Abstract
Background and objective
Late-life depression (LLD) and amnestic mild cognitive impairment (aMCI) are associated with white matter (WM) disruptions of the fronto-limbic and interhemispheric tracts implicated in mood regulation and episodic memory functions. This work investigates the extent of these WM abnormalities in patients LLD and aMCI when these diseases occur alone and when they coexist.
Materials and methods
Eighty-four subjects separated into cognitively normal (n = 33), LLD (n = 20), aMCI (n = 18), and comorbid aMCI and LLD (n = 13) completed Diffusion Tensor Imaging (DTI) scans. Tract-Based Spatial Statistics was employed to skeletonize multiple DTI indices of the cingulum, corpus callosum, fornix and uncinate fasciculus. Analysis of covariance and post-hoc tests compared group differences. Multiple linear regressions were performed between DTI and behavioral measures for the whole sample and within individual patient groups.
Results
Divergent microstructural disruptions were identified in LLD- and aMCI-only groups, whereas the comorbid group showed widespread abnormalities especially in the hippocampal cingulum and fornix tracts. The LLD groups also showed significant disruptions in the uncinate fasciculus and corpus callosal tracts. Higher depressive symptom and lower episodic memory scores were associated with increased diffusivity measures in the fornix and hippocampal cingulum across all subjects.
Conclusions
Widespread WM microstructural disruptions are present when LLD and aMCI are comorbid – especially in the medial temporal lobe tracts. These WM disruptions may be a marker of disease severity. Also, multiple DTI parameters should be used when evaluating the WM fiber integrity in LLD and aMCI.
Keywords: Late-life depression, mild cognitive impairment, diffusion tensor imaging, white matter, TBSS, Alzheimer’s disease
1. Introduction
With the rapidly aging population, Late-Life Depression (LLD) is a growing public health concern in the United States and around the world. LLD is associated with poorer treatment response, greater caregiver burden, increased risk of mortality and incident Alzheimer’s disease (AD) [1]. LLD often coexists with amnestic mild cognitive impairment (aMCI), and their comorbidity further increases the risk of developing AD [2, 3]. Structural magnetic resonance imaging (MRI) investigations in LLD and aMCI have separately identified frontal and medial temporal lobe (MTL) atrophy and macrostructural abnormalities in the related white matter (WM) [4, 5]. In LLD, increased severity of WM hyperintensities and subclinical strokes on T2-weighted imaging are associated with poorer cognitive outcomes and incident dementia [6]. In aMCI, the subclinical cerebrovascular disease also accelerates conversion to AD [7].
More recently, Diffusion Tensor Imaging (DTI) has been increasingly utilized to investigate the microstructural WM changes in normal aging and diseases, including LLD and aMCI [5, 8]. However, it is unclear if shared or divergent microstructural abnormalities are associated with LLD and aMCI in the elderly, when these disorders occur alone or coexist. The majority of the DTI studies has estimated fractional anisotropy (FA) and mean diffusivity (MD) indices as complementary measures of water diffusion in the biological tissue, thereby permitting the mapping of WM integrity in vivo. FA measures the degree of anisotropy of water molecules, whereas MD quantifies the average of overall water diffusivity in all WM fiber directions. These DTI indices are derived from the three eigenvalues (λ1, λ2, and λ3) of the diffusion tensor [9, 10]. Using the traditional a priori-defined region-of-interest (ROI) and whole-brain voxelwise analyses, decreased FA and increased MD primarily in the frontal and temporal regions, have been demonstrated in LLD [11–14], suggesting compromised integrity of WM fibers related to emotion processing and cognitive functions (i.e., uncinate fasciculus, cingulum) and interhemispheric connections (i.e., corpus callosum). Similar FA and MD alterations involving these WM regions are also found in persons with aMCI [8, 15]. In addition, alterations in the fornix, the predominant outflow tract of the hippocampus and an integral component of the Papez circuit, are also demonstrated in aMCI [16]. However, conflicting FA and MD findings have been reported in LLD [13] and aMCI [17] patients. Some have suggested that FA is both insensitive at detecting early WM disruptions in AD, and insensitive at predicting those with aMCI who are at higher risk of AD conversion [18, 19].
The inconsistent findings are likely driven by the fact that FA and MD values are influenced by several factors, including axon density and diameter, axonal membrane integrity, myelination and intravoxel coherence of fiber orientation [10, 20]. However, individual eigenvalues may provide unique information about the specifics of the underlying WM damage [21]. Axial diffusivity (DA), which represents the diffusion along the direction of the highest eigenvalue (λ1), is associated with axonal fiber integrity [22]. Radial diffusivity (DR), which refers to the direction of diffusion perpendicular to the fibers, is calculated by averaging the second and the third eigenvalues (λ2, and λ3), and has been related to myelin alterations [23]. Therefore, a comprehensive analysis of multiple DTI indices may improve our understanding regarding the microstructural WM fiber architecture associated with LLD and aMCI.
The traditional ROI and voxelwise DTI approaches are prone to operator selection bias, partial volume effects and image registration errors, which can lead to variable results. Tract-based spatial statistics (TBSS) is a recently developed DTI postprocessing technique that minimizes the concerns of misalignment and partial volume effects by projecting the relevant DTI values onto a skeletonized WM map, which contains the center of the major WM tract common to all subjects [24]. By doing so, DTI values across subjects become more Gaussian and less variable than the traditional voxel-based morphometry approach, as previously demonstrated. Moreover, there is growing evidence that supports using multiple diffusion tensor parameters measured by TBSS methods to understand the WM tract involvement in LLD, aMCI and early AD [19, 25–31].
The primary objective of this study was to determine the microstructural abnormalities in LLD and aMCI, when these syndromes occur alone or in combination, using the TBSS method. Since WM fiber abnormalities associated with episodic memory and mood regulating functions (cingulum, fornix and uncinate fasciculus), and interhemispheric connections (corpus callosum) have been reported in LLD and aMCI, we limited our investigations to the related major WM tracts. We hypothesized that 1) in the episodic memory-related MTL tracts (i.e. hippocampal cingulum and fornix), those with aMCI and LLD comorbidity will show the greatest WM disruptions, followed by the aMCI and LLD subject groups; and 2) in the interhemispheric and mood regulating circuitry-related WM tracts, the depressed groups (LLD and the comorbid groups) will show more significant abnormalities followed by aMCI and control subject groups. We also determined whether the microstructural alterations would be associated with depressive symptom severity and episodic memory performance within each study groups.
