Abstract
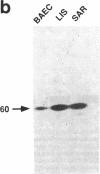
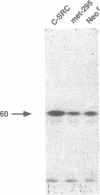
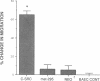
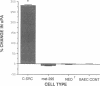
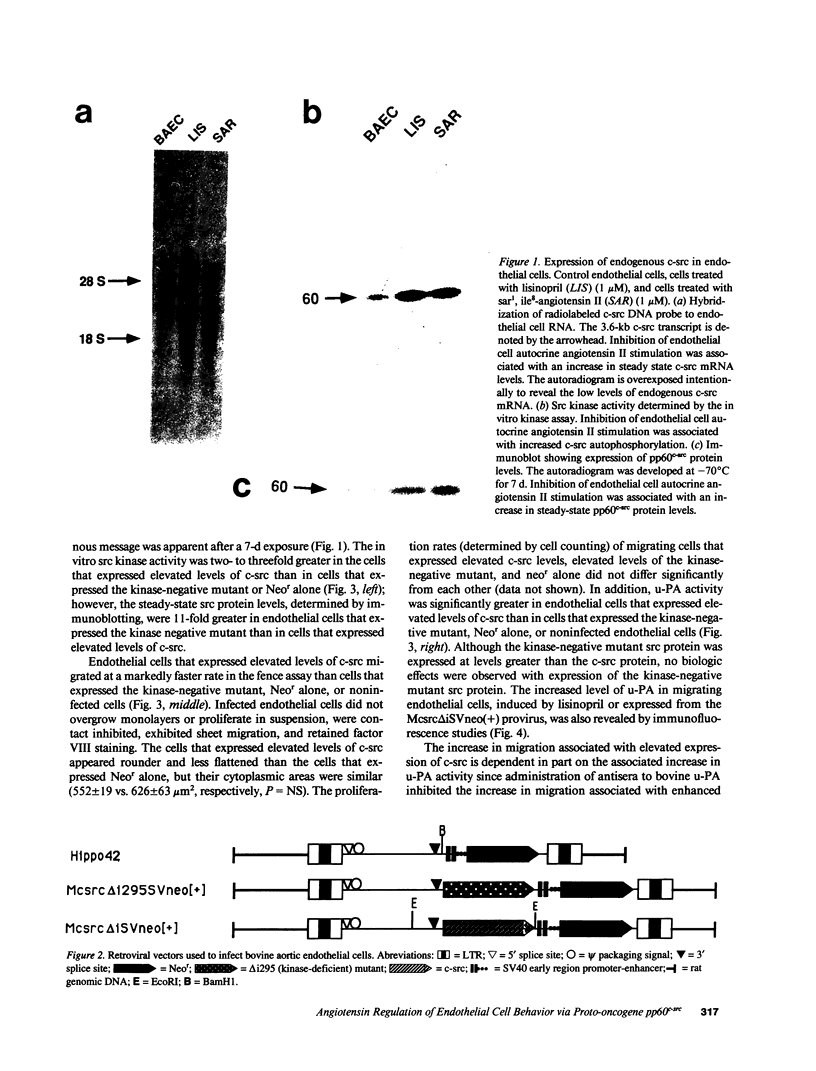
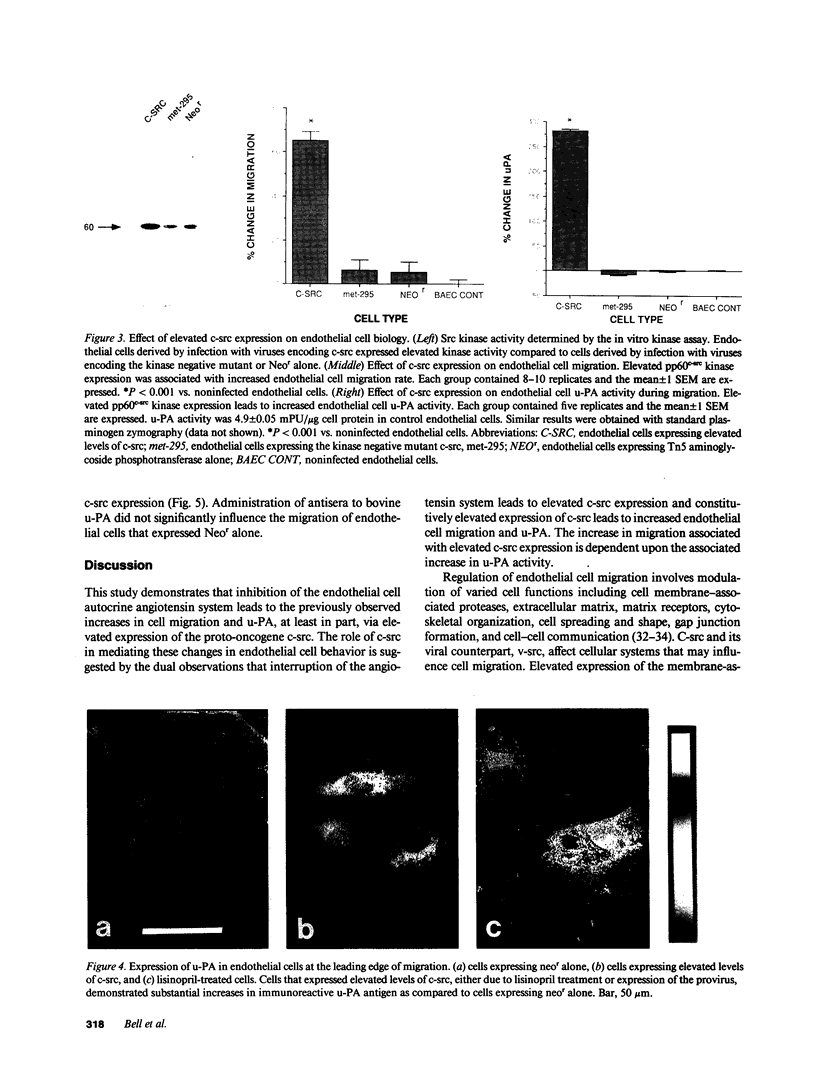
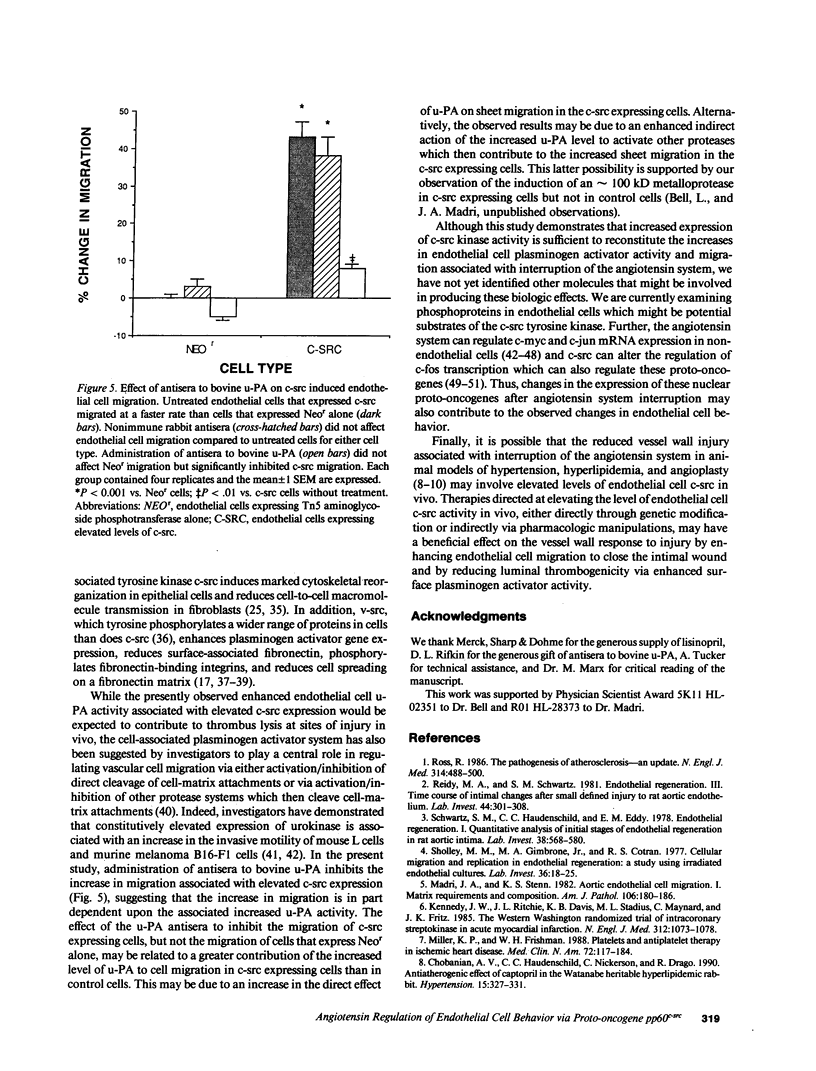
Rapid endothelial cell migration and inhibition of thrombosis are critical for the resolution of denudation injuries to the vessel wall. Inhibition of the endothelial cell autocrine angiotensin system, with either the angiotensin-converting enzyme inhibitor lisinopril or the angiotensin II receptor antagonist sar1, ile8-angiotensin II, leads to increased endothelial cell migration and urokinase-like plasminogen activator (u-PA) activity (Bell, L., and J. A. Madri. 1990. Am. J. Pathol. 137:7-12). Inhibition of the autocrine angiotensin system with the converting-enzyme inhibitor or the receptor antagonist also leads to increased expression of the proto-oncogene c-src: pp60c-src mRNA increased 7-11-fold, c-src protein 3-fold, and c-src kinase activity 2-3-fold. Endothelial cell expression of c-src was constitutively elevated after stable infection with a retroviral vector containing the c-src coding sequence. Constitutively increased c-src kinase activity reconstituted the increases in migration and u-PA observed with angiotensin system interruption. Antisera to bovine u-PA blocked the increase in migration associated with increased c-src expression. These data suggest that increases in endothelial cell migration and plasminogen activator after angiotensin system inhibition are at least partially pp60c-src mediated. Elevated c-src expression with angiotensin system inhibition may act to enhance intimal wound closure and to reduce luminal thrombogenicity in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Kawahara Y., Kariya K., Sunako M., Tsuda T., Fukuzaki H., Yoshimi T. Stimulation of platelet-derived growth factor-induced DNA synthesis by angiotensin II in rabbit vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 Apr 16;168(1):350–357. doi: 10.1016/0006-291x(90)91715-5. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Bell L., Madri J. A. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989 Oct;65(4):1057–1065. doi: 10.1161/01.res.65.4.1057. [DOI] [PubMed] [Google Scholar]
