Abstract
Purpose.
To determine whether a novel automatic segmentation program, the Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP), can be applied to spectral-domain optical coherence tomography (SD-OCT) images obtained from different commercially available SD-OCT in eyes with diabetic macular edema (DME).
Methods.
A novel segmentation framework was used to segment the retina, inner retinal pigment epithelium, and Bruch's membrane on images from eyes with DME acquired by one of two SD-OCT systems, Spectralis or Cirrus high definition (HD)-OCT. Thickness data obtained by the DOCTRAP software were compared with those produced by Spectralis and Cirrus. Measurement agreement and its dependence were assessed using intraclass correlation (ICC).
Results.
A total of 40 SD-OCT scans from 20 subjects for each machine were included in the analysis. Spectralis: the mean thickness in the 1-mm central area determined by DOCTRAP and Spectralis was 463.8 ± 107.5 μm and 467.0 ± 108.1 μm, respectively (ICC, 0.999). There was also a high level agreement in surrounding areas (out to 3 mm). Cirrus: the mean thickness in the 1-mm central area was 440.8 ± 183.4 μm and 442.7 ± 182.4 μm by DOCTRAP and Cirrus, respectively (ICC, 0.999). The thickness agreement in surrounding areas (out to 3 mm) was more variable due to Cirrus segmentation errors in one subject (ICC, 0.734–0.999). After manual correction of the errors, there was a high level of thickness agreement in surrounding areas (ICC, 0.997–1.000).
Conclusions.
The DOCTRAP may be useful to compare retinal thicknesses in eyes with DME across OCT platforms.
Keywords: diabetic macular edema (DME), spectral-domain optical coherence tomography (SD-OCT), segmentation
A novel automatic segmentation program, DOCTRAP, was applied to the images from different SD-OCT in eyes with DME. The mean thickness showed a high level of agreements between DOCTRAP and Spectralis/Cirrus. DOCTRAP may be useful to compare measurements across OCT platforms.
Introduction
Diabetic macular edema (DME) is the primary cause of decreased visual acuity in eyes with diabetic retinopathy and is the main cause of blindness in working age individuals.1 The Early Treatment of Diabetic Retinopathy Study (ETDRS) found that clinically significant macular edema (CSME) led to moderate visual loss in 25% of patients within 3 years, and that grid or focal laser photocoagulation reduced the risk.2 Recently, it has been shown that intravitreal corticosteroid and antivascular endothelial growth factor injections with focal laser photocoagulation improve visual acuity in individuals with DME.3 To effectively manage these patients, it is essential to monitor macular edema on an ongoing basis.
Optical coherence tomography (OCT) is a noninvasive imaging technique for obtaining high-resolution cross-sectional images of the retina and has been widely used to monitor changes in retinal thickness and morphology in eyes with DME.4 Accurate measurement of retinal layer thickness depends on well-resolved images obtained by the OCT system and accurate segmentation of retinal boundaries by the OCT instrument's analysis software. Currently, many types of OCT systems are used clinically, including the Stratus time domain OCT (TD-OCT) system (Carl Zeiss Meditec, Dublin, CA) as well as spectral-domain OCT (SD-OCT) systems such as the Cirrus HD-OCT (Carl Zeiss Meditec) and Spectralis (Heidelberg Engineering, Heidelberg, Germany) systems. Although each OCT system possesses its own intrinsic software to segment the retina, each OCT system's software segmentation algorithm uses a different reference line to identify the outer retinal boundary.5–8
Retinal thickness measurement differences produced by different OCT software algorithms are important and can adversely affect the interpretation of DME retinal thickness changes in response to therapy when applied to clinical practice and trial outcomes. For example, in DME multicenter clinical trials, it is not possible to directly compare retinal thicknesses among eyes in which thickness measurements have been obtained by different systems. In the clinic, it may be difficult to assess retinal thickness changes when a patient has been referred from an outside practitioner's office where retinal thickness was determined on a different OCT system. If the outer retinal segmentation line is adjusted manually by the OCT system's software to a common outer retinal boundary, then these difficulties can be obviated. However, this approach is slow and inefficient for clinical trials and may not be practical in the clinic.
