Abstract
Background
Type I collagen is an abundant natural polymer with several applications in medicine as matrix to regenerate tissues. Silver nanoparticles is an important nanotechnology material with many utilities in some areas such as medicine, biology and chemistry. The present study focused on the synthesis of silver nanoparticles (AgNPs) stabilized with type I collagen (AgNPcol) to build a nanomaterial with biological utility. Three formulations of AgNPcol were physicochemical characterized, antibacterial activity in vitro and cell viability assays were analyzed. AgNPcol was characterized by means of the following: ultraviolet–visible spectroscopy, dynamic light scattering analysis, Fourier transform infrared spectroscopy, atomic absorption analysis, transmission electron microscopy and of X-ray diffraction analysis.
Results
All AgNPcol showed spherical and positive zeta potential. The AgNPcol at a molar ratio of 1:6 showed better characteristics, smaller hydrodynamic diameter (64.34 ± 16.05) and polydispersity index (0.40 ± 0.05), and higher absorbance and silver reduction efficiency (0.645 mM), when compared with the particles prepared in other mixing ratios. Furthermore, these particles showed antimicrobial activity against both Staphylococcus aureus and Escherichia coli and no toxicity to the cells at the examined concentrations.
Conclusions
The resulted particles exhibited favorable characteristics, including the spherical shape, diameter between 64.34 nm and 81.76 nm, positive zeta potential, antibacterial activity, and non-toxicity to the tested cells (OSCC).
Electronic supplementary material
The online version of this article (doi:10.1186/s12951-014-0036-6) contains supplementary material, which is available to authorized users.
Keywords: Silver nanoparticles, Collagen, Antimicrobial activity, Cell viability
Background
Collagen is the most abundant protein constituting to the 30% of total protein and 6% of animal body weight [1,2]. Type I collagen, a natural polymer, is a major extracellular matrix protein in mammals and exhibits favorable characteristics for promoting cell proliferation [3-5]. It can influence the cell physiology and morphology [4,6], create a good matrix for endothelial cells in vitro, induce platelet aggregation, promote blood clotting, and consequently accelerate the healing of skin wounds [7].
Since 1980s, some scientists have been using collagen as a matrix to regenerate tissues for repairing skin [8], bone [9], knee meniscal [10], joint cartilage [11], esophagus [12], dura mater [13], muscle [14] and nervous system [15]. The use of collagen combined with glycosaminoglycans as a skin implant has been already tested [16,17]. The ability of collagen gel to regenerate cornea and nerves has been also demonstrated by recent animal studies and clinical trials [18,19]. Furthermore, it has been shown that the combined collagen and hyaluronic acid can promote the revascularization of tissues in animal models [20].
In the field of nanotechnology, collagen scaffold has been widely used in biological experiments for introducing chemical and pharmaceutical substances. Bakare et al. [21] proposed a method for constructing a film by using poly(hydroxybutyrate valerate) (PHBV) grafted with scaffold tipo I collagen to support silver nanoparticles (AgNPs). Jithendra et al. [22] suggested a blend of Aloe Vera with collagen and chitosan scaffold for tissue engineering applications.
Metal nanoparticle, especially those made of noble metals, show excellent properties for biotechnology applications [23–25]. In particular, AgNPs have established a broad range of applications in the majority of biomedical studies [26], due to their antibacterial ability and selective toxicity to microorganisms [27].
In addition, AgNPs are widely used in various medical and industrial fields for venous catheters coating; vascular prostheses manufacturing; wound dressing manufacturing; treatment for chronic wounds and ulcers [25]; or as a constituent incorporated into cement for the realignment of bone fractures [27], in to water purification filter [28] and into wall paint for providing an aseptic environment to hospital patients [29].
The ability of AgNPs to control bacterial activity relies on the interactions with three major structural components of the bacteria: namely peptidoglycan in the cell wall, DNA, and proteins, by mainly affecting the enzymes involved in the electron transport chain [30–33].
The ideal properties of AgNPs for biomedical applications include prolonged effectiveness, high levels of bactericidal and bacteriostatic activity, ability to prevent a broad spectrum of bacteria, high biocompatibility, and low toxicity in vivo [33]. In particular, the shape and concentration of AgNPs in solutions are important factors in ensuring the effective contact of the particles with the bacterial membranes and in determining the amount of AgNPs for effectively inhibiting the targeting bacteria [34].
Some literatures reported the application of AgNPs for treating the wounds of mice, and these particles showed excellent tensile properties and resulted in improved alignment of fibers for skin repair [35,36].
Based on the previously discussed properties and applications of collagen and AgNPs, we designed and synthesized three types of AgNPs stabilized with type I collagen (AgNPcols) by using a chemical synthesis route in the present study. This article presents their chemical synthesis, physicochemical characterization, analysis of activity against gram-positive and gram-negative bacteria, and in vitro cell viability assays.