- Bell L., Madri J. A. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990 Jul;137(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Bell S. M., Brackenbury R. W., Leslie N. D., Degen J. L. Plasminogen activator gene expression is induced by the src oncogene product and tumor promoters. J Biol Chem. 1990 Jan 25;265(3):1333–1338. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cajot J. F., Schleuning W. D., Medcalf R. L., Bamat J., Testuz J., Liebermann L., Sordat B. Mouse L cells expressing human prourokinase-type plasminogen activator: effects on extracellular matrix degradation and invasion. J Cell Biol. 1989 Aug;109(2):915–925. doi: 10.1083/jcb.109.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V., Haudenschild C. C., Nickerson C., Drago R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990 Mar;15(3):327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Salvo J., Gifford D., Kokkinakis A. pp60c-src kinase activity in bovine coronary extracts is stimulated by ATP. Biochem Biophys Res Commun. 1988 May 31;153(1):388–394. doi: 10.1016/s0006-291x(88)81236-1. [DOI] [PubMed] [Google Scholar]
- Dorai T., Wang L. H. An alternative non-tyrosine protein kinase product of the c-src gene in chicken skeletal muscle. Mol Cell Biol. 1990 Aug;10(8):4068–4079. doi: 10.1128/mcb.10.8.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Kornbluth S., Hanafusa H. Enzymatically inactive p60c-src mutant with altered ATP-binding site is fully phosphorylated in its carboxy-terminal regulatory region. Cell. 1987 Sep 11;50(6):937–943. doi: 10.1016/0092-8674(87)90520-4. [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Sunako M., Tsuda T., Fukuzaki H., Fukumoto Y., Takai Y. Angiotensin II induces expression of the c-fos gene through protein kinase C activation and calcium ion mobilization in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):52–59. doi: 10.1016/0006-291x(88)90485-8. [DOI] [PubMed] [Google Scholar]
- Kellie S., Patel B., Mitchell A., Critchley D. R., Wigglesworth N. M., Wyke J. A. Comparison of the relative importance of tyrosine-specific vinculin phosphorylation and the loss of surface-associated fibronectin in the morphology of cells transformed by Rous sarcoma virus. J Cell Sci. 1986 Jun;82:129–142. doi: 10.1242/jcs.82.1.129. [DOI] [PubMed] [Google Scholar]
- Kennedy J. W., Ritchie J. L., Davis K. B., Stadius M. L., Maynard C., Fritz J. K. The western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction. A 12-month follow-up report. N Engl J Med. 1985 Apr 25;312(17):1073–1078. doi: 10.1056/NEJM198504253121701. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Nachman R. L. Matrix plasminogen activator inhibitor. Modulation of the extracellular proteolytic environment. J Biol Chem. 1988 Jul 5;263(19):9476–9481. [PubMed] [Google Scholar]