In the present study, a fully automatic segmentation program, the Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP) software, was used to segment the retina, RPE, and Bruch's membrane and to calculate retinal thickness on images acquired from eyes with DME by two different SD-OCT systems (Cirrus and Spectralis). We also assessed whether the automatically generated retinal layer thickness values produced by DOCTRAP match corresponding values attained by automatic and manually corrected semi-automatic segmentation using the Spectralis and Cirrus native software.
Methods
Study Dataset
SD-OCT images of eyes from subjects with DME enrolled in a randomized prospective DME clinical trial were evaluated. The Duke University Review Board approved this study, which adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants.
Volumetric scans were acquired using two SD-OCT imaging systems, Spectralis with Spectralis Viewing Module (Version 5.3.0.15) and Cirrus HD-OCT with review software (Version 5.2.0.210). For images obtained by Spectralis SD-OCT, a custom 20° × 20° volume acquisition protocol was used to obtain one set of high-speed scans from each eye. With this protocol, 49 cross-sectional B-scan images were obtained, each composed of 512 A-scans. For images obtained by Cirrus HD-OCT, the 512 × 128 Macular Cube scan protocol was used. With this protocol, 128 cross-sectional B-scan images were obtained, each composed of 512 A-scans.
A Duke Reading Center–certified reader determined scan quality. Readers classified image quality as high or low based on our previously published criteria.9 For example, images that were well saturated, well resolved, and free of artifacts were deemed high quality; images with low resolution, low saturation, or with artifacts produced by eye motion or loss of fixation were denoted low-quality images. We then randomly selected 40 subjects (20 imaged by Spectralis and 20 imaged by Cirrus) with high-quality images to validate the segmentation algorithm.
Guidelines for Retinal Layer Identification on Images With DME
Prior to any manual segmentation and algorithm development, we constructed a set of qualitative guidelines based on previous literature, expertise from the Duke Reading Center, and representative images to identify layer boundaries on images with DME. These guidelines were established to maintain a consistent and unbiased interpretation of each retinal boundary and are as follows:
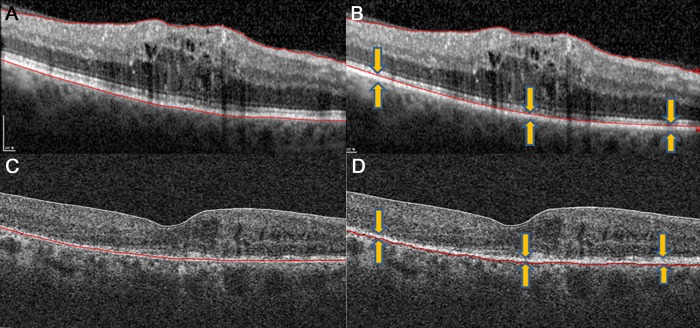
The retina/RPE thickness was defined as the region of tissue between the inner limiting membrane (ILM) and the outer RPE boundary for both Spectralis and Cirrus images (Figs. 1A, 1D);
On images acquired by Spectralis, the retinal thickness was defined as the region of tissue between the ILM and the inner RPE boundary (Fig. 1B); and
On images acquired by Cirrus, the retinal thickness was defined as the region of tissue between the ILM and a position just external to the inner RPE boundary. This definition was used because the Cirrus software segments the outer boundary at this location rather than at the inner RPE boundary (Fig. 1C).
Figure 1.
(A) Unaltered Spectralis SD-OCT segmentation of the retina/RPE (lines at ILM and outer RPE/Bruch's membrane). (B) Outer segmentation line moved by the reader to the inner RPE border to isolate the retina. (C) Unaltered Cirrus SD-OCT segmentation of the inner retina (lines at the ILM and a region just external to the inner RPE). (D) Outer segmentation line moved by the reader to the outer RPE/Bruch's membrane to isolate the retina/RPE.
Automatic Segmentation and Manual Correction by Commercial Software
Using the automatic software algorithms corresponding to each OCT system, the Spectralis system segmented the retina/RPE, and the Cirrus system segmented the retina (Figs. 1A, 1C). Both systems generated topographic surface maps for each patient as defined by the ETDRS. In cases where the image was not centered within the grid, the grid was moved manually with the system software to center the images (Fig. 2). Based on automatic segmentation, the Spectralis software determined average retina/RPE thickness measurements, and the Cirrus software calculated retinal thickness values, for each of five ETDRS grid map sectors: the center 1 mm, and the superior, inferior, nasal, and temporal sectors extending 3 mm from the center of the ETDRS map.
