Abstract
Hispanic women have higher breast cancer mortality compared to non-Hispanic whites. We evaluated for Proliferation Axis Score differences, as determined by Oncotype Dx, in Hispanic and non-Hispanic white women with newly diagnosed breast cancer. We matched 219 women, based upon age, stage, and nodal status. Compared to non-Hispanic whites, Hispanic women with hormone-sensitive, HER2-negative early-stage breast cancer had a higher Proliferation Axis Score. No differences were seen in Recurrence Score, ER, PR, or HER2 by Oncotype DX. CCNB1 and AURKA were significantly higher in Hispanic women. These tumor differences may help explain breast cancer outcome differences between the two ethnicities.
Keywords: Breast cancer, Oncotype DX, Hispanic, Non-Hispanic white, Tumor proliferation, Proliferation axis score
INTRODUCTION
In the United States, the Hispanic population is the fastest growing major demographic group (1). This ethnicity is comprised of a heterogeneous group of nationalities and countries of origin, ranging from the Caribbean and Central/South America to Europe. While breast cancer incidence is lower among Hispanic women as compared to non-Hispanic white women (1), Hispanics have a 1.2–1.7 fold increase in breast cancer mortality risk (2–4). This increased risk of breast cancer death persists after adjusting for tumor stage, hormone receptor status, socioeconomic status, surgical, and radiation treatment (5). It is possible that differences in tumor biology may also be contributing to the disparity in breast cancer outcome.
In patients with hormone receptor positive (HR+) breast cancer, the Oncotype DX® assay is a commercially available validated predictor of distant breast cancer recurrence (6, 7). A 21-gene score, known as the Recurrence Score, is determined by a reverse-transcriptase polymerase chain reaction (RT-PCR)-based assay consisting of 16 cancer-related genes and five reference genes (6, 7). The 16 genes are involved in invasion, the estrogen receptor (ER) and HER2 signaling, and proliferation. Of these genes, five of them provide the Proliferation Axis Score [i.e., BIRC5 (Survivin), MKI67 (Ki-67), CCNB1 (Cyclin B1), MYBL2, and AURKA (STK15)]. In addition to other Group Scores such as the HER2, ER, and Invasion Group Scores, the Proliferation Axis Score is then used to calculate the Recurrence Score (7). Of the Group Scores, the Proliferation Axis Score is the one that is most heavily weighted in the Recurrence Score calculation (7). In node-negative breast cancer, Oncotype DX is used for clinical decision-making about adjuvant chemotherapy for patients who will receive anti-estrogen therapy (6, 7). The Recurrence Score has also demonstrated prognostic value in lymph node-positive breast cancer (8, 9).
Similar to Hispanic women, black women have a worse breast cancer-specific survival as compared to non-Hispanic whites (3, 4). Racial differences in outcome are thought to be partially the result of differences in tumor biology (10). In a small retrospective analysis, breast tumor samples from black women (n = 27) demonstrated no differences in the 21-gene Recurrence Score as compared to other races (p = .60) (10). However, black women were noted to have a significantly higher expression of the Proliferation Axis Score (p = .004). We conducted a matched case-control study to assess genomic differences in Oncotype DX-derived tumor characteristics between Hispanic and non-Hispanic white women with early-stage, HR+ breast cancer. Our hypothesis was that there will be a higher expression of the Proliferation Axis Score in the Hispanic population as compared to matched non-Hispanic white women.