Results and discussion
Type I collagen is the most abundant protein in mammals and is present during tissue repair [1–5,7]. Although collagen has been used in biomedical research for several years, AgNPs stabilized with collagen, as well as their biocompatibility and antibacterial properties, have been recently reported by Alarcon et al. [37]. The authors used a photochemical route for fabricating AgNPs from silver nitrate (AgNO3), and this route was different from the chemical route employed in this study, where a reducing agent, sodium borohydride (NaBH4), was involved. Because NaBH4 is unstable when being in contact with water at room temperature, it is necessary to stabilize NaBH4 by using ultra-pure water at low temperature (4°C) and keep the solution refrigerated until use. In addition, Sun et al. [38] reported the use of NaBH4 for the synthesis AgNPs associated to a trisodium citrate solution. Thereafter, a multilayer film consisting of AgNPs and collagen in a layer-by-layer (LbL) configuration is generally constructed for stabilizing the particles.
An exclusive study on AgNPs stabilized by collagen has been reported [37]. Based on this study, we designed and synthesized three different formulations of AgNPs, at AgNO3 to NaBH4 molar ratios of 1:1, 1:6, and 1:15, by varying the concentration of NaBH4 to obtain the best silver (Ag0) reduction result in solution. The solution at the AgNO3/NaBH4 molar ratio of 1:6 resulted in a final Ag0 concentration of 0.64 mM (Table 1), as confirmed by the atomic absorption test. In the ratio of 1:1 between AgNO3 and NaBH4, the amount of reducer was not sufficient to reduce all molecules of silver. At ratio of 1:6 was obtained the best concentration for the chemical reaction, probably the molecules amount of AgNO3 and NaBH4 reached an optimum value for reduction. However, the ratio was 1:15 excess NaBH4 causing release of ions in solution and forming nanoparticles with hydrodynamic diameter higher by aggregation [39].
Table 1.
Diameter, zeta potential, PDI of AgNPcols (mean ± standard deviation) and molar concentration of silver in the solution
| Diameter (nm) | Zeta Potential (mv) | PDI* | [Ag] (mM) | |
|---|---|---|---|---|
| AgNPcol (1:1) | 78.87 ± 12.89 | 31.8 ± 0.62 | 0.60 ± 0.02 | 0.434 |
| AgNPcol (1:6) | 64.34 ± 16.05 | 24.9 ± 0.79 | 0.10 ± 0.05 | 0.645 |
| AgNPcol (1:15) | 81.76 ± 18.22 | 19.9 ± 0.4 | 0.77 ± 0.17 | 0.345 |
*PDI: polydisperity index.
All synthesized solutions were characterized in terms of particle size, zeta potential, and polydispersity index (PDI) by dynamic light scattering (DLS) analysis. A positive potential (19.9–31.8 mV) was obtained for all AgNPcols (Table 1). This occurs due to amino group carries a positive charge and is present in AgNPcol [40,41] (Figure 1D). The hydrodynamic diameter of the nanoparticles was between 64.34 nm and 81.76 nm and PDI value was between 0.40 and 0.77. The AgNPs associated with titanium dioxide synthesized by Desai and Kowshik [42] showed PDI value of 0.47, which was very close to the result obtained from this study, although other PDI values were also reported [43–45]. The value of PDI is one of the major parameters used for selecting a low-polydispersity solution for the subsequent cell viability test.
Figure 1.
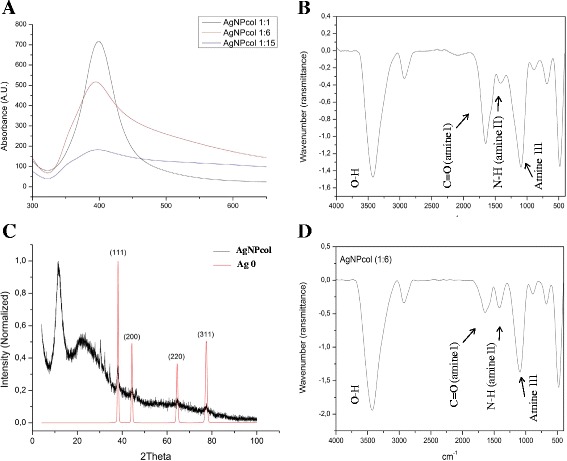
AgNPcol characterization. (A) Absorbance spectra of AgNPcols at three different NaBH4 to AgNO3 molar ratios; (B) FTIR spectra of collagen; (C) XDR patterns of AgNPcol (1:6 molar ratio); (D) FTIR spectra of AgNPcol (1:6 molar ratio).
The presence of a positive zeta potential favors the interaction between the particles and Gram-negative and Gram-positive bacteria [46]. The efficiency of ionic silver against bacteria with negatively charged membranes is related to the electrostatic attraction caused by the positive potentials of the particles [47]. In the present study, the positive zeta potential of AgNPcols is one of the aspects that may explain the favorable result of its acting against E. coli and S. aureus. Hamouda and Baker [48] reported that the opposite surface charges could promote the interactions between the bacterial membranes and AgNPs. They also mentioned that, due to the small size of nanoparticles, the tested solutions could easily permeate the membranes of the bacteria and promote their death [34,49]. Furthermore, Baker et al. [50] reported that small particles with large contact areas showed increased efficiency against bacteria, as compared with the particles with large sizes. Saptarshi et al. [51] suggests that associate protein with nanoparticle, there are better cell absorption because the protein favors interaction with cell membrane facilitating interaction to nanoparticle with bacteria and another live cells.