Figure 2.
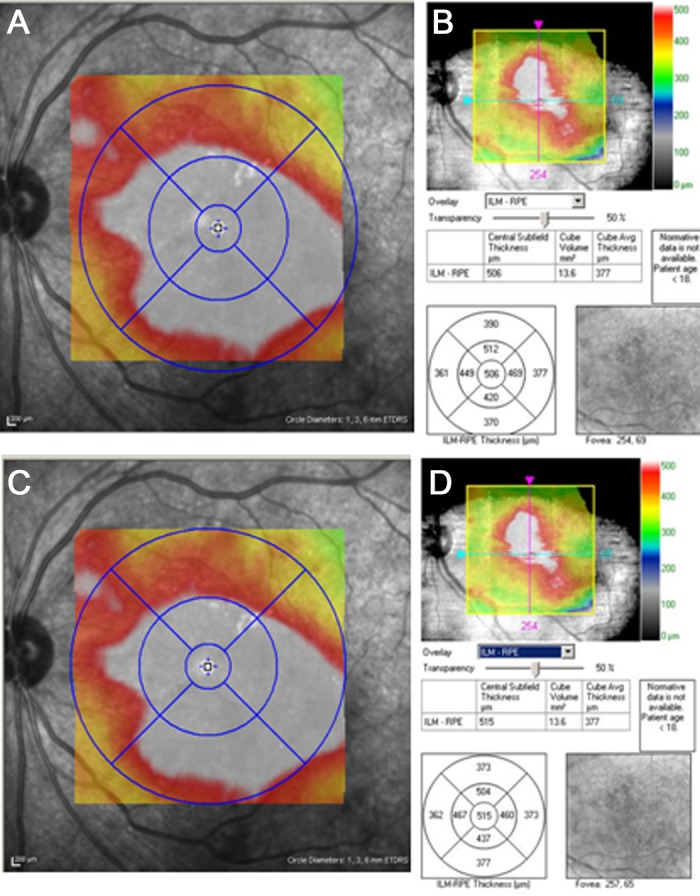
Unaltered Spectralis (A) and Cirrus (B) SD-OCT thickness maps before ETDRS grid adjustment (C, D).
To correct for errors in the Spectralis and Cirrus software segmentation, a Duke Reading Center–certified reader manually adjusted retinal layer boundary positions using the respective system's software. Using the manually corrected segmentation, thickness measurements were redetermined.
The outer segmentation lines were also manually adjusted by readers to the inner aspect of the RPE on Spectralis and to the outer aspect of the RPE/Bruch's membrane on Cirrus to provide thickness data that could be compared with the DOCTRAP fully automatic software, using two different common reference boundaries (Figs. 1B, 1D).
Automatic Segmentation by DOCTRAP Software
All 49 B-scans from each subject imaged on the Spectralis system were exported as bitmap files, and all 128 B-scans from each subject imaged on the Cirrus system were exported as IMG files. Cirrus IMG files were then converted to bitmap files using MATLAB (Mathworks, Natick, MA). We then used the DOCTRAP automatic segmentation software to segment the ILM and the inner and outer RPE boundaries on the bitmap images. Unlike the commercial software segmentation results, no manual correction was performed to alter the fully automatic lines output by DOCTRAP.
Our new three-retinal layer boundary segmentation algorithm for SD-OCT images with DME was developed based on the generalized graph theory and dynamic programming framework that we previously introduced for a normal retina and eyes with drusen and geographic atrophy secondary to nonneovascular AMD.9,10 In these previous publications, images were acquired by the Bioptigen SD-OCT system (Bioptigen, Inc., Research Triangle Park, NC). An outline of the new algorithm flow is shown in Figure 3, and the key components needed to adapt this method for Spectralis and Cirrus images of eyes with macular edema are described below. In this study, the same DOCTRAP implementation and corresponding parameters were used for all SD-OCT images captured with a given SD-OCT system, Cirrus or Spectralis.
Figure 3.
