 10.1006/exnr.1996.0017. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 155.Louw DF, Masada T, Sutherland GR. Ischemic neuronal injury is ameliorated by astrocyte activation. Can J Neurol Sci. 1998 May;25(2):102–107. doi: 10.1017/s0317167100033692. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 156.Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994 May;53(3):213–220. doi: 10.1097/00005072-199405000-00001. [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. Review]. [DOI] [PubMed] [Google Scholar]
- 157.McConnell GC, Schneider TM, Owens DJ, Bellamkonda RV. Extraction Force and Cortical Tissue Reaction of Silicon Microelectrode Arrays Implanted in the Rat Brain. IEEE Trans Biomed Eng. 2007;54(6):1097–1107. doi: 10.1109/TBME.2007.895373. [DOI] [PubMed] [Google Scholar]
- 158.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007 Dec;4(4):410–423. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 159.Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007 Apr 5; doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 160.Ravikumar m, Hageman DJ, Tomaszewski WH, Chandra GM, Skousen JL, Capadona JR. The effect of residual endotoxin contamination on the neuroinflammatory response to sterilized intracortical microelectrodes. J Mater Chem B. 2014;2:2517–2529. doi: 10.1039/C3TB21453B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McConnell GC, Rees HD, Levey AI, Gutekunst C-A, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6(5):056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 162.Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials. 2013;34(20):4703–4713. doi: 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 163.Jones LLYY, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci Methods. 2002;22(7):2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jones LLMR, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182(2):399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 165.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 166.Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007 May 7;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The Neuronal Chondroitin Sulfate Proteoglycan Neurocan Binds to the Neural Cell Adhesion Molecules Ng-CAM/L1/NILE and N-CAM and Inhibits Neuronal Adhesion and Neurite Outgrowth. The Journal of Cell Biology. 1994;125(3):669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gopalakrishnan SM, Teusch N, Imhof C, Bakker MH, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res. 2008 Aug 1;86(10):2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- 169.Hynds DL, Snow DM. Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: stalling/stopping exceeds turning in human neuroblastoma growth cones. Exp Neurol. 1999 Nov;160(1):244–255. doi: 10.1006/exnr.1999.7212. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 170.Iijima N, Oohira A, Mori T, Kitabatake K, Kohsaka S. Core protein of chondroitin sulfate proteoglycan promotes neurite outgrowth from cultured neocortical neurons. J Neurochem. 1991 Feb;56(2):706–708. doi: 10.1111/j.1471-4159.1991.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 171.Kuffler DP, Sosa IJ, Reyes O. Schwann cell chondroitin sulfate proteoglycan inhibits dorsal root ganglion neuron neurite outgrowth and substrate specificity via a soma and not a growth cone mechanism. J Neurosci Res. 2009 Oct;87(13):2863–2871. doi: 10.1002/jnr.22132. [DOI] [PubMed] [Google Scholar]
- 172.Nakanishi K, Aono S, Hirano K, Kuroda Y, Ida M, Tokita Y, Matsui F, Oohira A. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. The Journal of Biological Chemistry. 2006 Aug 25;281(34):24970–24978. doi: 10.1074/jbc.M601498200. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 173.Yamada H, Fredette B, Shitara K, Hagihara K, Miura R, Ranscht B, Stallcup WB, Yamaguchi Y. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997 Oct 15;17(20):7784–7795. doi: 10.1523/JNEUROSCI.17-20-07784.1997. [Research Support, U.S. Gov't, P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aihara N, Tanno H, Hall JJ, Pitts LH, Noble LJ. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- 175.Azzi G, Bernaudin JF, Bouchaud C, Bellon B, Fleury-Feith J. Permeability of the normal rat brain, spinal cord and dorsal root ganglia microcirculations to immunoglobulins G. Biol Cell. 1990;68(1):31–36. doi: 10.1111/j.1768-322x.1990.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 176.Seitz RJ, Heininger K, Schwendemann G, Toyka KV, Wechsler W. The mouse bloodbrain barrier and blood-nerve barrier for IgG: a tracer study by use of the avidin-biotin system. Acta Neuropathol. 1985;68(1):15–21. doi: 10.1007/BF00688950. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 177.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011 Dec 1;585(23):3770–3780. doi: 10.1016/j.febslet.2011.04.066. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 178.Lassmann H. A dynamic view of the blood-brain barrier in active multiple sclerosis lesions. Ann Neurol. 2011 Jul;70(1):1–2. doi: 10.1002/ana.22494. [Comment Editorial]. [DOI] [PubMed] [Google Scholar]
- 179.Rennaker RL, Miller J, Tang H, Wilson DA. Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng. 2007 Jun;4(2):L1–L5. doi: 10.1088/1741-2560/4/2/L01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee SM, Yune TY, Kim SJ, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem. 2004;91(3):568–578. doi: 10.1111/j.1471-4159.2004.02780.x. [DOI] [PubMed] [Google Scholar]