2. Methods
2.1. Participants
A total of 84 participants aged 60 and older, including cognitively normal (CN: n = 33) individuals, and those with late-life depression (LLD: n = 20) and amnestic mild cognitive impairment (aMCI: n = 18), and coexisting aMCI and LLD (aMCI-LLD: n = 13) subjects, were enrolled in this study. All patients diagnosed with LLD and/or aMCI were recruited from the Geriatric Psychiatry and Memory Disorder Clinics at the Medical College of Wisconsin (MCW). Control participants were recruited from the community. All participants provided written informed consent and the MCW Institutional Review Board approved the study protocol.
The study participants received detailed clinical and neuropsychiatric assessments as published previously [32], including Mini-Mental State Examination (MMSE) (all subjects had to score ≥ 24); Mattis Dementia Rating Scale-2 (MDRS-2) (age- and education-corrected MOANS scaled score of ≥ 5); education adjusted Logical Memory II Delayed Paragraph Recall (LMII-DR) subscale from the Wechsler Memory Scale-Revised; Lawton Physical Self-Maintenance Scale/Instrumental Activities of Daily Living (PSMS/IADL); 30-item Yesavage Geriatric Depression Scale (GDS); diagnostic assessment for Axis 1 disorders including the depression module from the Structured Clinical Interview for DSM IV (SCID); and Hamilton Anxiety Scale (HAM-A). The modified Hachinski Ischemic Scale (HIS) was completed and all participants had to score ≤ 4. All clinical data were reviewed during the weekly conference attended by at least two neurologists with dementia expertise, one neuropsychologist, one geriatric psychiatrist, and study personnel and consensus diagnoses were reached.
Inclusion criteria for LLD (n = 20) included a GDS score of 10 or above, MMSE ≥ 26, PSMS ≤ 6 and IADL ≤ 9, score above the education-adjusted cutoff on the LMII-DR (Delayed recall score > 8 for 16 or more years of education or score > 4 for 8–15 years of education), and SCID depression module positive for major depression. Since clinical significant anxiety often coexists with LLD, we did not exclude patients with HAM-A scores ≥ 17, if the study psychiatrist determined that the primary diagnosis was a depressive disorder.
aMCI (n = 18) was operationally defined according to the established criteria [33]: subjective report of cognitive decline, objective cognitive impairment that includes scoring 1.5 SD below on memory measures, intact activities of daily living (ADLs) and relatively preserved instrumental ADLs (IADLs) and no dementia. For meeting criteria for objective cognitive impairment, participants had to score below the education-adjusted cutoff on the LMII-DR (i.e., ≤ 8 for 16 or more years of education, and ≤ 4 for 8–15 years of education), and score below 1.5 SD below the mean on one or more subscales (one of the impairments had to be memory) of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [34].
All participants with aMCI who endorsed depression (i.e., GDS ≥ 10) were included in the aMCI-LLD group (Major depression (n = 12). Also, one aMCI subject who scored 9 on the GDS met the SCID criteria for dysthymic disorder was included in this group. To be included in this group, participants had to meet the criteria for aMCI prior to being diagnosed with the current depressive episode. One subject in this group had a HAM-A score ≥ 17.
The eligibility criteria for the control (n = 33) group (CN) were similar to that used for the LLD cohort, except these subjects could not meet criteria for any Axis 1 disorders.
Exclusion criteria included past or current history of concurrent Axis 1 psychiatric disorders, such as psychotic or bipolar disorders; alcohol or substance abuse/dependence during the past five years; active suicidality; MMSE scores < 24; history of neurological disease, including Parkinson’s disease, dementia, multiple sclerosis, seizures, or stroke; head injury with loss of consciousness; MRI contraindications and unstable chronic medical conditions.
2.2. Image acquisition
A 3T GE Signa scanner (Waukesha, Wisconsin) with a standard transmit-receive head coil acquired MRI scans. These included high-resolution anatomical images and DTI. The high-resolution T1-weighted anatomical image was acquired with 3D spoiled gradient-recalled echo (SPGR) sequence (TR = 10 ms, TE = 4 ms, TI = 450 ms, field of view = 24 × 24 cm2, flip angle = 12°, number of slices = 144, matrix size = 256 × 192, and voxel size = 0.983 × 0.983 × 1 mm3) in axial slice orientation. The whole-brain DTI was acquired with echo-planar imaging (EPI) using a twice-refocused diffusion prepared sequence (TR = 10000 ms, TE =86.5 ms, 25 diffusion directions with b-value = 1000 s/mm2, three images without diffusion weighting, number of slices = 35, and voxel size = 2 × 2 × 4 mm3) in axial slice orientation. The scan time for anatomical MRI was 6 min and for DTI was 5 min. The subject motion was monitored in real-time during the scans. The images with translational motion larger than 1 mm or rotational motion exceeding 1° were subjected to rescan.
2.3. Data processing
Image distortions in DTI scans caused by eddy currents and head motions were corrected using the programs provided in the FMRIB Diffusion Toolbox (FSL software, www.fmrib.ox.ac.uk/fsl). Diffusion tensors were estimated using the FSL’s Linear Least Squares (LLS) method and then fitted to the data to obtain FA, MD, DA and DR.
The TBSS method was used to evaluate FA, MD, DA, and DR data [24] in the skeletonized WM. First, participants’ FA images were skull stripped and spatially registered to a target image (FMRIB58_FA) in standard space using a nonlinear transformation tool FNIRT. The mean FA image was thresholded at 0.2 and skeletonized to contain only the centers of major WM tracts in standard space. The spatially normalized FA, MD, DA and DR images for each subject were then projected onto the skeleton using the derived registration and projection parameters.
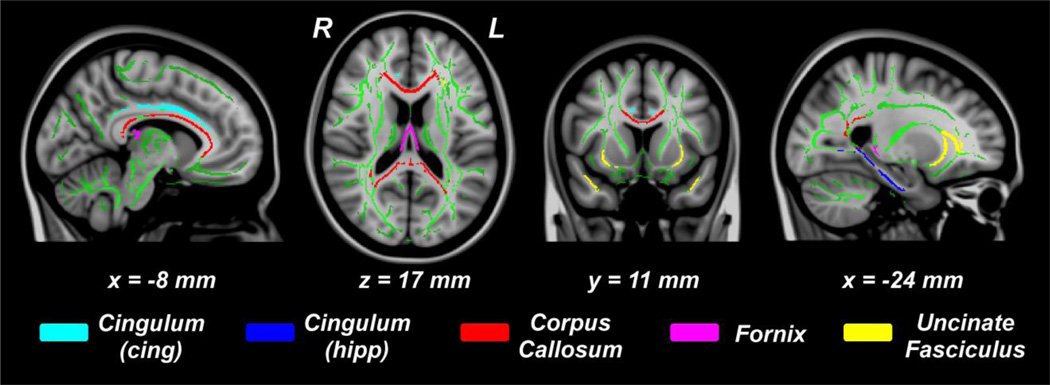
Five major WM tracts of interest (TOIs), including cingulum-cingulate part (cingulum-cing), cingulum-hippocampus part (cingulum-hipp), corpus callosum (CC), fornix, and uncinate fasciculus (Unc), were selected by masking the skeletonized FA and diffusivity images with the predefined WM template masks (JHU white-matter tractography atlas, FSL) (Figure 1). The mean values of each DTI measures for each TOI were then extracted for all participants and converted to standardized z-scores.