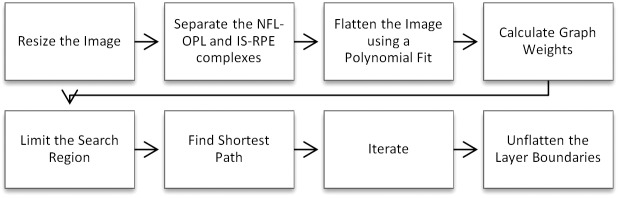
Automatic segmentation flow chart to segment Spectralis and Cirrus SD-OCT images of eyes with DME.
First, instead of flattening the retinal structures based on the convex hull of the estimated inner retinal pigment epithelium and drusen complex (RPEDC) boundary,9,10 the image was flattened based on a pilot estimate of Bruch's membrane. We fitted the pilot estimate of Bruch's membrane to both a second- and third-order polynomial, and the polynomial with the lower norm of residuals was used to flatten the image. Second, the graph weights were changed; we segmented Bruch's membrane using a combination of gradient, intensity, and distance weights, and the inner RPE was segmented using only gradient weights. These weights were fixed for all images of all patients captured by both the Spectralis and Cirrus systems. Third, we utilized the lateral (horizontal) and axial (vertical) pixel resolutions imported from the Spectralis and Cirrus systems so that the algorithm could segment images of any resolution. This allowed us to apply the exact same algorithm to segment both Spectralis and Cirrus images.
Comparison of DOCTRAP and Commercial Software Segmentation
To demonstrate that the automatic DOCTRAP software was able to accurately match the segmentation output by both the Spectralis and Cirrus software, we compared the following for all patients:
Average retina/RPE thickness generated automatically by DOCTRAP and Spectralis (with and without manual correction of Spectralis segmentation lines);
Average retinal thickness generated automatically by DOCTRAP and semi-automatically (manual placement of the inner RPE boundary) by Spectralis;
Average retinal thickness generated automatically by DOCTRAP and Cirrus (with and without manual correction of Cirrus segmentation lines); and
Average retina/RPE thickness generated automatically by DOCTRAP and semi-automatically (manual placement of the outer RPE/Bruch's membrane boundary) by Cirrus.
We reported these average thickness values for five of nine ETDRS sectors, including the center 1 mm and the superior, temporal, inferior, and nasal areas within the inner circle.
This analysis revealed that there was a constant offset in the position of the segmented inner and outer RPE boundaries when comparing DOCTRAP with the Spectralis and Cirrus software. To adjust for these differences, the DOCTRAP inner RPE line was adjusted 1 pixel externally and the DOCTRAP outer RPE line was adjusted 1 pixel internally to correspond to the segmentation lines placed by the Spectralis software. Similarly, when comparing the DOCTRAP segmentation lines with those placed by the Cirrus software, we adjusted the DOCTRAP inner RPE line 5 pixels externally and we adjusted the DOCTRAP outer RPE line 1 pixel externally.
Statistical Analysis
Statistical analysis was performed using JMP 9.0.0 (SAS Institute, Cary, NC) and IBM SPSS Statistics 20 (SPSS, Inc., Chicago, IL). The intraclass correlation (ICC) was used to determine agreement between retinal thickness measurements obtained by DOCTRAP and Spectralis software, and between DOCTRAP and Cirrus software. Bland-Altman plots with 95% limits of agreement for each comparison were determined to evaluate whether differences in thickness measurements in the different regions depended on the magnitude of the measured thickness.
Results
Spectralis
Automatic segmentation by DOCTRAP and Spectralis software is shown in Figures 4A and 4C. The retina/RPE thickness measurements determined automatically by DOCTRAP, automatically by Spectralis software, and by Spectralis software with manual correction were all very similar to one another. Tables 1 and 2 show the mean retina/RPE thickness for the 1-mm diameter central foveal area and the surrounding innermost superior, nasal, inferior, and temporal regions on the ETDRS grid. The mean retina/RPE thickness of the 1-mm diameter central foveal subfield was 463.8 ± 107.5 μm, 467.0 ± 108.1 μm, and 467.2 ± 109.3 μm as determined by the DOCTRAP automatic segmentation software, Spectralis automatic segmentation software, and Spectralis software with manual correction, respectively. There was also a high degree of agreement for the mean thickness in the surrounding quadrants determined by both software products (Table 1).