- 181.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death and Disease. 2013;4(3):e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11(2):186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- 183.Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp Neurol. 2005;194(2):289–300. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 184.Zhong Y, McConnell GC, Ross JD, DeWeerth SP, Bellamkonda RV, editors. A Novel Dexamethasone-releasing, Anti-inflammatory Coating for Neural Implants. Neural Engineering, 2005 Conference Proceedings 2nd International IEEE EMBS Conference on; 2005 16–19 March.2005. [Google Scholar]
- 185.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Release. 2006 Feb 21;110(3):531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 186.Girbovan C, Morin L, Plamondon H. Repeated resveratrol administration confers lasting protection against neuronal damage but induces dose-related alterations of behavioral impairments after global ischemia. Behav Pharmacol. 2012 Feb;23(1):1–13. doi: 10.1097/FBP.0b013e32834eafa3. [DOI] [PubMed] [Google Scholar]
- 187.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011 Jan;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [Review]. [DOI] [PubMed] [Google Scholar]
- 188.Khodagholy D, Doublet T, Quilichini P, Gurfinkel M, Leleux P, Ghestem A, Ismailova E, Hervé T, Sanaur S, Bernard C, Malliaras GG. In vivo recordings of brain activity using organic transistors. Nature Communications. 2013;4:1575. doi: 10.1038/ncomms2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013 May 16;497(7449):332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harris JP. The Glia-Neuronal Response to Cortical Electrodes: Interactions with Substrate Stiffness and Electrophysiology: Case Western Reserve University. 2011 [Google Scholar]
- 191.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes and Infection. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 192.Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011 Jan;25(1):99–110. doi: 10.1096/fj.10-164152. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lund S, Christensen K, Hedtjarn M, Mortensen A, Hagberg H, Falsig J, Hasseldam H, Schrattenholz A, Porzgen P, Leist M. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180(1–2):71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 194.Winslow BD, Tresco PA. Quantitative analysis of the tissue response to chronically implanted microwire electrodes in rat cortex. Biomaterials. 2010 Mar;31(7):1558–1567. doi: 10.1016/j.biomaterials.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 195.Gutowski SM, Templeman KL, South AB, Gaulding JC, Shoemaker JT, Laplaca MC, Bellamkonda RV, Lyon LA, Garcia AJ. Host response to microgel coatings on neural electrodes implanted in the brain. J Biomed Mater Res A. 2013 May 13; doi: 10.1002/jbm.a.34799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sharp AA, Ortega AM, Restrepo D, Curran-Everett D, Gall K. In vivo penetration mechanics and mechanical properties of mouse brain tissue at micrometer scales. IEEE Trans Biomed Eng. 2009 Jan;56(1):45–53. doi: 10.1109/TBME.2008.2003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jensen W, Yoshida K, Hofmann UG. In-Vivo Implant Mechanics of Flexible, Silicon-Based ACREO Microelectrode Arrays in Rat Cerebral Cortex. Biomedical Engineering, IEEE Transactions on. 2006;53(5):934–940. doi: 10.1109/TBME.2006.872824. [DOI] [PubMed] [Google Scholar]
- 198.Jensen W, Hofmann UG, Yoshida K, editors. Assessment of subdural insertion force of single-tine microelectrodes in rat cerebral cortex; Engineering in Medicine and Biology Society, 2003 Proceedings of the 25th Annual International Conference of the IEEE; 2003. [Google Scholar]
- 199.Egert D, Peterson RL, Najafi K, editors. Parylene microprobes with engineered stiffness and shape for improved insertion. Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), 2011 16th International; 2011 5–9 June.2011. [Google Scholar]
- 200.ChunXiang T, Jiping H, editors. Monitoring Insertion Force and Electrode Impedance during Implantation of Microwire Electrodes. Engineering in Medicine and Biology Society, 2005 IEEE-EMBS 2005 27th Annual International Conference of the; 17–18 Jan 2006; 2005. [DOI] [PubMed] [Google Scholar]
- 201.Wester BA, Lee RH, LaPlaca MC. Development and characterization of in vivo flexible electrodes compatible with large tissue displacements. J Neural Eng. 2009 Apr;6(2):024002. doi: 10.1088/1741-2560/6/2/024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee H, Bellamkonda RV, Sun W, Levenston ME. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J Neural Eng. 2005;2(4):81–89. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- 203.Subbaroyan J. Investigations of tethering induced injury response in brain tissue by intracortical implants through modeling and in vivo experiments. Ann Arbor, MI: University of Michigan; 2007. [Google Scholar]
- 204.Zhu RYH, Varadan VK, Smith CS, Huang GL. Biomechanical Strain Analysis at the Interface of Brain and Nanowire Electrodes on a Neural Probe. Journal of Nanotechnology in Engineering and Medicine. 2012;2(3) [Google Scholar]
- 205.Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004 May;25(12):2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 206.Markwardt NT, Stokol J, Rennaker RL., 2nd Sub-meninges implantation reduces immune response to neural implants. J Neurosci Methods. 2013 Apr 15;214(2):119–125. doi: 10.1016/j.jneumeth.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Subbaroyan J, Kipke DR, editors. Conf Proc IEEE Eng Med Biol Soc. 2006. The role of flexible polymer interconnects in chronic tissue response induced by intracortical microelectrodes--a modeling and an in vivo study. [DOI] [PubMed] [Google Scholar]