The increase in the proportion of NaBH4 during particle synthesis could reduce the zeta potential of the resulting particles. Zhang and Wu [39] reported the same behavior of gold nanoparticles and claimed that this was due to the aggregation of metal particles. The AgNPcols produced in our study demonstrated similar behaviors, as indicated by the decrease in the amount of nanoparticles in the solution. This is because that the aggregated Ag0 formed larger particles, resulting in a polydisperse solution and precipitation during centrifugation. The relationship between the increases of the ratio of NaBH4 in relation to the increase in diameter of the nanoparticles is due to the release of electrons caused by NaBH4. Because when an increase occurs in the concentration of NaBH4 increases the number of free electrons in the solution and decreases the zeta potential favors the aggregation of silver [39].
After synthesis, all solutions were characterized by using ultraviolet–visible (UV–vis) spectroscopy analysis, which was efficient for detecting the sensitive AgNPs that could display a strong absorption peak [52,53]. In this study, we found a wider plasmon band and lower absorption peak intensity for the solution at a higher NaBH4 to AgNO3 molar ratio, as compared with the solutions at lower molar ratios (Figure 1A). It is believed that this is due to the aggregation of Ag0 molecules [53,54], as indicated by the values of zeta potential described above and the presence of large particles in AgNPcol at molar ratio of 1:15. We can also notice the presence of the plasmon band between 380 nm and 450 nm, and this is indicative of the spherical shape of AgNPs [55], which can be further confirmed by transmission electron microscopy (TEM) analysis (Figure 2). Some researchers [35,56] reported that the spherical shape is the optimal morphology for nanoparticles against bacteria, as it can facilitate the interaction between the particles and the bacterial membranes.
Figure 2.
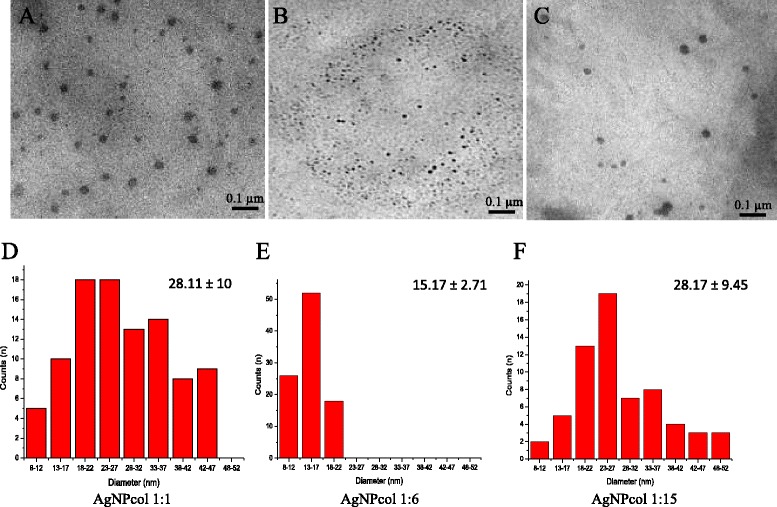
Images of Silver nanoparticle stabilized with collagen. TEM images of AgNPcol at AgNO3/NaBH4 molar ratio of (A) 1:1, (B) 1:6, and (C) 1:15 molar ratio. Histograms showing the particle size distribution of AgNPcol at molar ratio of (D) 1:1 (28.11 ± 10 nm), (E) 1:6 (15.17 ± 2.71 nm), and (F) 1:15 (28.17 ± 9.45 nm) (scale bar = 0.1 μm).
The difference in size AgNPcol found between the results of Table 1 (DLS) and Figure 2 (MET) occurs because the DLS diameter is measured in solution (hydrodynamic diameter value). Already in the TEM, the nanoparticle is no in solution and the result is a projected estimate of the diameter of the nanoparticle. The hydrodynamic diameter of the nanoparticles (DLS) was highlight, because this nanoparticle was developed for use in a biological environment and will be in solution [57].
From the Fourier transform infrared spectra (FTIR) of collagen and AgNPcol (1:6 molar ratio) (Figure 1B and D), we can observe the presence of C = O (amine I at 1652.84 cm−1) and NH bands (amine II at 1571.64 cm−1) in both samples of collagen and AgNPcol. However, the band due to C = O (amine I at 1652.84 cm−1) in the spectrum of AgNPcol showed a low intensity, indicating that this group was possibly involved in the reduction and stabilization of AgNPs. Sun et al. [38] suggested that these changes were due to the association of collagen molecules (i.e., amines) with AgNPs.