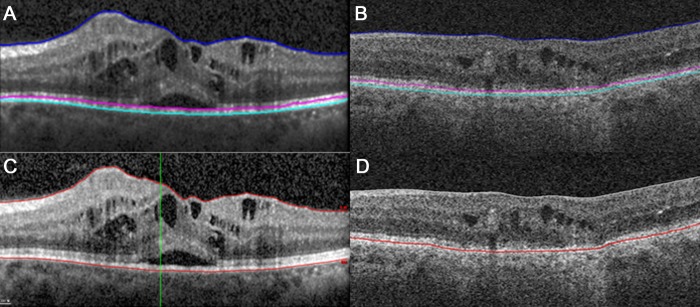
Figure 4.
Automatic segmentation results. (A, B) DOCTRAP segmentation of a (C) Spectralis and a (D) Cirrus image.
Table 1.
Mean Retina/RPE Thickness Determined by DOCTRAP Automatic Software, Spectralis Automatic Software, and Spectralis Software With Manual Correction
|
Retina/RPE Thickness |
|||||||||
|
Mean Thickness, μm |
DOCTRAP Software vs. Spectralis Automatic Software |
DOCTRAP Software vs. Spectralis Software With Manual Correction |
|||||||
|
DOCTRAP Automatic Software, Mean ± SD* |
Spectralis Automatic Software, Mean ± SD* |
Spectralis Software With Manual Correction, Mean ± SD* |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
|
| 1-mm diameter central foveal area | 463.8 ± 107.3 | 467.0 ± 108.1 | 467.2 ± 109.3 | −3.22 ± 1.14 | 0.999 (0.997–0.999) | −5.61 to −0.82 | −3.42 ± 0.67 | 1.000 (0.999–1.000) | −4.83 to −2.01 |
| Surrounding area, out to 3 mm | |||||||||
| Superior | 444.1 ± 90.3 | 442.9 ± 88.6 | 442.9 ± 89.2 | 1.25 ± 1.37 | 0.998 (0.994–0.999) | 1.20 to 1.32 | 1.20 ± 1.32 | 0.998 (0.995–0.999) | −1.33 to 2.28 |
| Nasal | 420.7 ± 68.7 | 418.3 ± 66.6 | 418.9 ± 67.5 | 2.36 ± 1.66 | 0.994 (0.985–0.998) | 1.81 to 1.58 | 1.81 ± 1.58 | 0.995 (0.986–0.998) | 1.85 to 9.63 |
| Inferior | 410.1 ± 63.7 | 406.5 ± 59.2 | 405.9 ± 59.8 | 3.71 ± 1.66 | 0.993 (0.982–0.997) | 4.26 to 1.60 | 4.26 ± 1.60 | 0.993 (0.983–0.997) | 6.92 to 16.2 |
| Temporal | 456.9 ± 103.9 | 457.0 ± 102.7 | 455.7 ± 103.1 | −0.08 ± 1.62 | 0.998 (0.994–0.999) | 1.22 to 1.65 | 1.22 ± 1.65 | 0.997 (0.994–0.999) | 2.15 to 8.94 |
CI, confidence interval.
n = 20.
Table 2.
Mean Retinal Thickness Determined by DOCTRAP Automatic Software and Spectralis Semi-Automatic Software (Manual Placement of Inner RPE Boundary)
|
Retinal Thickness |
|||||
|
Mean Thickness, μm |
DOCTRAP Automatic Software vs. Spectralis Semi-Automatic Software |
||||
|
DOCTRAP Automatic Software, Mean ± SD* |
Spectralis Semi-Automatic Software, Mean ± SD* |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
|
| 1-mm diameter central foveal area | 441.9 ± 107.9 | 443.9 ± 108.6 | −2.02 ± 1.35 | 0.998 (0.996–0.9999) | −4.84 to 0.80 |
| Surrounding area, out to 3 mm | |||||
| Superior | 424.3 ± 90.1 | 422.3 ± 88.1 | 1.99 ± 1.53 | 0.997 (0.993–0.999) | −1.21 to 5.19 |
| Nasal | 399.0 ± 68.8 | 396.4 ± 66.1 | 2.64 ± 1.79 | 0.993 (0.982–0.997) | −1.12 to 6.39 |
| Inferior | 389.3 ± 63.3 | 385.2 ± 58.5 | 4.14 ± 2.20 | 0.987 (0.967–0.995) | −0.45 to 8.74 |
| Temporal | 436.4 ± 103.9 | 435.5 ± 102.7 | 0.87 ± 1.50 | 0.998 (0.995–0.999) | −2.26 to 4.01 |
n = 20.