- 208.Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2001;48(3):361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 209.Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K, Suzuki T. Parylene flexible neural probes integrated with microfluidic channels. Lab on a Chip. 2005;5(5):519–523. doi: 10.1039/b417497f. [DOI] [PubMed] [Google Scholar]
- 210.Fernandez LJ, Altuna A, Tijero M, Gabriel G, Villa R, Rodriguez MJ, Batlle M, Vilares R, Berganzo J, Blanco FJ. Study of functional viability of SU-8-based microneedles for neural applications. J Micromech Microeng. 2009;19(2):025007. [Google Scholar]
- 211.Mercanzini A, Colin P, Bensadoun JC, Bertsch A, Renaud P. In vivo electrical impedance spectroscopy of tissue reaction to microelectrode arrays. IEEE Trans Biomed Eng. 2009 Jul;56(7):1909–1918. doi: 10.1109/TBME.2009.2018457. [DOI] [PubMed] [Google Scholar]
- 212.Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY. Poly(vinyl alcohol)/poly(acrylic acid) hydrogel coatings for improving electrode-neural tissue interface. Biomaterials. 2009;30(25):4143–4151. doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 213.Lacour S, Benmerah S, Tarte E, FitzGerald J, Serra J, McMahon S, Fawcett J, Graudejus O, Yu Z, Morrison B., III Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. [2010/10/01];Med Biol Eng Comput. 2010 48(10):945–954. doi: 10.1007/s11517-010-0644-8. [DOI] [PubMed] [Google Scholar]
- 214.Clement RS, Singh A, Olson B, Lee K, He J, editors. Neural Recordings from a Benzocyclobutene (BCB) Based Intra-cortical Neural Implant in an Acute Animal Model. Proceedings of the 25* Annual International Conference of the IEEE EMBS; Cancun, Mexico *. 2003. [Google Scholar]
- 215.Altuna A, Bellistri E, Cid E, Aivar P, Gal B, Berganzo J, Gabriel G, Guimera A, Villa R, Fernandez LJ, Menendez de la Prida L. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab on a Chip. 2013;13(7):1422–1430. doi: 10.1039/c3lc41364k. [10.1039/C3LC41364K]. [DOI] [PubMed] [Google Scholar]
- 216.Vernekar VN, Cullen DK, Fogleman N, Choi Y, Garcia AJ, Allen MG, Brewer GJ, LaPlaca MC. SU-8 2000 rendered cytocompatible for neuronal bioMEMS applications. Journal of Biomedical Materials Research Part A. 2009 Apr;89A(1):138–151. doi: 10.1002/jbm.a.31839. [DOI] [PubMed] [Google Scholar]
- 217.Lee K, Singh A, He J, Massia S, Kim B, Raupp G. Polyimide based neural implants with stiffness improvement. Sensors and Actuators B: Chemical. 2004;102(1):67–72. [Google Scholar]
- 218.Lee K-K, He J, Singh A, Massia S, Ehteshami G, Kim B, Raupp G. Polyimide-based intracortical neural implant with improved structural stiffness. J Micromech Microeng. 2004;14(1):32–37. [Google Scholar]
- 219.Lee K, He J, Wang L. Benzocyclobutene (BCB) based neural implants with microfluidic channel. P Ann Int Ieee Embs. 2004;26:4326–4329. doi: 10.1109/IEMBS.2004.1404204. [DOI] [PubMed] [Google Scholar]
- 220.Lee K, He J, Clement R, Massia S, Kim B. Biocompatible benzocyclobutene (BCB)-based neural implants with micro-fluidic channel. Biosens Bioelectron. 2004;20(2):404–407. doi: 10.1016/j.bios.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 221.Lewitus D, Smith KL, Shain W, Kohn J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011 Jun;7(6):2483–2491. doi: 10.1016/j.actbio.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 222.Lewitus DY, Smith KL, Shain W, Bolikal D, Kohn J. The fate of ultrafast degrading polymeric implants in the brain. Biomaterials. 2011 Aug;32(24):5543–5550. doi: 10.1016/j.biomaterials.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lewitus D, Vogelstein RJ, Zhen GH, Choi YS, Kohn J, Harshbarger S, Jia XF. Designing Tyrosine-Derived Polycarbonate Polymers for Biodegradable Regenerative Type Neural Interface Capable of Neural Recording. Ieee T Neur Sys Reh. 2011 Apr;19(2):204–212. doi: 10.1109/TNSRE.2010.2098047. [DOI] [PubMed] [Google Scholar]
- 224.Kato Y, Saito I, Hoshino T, Suzuki T, Mabuchi K. Preliminary study of multichannel flexible neural probes coated with hybrid biodegradable polymer. 2006 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vols 1–15; 2006. pp. 5444–5447. [DOI] [PubMed] [Google Scholar]
- 225.Gilgunn PJ, Khilwani R, Kozai TDY, Weber DJ, Cui XT, Erdos G, Ozdoganlar OB, Fedder GK, editors. An ultra-compliant, scalable neural probe with molded biodissolvable delivery vehicle. IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS); Jan. 29 2012–Feb. 2 2012.2012. [Google Scholar]
- 226.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 227.Rao L, Zhou H, Li T, Li C, Duan YY. Polyethylene glycol-containing polyurethane hydrogel coatings for improving the biocompatibility of neural electrodes. Acta Biomater. 2012;8(6):2233–2242. doi: 10.1016/j.actbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 228.Kim D-H, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim Y-S, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang K-C, Zakin MR, Litt B, Rogers JA. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater. 2010 doi: 10.1038/nmat2745. [10.1038/nmat2745]. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomedical Materials. 2009 Apr;4(2):022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 230.Alarcon CdlH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chemical Society Reviews. 2005;34(3):276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 231.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nature Materials. 2010 Feb;9(2):101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 232.Urban MW, editor. Handbook of Stimuli-Responsive Materials. 1 ed. Wiley-VCH: 2011. [Google Scholar]