The phases of the samples were determined by X-ray powder diffraction (XDR) analysis by searching against databases. In Figure 1C, it can see the silver peaks (ICSD: 44387-Ag0), with reflections identified. Traces of silver oxides (ICSD: 35540-Ag2O, 27659-AgO, 15999/59193-Ag2O3, 202218-Ag3O4) were also identified from the sample. Silver oxides reflections can be found in [Additional file 1]. This result is consistent with our expectation that oxidation products can be formed on the surface of pure silver. In addition, we found that the amorphous phase of collagen affected the sample’s crystallinity, resulting in its semi-crystalline state.
The activity of AgNPcols against the gram-positive bacterium, Staphylococcus aureus (ATCC 29213), and gram-negative bacterium, E. coli (ATCC 25922), was tested by using an AgNO3 solution as control. Based on the results of the atomic absorption spectrometric study, the concentration of AgNPcols was corrected for the antimicrobial assays. It was found that the behavior of AgNPcol (1:6 molar ratio) for inhibiting the growth of bacteria was comparable to that of AgNO3 (Table 2). Thus, we may assume that the silver particle can maintain its antimicrobial property when being incorporated into the collagen-stabilized nanoparticles. In Alarcon’s study [37] about AgNPs stabilized with collagen, was lower than that of AgNO3 used as control.
Table 2.
Minimum inhibitory concentrations (MICs) of AgNPcol (μg Ag/mL), AgNO 3 (μg Ag/mL), and standard antibiotics (μg/mL) for inhibiting Staphylococcus aureus and Escherichia coli
| AgNPcol (μgAg/mL) | Controls | ||||
|---|---|---|---|---|---|
| Bacterial strains | 1:1 | 1:6 | 1:15 | AgNO3 (μgAg/mL) | Antibiotic (μg/mL) |
| S. aureus | 11.7 | 17.4 | 11.7 | 13.5 | <0.5a |
| E. coli | 11.7 | 8.7 | 11.7 | 6.75 | <0.5b |
aOxacilin.
bMeropenen.
For the cell viability test, we chose only one synthesized solution by analyzing the relevant characterization data. AgNPcol at the molar ratio of 1:6 was chosen for the test, due to its smaller particle size, lower PDI, and a higher percentage of Ag0 than those of other samples, as determined by atomic absorption spectroscopy. The results (Figure 3) indicated that the AgNPcol solution at the tested concentrations did no cause significant differences in cell viability as compared with the control (CT). In addition, AgNO3 and collagen (Col) were also used for comparison. It is known that collagen does not show any cytotoxicity towards cells, as this can be evidenced by its abundance in animals and the human body. Although AgNO3 solution was reported to be toxic to cells, the AgNPcol at the approximate Ag concentration as that of AgNO3 did not show any toxicity to the cells tested in this study. We attempted to use higher concentrations of AgNPcol to evaluate its cytotoxicity; however, precipitation occurred in the solution before incubation under the physiological pH and ambient temperature conditions.
Figure 3.
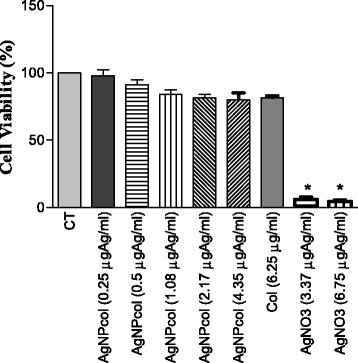
Cell viability. Results of cell viability test of AgNPcol and control solutions (Col and AgNO3). All data were expressed as mean ± SEM values of three independent experiments. A value of *p < 0.05 was considered statistically significance.
Gurunathan et al. [58] performed the cell viability test by using breast cancer cells (MDA-MB-23) and AgNP at 5 μg/ml, which did not exhibit cytotoxicity as compared with the control. In addition, Prokopovich et al. [59] synthesized the AgNPs by using NaBH4, and the produced nanoparticles were incorporated into the bone cement, which did not show cytotoxicity to osteoblast cells (MC 3TC) either.
The results of the present study showed that the synthesized AgNPcol was effective against the tested bacteria and was non-toxic to the examined cells. Further tests will be conducted to evaluate the in vivo cytotoxicity and healing ability of AgNPcol by using biological tissues and animal samples.
Conclusion
In the present study, we demonstrated the synthesis of an AgNP solution stabilized with type I collagen by using NaBH4 as a reducing agent. The resulted particles exhibited favorable characteristics, including the spherical shape, diameter between 64.34 nm and 81.76 nm, positive zeta potential, antibacterial activity, and non-toxicity to the tested cells (OSCC). It is found that the activity against bacterium is facilitated by the electrostatic interaction between the positively charged AgNPcols and the negatively charged bacterial membranes. Probably the shape, size and positive zeta potential of AgNPcols facilitates the activity against gram negative bacterium and gram positive bacterium. Furthermore, the cell viability test provides the basics for the future study that aims to investigate the in vivo behaviors of AgNPcols by using biological tissues.
Methods
Synthesis of collagen-based silver nanoparticles (AgNPcols)
A solution of silver nitrate (AgNO3) at a concentration of 108 μgAg/mL, a collagen type I from rat tail (Santa Cruz Biotechnology) solution at a concentration of 0.1 mg/ml and a solution of borohydride (NaBH4) at 3.78 mg/ml, prepared using ultrapure water at 4°C were used to carry out the synthesis of nanoparticles.