We next determined mean retinal thickness measurements after repositioning the outer Spectralis segmentation line to the inner aspect of the RPE. With this segmentation line placement, the mean 1-mm diameter central foveal subfield retinal thickness determined by DOCTRAP and Spectralis software also agreed with one another; the mean paired difference was −2.02 ± 1.35 μm (ICC, 0.998). There was also good agreement for the mean retinal thickness in the surrounding superior, nasal, inferior, and temporal quadrants (Table 2).
Cirrus
Automatic segmentation by DOCTRAP and Cirrus software is shown in Figures 4B and 4D. The retinal thickness measurements determined automatically by DOCTRAP, automatically by Cirrus software, and by Cirrus software with manual correction were all very similar to one another. The mean 1-mm diameter central foveal subfield retinal thickness was 440.8 ± 183.4 μm, 442.7 ± 182.4 μm, and 442.5 ± 182.9 μm as determined by the DOCTRAP automatic segmentation software, Cirrus automatic segmentation software, and Cirrus software with manual correction, respectively. Furthermore, the mean retinal thicknesses of the regions surrounding the central subfield out to 3 mm were all similar to one another except for the superior region, which was more variable (ICC for all regions except the superior region, 0.993–0.999). In the superior region, the average difference between the automatic Cirrus measurements and automatic DOCTRAP measurements was 19.47 μm (ICC, 0.734) (Table 3).
Table 3.
Mean Retinal Thickness Determined by DOCTRAP Automatic Software, Cirrus Automatic Software, and Cirrus Software With Manual Correction
|
Retinal Thickness |
|||||||||
|
Mean Thickness, μm |
DOCTRAP Software vs. Cirrus Automatic Software |
DOCTRAP Software vs. Cirrus Manual Correction |
|||||||
|
DOCTRAP Automatic Software, Mean ± SD* |
Cirrus Automatic Software, Mean ± SD* |
Cirrus Software With Manual Correction, Mean ± SD* |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
|
| 1-mm diameter central foveal area | 440.8 ± 183.4 | 442.7 ± 182.4 | 442.5 ± 182.9 | −1.84 ± 1.65 | 0.999 (0.998–1.000) | −5.30 to 1.61 | −2.24 ± 1.16 | 1.000 (0.999–1.000) | −4.67 to 0.18 |
| Surrounding area, out to 3 mm | |||||||||
| Superior | 410.7 ± 142.7 | 391.3 ± 139.9 | 411.2 ± 139.9 | 19.47 ± 19.72 | 0.734 (0.441–0.886) | −21.80 to 60.75 | −0.48 ± 1.57 | 0.999 (0.997–1.000) | −3.77 to 2.81 |
| Nasal | 409.2 ± 131.5 | 408.6 ± 128.3 | 408.7 ± 128.2 | 0.61 ± 1.70 | 0.998 (0.996–0.999) | −2.94 to 4.17 | 0.46 ± 1.78 | 0.998 (0.995–0.999) | −3.25 to 4.18 |
| Inferior | 405.4 ± 148.6 | 402.6 ± 142.8 | 401.8 ± 141.0 | 2.83 ± 2.10 | 0.998 (0.995–0.999) | −1.57 to 7.24 | 3.63 ± 2.49 | 0.997 (0.993–0.999) | −1.57 to 8.84 |
| Temporal | 416.2 ± 170.3 | 411.0 ± 154.7 | 415.5 ± 165.7 | 5.16 ± 4.16 | 0.993 (0.984–0.997) | −3.55 to 13.9 | 0.71 ± 1.68 | 0.999 (0.997–1.000) | −2.80 to 4.23 |
n = 20.
We next determined mean retina/RPE thickness measurements after repositioning the outer Cirrus segmentation line to the outer RPE/Bruch's membrane. With this segmentation line placement, the mean 1-mm diameter central foveal subfield retina/RPE thickness determined by DOCTRAP and Cirrus software was comparable clinically. The mean paired difference was −4.05 ± 1.45 μm (ICC, 0.999). There was also good agreement for the mean retina/RPE thickness in the surrounding superior, nasal, inferior, and temporal quadrants (ICC, 0.996–0.999; Table 4).