- 233.Mano JF. Stimuli-responsive polymeric systems for biomedical applications. Adv Eng Mater. 2008 Jun;10(6):515–527. [Google Scholar]
- 234.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci. 2010;35(1–2):278–301. [Google Scholar]
- 235.Nguyen JK, Park DJ, Skousen JL, Hess-Dunning A, Tyler DJ, Rowan SJ, Weder C, Capadona JR. Mechanically-Compliant Intracortical Implants Reduce the Neuroinflammatory Response. J Neural Eng. 2014;11:056014. doi: 10.1088/1741-2560/11/5/056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ware T, Simon D, Hearon K, Liu C, Shah S, Reeder J, Khodaparast N, Kilgard MP, Maitland DJ, Rennaker RL, Voit WE. Three-Dimensional Flexible Electronics Enabled by Shape Memory Polymer Substrates for Responsive Neural Interfaces. Macromolecular Materials and Engineering. 2012;297(12):1193–1202. doi: 10.1002/mame.201200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sharp AA, Panchawagh HV, Ortega A, Artale R, Richardson-Burns S, Finch DS, Gall K, Mahajan RL, Restrepo D. Toward a self-deploying shape memory polymer neuronal electrode. J Neural Eng. 2006 Dec;3(4):L23–L30. doi: 10.1088/1741-2560/3/4/L02. [DOI] [PubMed] [Google Scholar]
- 238.Tien LW, Wu F, Tang-Schomer MD, Yoon E, Omenetto FG, Kaplan DL. Silk as a Multifunctional Biomaterial Substrate for Reduced Glial Scarring around Brain-Penetrating Electrodes. Adv Funct Mater. 2013 n/a-n/a. [Google Scholar]
- 239.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983(1–2):23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 240.Stice P, Gilletti A, Panitch A, Muthuswamy J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J Neural Eng. 2007 Jun;4(2):42–53. doi: 10.1088/1741-2560/4/2/005. [DOI] [PubMed] [Google Scholar]
- 241.Saddow SE. Silicon Carbide Biotechnology. First ed. Waltham, MA: Elsevier Inc; 2012. [Google Scholar]
- 242.Raynor JE, Capadona JR, Collard DM, Petrie TA, Garcia AJ. Polymer brushes and self-assembled monolayers: Versatile platforms to control cell adhesion to biomaterials (Review) Biointerphases. 2009 Jun;4(2):FA3–FA16. doi: 10.1116/1.3089252. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 243.Leung BK, Biran R, Underwood CJ, Tresco PA. Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials. 2008 Aug;29(23):3289–3297. doi: 10.1016/j.biomaterials.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 244.Sommakia S, Gaire J, Rickus JL, Otto KJ. Resistive and reactive changes to the impedance of intracortical microelectrodes can be mitigated with polyethylene glycol under acute in vitro and in vivo settings. Front Neuroengineering. 2014;7(33):1–8. doi: 10.3389/fneng.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, Lee IS. Culture of neural cells on silicon wafers with nano-scale surface topograph. J Neurosci Methods. 2002 Oct 15;120(1):17–23. doi: 10.1016/s0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 246.Green RA, Baek S, Poole-Warren LA, Martens PJ. Conducting polymer-hydrogels for medical electrode applications. Sci Technol Adv Mat. 2010 Feb;11(1) doi: 10.1088/1468-6996/11/1/014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dalby MJ. Cellular response to low adhesion nanotopographies. International journal of nanomedicine. 2007;2(3):373–381. [PMC free article] [PubMed] [Google Scholar]
- 248.Moxon KA, Kalkhoran NM, Markert M, Sambito MA, McKenzie JL, Webster JT. Nanostructured surface modification of ceramic-based microelectrodes to enhance biocompatibility for a direct brain-machine interface. IEEE Trans Biomed Eng. 2004;51(6):881–889. doi: 10.1109/TBME.2004.827465. [DOI] [PubMed] [Google Scholar]
- 249.Moxon KA, Leiser SC, Gerhardt GA, Barbee KA, Chapin JK. Ceramic-based multisite electrode arrays for chronic single-neuron recording. IEEE Trans Biomed Eng. 2004 Apr;51(4):647–656. doi: 10.1109/TBME.2003.821037. [DOI] [PubMed] [Google Scholar]
- 250.Moxon KA, Hallman S, Aslani A, Kalkhoran NM, Lelkes PI. Bioactive properties of nanostructured porous silicon for enhancing electrode to neuron interfaces. J Biomater Sci Polym Ed. 2007;18(10):1263–1281. doi: 10.1163/156856207782177882. [DOI] [PubMed] [Google Scholar]
- 251.Ereifej ES, Matthew HW, Newaz G, Mukhopadhyay A, Auner G, Salakhutdinov I, VandeVord PJ. Nanopatterning effects on astrocyte reactivity. Journal of Biomedical Materials Research Part A. 2013;101A(6):1743–1757. doi: 10.1002/jbm.a.34480. [DOI] [PubMed] [Google Scholar]
- 252.Capadona JR, Petrie TA, Fears KP, Latour RA, Collard DM, García AJ. Surface-nucleated assembly of fibrillar extracellular matrices. Adv Mater. 2005;17:2604–2608. [Google Scholar]
- 253.Bridges AW, García AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. Journal of diabetes science and technology. 2008 Nov;2(6):984–994. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Leach J, Achyuta AKH, Murthy SK. Bridging the divide between neuroprosthetic design, tissue engineering and neurobiology. Frontiers in Neuroengineering. 2010 Feb 8;2 doi: 10.3389/neuro.16.018.2009. [Review]. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.He W, McConnell GC, Schneider TM, Bellamkonda RV. A Novel Anti-inflammatory Surface for Neural Electrodes. Adv Mater. 2007;19(21):3529–3533. [Google Scholar]
- 256.Azemi E, Lagenaur CF, Cui XT. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011;32(3):681–692. doi: 10.1016/j.biomaterials.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT. Surface immobilization of neural adhesion molecule L1 for improving the biocompatibility of chronic neural probes: In vitro characterization. Acta Biomater. 2008 Mar 20; doi: 10.1016/j.actbio.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 258.Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts Biomaterials. 2001;22:1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 259.Taub AH, Hogri R, Magal A, Mintz M, Shacham-Diamand Y. Bioactive antiinflammatory coating for chronic neural electrodes. Journal of Biomedical Materials Research Part A. 2012;100A(7):1854–1858. doi: 10.1002/jbm.a.34152. [DOI] [PubMed] [Google Scholar]