MATERIAL AND METHODS
Patient population and data source
Women with stage I–III, HR+/HER2 negative (HER2−) breast cancer diagnosed between 2005 and 2011 at either Columbia University Medical Center (CUMC) or Albert Einstein Medical Center (AEMC) who underwent Oncotype DX testing were identified. After obtaining CUMC and AEMC institutional review board approval, patient charts were reviewed for self-reported ethnicity (Hispanic or non-Hispanic white). Hispanics, any race, were matched to non-Hispanic whites in a 1:2 ratio. Patients were matched based upon the following characteristics: age at diagnosis (+/− 10 years), tumor stage, and lymph node status (negative or positive). In addition to these variables, the following demographic, clinical, and tumor characteristics were collected: country of origin (self-reported and documented in the electronic medical record), body mass index (BMI), lymphovascular invasion (LVI: absent or present), and tumor grade (well, moderately, or poorly differentiated). The immunohistochemical (IHC) scoring of the ER and progesterone receptor (PR) and HER2 status (IHC +/− in situ hybridization) were determined per standard ASCO-CAP guidelines (11, 12) and recorded from the pathology reports done at the time of diagnosis. The weighted Recurrence Score (6), corresponding 10-year Distant Recurrence Risk, ER, PR, and HER2 status as evaluated by RT-PCR were obtained by Oncotype Dx reports in the patient records. With assistance from Genomic Health, Incorporated, we obtained each patient’s composite Proliferation Axis Score and five individual proliferation genes that make up this score: BIRC5, MKI67, CCNB1, MYBL2, and AURKA.
Statistical analysis
The primary aim was to determine the association between the Proliferation Axis Score and ethnicity. Assuming equal variances between the two samples (SD = 1) (10), we anticipated > 90% power to detect a mean difference of 0.5 in Proliferation Axis Score in the Hispanic vs. non-Hispanic white women in 219 patients, with the significance level of 0.05.
Secondary aims were to evaluate ethnic differences in other clinical and tumor characteristics, such as BMI and 21-gene Recurrence Score, as well as differences in the individual proliferation genes. The Wilcoxon two-sample test was used to evaluate for differences in continuous variables between the two groups, and Chi-squared tests were used to evaluate for differences in categorical variables, such as presence of LVI and tumor grade. Descriptive statistics were generated for all data collected. All tests were two sided and considered to be statistically significant if the p-value was <.05. Variables determined to be significant in univariable analysis were selected for inclusion in multivariable linear regression analysis. All statistical analyses were conducted using SAS version 9.3.
RESULTS
We identified 74 Hispanic women who underwent Oncotype DX testing and had Oncotype DX data available in the medical record. Hispanic women were then matched to 145 non-Hispanic white women in a 1:2 ratio (Table 1), as 3 Hispanics had only a 1:1 non-Hispanic white match available. There was no significant difference in age or tumor size between the 2 groups. All but 9 patients had lymph node negative breast cancer (3 Hispanics, 6 non-Hispanic whites). By IHC and/or in situ hybridization, none were HER2+ or triple negative. By histology, 83% were invasive ductal carcinoma and 11% were invasive lobular carcinoma, with no statistical differences in histologic type between ethnicities (data not shown). Of the Hispanic women, 43% were from Dominican Republic and 41% from Puerto Rico.
Table 1.
Clinical and Pathologic Characteristics of Hispanic (n = 74) and Non-Hispanic White Women (n = 145) with Early Stage Breast Cancer
| Variable | Hispanic | Non-Hispanic White | P value* |
|---|---|---|---|
| Median Age, years (IQR)a | 58 (48–63) | 55 (49–63) | .58 |
| Median Tumor Size, cm (IQR) | 1.4 (1.0–1.8) | 1.3 (1.0–1.7) | .47 |
| Median ER% by IHCb | 95 (87.5–99.5) | 95 (90–100) | .91 |
| Median PR% by IHCc | 80 (15–90) | 80 (50–90) | .29 |
| Hormone Receptor Status, N (%)d | |||
| ER+/PR+/HER2− | 61 (82.4) | 132 (91.0) | .03 |
| ER+/PR−/HER2− | 13 (17.6) | 11 (7.6) | |
| ER−/PR+/HER2− | 0 | 0 | |
| Unknown | 0 | 2 (1.4) | |
| BMI, kg/m2e | 28 (25.8–32.6) | 26 (23–30.9) | .01* |
| LVI, N (%) f | |||
| Present | 12 (16.2) | 17 (11.7) | |
| Absent | 60 (81.1) | 123 (84.8) | .36 |
| Tumor Grade, N (%) g | .09 | ||
| Well Differentiated | 11 (14.9) | 25 (17.3) | |
| Moderately Differentiated | 41 (55.4) | 91 (62.8) | |
| Poorly Differentiated | 22 (29.7) | 28 (19.3) |
ER = estrogen receptor, PR = progesterone receptor, IHC = immunohistochemistry, BMI = body mass index, LVI = lymphovascular invasion, SD = standard deviation, HW = Hispanic women, NHW = non-Hispanic white women.