Table 4.
Mean Retina/RPE Thickness Determined by DOCTRAP Automatic Software and Cirrus Semi-Automatic Software (Manual Placement of Outer RPE/Bruch's Membrane Boundary)
|
Outer Retina |
|||||
|
Mean Thickness, μm |
DOCTRAP Automatic Software vs. Cirrus Semi-Automatic Software |
||||
|
DOCTRAP Automatic Software, Mean ± SD* |
Cirrus Semi-Automatic Software, Mean ± SD* |
Mean Paired Difference, Mean ± SE |
ICC (95% CI) |
95% Limits of Agreement |
|
| 1-mm diameter central foveal area | 459.0 ± 183.8 | 463 ± 184.8 | −4.05 ± 1.45 | 0.999 (0.998–1.000) | −7.08 to −1.01 |
| Surrounding area, out to 3 mm | |||||
| Superior | 428.9 ± 143.5 | 428.9 ± 140.6 | 0.00 ± 1.72 | 0.999 (0.996–0.999) | −3.61 to 1.72 |
| Nasal | 428.1 ± 131.7 | 428.3 ± 127.9 | −0.19 ± 2.61 | 0.996 (0.990–0.998) | −5.66 to 5.28 |
| Inferior | 423.2 ± 148.8 | 420.8 ± 141.2 | 2.44 ± 2.86 | 0.996 (0.990–0.998) | −3.56 to 8.43 |
| Temporal | 434.4 ± 171.0 | 435.0 ± 168.2 | −0.62 ± 1.74 | 0.999 (0.997–1.000) | −4.26 to 3.03 |
n = 20.
Discussion
In the present report, we have shown that the DOCTRAP automatic segmentation software can readily import universal image files (bitmap images) exported from two different SD-OCT systems and can identify specific inner and outer retinal layer boundaries in the central subfield and surrounding regions in eyes with DME. From the segmentation lines placed on these boundaries, retina/RPE and retinal thickness measurements were calculated automatically with this novel software. These measurements agreed well with those determined by commercially available software from two different manufacturers. Furthermore, there were no reproducible systematic thickness differences as a function of retinal thickness magnitude.
The thickness measurements were similar for all DOCTRAP and Spectralis and all DOCTRAP and Cirrus software comparisons, with the exception of the superior region surrounding the central subfield in the DOCTRAP–Cirrus retinal thickness comparison. This difference in the superior subfield was likely caused by significant errors in the Cirrus automated segmentation for one of the subjects (Fig. 5), as after manual correction of these errors, the mean retinal thicknesses determined by DOCTRAP and Cirrus software were nearly identical (ICC, 0.999; Table 3).
Figure 5.
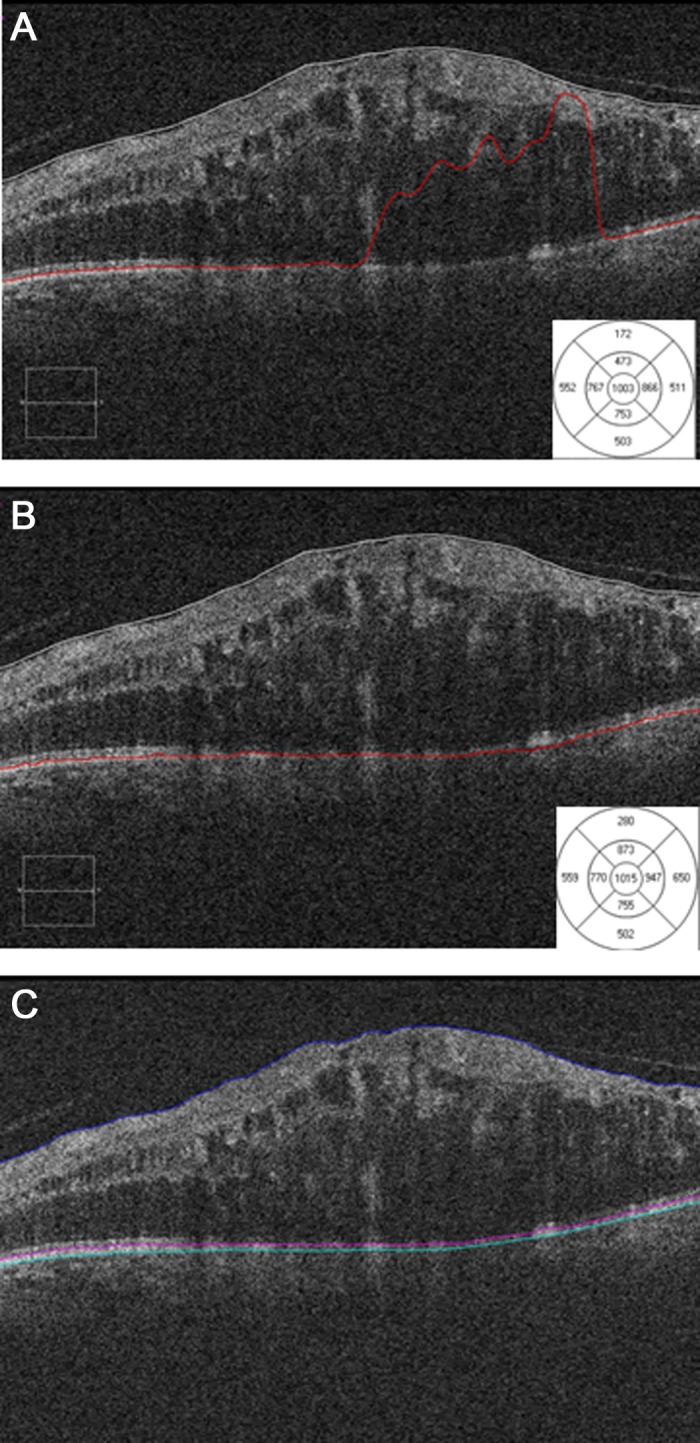
An example segmentation error produced by the Cirrus software. (A) Erroneous outer segmentation line results in an erroneous mean retinal thickness measurement of 473 μm in superior surrounding area. (B) Accurate outer segmentation line after manual correction results in a mean retinal thickness of 873 μm. (C) Accurate outer segmentation line placed automatically by DOCTRAP software.