- 260.He W, Bellamkonda RV. Nanoscale neuro-integrative coatings for neural implants. Biomaterials. 2005;26(16):2983–2990. doi: 10.1016/j.biomaterials.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 261.Kim DH, Richardson-Burns SM, Hendricks JL, Sequera C, Martin DC. Effect of Immobilized Nerve Growth Factor on Conductive Polymers: Electrical Properties and Cellular Response. Adv Funct Mater. 2007;17(1):79–86. [Google Scholar]
- 262.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006 May;27(15):3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 263.Zhong Y, Bellamkonda RV. Controlled release of anti-inflammatory agent α-MSH from neural implants. J Controlled Release. 2005;106(3):309–318. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 264.Abidian MR, Martin DC. Multifunctional Nanobiomaterials for Neural Interfaces. Adv Funct Mater. 2009;19(4):573–585. [Google Scholar]
- 265.Mercanzini A, Reddy ST, Velluto D, Colin P, Maillard A, Bensadoun JC, Hubbell JA, Renaud P. Controlled release nanoparticle-embedded coatings reduce the tissue reaction to neuroprostheses. J Controlled Release. 2010 Aug 3;145(3):196–202. doi: 10.1016/j.jconrel.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 266.Massia SP, Holecko MM, Ehteshami GR. In vitro assessment of bioactive coatings for neural implant applications. Journal of Biomedical Materials Research Part A. 2004;68A(1):177–186. doi: 10.1002/jbm.a.20009. [DOI] [PubMed] [Google Scholar]
- 267.Stauffer WR, Cui XT. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials. 2006;27(11):2405–2413. doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 268.He W, McConnell GC, Bellamkonda RV. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J Neural Eng. 2006 Dec;3(4):316–326. doi: 10.1088/1741-2560/3/4/009. [DOI] [PubMed] [Google Scholar]
- 269.Kolarcik CL, Bourbeau D, Azemi E, Rost E, Zhang L, Lagenaur CF, Weber DJ, Cui XT. In vivo effects of L1 coating on inflammation and neuronal health at the electrode–tissue interface in rat spinal cord and dorsal root ganglion. Acta Biomater. 2012 Oct;8(10):3561–3575. doi: 10.1016/j.actbio.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Green RA, Suaning GJ, Poole-Warren LA, Lovell NH, editors. Bioactive conducting polymers for neural interfaces application to vision prosthesis. Neural Engineering, 2009 NER '09 4th International IEEE/EMBS Conference on; April 29 2009–May 2 2009.2009. [Google Scholar]
- 271.Kang G, Borgens RB, Cho Y. Well-Ordered Porous Conductive Polypyrrole as a New Platform for Neural Interfaces. [2011/05/17];Langmuir. 2011 27(10):6179–6184. doi: 10.1021/la104194m. [DOI] [PubMed] [Google Scholar]
- 272.Potter-Baker KA, Nguyen JK, Kovach KM, Gitomer MM, Srail TW, Stewart WG, Skousen JL, Capadona JR. Development of Superoxide Dismutase Mimetic Surfaces to Reduce Accumulation of Reactive Oxygen Species Surrounding Intracortical Microelectrodes Journal of Materials Chemistry B. 2014;2:2248–2258. doi: 10.1039/C4TB00125G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen S, Allen MG. Extracellular matrix-based materials for neural interfacing. MRS Bulletin. 2012;37(06):606–613. [Google Scholar]
- 274.Heiduschka P, Romann I, Ecken H, Schöning M, Schuhmann W, Thanos S. Defined adhesion and growth of neurones on artificial structured substrates. Electrochimica Acta. 2001 Sep 1;47(1–2):299–307. [Google Scholar]
- 275.Massia SP, Rao SS, Hubbell JA. Covalently Immobilized Laminin Peptide Tyr-Ile-Gly-Ser-Arg (YIGSR) Supports Cell Spreading and Co-localization of the 67- Kilodalton Laminin Receptor with a-Actinin and Vinculin. The Journal of Biological Chemistry. 1993;268(11):8053–8059. [PubMed] [Google Scholar]
- 276.Green RA, Lovell NH, Poole-Warren LA. Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomaterials. 2009;30(22):3637–3644. doi: 10.1016/j.biomaterials.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 277.Green RA, Lovell NH, Poole-Warren LA. Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomater. 2010;6(1):63–71. doi: 10.1016/j.actbio.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 278.Green RA, Matteucci PB, Hassarati RT, Giraud B, Dodds CWD, Chen S, Byrnes-Preston PJ, Suaning GJ, Poole-Warren LA, Lovell NH. Performance of conducting polymer electrodes for stimulating neuroprosthetics. J Neural Eng. 2013;10(1):016009. doi: 10.1088/1741-2560/10/1/016009. [DOI] [PubMed] [Google Scholar]
- 279.Cutler S. Engineering cell adhesive surfaces that direct integrin α5β1 binding using a recombinant fragment of fibronectin. Biomaterials. 2003;24(10):1759–1770. doi: 10.1016/s0142-9612(02)00570-7. [DOI] [PubMed] [Google Scholar]
- 280.Tanaka J, Toku K, Sakanaka M, Maeda N. Morphological differentiation of microglial cells in culture: involvement of insoluble factors derived from astrocytes. Neurosci Res. 1999 Sep;34(4):207–215. doi: 10.1016/s0168-0102(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 281.Tanaka J, Maeda N. Microglial Ramification Requires Nondiffusible Factors Derived from Astrocytes. Exp Neurol. 1996;137:367–375. doi: 10.1006/exnr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 282.Wolchok JC, Tresco PA. The isolation of cell derived extracellular matrix constructs using sacrificial open-cell foams. Biomaterials. 2010 Dec;31(36):9595–9603. doi: 10.1016/j.biomaterials.2010.08.072. [DOI] [PubMed] [Google Scholar]