Figure 1.
Tracts of interest (TOI) overlaid on skeletonized white matter (green). TOIs: cingulum cingulate-part (cyan), cingulum hippocampus-part (blue), corpus callosum (red), fornix (magenta), and uncinate fasciculus (yellow).
2.4. Statistical Analysis
To test the group differences and correlations with neuropsychiatric measurements, TOI analyses on the skeletonized FA and diffusivity images were carried out. Analysis of covariance (ANCOVA) and post hoc analyses were performed using the Fisher’s Least Significant Difference, to compare group differences in the standardized DTI measures within each WM tract, after controlling for age, gender and education. Since five TOIs are examined, Bonferroni corrected p value <0.01 was used to control for type 1 error. Further, since four diffusion parameters were compared between the subject groups on five TOIs, a more conservative p value of <0.0025 was also considered.
To examine the localized differences in DTI measures on WM TOIs, tract-specific voxelwise ANCOVA and post hoc analyses were used to compare FA and diffusivities on the five WM TOIs, after adjusting for age, gender and education. A Monte Carlo simulation-based multiple comparison correction approach (3dClustSim, program, http://afni.nimh.nih.gov) was used to control for the type I errors by estimating the probability of false positive clusters (p <0.05, cluster size > 45 mm3), based on 5,000 iterations.
Finally, multiple linear regression analyses between the DTI and dimensional (GDS and LMII-DR) measures were performed for the entire sample first and also in each subject group separately, after controlling for age, gender and education. To reduce the number of comparisons, we limited this set of analyses to the TOIs where we found the most significant group differences (i.e., cing-hipp and fornix TOIs).
3. Results
3.1. Demographics and Neuropsychiatric Assessments
While no significant differences in gender and education among the four groups (p > 0.05) were found, the age of the LLD group was significantly younger than the aMCI group (p = 0.037) (Table 1). Differences in the neuropsychiatric measures are summarized in Table 1.
Table 1.
Demographic data and neuropsychiatric characteristics
| CN (n = 33) | LLD (n = 20) | aMCI (n =18) | aMCI-LLD (n = 13) | p-value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 72.33 ± 8.31 | 68.50 ± 5.96 | 75.39 ± 6.53 | 71.62 ± 6.49 | 0.037* |
| Gender (F/M) | 17/16 | 16/4 | 7/11 | 7/6 | 0.152 |
| Education | 15.06 ± 2.87 | 14.65 ± 2.74 | 13.61 ± 2.09 | 14.08 ± 2.93 | 0.297 |
| Neuropsychiatric measurements | |||||
| GDS | 2.09 ± 2.05 | 16.80 ± 3.56 | 4.11 ± 2.78 | 16.00 ± 6.47 | <0.001acdf |
| MMSE | 28.79 ± 1.32 | 28.20 ± 1.20 | 27.33 ± 1.78 | 26.85 ± 1.86 | 0.001bce |
| HAM-A | 1.25 ± 1.11 | 10.30 ± 5.09 | 2.25 ± 1.57** | 8.92 ± 4.11 | <0.001acdf |
| LMII Recall Scores | |||||
| Immediate | 14.27 ± 3.40 | 14.10 ± 3.97 | 8.56 ± 3.54 | 7.08 ± 3.62 | <0.001acde |
| Delayed | 12.70 ± 3.58 | 12.35 ± 4.07 | 2.50 ± 3.20 | 3.23 ± 3.00 | <0.001acde |
| DRS-2 raw scores | |||||
| Attention | 36.53 ± 0.51 | 36.45 ± 0.60 | 35.72 ± 0.75 | 36.08 ± 1.19 | 0.002bd |
| Init/Pers | 36.38 ± 1.31 | 36.30 ± 2.00 | 35.00 ± 3.55 | 32.46 ± 5.32 | 0.001cef |
| Construct | 5.97 ± 0.18 | 5.95 ± 0.22 | 5.94 ± 0.24 | 5.85 ± 0.55 | 0.621 |
| Conceptual | 37.69 ± 1.31 | 36.65 ± 3.65 | 35.89 ± 2.37 | 35.54 ± 3.02 | 0.023bc |
| Memory | 23.63 ± 1.07 | 24.05 ± 1.10 | 18.89 ± 2.70 | 19.69 ± 3.52 | <0.001acde |
| Total | 140.25 ± 2.70 | 139.95 ± 3.12 | 131.00 ± 5.35 | 130.00 ± 6.53 | <0.001acde |
| Current antidepressants (%) | |||||
| No antidepressant | N/A | 1 (5.0) | 16 (88.9) | 2 (15.4) | |
| SSRI monotherapy | N/A | 3 (15.0) | 1 (5.6) | 3 (23.1) | |
| SNRI monotherapy | N/A | 5 (25.0) | 1 (5.6) | 2 (15.4) | |
| Other | N/A | 3 (15.0) | N/A | 1 (7.7) | |
| Combination treatment | N/A | 8 (40.0) | N/A | 5 (38.5) | |
| Current cognitive enhancers (%) | |||||
| No cognitive enhancer | N/A | N/A | 8 (44.4) | 9 (69.2) | |
| ChEI monotherapy | N/A | N/A | 8 (44.4) | 2 (15.4) | |
| Memantine monotherapy | N/A | N/A | 1 (5.6) | 2 (15.4) | |
| Combination treatment | N/A | N/A | 1 (5.6) | N/A | |
Notes -
: Significant difference in age (p < 0.05) was found between LLD group and aMCI group.
: Two aMCI subjects did not have HAM-A scores; the mean and SD are based on the remaining 16 subjects. ANOVA showed significant differences in neuropsychiatric measurements in GDS, MMSE, HAM-A, Recall scores (immediate and delayed), and DRS-2 raw scores (except for Construct).
: Post-hoc analyses revealed the source of ANOVA (a: CN vs LLD, b: CN vs aMCI, c: CN vs aMCI-LLD, d: LLD vs aMCI, e: LLD vs aMCI-LLD, f: aMCI vs aMCI-LLD).