Statistically significant, p < .05, Wilcoxon Two-Sample Test.
HW and NHW were matched on age, tumor size, and lymph node status.
Missing data: 2 NHW with ER + breast cancer by IHC without ER%.
Missing data: 1 NHW with PR + breast cancer by IHC without PR%.
Defined as ER and/or PR > 1%.
Missing data: 6 NHW without BMI.
Comparing presence of LVI in HW vs. NHW. Unknown: 2 HW and 5 NHW.
Comparing poorly differentiated breast tumors in HW vs. NHW. Unknown: 0 HW and 1 NHW.
Hispanic women had a higher BMI than non-Hispanic white women (28.0 kg/m2 vs. 26.0 kg/m2, p = .01). While Hispanic women had numerically more poorly differentiated tumors (29.7% vs. 19.3%) and tumors with LVI (16.2% vs. 11.7%), this difference was not statistically significant (p = .09 and .36, respectively).
There was no ethnic difference in ER, PR, or HER2 score, as derived by Oncotype DX assessment (Table 2). While there was a statistically significant difference in the rate of ER+/PR+/HER2− vs. ER+/PR−/HER2− breast tumors in Hispanic and non-Hispanic IHC (Table 1: p = .03), this was not statistically different by Oncotype DX analysis (Table 2: p = .08). One non-Hispanic white woman had HER2+ breast cancer as defined by Oncotype DX. There was no statistically significant difference in median Recurrence Score between Hispanic and non-Hispanic white women (17.0 vs. 15.0, p = .49). No difference was identified when Recurrence Score was evaluated as a categorical value [low risk: <18, intermediate risk: 18–30, high risk >30): p = .88]. As shown in Figure 1, Hispanic women had tumors with significantly higher proliferation scores, as measured by the composite median Proliferation Axis Score (5.4 vs. 5.2, p = .03).
Table 2.
Oncotype DX results in Hispanic (n = 74) and Non-Hispanic White Women (n = 145) with Early Stage Breast Cancer
| Median Variable (IQR) | Hispanic | Non-Hispanic White | P value* |
|---|---|---|---|
| ER Score (Positive ≥ 6.5) | 9.8 (9.1–10.8) | 9.8 (9.1–10.8) | .57 |
| PR Score (Positive ≥ 5.5) | 7.7 (6.4–8.6) | 7.65 (6.8–8.5) | .64 |
| HER2 Score (Positive ≥ 11.5) | 9.0 (8.6–9.4) | 9.0 (8.6–9.4) | .85 |
| Hormone Receptor Status, N (%) | |||
| ER+/PR+/HER2− | 52 (70.2) | 106 (73.1) | .08 |
| ER+/PR−/HER2– | 11 (14.9) | 8 (5.6) | |
| ER−/PR+/HER2− | 0 | 0 | |
| ER+/PR+/HER2+ | 0 | 1 (0.1) | |
| Unknown | 11 (14.9) | 30 (21.0) | |
| Recurrence Score | 17.0 (11–22) | 15.0 (11–20) | .49 |
| 10-year Distant Recurrence Score, % | 10.5 (7–14) | 10.0 (7–13) | .51 |
ER = estrogen receptor, PR = progesterone receptor, SD = standard deviation, HW = Hispanic women, NHW = non-Hispanic white women.