- 283.McClelland R, Wauthier E, Uronis J, Reid L. Gradients in the liver's extracellular matrix chemistry from periportal to pericentral zones: influence on human hepatic progenitors. Tissue engineering Part A. 2008 Jan;14(1):59–70. doi: 10.1089/ten.a.2007.0058. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 284.Huet C, Pisselet C, Mandon-Pepin B, Monget P, Monniaux D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol. 2001 May;169(2):347–360. doi: 10.1677/joe.0.1690347. [Comparative Study]. [DOI] [PubMed] [Google Scholar]
- 285.Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007 Sep;13(9):2301–2310. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 286.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009 Oct;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, Soker S, Van Dyke M. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009 Apr;30(12):2393–2399. doi: 10.1016/j.biomaterials.2008.12.069. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009 Aug;30(23–24):4021–4028. doi: 10.1016/j.biomaterials.2009.04.005. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. The heart surgery forum. 2003;6(2):E20–E26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 290.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001 Jun 15;71(11):1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 292.Allaire E, Bruneval P, Mandet C, Becquemin JP, Michel JB. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997 Jul;122(1):73–81. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 293.Allaire E, Mandet C, Bruneval P, Bensenane S, Becquemin JP, Michel JB. Cell and extracellular matrix rejection in arterial concordant and discordant xenografts in the rat. Transplantation. 1996 Sep 27;62(6):794–803. doi: 10.1097/00007890-199609270-00017. [DOI] [PubMed] [Google Scholar]
- 294.Allaire E, Guettier C, Bruneval P, Plissonnier D, Michel JB. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994 Mar;19(3):446–456. doi: 10.1016/s0741-5214(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 295.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997 Mar;40(3):262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 296.Winn SR, Aebischer P, Galletti PM. Brain tissue reaction to permselective polymer capsules. J Biomed Mater Res. 1989 Jan;23(1):31–44. doi: 10.1002/jbm.820230104. [DOI] [PubMed] [Google Scholar]
- 297.Jaeger CB, Winn SR, Tresco PA, Aebischer P. Repair of the blood-brain barrier following implantation of polymer capsules. Brain Res. 1991 Jun 14;551(1–2):163–170. doi: 10.1016/0006-8993(91)90929-p. [DOI] [PubMed] [Google Scholar]
- 298.Winn SR, Tresco PA, Zielinski B, Greene LA, Jaeger CB, Aebischer P. Behavioral recovery following intrastriatal implantation of microencapsulated PC12 cells. Exp Neurol. 1991 Sep;113(3):322–329. doi: 10.1016/0014-4886(91)90022-5. [DOI] [PubMed] [Google Scholar]
- 299.Bridge MJ, Tresco PA. In Vivo Solute Diffusivity in Brain Tissue Surrounding Indwelling Neural Implants. In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton (FL): 2008. [PubMed] [Google Scholar]
- 300.La Flamme KE, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials. 2007 Jun;28(16):2638–2645. doi: 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lopez CA, Fleischman AJ, Roy S, Desai TA. Evaluation of silicon nanoporous membranes and ECM-based microenvironments on neurosecretory cells. Biomaterials. 2006 Jun;27(16):3075–3083. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 302.Galimberti D, Baron P, Meda L, Prat E, Scarpini E, Delgado R, Catania A, Lipton JM, Scarlato G. α-MSH Peptides Inhibit Production of Nitric Oxide and Tumor Necrosis Factor-α by Microglial Cells Activated with β-Amyloid and Interferon γ. Biochem Biophys Res Commun. 1999 Sep 16;263(1):251–256. doi: 10.1006/bbrc.1999.1276. [DOI] [PubMed] [Google Scholar]
- 303.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Progress in Polymer Science. 2007;32(8–9):876–921. [Google Scholar]
- 304.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface. 2010 Oct 6;7(Suppl 5):S559–S579. doi: 10.1098/rsif.2010.0120.focus. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cui X, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sensors and Actuators A: Physical. 2001 Aug 25;93(1):8–18. [Google Scholar]
- 306.Buchko CJ, Kozloff KM, Martin DC. Surface characterization of porous, biocompatible protein polymer thin films. Biomaterials. 2001;22:1289–1300. doi: 10.1016/s0142-9612(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 307.Yang J, Martin DC. Microporous conducting polymers on neural microelectrode arrays: I Electrochemical deposition. Sensors and Actuators B: Chemical. 2004 Jun 15;101(1–2):133–142. [Google Scholar]
- 308.Harris AR, Morgan SJ, Chen J, Kapsa RMI, Wallace GG, Paolini AG. Conducting polymer coated neural recording electrodes. J Neural Eng. 2013;10(1):016004. doi: 10.1088/1741-2560/10/1/016004. [DOI] [PubMed] [Google Scholar]
- 309.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials. 2008;29(24–25):3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 310.Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton (FL): CRC Press; 2008. [PubMed] [Google Scholar]
- 311.Kim J, Choi S, Lillehei P, King G, Watt G, Chu S, Park Y, Thibeault S, editors. Smart Structures and Materials 2004: Smart Electronics, MEMS, BioMEMS, and Nanotechnology. SPIE; 2004. Electrochemical reconstitution of biomolecules for applications as electrocatalysts for the bionanofuel cell. [Google Scholar]