Abbreviations: CN, cognitively normal; LLD, late-life depression; aMCI, amnestic mild cognitive impairment; aMCI-LLD, amnestic mild cognitive impairment with depression; SD, standard deviation; F/M, female/male; GDS, geriatric depression scale; MMSE, mini-mental state examination; HAM-A, Hamilton anxiety; LMII, logical memory II, DRS-2, dementia rating scale-2; Init/Pers, Initiation/Preservation; SSRI: selective serotonin re-uptake inhibitors; SNRI: serotonin norepinephrine re-uptake inhibitors; ChEI: cholinesterase inhibitors. Other antidepressants include: bupropion, mirtazapine, and trazodone.
3.2. DTI differences
Average FA differences
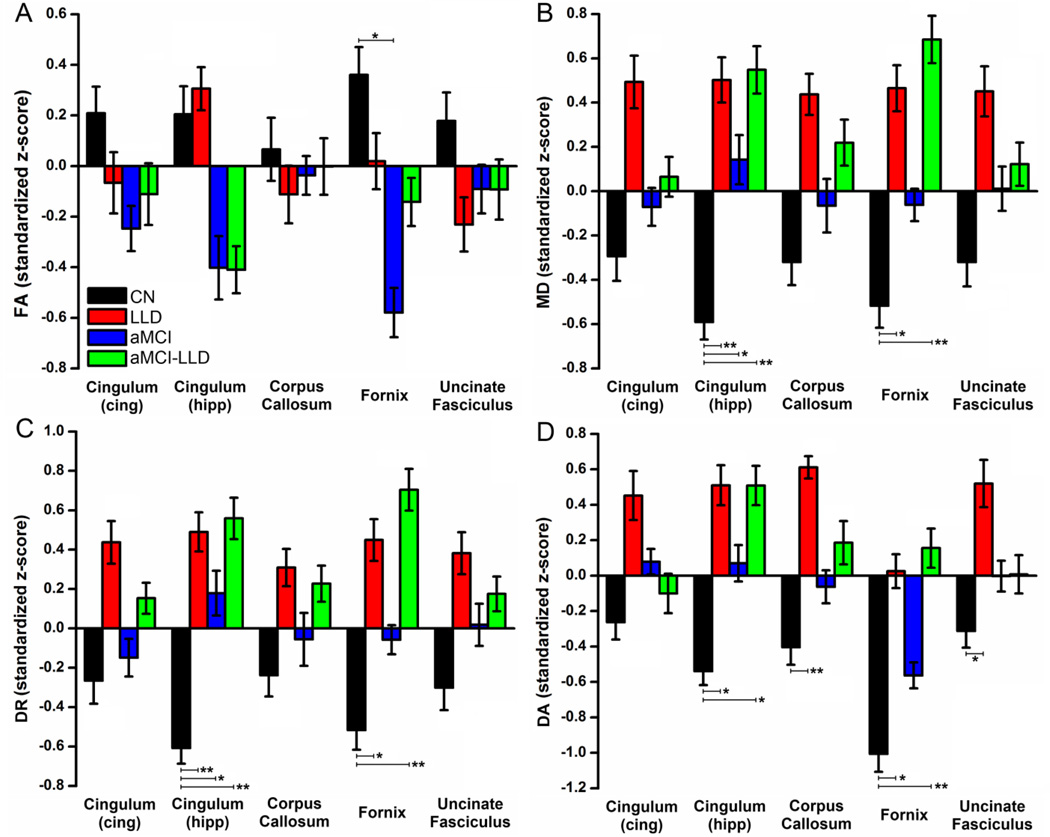
TOI analyses revealed decreased fornix FA in aMCI compared to CN (p < 0.01) (Figure 2A; Table 2).
Figure 2.
Group average (A) fractional anisotropy, (B) mean diffusivity, (C) radial diffusivity, and (D) axial diffusivity for the five tracts of interest, including cingulum (cingulate), cingulum (hippocampus), corpus callosum, fornix, and uncinate fasciculus, in CN (black), LLD (red), aMCI (blue), and aMCI-LLD (green) groups. The data values are standardized z-scores (error bars indicate the standard error of the mean). Group differences in the five tracts of interest are obtained using ANCOVA and post-hoc analyses, adjusting for age, gender, and education. Statistically significant betweengroup differences are indicated by * (p < 0.01), and ** (p < 0.0025). FA: fractional anisotropy, MD: mean diffusivity, DR: radial diffusivity, DA: axial diffusivity.
Table 2.
Significant average tract of interest results for the diffusion tensor indices.
| FA | MD | DR | DA | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOI | aMCI-LLD | aMCI | LLD | aMCI-LLD | aMCI | LLD | aMCI-LLD | aMCI | LLD | aMCI-LLD | aMCI | LLD | ||||||||||||
| CN | aMCI | LLD | CN | LLD | CN | CN | aMCI | LLD | CN | LLD | CN | CN | aMCI | LLD | CN | LLD | CN | CN | aMCI | LLD | CN | LLD | CN | |
| Cingulum cing) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cingulum (hipp) | - | - | - | - | - | - | ↑↑ | - | - | ↑ | - | ↑↑ | ↑↑ | - | - | ↑ | - | ↑↑ | ↑ | - | - | - | - | ↑ |
| Corpus Callosum | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑↑ |
| Fornix | - | - | - | ↓ | - | - | ↑↑ | - | - | - | - | ↑ | ↑↑ | - | - | - | - | ↑ | ↑↑ | - | - | - | - | ↑ |
| Uncinate Fasciculus | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑ |
Notes. Significant increases (red arrow) and decreases (blue arrow) are summarized, based on the ANCOVA and post-hoc analyses results (Bonferroni corrected; single arrow: p value <0.01; double arrows: p value < 0.0025).
Abbreviations: CN, cognitively normal; LLD, late-life depression; aMCI, amnestic mild cognitive impairment; aMCI-LLD, amnestic mild cognitive impairment with depression; ANCOVA, Analysis of covariance; TOIs, Tracts of interest.
Average MD differences
The MD was increased in the cingulum-hipp (p < 0.0025) and fornix (p < 0.01) tracts in LLD compared to the CN (Figure 2B; Table 2). The MD also was increased in the aMCI-LLD group in cingulum-hipp and fornix tracts compared to the CN (p < 0.0025). Increased MD in aMCI group was found only in the cingulum-hipp tract, relative to CN (p < 0.01).
Average DA and DR differences
DA and DR of the cingulum-hipp and fornix tracts were increased in the LLD and the aMCI-LLD groups, relative to CN. In aMCI subjects, DR measures were significantly increased in the cingulum-hipp only, compared to CN group (Figure 2C–D). In addition, LLD subjects showed increased DA in corpus callosum and Unc tracts, relative to CN (Figure 2D; Table 2).