The AgNO3 solution was added to the collagen, both with the same volume and remained under agitation to homogenize for 10 min. The NaBH4 solution was added later, in the form of jet, for any solution of NaBH4, came in contact with the Beker solutions quickly and completely. This solution was stirred for 10 minutes to homogenize. Subsequently, the reaction mixture was centrifuged at 3600 rpm for 15 minutes and finally separated from the supernatants of the final solution present in the container. In the present study three different proportions of borohydride solution regarding Silver Nitrate (AgNO3) (molar ratio: 1:1, 1:6 e 1:15) were selected.
Physicochemical characterization of AgNPcols
The AgNPcols were characterized by UV–vis spectroscopy using a Shimadzu (UV 1800) spectrophotometer. Were subsequently characterized according to their size, electrical potential and PDI using the DLS (Malvern Zetasizer Nano ZS Model 3600) with laser with a wavelength of 633 nm and scattering angle of 90° all measurements were performed in triplicate. To verify the shape and confirm the diameter of the nanoparticles non-diluted samples were placed on two screens (20 μL) for transmission electron microscopy (TEM) previously coated with Formvar. After drying for 2 h at room temperature (25 ± 2°C) screens were analyzed in a Jeol JEM-1010 electron microscope and photomicrographed by an UltraScan® with Digital Micrograph 3.6.5 software (Gatan/USA) [25].
In order to quantify the percentage of silver in solution, the atomic absorption spectroscopy (Varian - Model AA240FS) was used, with a wavelength of 328.1 nm and multielement lamp (Varian No. 5610108700). The reading was held in atomic absorption flame with Oxygen and Acetylene gases.
XRD data were obtained at the Laboratory of X-ray Crystallography of IFSC/USP using a Rigaku Rotaflex diffractometer equipped with graphite monochromator and rotating anode tube, operating with Cu Ka, 50 kV and 100 mA. Powder diffraction patterns were obtained in step scanning mode, 2θ = 5–100°, step of 0.02° and 5 s/step. Peak Fitting Module program [60] was used for the peak decomposition of the semicrystalline pattern and determination of area due to the amorphous phase.
Evaluation of antibacterial activity of AgNPcols
To study the antibacterial properties of AgNPcols, by the determination of Minimum Inhibitory Concentration (MIC), two bacterial strains were selected: Staphylococcus aureus ATCC 29213 (Gram-positive) and Escherichia coli ATCC 25922 (Gram-negative). The microorganisms were cultured in Mueller-Hinton agar at 37°C for 24 hours in aerobic conditions. Then a suspension of bacterial strains with an optical density of McFarland of 0.5 (1 × 108 CFU/mL) was made in an isotonic sodium chloride 0.85% solution. Later in time, this solution was diluted ten times (1 × 107 CFU/mL) and used as inoculum in the experiment. MIC was determined according to protocols previously described [61–63] using 96-well microdilution plate with Mueller-Hinton broth where the strains (concentration of 5 × 105 CFU/mL) were exposed to two-fold dilution series of the AgNPcols with concentrations ranging from 34,8 to 0,36 μgAg/mL. The same procedure was used to determine the MIC of the following controls: collagen, AgNO3, and standard antibiotics effective against the tested bacterial strains with concentrations ranging from 27 to 0.42 μgAg/mL for AgNO3; 50 to 3,12 μg/mL for collagen and 32 to 0.5 μg/mL for antibiotics. Sterile Mueller-Hinton broth was used as the negative control and inoculated broth was used as the positive control. MIC was defined as the lowest concentration of agent that restricted the visual bacterial growth in the culture media.
Cell viability
For the study of cell viability, the AgNPcol 1:6 was diluted four times with the ratio of two, starting at a concentration of 4.35 μgAg/ml. The cell lines used in this study was oral squamous cell carcinoma (OSCC) obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in 75 cm2 flasks and maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, streptomycin/penicillin antibiotics and non-essential aminoacids. For the experiment the cells were seeded in a 96-well plate (5,000 cel./Well) and kept in an incubator (atmosphere at 37°C and humidified 5% CO2) for 24 hours. In a 96-well plate was added 5 different concentrations AgNPcol solutions and control solutions (medium, collagen - 6.25 μg/ml, AgNO3 - 3.37 μgAg/ml and AgNO3 - 6.75 μgAg/ml). The plates were kept in an incubator for 3 hours. Subsequently, the added solutions were removed and placed in Hank's buffer for 5 minutes. The buffer was removed and fresh medium was added. After 24 hours the medium was removed and added to a solution of DMEM without phenol and MTT ([3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (15%) to each well. The plates were incubated for 4 hours. Thereafter all the medium with MTT was removed with care to do not remove formazam produced by living cells. Finally, isopropanol was added to each well to solubilize the formazam and taken to review the reader Safire (TECAN EUA Inc., Durham, NC). Statistical analysis was performed using Prism 5.0 (GraphPad Software) by ANOVA and Tukey test. All data were expressed as mean and standard deviation of three independent experiments. We used the statistical significance of p <0.05 for this study [64].