- 312.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005 Jun;26(17):3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 313.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proceedings of the National Academy of Sciences. 1997 Aug 19;94(17):8948–8953. doi: 10.1073/pnas.94.17.8948. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Schlenoff JB, Xu H. Evolution of Physical and Electrochemical Properties of Polypyrrole during Extended Oxidation. J Electrochem Soc. 1992 Sep;139(9):2397–2401. [Google Scholar]
- 315.Cui X, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensors and Actuators B. 2003;89:92–102. [Google Scholar]
- 316.Xiao Y, Cui X, Hancock JM, Bouguettaya M, Reynolds JR, Martin DC. Electrochemical polymerization of poly(hydroxymethylated-3,4-ethylenedioxythiophene) (PEDOT-MeOH) on multichannel neural probes. Sensors and Actuators B: Chemical. 2004;99(2–3):437–443. [Google Scholar]
- 317.Yang J, Kim DH, Hendricks JL, Leach M, Northey R, Martin DC. Ordered surfactanttemplated poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomater. 2005;1:125–136. doi: 10.1016/j.actbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 318.Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim D-H, Martin DC. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28(8):1539–1552. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Richardson-Burns SM, Hendricks JL, Martin DC. Electrochemical polymerization of conducting polymers in living neural tissue. J Neural Eng. 2007 Jun;4(2):L6–L13. doi: 10.1088/1741-2560/4/2/L02. [DOI] [PubMed] [Google Scholar]
- 320.Ludwig KA, Langhals NB, Joseph MD, Richardson-Burns SM, Hendricks JL, Kipke DR. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J Neural Eng. 2011 Feb;8(1):014001. doi: 10.1088/1741-2560/8/1/014001. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kozai TDY, Langhals NB, Patel PR, Deng XP, Zhang HN, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nature Materials. 2012 Dec;11(12):1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yamato H, Ohwa M, Wernet W. Stability of polypyrrole and poly(3,4-ethylenedioxythiophene) for biosensor application. Journal of Electroanalytical Chemistry. 1995 Nov;397(1–2):163–170. [Google Scholar]
- 323.Mandal HS, Knaack GL, Charkhkar H, McHail DG, Kastee JS, Dumas TC, Peixoto N, Rubinson JF, Pancrazio JJ. Improving the performance of poly(3,4-ethylenedioxythiophene) for brain–machine interface applications. Acta Biomater. 2014;10(6):2446–2454. doi: 10.1016/j.actbio.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 324.Aguilar Z. Nanomaterials for Medical Applications. 1 ed. Elsevier; 2012. [Google Scholar]
- 325.Mozafari MR, editor. Nanomaterials and Nanosystems for Biomedical Applications. 1 ed. Springer; 2007. [Google Scholar]
- 326.Michael Giersig, Khomutov GB, et al., editors. Nanomaterials for Application in Medicine and Biolog. 1 ed. Springer; 2008. [Google Scholar]
- 327.Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L. Nanomaterials for Neural Interfaces. Adv Mater. 2009;21(40):3970–4004. [Google Scholar]
- 328.Pancrazio JJ. Neural interfaces at the nanoscale. Nanomedicine. 2008 Dec;3(6):823–830. doi: 10.2217/17435889.3.6.823. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005 Feb 15;141(2):171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 330.Silva GA. Neuroscience nanotechnology: Progress, opportunities and challenges. Nat Rev Neurosci. 2006 Jan;7(1):65–74. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
- 331.Mohammed JS, DeCoster MA, McShane MJ. Micropatterning of nanoengineered surfaces to study neuronal cell attachment in vitro. Biomacromolecules. 2004 Sep-Oct;5(5):1745–1755. doi: 10.1021/bm0498631. [DOI] [PubMed] [Google Scholar]
- 332.Ai H, Meng H, Ichinose I, Jones SA, Mills DK, Lvov YM, Qiao X. Biocompatibility of layer-by-layer self-assembled nanofilm on silicone rubber for neurons. J Neurosci Methods. 2003 Sep 30;128(1–2):1–8. doi: 10.1016/s0165-0270(03)00191-2. [DOI] [PubMed] [Google Scholar]
- 333.Alivisatos AP, Andrews AM, Boyden ES, Chun M, Church GM, Deisseroth K, Donoghue JP, Fraser SE, Lippincott-Schwartz J, Looger LL, Masmanidis S, McEuen PL, Nurmikko AV, Park H, Peterka DS, Reid C, Roukes ML, Scherer A, Schnitzer M, Sejnowski TJ, Shepard KL, Tsao D, Turrigiano G, Weiss PS, Xu C, Yuste R, Zhuang X. Nanotools for Neuroscience and Brain Activity Mapping. [2013/03/26];ACS Nano. 2013 7(3):1850–1866. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Voge CM, Stegemann JP. Carbon nanotubes in neural interfacing applications. J Neural Eng. 2011 Feb;8(1):011001. doi: 10.1088/1741-2560/8/1/011001. [Review]. [DOI] [PubMed] [Google Scholar]
- 335.Hanein Y, Bareket-Keren L. Carbon nanotube based multi electrode arrays for neuronal interfacing: progress and prospects. Frontiers in Neural Circuits. 2013 Jan 9;6 doi: 10.3389/fncir.2012.00122. [Review]. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mattson M, Haddon R, Rao A. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. [2000/06/01];J Mol Neurosci. 2000 14(3):175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 337.Gheith MK, Pappas TC, Liopo AV, Sinani VA, Shim BS, Motamedi M, Wicksted JP, Kotov NA. Stimulation of Neural Cells by Lateral Currents in Conductive Layer-by-Layer Films of Single-Walled Carbon Nanotubes. Adv Mater. 2006;18(22):2975–2979. [Google Scholar]
- 338.Gheith MK, Sinani VA, Wicksted JP, Matts RL, Kotov NA. Single-Walled Carbon Nanotube Polyelectrolyte Multilayers and Freestanding Films as a Biocompatible Platform for Neuroprosthetic Implants. Adv Mater. 2005;17(22):2663–2670. [Google Scholar]