3.3. Voxelwise analyses
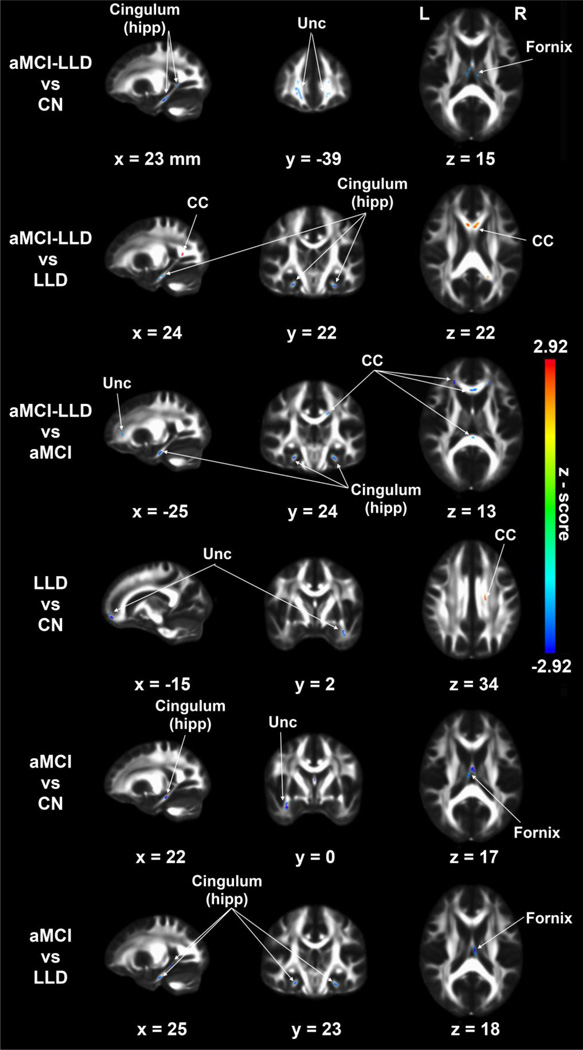
Decreased bilateral cingulum-hipp FA was seen in aMCI-LLD compared to the other groups. Decreased fornix and Unc FA also were found in the two aMCI groups, relative to controls. Nondepressed aMCI group also showed decreased FA in the cingulum-hipp compared to the control group. The Unc FA was significantly decreased in the LLD group, relative to CN. Furthermore, Unc and CC FA were decreased in the aMCI-LLD group, relative to aMCI subjects. Interestingly, increased FA on the corpus callosum tracts was found in the LLD group relative to CN; and in the aMCI-LLD groups relative to LLD subjects (p < 0.05, corrected) (Figure 3; Table 3).
Figure 3.
Voxelwise fractional anisotropy (FA) group differences (p < 0.05, corrected for multiple comparisons) in the five tracts of interests, including cingulum (cingulate), cingulum (hippocampus), corpus callosum, fornix, and uncinate fasciculus using ANCOVA and post hoc analyses adjusted for age, gender, and education. CC: corpus callosum, Unc: uncinate fasciculus.
Table 3.
Significant voxelwise results for the diffusion tensor indices.
| FA | MD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOI | aMCI-LLD | aMCI | LLD | aMCI-LLD | aMCI | LLD | ||||||
| CN | aMCI | LLD | CN | LLD | CN | CN | aMCI | LLD | CN | LLD | CN | |
| Cingulum (cing) | - | - | - | - | - | - | - | - | ↓ | - | - | - |
| Cingulum (hipp) | ↓ | ↓ | ↓ | ↓ | ↓ | - | ↑ | - | - | ↑ | ↑ | ↑ |
| Corpus Callosum | - | ↓ | ↑ | - | - | ↑ | - | ↑ | - | - | ↓ | ↑ |
| Fornix | ↓ | - | - | ↓ | ↓ | - | ↑ | ↑ | - | ↑ | - | ↑ |
| Uncinate Fasciculus | ↓ | ↓ | - | ↓ | - | ↓ | ↑ | - | ↓ | - | ↑ | - |
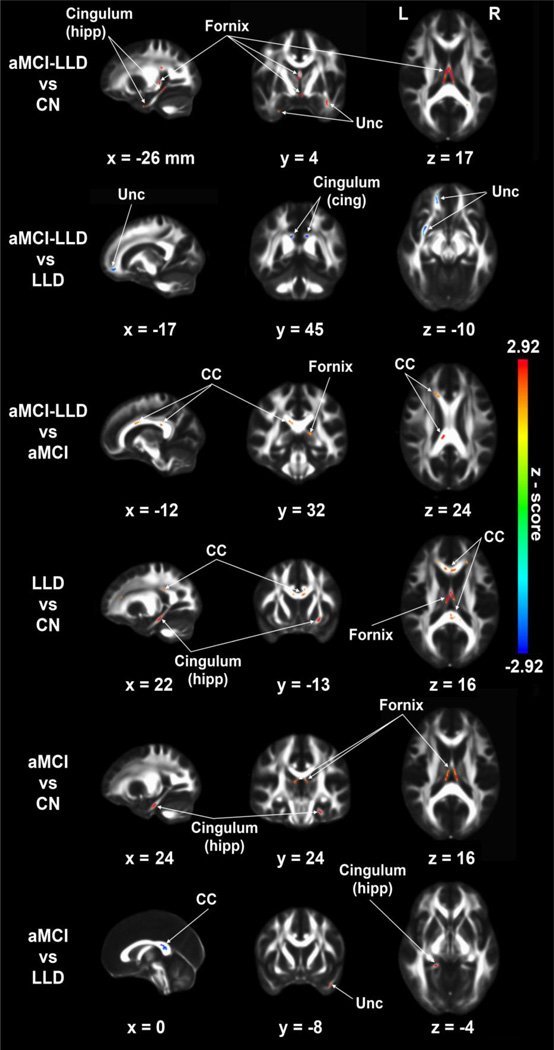
Notes. Significant increases (red arrow) and decreases (blue arrow) are summarized, based on the post-hoc analyses (3dClustSim, p <0.05, cluster size > 45 mm3).
Abbreviations: CN, cognitively normal; LLD, late-life depression; aMCI, amnestic mild cognitive impairment; aMCI-LLD, amnestic mild cognitive impairment with depression.
Increased MD was found across the cingulum-hipp and fornix tracts in all three diseased groups compared to CN. In the aMCI-LLD group, increased MD also was found in the Unc tract (vs. CN), and in the CC and fornix (vs. aMCI subjects). In aMCI subjects, increased MD was seen in the cingulum-hipp and Unc, relative to LLD patients. Conversely, significantly decreased MD was found in the Unc and cingulumcing tracts in aMCI-LLD compared to the LLD group. Similarly, decreased MD in the CC was seen in the aMCI compared to the LLD group (p < 0.05, corrected) (Figure 4; Table 3).
Figure 4.