Acknowledgements
We thank the Laboratory of Photobiology and Photomedicine of University of São Paulo, the Laboratory of Microscopy of University of Brasília and the Institute of Physics of São Carlos to contribute with this paper. ACM are grateful to FAPESP (2014/02282-6). YPM are grateful to CAPES (AUX-PERM-705/2009).
Abbreviations
- AgNPs
Silver nanoparticles
- AgNPcols
Silver nanoparticles stabilized with type I collagen
- AgNO3
Silver nitrate
- NaBH4
Sodium borohydride
- LbL
Layer-by-layer
- Ag0
Silver
- mM
Millimolar
- PDI
Polydispersity index
- UV–vis
Ultraviolet–visible
- DLS
Dynamic light scattering
- TEM
Transmission electron microscopy
- FTIR
Fourier transform infrared spectra
- XDR
X-ray powder diffraction
- MICs
Minimum inhibitory concentrations
- CT
Control
- Col
Collagen
Additional file
Additional information about XRD result.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VSC contributed to the organization and drafting of this article. PVQ, AA, FLP, GGG, ACT, ACM, YPM, JRC, SASK, CE, JRSA, DS and JRSJ contributed to the selection of methodology, analysis and discussion of the results. All authors read and approved the final manuscript.
Contributor Information
Vinicius S Cardoso, Email: vscfisio@ufpi.edu.br.
Patrick V Quelemes, Email: pquelemes@gmail.com.
Adriany Amorin, Email: adriany1210@gmail.com.
Fernando Lucas Primo, Email: fernandolucasprimo@gmail.com.
Graciely Gomides Gobo, Email: gragobbo@yahoo.com.br.
Antonio C Tedesco, Email: atedesco@usp.br.
Ana C Mafud, Email: mafud@usp.br.
Yvonne P Mascarenhas, Email: yvonne@if.sc.usp.br.
José Raimundo Corrêa, Email: joseraimundocorrea@gmail.com.
Selma AS Kuckelhaus, Email: selmask@gmail.com.
Carla Eiras, Email: carla.eiras.ufpi@gmail.com.
José Roberto SA Leite, Email: jrsaleite@gmail.com.
Durcilene Silva, Email: durcileneas@yahoo.com.br.
José Ribeiro dos Santos Júnior, Email: jribeiro@ufpi.edu.br.
References
- 1.Dornelles C, Costa S. Estudo comparativo da dissolução de três diferentes marcas de colágeno utilizadas em técnicas cirúrgicas otológicas. Rev Bras Otorrinolaringol. 2003;69:744–751. doi: 10.1590/S0034-72992003000600004. [DOI] [Google Scholar]
- 2.Tonhi E, Plepis AMG. Obtenção e caracterização de blendas colágeno-quitosana. Qim nova. 2002;25:943–948. doi: 10.1590/S0100-40422002000600011. [DOI] [Google Scholar]
- 3.Lin YC, Tan FJ, Marra KG, Jan SS, Liu DC. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009;5:2591–2600. doi: 10.1016/j.actbio.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, Van der Werf M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials. 2003;24:3213–3220. doi: 10.1016/S0142-9612(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 5.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, Sledge CB, Yannas IV, Spector M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/S0142-9612(97)00001-X. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa AK, Taira T, Yoshizato K. In vitro maturation of collagen fibrils modulates spreading, DNA synthesis, and collagenolysis of epidermal cells and fibroblasts. Exp Cell Res. 1987;171:164–177. doi: 10.1016/0014-4827(87)90259-X. [DOI] [PubMed] [Google Scholar]
- 7.Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, Jordan M, McManus W, Solem L, Warden G, Zawacki B. Artificial dermis for major burns. Ann Surg. 1988;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vries HJC, Middelkoop E, Mekkes JR, Dutrieux RP, Wildevuur CHR, Westerhof W. Dermal regeneration in native noncross-linked collagen sponges with diferent extracellular matrix molecules. Wound Repair Regen. 1994;2:37–47. doi: 10.1046/j.1524-475X.1994.20107.x. [DOI] [PubMed] [Google Scholar]
- 9.Nevins M, Kirkerhead C, Nevins M, Wozney JA, Palmer R. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodont Restorative Dent. 1996;16:9–19. [PubMed] [Google Scholar]
- 10.Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of a meniscal cartilage with use of a collagen scaffold: analysis of preliminary data. J Bone Jt Surg. 1997;79A:1770–1777. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Speer DP, Chvapil M, Volz RG, Holmes MD. Enhancement of healing in osteochondral defects by collagen sponge implants. Clin Orthop Relat Res. 1979;144:326–335. [PubMed] [Google Scholar]
- 12.Natsume T, Ike O, Okada T, Takimoto N, Shimizu Y, Ikada Y. Porous collagen sponge for esophageal replacement. J Biomed Mater Res. 1993;27:867–875. doi: 10.1002/jbm.820270705. [DOI] [PubMed] [Google Scholar]
- 13.Narotam PK, Van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995;82:406–412. doi: 10.3171/jns.1995.82.3.0406. [DOI] [PubMed] [Google Scholar]