Statistically Significant, p < .05, Wilcoxon Two-Sample Test.
Figure 1.
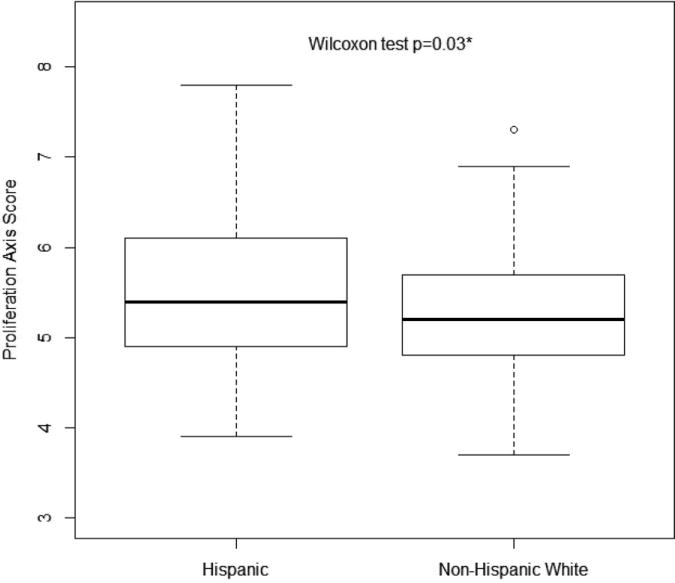
Proliferation axis score in Hispanic vs. non-Hispanic white with early stage breast cancer.
Each of the five genes that make up the Proliferation Axis Score was assessed individually (Figure 2). As demonstrated in Table 2, Hispanic women had significantly higher median scores for CCNB1 and AURKA as compared to non-Hispanic whites, (p = .01 and .03 respectively). While the differences for the three additional proliferation genes (BIRC5, MKI67, and MYBL2) were numerically higher in Hispanic women, the differences between ethnic groups were not found to be statistically significant.
Figure 2.
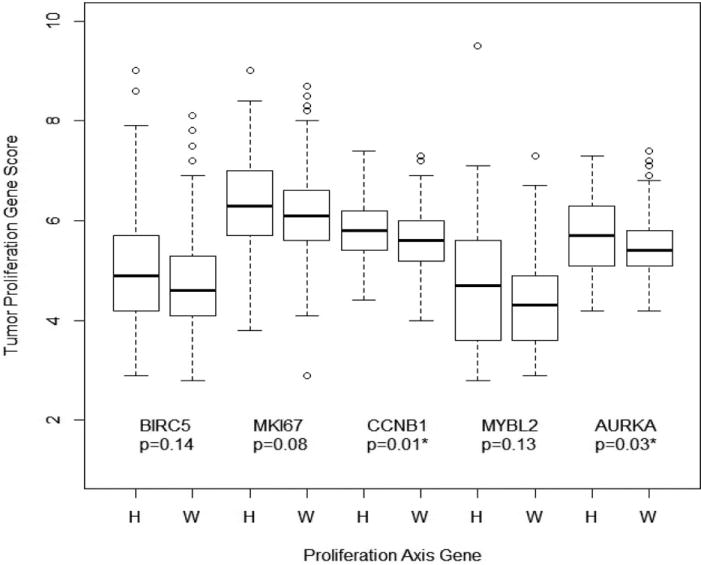
Differences in tumor proliferation genes between Hispanic (H) and non-Hispanic white (W) women.
In order to determine whether ethnicity independently predicts proliferation in breast tumors, a multivariable linear analysis was conducted with Proliferation Axis Score as the outcome variable. In a model including only variables significant in the univariable analysis (i.e., ethnicity and BMI), ethnicity independently predicted the Proliferation Axis Score, (p = .04), with Hispanic women associating with a higher proliferation score. As a continuous variable, BMI was no longer an independent predictor (p = .60). In a model including variables for which the patients were matched (i.e., age, lymph node status, and tumor stage), ethnicity was no longer a significant predictor of the Proliferation Axis Score (p = .08). A similar analysis was conducted with Recurrence Score as the outcome variable, and neither ethnicity (p = .16) nor BMI (p = .83) were independent predictors of outcome.