Voxelwise mean diffusivity (MD) group differences (p < 0.05, corrected for multiple comparisons) in the five tracts of interests, including cingulum (cingulate), cingulum (hippocampus), corpus callosum, fornix, and uncinate fasciculus using ANCOVA and post hoc analyses adjusted for age, gender, and education. CC: corpus callosum, Unc: uncinate fasciculus.
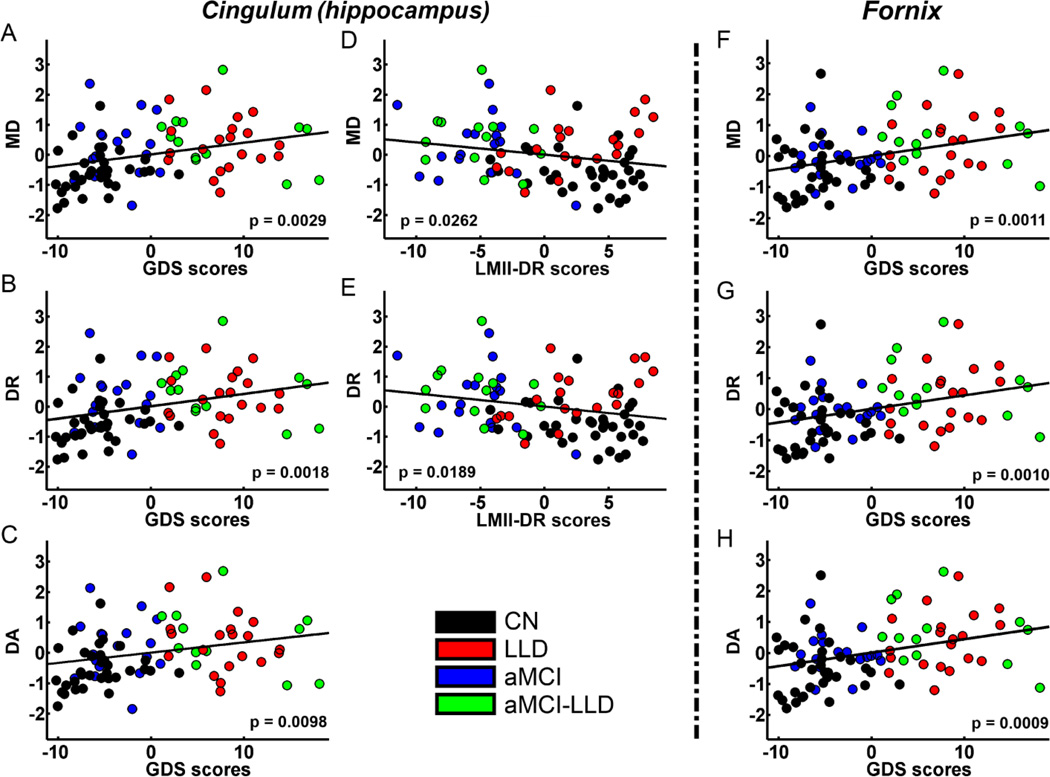
3.4. Correlations between DTI measures and behaviors
The MD, DA and DR indices were positively correlated with the GDS in cingulumhipp (Figure 5A–C) and fornix (Figure 5F–H) tracts for the whole sample. MD and DR measures were negatively correlated with the LMII-DR in the cingulum-hipp tract (Figure 5D, E) across all subjects. Correlations between GDS and mean, axial and radial diffusivity measures are positive, and correlations between LMII-DR and diffusivities are negative in these TOIs. The correlations of FA with GDS and LMII-DR were insignificant in these TOIs. No statistically significant results were found in the within-group linear regression analyses between the DTI measures (FA, MD, DR and DA) and behavioral (LMII-DR and GDS) measures in these TOIs.
Figure 5.
Behavior significance of mean diffusivity (MD), radial diffusivity (DR), and axial diffusivity (DA) in the cingulum (hippocampus) (A ~ E) and fornix (F ~ H) tracts after adjusting for age, gender, and education. All diffusivity measures are positively correlated with GDS scores in the cingulum (hippocampus) and fornix tracts. MD and DR in the cingulum (hippocampus) but not in the fornix tract are negatively correlated with LMII-DR scores. Colored circles indicate individual participants belonging to CN (black), LLD (red), aMCI (blue), and aMCI-LLD (green) groups. MD: mean diffusivity, DR: radial diffusivity, DA: axial diffusivity, GDS: geriatric depression scale, LMII-DR: logical memory II - delayed recall.
4. Discussion
This study yielded four principal findings, which we will describe in turn. First, the average FA on the TOIs only showed minimal group differences, whereas significant abnormalities in the average mean and principal diffusivities involving the WM tracts associated with mood regulation and episodic memory functions were detected, when LLD occur alone or in combination with aMCI. Specifically, while increases in the average mean and principal diffusivities in the hippocampal cingulum and fornix TOIs were seen in both the depressed groups, cognitively normal LLD group showed differences in the corpus callosal and uncinate fasciculus tracts limited only to the average DA measure. Second, in those with aMCI, the MTL-associated WM tracts (i.e., hippocampal cingulum and the fornix) were the most affected, with the comorbid group showing the greatest damage followed by the aMCI-only group. Third, in those with LLD on the other hand, significant disruptions in the interhemispheric (i.e., corpus callosal) and mood regulation-related (i.e., uncinate fasciculus) WM tracts were also seen, with LLD-only group showing the greatest disruptions followed by the comorbid group. Finally, greater depressive symptom severity and episodic memory deficits were related to diffusivity measures in the MTL-associated tracts in the whole sample. Assessing multiple DTI indices can better illustrate the underlying WM pathology in LLD and aMCI more precisely than any single index.
4.1. Average fractional anisotropy findings
The mean FA reductions observed in the aMCI groups, primarily affecting the fornix fibers, is consistent with previous studies [16, 18, 35]. However, null FA findings in the other TOIs are inconsistent with prior LLD and aMCI studies [25, 27, 35, 36]. There are two potential explanations for these discrepancies. First, the overall FA differences may only reach statistical significance when the disruptions are extensive. It is conceivable that subtle FA changes within subregions of these tracts could have been missed with TOIs, which can be identified using voxelwise analyses [37]. Second, distinct pathophysiologic mechanisms may differentially contribute to the underlying disruptions in WM tissue integrity, and FA alone may lack sensitivity in capturing such damage [18, 19, 28, 36].