- 339.Malarkey EB, Fisher KA, Bekyarova E, Liu W, Haddon RC, Parpura V. Conductive Single-Walled Carbon Nanotube Substrates Modulate Neuronal Growth. [2009/01/14];Nano Letters. 2008 9(1):264–268. doi: 10.1021/nl802855c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. [2005/06/01];Nano Letters. 2005 5(6):1107–1110. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- 341.Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically Functionalized Carbon Nanotubes as Substrates for Neuronal Growth. [2004/03/01];Nano Letters. 2004 4(3):507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang K, Fishman HA, Dai H, Harris JS. Neural Stimulation with a Carbon Nanotube Microelectrode Array. [2006/09/01];Nano Letters. 2006 6(9):2043–2048. doi: 10.1021/nl061241t. [DOI] [PubMed] [Google Scholar]
- 343.Mazzatenta A, Giugliano M, Campidelli S, Gambazzi L, Businaro L, Markram H, Prato M, Ballerini L. Interfacing neurons with carbon nanotubes: Electrical signal transfer and synaptic stimulation in cultured brain circuits. J Neurosci. 2007 Jun 27;27(26):6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M, Scaini D, Gelain F, Casalis L, Prato M, Giugliano M, Ballerini L. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009 Feb;4(2):126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 345.Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nat Nanotechnol. 2008 Jul;3(7):434–439. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- 346.Luo X, Weaver CL, Zhou DD, Greenberg R, Cui XT. Highly stable carbon nanotube doped poly(3,4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials. 2011;32(24):5551–5557. doi: 10.1016/j.biomaterials.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Baranauskas G, Maggiolini E, Castagnola E, Ansaldo A, Mazzoni A, Angotzi GN, Vato A, Ricci D, Panzeri S, Fadiga L. Carbon nanotube composite coating of neural microelectrodes preferentially improves the multiunit signal-to-noise ratio. J Neural Eng. 2011 Dec;8(6):066013. doi: 10.1088/1741-2560/8/6/066013. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 348.Lu Y, Li T, Zhao XQ, Li M, Cao YL, Yang HX, Duan YWY. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials. 2010 Jul;31(19):5169–5181. doi: 10.1016/j.biomaterials.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 349.Lin C-M, Lee Y-T, Yeh S-R, Fang W. Flexible carbon nanotubes electrode for neural recording. Biosens Bioelectron. 2009 May 15;24(9):2791–2797. doi: 10.1016/j.bios.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 350.Hsu H-L, Teng IJ, Chen Y-C, Hsu W-L, Lee Y-T, Yen S-J, Su H-C, Yeh S-R, Chen H, Yew T-R. Flexible UV-Ozone-Modified Carbon Nanotube Electrodes for Neuronal Recording. Adv Mater. 2010;22(19):2177–2181. doi: 10.1002/adma.200903413. [DOI] [PubMed] [Google Scholar]
- 351.Chen Y-C, Hsu H-L, Lee Y-T, Su H-C, Yen S-J, Chen C-H, Hsu W-L, Yew T-R, Yeh SR, Yao D-J, Chang Y-C, Chen H. An active, flexible carbon nanotube microelectrode array for recording electrocorticograms. J Neural Eng. 2011;8(3):034001. doi: 10.1088/1741-2560/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 352.Gabay T, Ben-David M, Kalifa I, Sorkin R, Abrams ZeR, Ben-Jacob E, Hanein Y. Electro-chemical and biological properties of carbon nanotube based multi-electrode arrays. Nanotechnology. 2007;18(3):035201. doi: 10.1088/0957-4484/18/3/035201. [DOI] [PubMed] [Google Scholar]
- 353.Hanein Y. Carbon nanotube integration into MEMS devices. physica status solidi (b) 2010;247(11–12):2635–2640. [Google Scholar]
- 354.Nguyen-Vu TDB, Chen H, Cassell AM, Andrews R, Meyyappan M, Li J. Vertically Aligned Carbon Nanofiber Arrays: An Advance toward Electrical–Neural Interfaces. Small. 2006;2(1):89–94. doi: 10.1002/smll.200500175. [DOI] [PubMed] [Google Scholar]
- 355.Li J, Nguyen-Vu TDB, Chen H, Cassell AM, Andrews RJ, Meyyappan M. Vertically aligned carbon nanofiber architecture as a multifunctional 3-D neural electrical interface. IEEE Trans Biomed Eng. 2007 Jun;54(6):1121–1128. doi: 10.1109/TBME.2007.891169. [DOI] [PubMed] [Google Scholar]
- 356.Agarwal S, Zhou X, Ye F, He Q, Chen GCK, Soo J, Boey F, Zhang H, Chen P. Interfacing Live Cells with Nanocarbon Substrates. [2010/02/16];Langmuir. 2010 26(4):2244–2247. doi: 10.1021/la9048743. [DOI] [PubMed] [Google Scholar]
- 357.Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, Liu L, Jin G, Tang M, Cheng G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32(35):9374–9382. doi: 10.1016/j.biomaterials.2011.08.065. [DOI] [PubMed] [Google Scholar]
- 358.Lv M, Zhang Y, Liang L, Wei M, Hu W, Li X, Huang Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale. 2012;4(13):3861–3866. doi: 10.1039/c2nr30407d. [DOI] [PubMed] [Google Scholar]
- 359.Chen CH, Lin CT, Chen JJ, Hsu WL, Chang YC, Yeh SR, Li LJ, Yao DJ, editors. A graphene-based microelectrode for recording neural signals. Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), 2011 16th International; 5–9 June 2011.2011. [Google Scholar]
- 360.Luo X, Weaver CL, Tan S, Cui XT. Pure Graphene Oxide Doped Conducting Polymer Nanocomposite for Bio-Interfacing. Journal of Materials Chemistry B. 2013;1(9):1340–1348. doi: 10.1039/C3TB00006K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29(9):1273–1283. doi: 10.1016/j.biomaterials.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly(3,4-ethylenedioxythiophene) Nanotubes. Adv Mater. 2009;21(37):3764–3770. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Abidian MR, Corey JM, Kipke DR, Martin DC. Conducting-Polymer Nanotubes Improve Electrical Properties, Mechanical Adhesion, Neural Attachment, and Neurite Outgrowth of Neural Electrodes. Small. 2010 Feb 5;6(3):421–429. doi: 10.1002/smll.200901868. [DOI] [PMC free article] [PubMed] [Google Scholar]