DISCUSSION
Based on RT-PCR assessment by the Oncotype DX assay, the 5-gene composite Proliferation Axis Score is significantly higher in Hispanic women with breast cancers compared to non-Hispanic white women. Our results are similar to findings among black women (10), in whom a higher rate of tumor proliferation genes was observed despite no differences in the weighted Recurrence Score. It is possible that differences in tumor proliferation may contribute to the ethnic disparities in breast cancer mortality (2–5).
While this study demonstrated that HR+/HER2− tumors in Hispanic women have higher proliferative scores compared to non-Hispanic whites, only two of the five proliferation genes were found to be significantly different. CCNB1 is the gene that encodes for the protein Cyclin B1, which binds to cyclin-dependent kinase 1 (CDK1) and ultimately results in high proliferation of human mammary carcinomas (13). AURKA encodes for Aurora Kinase A, which is a cell cycle regulator (14). High AURKA is an independent prognostic indicator in the HR+/HER2− subtype for worse breast cancer metastasis-free survival (15). While BIRC5, MKI67, and MYBL2 were not statistically different between Hispanic and non-Hispanic white women, the point estimate between these ethnic groups for each gene was similar to CCNB1 and AURKA. With a larger population, it is possible that BIRC5, MKI67, and MYBL2 may have reached statistical significance. In addition, the median Recurrence Score was numerically higher but not statistically different in Hispanic women as compared to non-Hispanic whites (17.0 vs. 15.0). It is possible that, despite the proliferation differences between ethnicities, there may be other genomic factors unaccounted for with the available data, which may be impacting the Recurrence Score. As no differences were identified in the ER, PR, or HER2 score between the two ethnicities, other genes, such as those associated with invasion, may also be different between ethnicities and affecting the Recurrence Score calculation.
Ultimately, the findings from this study may have treatment implications. Aurora Kinase A inhibitors are in various stages of drug development (16–18) and have shown induction of G2 cell-cycle arrest in breast cancer cell lines (16). In addition, CDKs mediate cell cycle progression and impact tumor proliferation (19). In an ex vivo breast cancer model, treatment with the CDK4/6 inhibitor PD-0332991 revealed a greater than 5-fold suppression of tumor proliferation, as measured by ki-67 staining (20). It is possible that cell cycle inhibitors will demonstrate greater efficacy in women with tumors that have increased expression of proliferation genes.
We observed that BMI was higher among Hispanic women. Others have reported higher rates of obesity and metabolic syndrome in Hispanic women as well (21, 22). Obesity at breast cancer diagnosis has a prognostic implication, as increasing BMI is associated with worse breast cancer outcome (23), specifically in patients with HR+/HER2− breast cancer (24). In addition, obese patients are diagnosed with breast tumors with higher cellular proliferation, as measured by high ki-67, as compared to normal-weight women (25, 26). However, despite BMI being higher in Hispanics, BMI was not a significant predictor of the Proliferation Axis Score after adjusting for ethnicity.