4.2. Voxelwise FA analysis
In line with previous findings, we also identified widespread MTL-associated tract voxelwise FA differences in the aMCI groups, and fewer, but significant areas of Unc FA alterations, relative to controls [27, 35]. Interestingly, the comorbid group showed the most extensive injury, followed by the nondepressed aMCI subjects. aMCI-LLD subjects also showed greater callosal disruptions in the genu and splenium than their nondepressed counterparts. Greater FA decreases in the genu of LLD patients than those with comorbidity were also seen. While the callosal FA abnormalities were limited to the genu in one aMCI study [38], others have reported more extensive involvement affecting the body and the splenium [15, 27]. In LLD, FA reductions have been reported primarily in the splenium, and were more pronounced in poor antidepressant responders [25]. LLD subjects also showed some areas with decreased FA along the Unc compared to controls, which is similar to prior observations [29]. Our results demonstrate that comorbid patients have greater detrimental effects on the MTL WM tissue than when either disorder occurs alone. Since the coexistence of depressive symptoms and aMCI is associated with greater GM atrophy of areas implicated in emotion processing and multidomain cognitive functions and increased incidence of AD and vascular dementia [2, 32, 39], it is plausible that the comorbidity may be a marker of disease severity.
4.3. Mean, axial and radial diffusivity findings
Greater average mean and principal diffusivities in the hippocampal part of the cingulum and fornix were identified predominantly in the comorbid and LLD only groups. Moreover, the increased average DA measures of the CC and Unc tracts were limited to LLD patients. On the other hand, the voxelwise abnormalities were greatest in the comorbid aMCI-LLD and LLD groups, followed by the aMCI-only subjects. There are only a handful of studies that have utilized TBSS methodology in LLD, and our findings are mostly consistent with the literature [25, 29, 30]. We further extend the results from previous LLD studies to also demonstrate changes in the diffusivity indices when LLD and aMCI coexist among older adults. An LLD study that employed a similar TBSS approach found that the increases in average MD and DR were more extensive than FA differences in the corpus callosum, cingulum and Unc tracts, independent of WM hyperintensities [25]. FA reductions were also accompanied by corresponding DR increases in the fronto-subcortical and fronto-limbic tracts in the absence of GM atrophy in another study [30]. Neither of these investigations found DA differences, however, in contrast to our results.
Widespread MD increases in the CC, cingulum bundle, fornix and Unc bilaterally have been reported in aMCI [31], though these findings are not universal [27]. We observed greater MD changes in the comorbid subjects than the nondepressed aMCI group in the fornix and CC tracts in our voxelwise analyses. Interestingly, increased MD was found in different parts of the cingulum-hipp in the LLD (front) and aMCI (back), while the comorbidity (aMCI-LLD) group showed increased MD in the front and back of the cingulum-hipp tract, relative to CN (Figure 4). Increased DR is a consistent finding in LLD and prodromal AD [25, 26]; in contrast, increased, decreased and no changes in DA are reported in aMCI [27, 31, 38, 40, 41]. Since reductions in DA are reflective of axonal damage in animal models [22], the mechanisms that lead to increased DA in LLD with and without comorbid aMCI remain unclear. DA increases may be dependent on the microglial activation and clearance of axonal fragments, as well as degeneration affecting only one tract in areas with crossing fibers [42, 43]. Other potential factors that can increase DA might include increased extra-axonal space and fluid due to reduced axonal diameter, myelin thinning or both [44]. In LLD with and without aMCI, all three principal diffusivities were abnormal in the MTL tracts, whereas callosal and Unc fiber alterations were limited to the DA measure in the LLD group only. Increases in the DA indices may be more sensitive indicators of earliest WM changes, and FA and DR may be superior measures of disease progression and more advanced WM degeneration, as recently demonstrated in aMCI and early AD [19, 28, 36]. Investigating the full tensor model in LLD and aMCI may provide critical information regarding the predilection of different pathologies on various fiber tracts and stage-specific changes within a single disorder.
4.4. Relationship of depressive symptom severity and episodic memory measures with DTI indices
In this study, greater severity of depressive symptoms was associated with increased diffusion parameters in the cingulum-hipp and fornix. Our lack of correlations of FA with depressive symptom severity is consistent with a previous study [45]. In contrast, greater episodic memory deficits were related to increases in the MD and DR measures, but only in the cingulum-hipp and not fornix, which is different from previous LLD and aMCI studies [31, 45]. The differences in the study populations and the reduced statistical power due to smaller sample sizes may explain these inconsistencies as well as the lack of correlations of the DTI indices with depressive symptoms and memory measures within the individual subject groups. Moreover, the effects of various cognitive functions other than episodic memory on DTI indices were not investigated in this study [25, 45].
4.5. Potential pathophysiologic mechanisms
LLD has been linked to neurodegeneration from amyloid and tau pathology, glucocorticoid toxicity, inflammation and oxidative stress [46]. The observation that the compromised WM integrity is largely driven by DA and DR abnormalities in LLD point to the possibility that axonal and myelin breakdown from chronic ischemic WM disease may be present. In the preclinical and prodromal AD stages, pathological WM changes have been demonstrated early in the disease course [47]. Two putative theories have been hypothesized as potential explanations underlying the microstructural abnormalities in aMCI. The first theory, termed retrogenesis, postulates that WM degeneration reflect myelin breakdown that initially affect late-myelinating association fibers, such as the MTL, limbic and commissural tracts in the early stages of AD [41, 48]. The second theory, wallerian degeneration, posits that the axonal damage mirror adjacent GM atrophy and the progression of WM tissue vulnerability parallel GM pathology [49]. Wallerian degeneration, and to a lesser extent, retrogenesis, may explain the WM degeneration patterns in aMCI and AD [31, 38, 40, 41], and these hypotheses may also hold true in LLD, especially in those individuals with comorbid cognitive impairment. Also, while abnormal DR in light of no DA changes is suggestive of myelin injury based on animal studies, increased DR in the presence of chronic axonal damage may represent demyelination, neurodegeneration, microglial activation or a combination of multiple pathologies [23], and is complicated by crossing fibers [50]. Our study was not designed to specifically test various theories of WM injury; therefore, future experiments should elucidate these hypotheses with joint assessments of adjacent GM and WM tissue [26].