- Lilly L. S., Pratt R. E., Alexander R. W., Larson D. M., Ellison K. E., Gimbrone M. A., Jr, Dzau V. J. Renin expression by vascular endothelial cells in culture. Circ Res. 1985 Aug;57(2):312–318. doi: 10.1161/01.res.57.2.312. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Bell L., Marx M., Merwin J. R., Basson C., Prinz C. Effects of soluble factors and extracellular matrix components on vascular cell behavior in vitro and in vivo: models of de-endothelialization and repair. J Cell Biochem. 1991 Feb;45(2):123–130. doi: 10.1002/jcb.240450202. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Stenn K. S. Aortic endothelial cell migration. I. Matrix requirements and composition. Am J Pathol. 1982 Feb;106(2):180–186. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M., Lin V. K. Comparison of two different hybridization systems in northern transfer analysis. Biotechniques. 1989 Apr;7(4):331-2, 334. [PubMed] [Google Scholar]
- Miller K. P., Frishman W. H. Platelets and antiplatelet therapy in ischemic heart disease. Med Clin North Am. 1988 Jan;72(1):117–184. doi: 10.1016/s0025-7125(16)30788-x. [DOI] [PubMed] [Google Scholar]
- Moalic J. M., Bauters C., Himbert D., Bercovici J., Mouas C., Guicheney P., Baudoin-Legros M., Rappaport L., Emanoil-Ravier R., Mezger V. Phenylephrine, vasopressin and angiotensin II as determinants of proto-oncogene and heat-shock protein gene expression in adult rat heart and aorta. J Hypertens. 1989 Mar;7(3):195–201. [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Eldridge C. S., Lin H. L., Dzau V. J. Angiotensin II induces c-fos expression in smooth muscle via transcriptional control. Hypertension. 1989 Jun;13(6 Pt 2):706–711. doi: 10.1161/01.hyp.13.6.706. [DOI] [PubMed] [Google Scholar]
- Owens G. K. Influence of blood pressure on development of aortic medial smooth muscle hypertrophy in spontaneously hypertensive rats. Hypertension. 1987 Feb;9(2):178–187. doi: 10.1161/01.hyp.9.2.178. [DOI] [PubMed] [Google Scholar]