- 14.Van-Wachem PB, Van-Luyn MJA, Costa MLP. Myoblast seeding in a collagen matrix evaluated in vitro. J Biomed Mater Res. 1996;30:353–360. doi: 10.1002/(SICI)1097-4636(199603)30:3<353::AID-JBM9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Ding T, Lu WW, Zheng Y, Li ZY, Pan HB, Luo Z. Rapid repair of rat sciatic nerve injury using a nanosilver-embedded collagen scaffold coated with laminin and fibronectin. Regen Med. 2011;6:437–447. doi: 10.2217/rme.11.39. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda K, Suzuki S, Isshiki N, Yoshioka K, Okada T, Ikada Y. Influence of glycosaminoglycans on the collagen sponge component of a bilayer artificial skin. Biomaterials. 1990;11:351–355. doi: 10.1016/0142-9612(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S, Gorham SD, French DA, Shivas AA, Courtney JM. In vivo evaluation and comparison of collagen, acetylated collagen and collagen/glycosaminoglycan composite films and sponges as candidate biomaterials. Biomaterials. 1990;11:155–161. doi: 10.1016/0142-9612(90)90148-J. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Deng C, McLaughlin CR, Fagerholm P, Lagali NS, Heyne B, Scaiano JC, Watsky MA, Kato Y, Munger R, Shinozaki N, Li F, Griffith M. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551–1559. doi: 10.1016/j.biomaterials.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Söderqvist M, Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 20.Perng CK, Wang YJ, Tsi CH, Ma H. In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. J Surg Res. 2011;168:9–15. doi: 10.1016/j.jss.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 21.Bakare RA, Bhan C, Raghavan D. Synthesis and characterization of collagen grafted Poly(hydroxybutyrate-valerate) (PHBV) scaffold for loading of bovine serum albumin capped silver (Ag/BSA) nanoparticles in the potential use of tissue engineering application. Biomacromolecules. 2014;15:423–435. doi: 10.1021/bm401686v. [DOI] [PubMed] [Google Scholar]
- 22.Jithendra P, Rajam AM, Kalaivani T, Mandal AB, Rose C. Preparation and characterization of aloe vera blended collagen-chitosan composite scaffold for tissue engineering applications. ACS Appl Mater Interfaces. 2013;5:7291–7298. doi: 10.1021/am401637c. [DOI] [PubMed] [Google Scholar]
- 23.Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Shang L, Wang Y, Huang L, Dong S. Preparation of DNA-silver nanohybrids in multilayer nanoreactors by in situ electrochemical reduction, characterization, and application. Langmuir. 2007;23:7738–7744. doi: 10.1021/la700700e. [DOI] [PubMed] [Google Scholar]
- 25.Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B. 2012;98:112–119. doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Neto EAB, Ribeiro C, Zucolotto V. Embrapa. 2008. Síntese de nanopartículas de prata para aplicação na sanitização de embalagens. [Google Scholar]
- 27.Wong KKY, Liu X. Silver nanoparticles-the real “silver bullet” in clinical medicine? Med Chem Commun. 2010;1:125–131. doi: 10.1039/c0md00069h. [DOI] [Google Scholar]
- 28.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ahamed M, Alsalhi MS, Siddiqui MKJ. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Lok C, Ho C, Chen R, He Q, Yu W, Sun H, Tam PK, Chiu J, Che C. Proteomic analysis of the mode of antibacterial action of silver research articles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 31.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Panácek A, Kvítek L, Prucek R, Kolář M, Veceřová R, Pizúrová N, Sharma VK, Nevěcná TJ, Zbořil R. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 33.Gnanadhas DP, Ben Thomas M, Thomas R, Raichur AM, Chakravortty D. Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrob Agents Chemother. 2013;57:4945–4955. doi: 10.1128/AAC.00152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 36.Kwan KHL, Liu X, To MKT, Yeung KWK, Ho C, Wong KKY. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomedicine. 2011;7:497–504. doi: 10.1016/j.nano.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Alarcon EI, Udekwu K, Skog M, Pacioni NL, Stamplecoskie KG, González-Béjar M, Polisetti N, Wickham A, Richter-Dahlfors A, Griffith M, Scaiano JC. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials. 2012;33:4947–4956. doi: 10.1016/j.biomaterials.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Wang L, Sun L, Guo C, Yang T, Liu Z, Xu F, Li Z. Fabrication, characterization, and application in surface-enhanced Raman spectrum of assembled type-I collagen-silver nanoparticle multilayered films. J Chem Phys. 2008;128:074704. doi: 10.1063/1.2832322. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Wu Y. Investigation of the NaBH4-induced aggregation of Au nanoparticles. Langmuir. 2010;26:9214–9223. doi: 10.1021/la904410f. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Douglas EP. Effects of various salts on structural polymorphism of reconstituted type I collagen fibrils. Colloids Surf B. 2013;112:42–50. doi: 10.1016/j.colsurfb.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 41.Sano S, Kato K, Ikada Y. Introduction of functional groups onto the surface of polyethylene for protein immobilization. Biomaterials. 1993;14:817–822. doi: 10.1016/0142-9612(93)90003-K. [DOI] [PubMed] [Google Scholar]