4.6. Limitations
A few limitations of this study should be acknowledged. First, our sample, especially in the comorbid group, is relatively small, and our preliminary results need to be replicated in larger studies. Second, while the LLD subjects were significantly younger than the nondepressed aMCI group, we did control for age in all our analytic models. Third, an ideal study design would be to include antidepressant- and cognitive enhancer-free participants to eliminate potential confounding effects of these medications. However, since the majority of our depressed participants had failed at least one therapeutic antidepressant trial and had moderate-to-severe depression, the ethical aspects restricted us from performing such a study. Also, while acetylcholinesterase inhibitors (ChEI) and memantine are not approved for treatment of aMCI, physicians commonly prescribe these medications off-label to those with aMCI with and without coexisting LLD [51–53]. Therefore, as long as aMCI patients were on stable doses and the dosages were not expected to change during the study participation, cognitive enhancer use was allowed. Fourth, vascular risk factors and disease burden were not controlled for, and may have influenced our results. Also, although all participants had to score within the normal range on HIS, microstructural damage observed in this study may be attributable to varying levels of WM hyperintensities across groups. Fifth, this study only obtained 25 diffusion gradient directions (DGD). In general, >20 DGD is considered sufficient for estimating DTI metrics (FA, MD, DR and DA), although there are slight discrepancies between studies [54–56]. Currently, there is no clear consensus with regards to the optimal acquisition protocol requirements (including the number of DGD needed) to calculate the various DTI indices for TBSS analyses. Considering the wide variety of acquisition schemes and analytic approaches for DTI and other models of the diffusion MRI study, future investigations will be essential to address these methodological considerations and application to detecting microstructural disruptions in LLD and aMCI. Finally, since this was a hypothesis driven study, we limited our analysis to only 5 major tracts. While it is plausible that other WM fibers may be affected by LLD and aMCI, extending our study to including more tracts may have resulted in type II errors.
4.7. Conclusions
In summary, the present results provide evidence suggesting the superiority of multiple tensor measurements than any single metric when studying the WM architecture in LLD and aMCI. Our results further underscore the widespread neurobiologic vulnerability posed by the coexistence of depression with aMCI on the fronto-limbic and interhemispheric structural connections in the elderly.
Acknowledgements
The authors thank Ms. Carrie M. O’Connor, M.A., for editorial assistance, Mr. Douglas Ward, M.S., for assistance with statistical analysis, Ms. Stacy Claesges for subject recruitment and Ms. Judi Zaferos-Pylant and Mr. Yu Liu, M.S., for MRI technical support.
Funding Support: This work was supported by Alzheimer’s Association New Investigator Research Grant NIRG-11-204070 (JSG), Advancing Healthier Wisconsin Endowment for Research to MCW (JSG), Extendicare Foundation grant (JSG and PGA), H Bader Foundation grant (PGA), Birnshein Foundation grant (PGA), NIH grant R01 AD20279 (SJL), and 1UL1RR031973 from the Clinical and Translational Science Award program of the National Center for Research Resources (JSG and SJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors report no potential conflict of interest.
References
- 1.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365(9475):1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 2.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 3.Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 4.Guzman VA, Carmichael OT, Schwarz C, Tosto G, Zimmerman ME, Brickman AM. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin S, Steffens DC. Structural neuroimaging of geriatric depression. Psychiatr Clin North Am. 2011;34(2):423–435. ix. doi: 10.1016/j.psc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15(10):839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 7.Defrancesco M, Marksteiner J, Deisenhammer E, Kemmler G, Djurdjevic T, Schocke M. Impact of White Matter Lesions and Cognitive Deficits on Conversion from Mild Cognitive Impairment to Alzheimer's Disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-122095. [DOI] [PubMed] [Google Scholar]
- 8.Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21(1):39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 11.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61(8):1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 13.Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60(12):1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 14.Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66(3):245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32(12):2322, e5–e18. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, et al. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement. 2012;8(2):105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243(2):483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- 18.Nowrangi MA, Lyketsos CG, Leoutsakos JM, Oishi K, Albert M, Mori S, et al. Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.05.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133(2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 21.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI. Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage. 2012;60(4):2182–2190. doi: 10.1016/j.neuroimage.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, et al. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging. 2010;31(2):244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves GS, O'Dwyer L, Jurcoane A, Oertel-Knochel V, Knochel C, Prvulovic D, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One. 2012;7(12):e52859. doi: 10.1371/journal.pone.0052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer's disease. PLoS One. 2012;7(11):e49072. doi: 10.1371/journal.pone.0049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colloby SJ, Firbank MJ, Thomas AJ, Vasudev A, Parry SW, O'Brien JT. White matter changes in late-life depression: a diffusion tensor imaging study. J Affect Disord. 2011;135(1–3):216–220. doi: 10.1016/j.jad.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, et al. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69(7):680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- 31.Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, et al. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Xie C, Li W, Chen G, Douglas Ward B, Franczak MB, Jones JL, et al. The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: voxel-based morphometry study. Behav Brain Res. 2012;235(2):244–250. doi: 10.1016/j.bbr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 34.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 35.Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, et al. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2011;32(9):1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 36.O'Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, Tanner C, et al. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer's disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preti MG, Lagana MM, Baglio F, Griffanti L, Nemni R, Cecconi P, et al. Comparison between skeleton-based and atlas-based approach in the assessment of corpus callosum damages in Mild Cognitive Impairment and Alzheimer Disease. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7808–7811. doi: 10.1109/IEMBS.2011.6091924. [DOI] [PubMed] [Google Scholar]
- 38.Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini AR, Sancesario G, et al. When, where, and how the corpus callosum changes in MCI and AD: a multimodal MRI study. Neurology. 2010;74(14):1136–1142. doi: 10.1212/WNL.0b013e3181d7d8cb. [DOI] [PubMed] [Google Scholar]
- 39.Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, et al. Late-Life Depression, Mild Cognitive Impairment, and Dementia. Arch Neurol. 2012:1–7. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol. 2007;28(10):1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, et al. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009;45(1):10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomalla G, Glauche V, Weiller C, Rother J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76(2):266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection". Neuroimage. 2010;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, et al. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med. 2012;42(6):1195–1202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Ajilore O, Kepe V, Barrio JR, Small G. Mood, cognition and in vivo protein imaging: the emerging nexus in clinical neuroscience. Int J Geriatr Psychiatry. 2008;23(6):555–563. doi: 10.1002/gps.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, et al. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6(5):394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- 49.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6(11):889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 50.Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 51.Roberts JS, Karlawish JH, Uhlmann WR, Petersen RC, Green RC. Mild cognitive impairment in clinical care: a survey of American Academy of Neurology members. Neurology. 2010;75(5):425–431. doi: 10.1212/WNL.0b013e3181eb5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds CF, 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68(1):51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClendon MJ, Hernandez S, Smyth KA, Lerner AJ. Memantine and acetylcholinesterase inhibitor treatment in cases of CDR 0.5 or questionable impairment. J Alzheimers Dis. 2009;16(3):577–583. doi: 10.3233/JAD-2009-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51(4):807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 55.Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol. 2006;27(8):1776–1781. [PMC free article] [PubMed] [Google Scholar]
- 56.Giannelli M, Cosottini M, Michelassi MC, Lazzarotti G, Belmonte G, Bartolozzi C, et al. Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. Journal of applied clinical medical physics / American College of Medical Physics. 2010;11(1):2927. doi: 10.1120/jacmp.v11i1.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]