A limitation of the study is that tumor measurement of ki-67 by IHC was not available to compare with the Proliferation Axis Score. While others have reported a significant strong correlation with ki-67 and the Recurrence Score (27), ki-67 has been observed to not be the only determinant of that score (28). The Proliferation Axis Score is not provided in the commercially available Oncotype Dx report, limiting direct comparisons of ki-67 by IHC directly to the 5-gene Proliferation Axis Score. However, while it would have been interesting to include the protein expression of ki-67, as a prior report showed no difference in ki-67 between ethnicities (29), there remains existing challenges with ki-67 assessment by IHC including pre-analytic and post-analytic variability between centers (28, 30, 31). Additionally, in our study, it is possible that we were under-powered to detect differences in other tumor characteristics. In addition, the identification of Hispanic vs. non-Hispanic white was based on self-report. In this study, differences between Hispanic ethnicity and other ethnicities, such as non-Hispanic blacks or Asians were not assessed. In addition, the follow-up time for patients in this study was 51.7 months, which is a too limited duration to determine whether these proliferative differences will result in differences in breast cancer outcome in a HR+/HER2− cohort. On the other hand, there are a number of strengths to this study. A relatively large number of Hispanic patients were evaluated in this matched case-control design. Approximately 85% of Hispanic women were from the Dominican Republic or Puerto Rico, limiting the heterogeneity of nationalities in this ethnicity. In addition, highly specific gene markers for tumor proliferation were evaluated in this study.
In summary, we found that Hispanic women with HR+/HER2− breast cancer are more likely to have tumors with increased proliferation compared to non-Hispanic white women, as determined by Oncotype Dx. It is possible that agents targeting tumor proliferation may improve ethnic disparities in breast cancer outcome.
Acknowledgments
The authors would like to acknowledge Genomic Health Incorporated for their collaboration in collecting the Oncotype DX data. In particular, the authors would like to thank Lauren Intagliata, Amy Sing, and Deborah Davison for their assistance and contributions to this project.
Financial Support: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081 and the Witten Breast Cancer Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Ethical Standards
All experiments in this manuscript comply with the current laws in the United States.
DECLARATION OF INTEREST
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA: A Cancer J Clin. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 2.Frost F, Tollestrup K, Hunt WC, Gilliland F, Key CR, Urbina CE. Breast cancer survival among New Mexico Hispanic, American Indian, and non-Hispanic white women (1973–1992) Cancer Epidem Biomar & Prevention. 1996;5(11):861–866. [PubMed] [Google Scholar]
- 3.Li CI, Malone KE, Daling JR. DIfferences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor Biologic Factors and Breast Cancer Prognosis Among White, Hispanic, and Black Women in the United States. J Natl Cancer I. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 5.Ooi S, Martinez M, Li C. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Tr. 2011;127(3):729–738. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller HT, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. New Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Wickerham DL, Wolmark N. Gene Expression and Benefit of Chemotherapy in Women With Node-Negative, Estrogen Receptor–Positive Breast Cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Bralow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. The Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S. Prediction of Risk of Distant Recurrence Using the 21-Gene Recurrence Score in Node-Negative and Node-Positive Postmenopausal Patients With Breast Cancer Treated With Anastrozole or Tamoxifen: A TransATAC Study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Hayes DF. Potential biologic causes of the racial survival disparity in adjuvant trials of ER-positive breast cancer. Journal of Clinical Oncology. 2010;28:15s. supplement; abstract 511. [Google Scholar]
- 11.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Whittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists Guideline Recommendations on ER/PgR and HER2 Testing in Breast Cancer. J Clin Oncol. 2011;29(15):e458. doi: 10.1200/JCO.2011.35.2245. [DOI] [PubMed] [Google Scholar]