- 42.Desai V, Kowshik M. Synthesis and characterization of fumaric acid functionalized AgCl/titania nanocomposite with enhanced antibacterial activity. J Nanosci Nanotechnol. 2013;13:2826–2834. doi: 10.1166/jnn.2013.7370. [DOI] [PubMed] [Google Scholar]
- 43.Prasad RY, McGee JK, Killius MG, Suarez DA, Blackman CF, DeMarini DM, Simmons SO. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: effect of size, surface coating, and intracellular uptake. Toxicol In Vitro. 2013;27:2013–2021. doi: 10.1016/j.tiv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Stevanović M, Bračko I, Milenković M, Filipović N, Nunić J, Filipič M, Uskoković DP. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity. Acta Biomater. 2014;10:151–162. doi: 10.1016/j.actbio.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Hebeish A, El-Rafie MH, El-Sheikh MA, Seleem AA, El-Naggar ME. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol. 2014;65:509–515. doi: 10.1016/j.ijbiomac.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 46.Silva T, Pokhrel LR, Dubey B, Tolaymat TM, Maier KJ, Liu X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ. 2014;468–469:968–976. doi: 10.1016/j.scitotenv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Hamouda T, Baker JR. Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. J Appl Microbiol. 2000;89:397–403. doi: 10.1046/j.1365-2672.2000.01127.x. [DOI] [PubMed] [Google Scholar]
- 49.Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol. 2014;12:5. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker C, Pradhan A, Parkstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5(2):244–249. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 51.Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11:26. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X, Gu G, Hu Z, Guo Y, Fu X, Song J. A simple method for preparation of silver dendrites. Colloids Surfaces A Physicochem Eng Asp. 2005;254:57–61. doi: 10.1016/j.colsurfa.2004.11.009. [DOI] [Google Scholar]
- 53.Sileikaitċ A, Prosyčevas I, Puišo J, Juraitis A, Guobienċ A. Analysis of silver nanoparticles produced by chemical reduction of silver salt solution. Mater Sci (Medziagotyra) 2006;12(4):287–291. [Google Scholar]
- 54.Yamamoto SY, Ujiwara KF, Atarai HW. Surface-enhanced Raman scattering from oleate-stabilized silver colloids at a liquid/liquid interface. Anal Sci. 2004;20(September):1347–1352. doi: 10.2116/analsci.20.1347. [DOI] [PubMed] [Google Scholar]
- 55.Zaheer K, Shaeel AA, Abdullah YO, Ziya AK, Abdulrahman AOA. Shape-directing role of cetyltrimethylammonium bromide in the preparation of silver nanoparticles. J Colloid Interface Sci. 2012;367:101–108. doi: 10.1016/j.jcis.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato H, Nakamura A, Takahashi K, Kinugasa S. Accurate size and size-distribution determination of polystyrene latex nanoparticles in aqueous medium using dynamic light scattering and asymmetrical flow field flow fractionation with multi-angle light scattering. Nanomaterials. 2012;2:15–30. doi: 10.3390/nano2010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurunathan S, Han JW, Eppakayala V, Jeyaraj M, Kim JH. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res Int. 2013;2013:535796. doi: 10.1155/2013/535796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prokopovich P, Leech R, Carmalt CJ, Parkin IP, Perni S. A novel bone cement impregnated with silver – tiopronin nanoparticles: its antimicrobial, cytotoxic, and mechanical properties. Int J Nanomed. 2013;8:2227–2237. doi: 10.2147/IJN.S42822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.PEAK Fitting Module. Northampton: OriginLab Corporation, One Roundhouse Plaza; 2002.
- 61.CLSI-Clinical Laboratory Standards Institute: Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. Available online: http://antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/03-CLSI-M07-A9-2012.pdf (accessed on 18 September 2013).
- 62.Quelemes PV, Araruna FB, de Faria BEF, Kuckelhaus SAS, da Silva DA, Mendonça RZ, Eiras C, Soares MJS, Leite JRSA. Development and antibacterial activity of cashew gum-based silver nanoparticles. Int J Mol Sci. 2013;14:4969–4981. doi: 10.3390/ijms14034969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzman M, Dille J, Godet S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine. 2012;8:37–45. doi: 10.1016/j.nano.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Falqueiro AM, Siqueira-Moura MP, Jardim DR, Primo FL, Morais PC, Mosiniewicz-Szablewska E, Suchocki P, Tedesco AC. In vitro cytotoxicity of Selol-loaded magnetic nanocapsules against neoplastic cell lines under AC magnetic field activation. J Appl Phys. 2012;111:07B335. doi: 10.1063/1.3680541. [DOI] [Google Scholar]