- Patel J. M., Yarid F. R., Block E. R., Raizada M. K. Angiotensin receptors in pulmonary arterial and aortic endothelial cells. Am J Physiol. 1989 May;256(5 Pt 1):C987–C993. doi: 10.1152/ajpcell.1989.256.5.C987. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Spray D. C., Chanson M., Montesano R., Orci L., Meda P. Junctional communication is induced in migrating capillary endothelial cells. J Cell Biol. 1989 Dec;109(6 Pt 1):3027–3038. doi: 10.1083/jcb.109.6.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Rohrschneider L., Reynolds S. Regulation of cellular morphology by the Rous sarcoma virus src gene: analysis of fusiform mutants. Mol Cell Biol. 1985 Nov;5(11):3097–3107. doi: 10.1128/mcb.5.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Ryan J. W., Whitaker C., Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Haudenschild C. C., Eddy E. M. Endothelial regneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest. 1978 May;38(5):568–580. [PubMed] [Google Scholar]
- Selden S. C., 3rd, Schwartz S. M. Cytochalasin B inhibition of endothelial proliferation at wound edges in vitro. J Cell Biol. 1979 May;81(2):348–354. doi: 10.1083/jcb.81.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholley M. M., Gimbrone M. A., Jr, Cotran R. S. Cellular migration and replication in endothelial regeneration: a study using irradiated endothelial cultures. Lab Invest. 1977 Jan;36(1):18–25. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Takimoto M., Quinn J. P., Farina A. R., Staudt L. M., Levens D. fos/jun and octamer-binding protein interact with a common site in a negative element of the human c-myc gene. J Biol Chem. 1989 May 25;264(15):8992–8999. [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Taubman M. B., Berk B. C., Izumo S., Tsuda T., Alexander R. W., Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem. 1989 Jan 5;264(1):526–530. [PubMed] [Google Scholar]
- Wang L. H., Iijima S., Dorai T., Lin B. Regulation of the expression of proto-oncogene c-src by alternative RNA splicing in chicken skeletal muscle. Oncogene Res. 1987 Jun;1(1):43–59. [PubMed] [Google Scholar]
- Warren S. L., Handel L. M., Nelson W. J. Elevated expression of pp60c-src alters a selective morphogenetic property of epithelial cells in vitro without a mitogenic effect. Mol Cell Biol. 1988 Feb;8(2):632–646. doi: 10.1128/mcb.8.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. R., Schultz R. M. Relationship between secreted urokinase plasminogen activator activity and metastatic potential in murine B16 cells transfected with human urokinase sense and antisense genes. Cancer Res. 1990 Dec 1;50(23):7623–7633. [PubMed] [Google Scholar]