- 13.Collecchi P, Santoni T, Gnesi E, Giuseppe Naccarato A, Passoni A, Rocchetta M, Danesi R, Bevilacqua G. Cyclins of phases G1, S and G2/M are overexpressed in aneuploid mammary carcinomas. Cytometry. 2000;42(4):254–260. doi: 10.1002/1097-0320(20000815)42:4<254::aid-cyto6>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi M, Fujiuchi N, Sasai K, Katayama H, Minamishima YA, Ongusaha PP, Deng C, Sen S, Lee SW, Ouchi T. BRCA1 Phosphorylation by Aurora-A in the Regulation of G2 to M Transition. J Biol Chem. 2004;279(19):19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- 15.Siggelkow W, Boehm D, Gebhard S, Battista M, Sicking I, Lebrecht A, Solbach C, Hellwig B, Rahnenführer J, Koelbl H, Gehrmann M, Marchan R, Cadenas C, Hengstler JG, Schmidt M. Expression of aurora kinase A is associated with metastasis-free survival in node-negative breast cancer patients. BMC Cancer. 2012;12(1):562. doi: 10.1186/1471-2407-12-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond JR, Eckhardt SG, Tan AC, Newton TP, Selby HM, Brunkow KL, Kachaeva MI, Varella-Garcia M, Pitts TM, Bray MR, Fletcher GC, Tentler JJ. Predictive Biomarkers of Sensitivity to the Aurora and Angiogenic Kinase Inhibitor ENMD-2076 in Preclinical Breast Cancer Models. Clin Cancer Res. 2013;19(1):291–303. doi: 10.1158/1078-0432.CCR-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiskus W, Hembruff S, Rao R, Sharma P, Balusu R, Venkannagari S, Smith JE, Peth K, Peiper SC, Bhalla K. Co-treatment with vorinostat synergistically enhances activity of Aurora kinase inhibitor against human breast cancer cells. Breast Cancer Res Tr. 2012;135(2):433–444. doi: 10.1007/s10549-012-2171-9. [DOI] [PubMed] [Google Scholar]
- 18.Jani JP, Arcari J, Bernardo V, Bhattacharya SK, Briere D, Cohen BD, Coleman K, Christensen JG, Emerson EO, Jakowski A, Hook K, Los G, Moyer JD, Pruimboom-Brees I, Pustilnik L, Rossi AM, Steyn SJ, Su C, Tsaparikos K, Wishka D, Yoon K, Jakubczak JL. PF-03814735, an Orally Bioavailable Small Molecule Aurora Kinase Inhibitor for Cancer Therapy. Mol Cancer Ter. 2010;9(4):883–894. doi: 10.1158/1535-7163.MCT-09-0915. [DOI] [PubMed] [Google Scholar]
- 19.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM, Sharpless NE. Multiple Roles of Cyclin-Dependent Kinase 4/6 Inhibitors in Cancer Therapy. J Natl Cancer I. 2012;104(6):476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, Knudsen ES. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11(14):2756–2761. doi: 10.4161/cc.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Beydoun MA. Obesity Epidemic in the United States—Gender, Age, Socioeconomic, Racial/Ethnic, and Geographic Characteristics: A Systematic Review and Meta-Regression Analysis. Epidemiol Rev. 2007;29(1):6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 22.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Gleiss LS, Gregg EW. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the U.S. Population: National Health and Nutrition Examination Survey 1999–2002. Diab Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 23.Protani M, Coory M, Martin J. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Tr. 2010;123(3):627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 24.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Fetting J, Davidson NE. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamineni A, Anderson M, White E, Taplin SH, Porter P, Ballard-Barbash R, Malone K, Buist DM. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Caus Control. 2013;24(2):305–312. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92(4):720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL. Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol. 2011;19(5):431–436. doi: 10.1097/PAI.0b013e318206d23d. [DOI] [PubMed] [Google Scholar]
- 28.Sahebjam S, Aloyz R, Pilavdzic D, Brisson ML, Ferrario C, Bouganim N, Bouganim N, Cohen V, Miller WH, Panasci LC. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer. 2011;105(9):1342–1345. doi: 10.1038/bjc.2011.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss SE, Tartter PI, Ahmed S, Brower ST, Brusco C, Bossolt K, Amberson JB, Bratton J. Ethnic differences in risk and prognostic factors for breast cancer. Cancer. 1995;76(2):268–74. doi: 10.1002/1097-0142(19950715)76:2<268::aid-cncr2820760217>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Colozza M, Sidoni A, Piccart-Gebhart M. Value of Ki67 in breast cancer: the debate is still open. Lancet Oncol. 2010;11(5):414–415. doi: 10.1016/S1470-2045(10)70089-9. [DOI] [PubMed] [Google Scholar]
- 31.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]