We, and others, have previously published the SD-OCT segmentation artifact rates in eyes with a variety of diseases, including those with diabetic macular edema. In this study, we randomly selected high-quality images for analysis to validate the segmentation algorithm. Accordingly, fewer segmentation line algorithm failures were observed than what would typically be seen in practice (with the exception of the error observed in one patient and illustrated in Fig. 5). In the future, it will be valuable to compare segmentation error rates in DOCTRAP with those produced by commercially available software. To conduct an appropriately detailed segmentation-error analysis is beyond the current study scope but is the subject of work currently in progress at our institution.
We specifically analyzed eyes with DME in this study; however, macular edema caused by other diseases such as retinal vein occlusion, uveitis, and neovascular AMD can also be assessed quantitatively with OCT11; thus we anticipate that our automatic software will be similarly useful in determining retinal thickness in eyes with other macular edema–related conditions. It will be of interest to apply our DOCTRAP segmentation analysis to eyes with macular edema from these various conditions.
In our previous studies, we showed that the DOCTRAP segmentation software could automatically segment the ILM, retinal nerve fiber layer, ganglion cell complex, external limiting membrane, inner segment/outer segment (IS/OS) junction, inner RPE, and Bruch's membrane in normal eyes and in eyes with dry AMD.9,10,12 In the present report, we used DOCTRAP to automatically segment the ILM and the inner and outer RPE boundaries to calculate the mean retina/RPE and retinal thickness in each ETDRS grid area in eyes with DME. Thickness abnormalities of the retinal nerve fiber layer and ganglion cell complex have been associated with visual function deficits in eyes with optic neuropathies such as optic neuritis, while abnormal external membrane and ellipsoid zone integrity, as well as abnormal thickness in the outer nuclear complex layer, have been associated with decreased visual acuity. An assessment of these layers in eyes with pathology is beyond the current scope of our study. However, studies to assess these boundary layers in eyes with diabetic macular edema and other retinal diseases are currently underway at our Reading Center.
This study has limitations. We selected relatively high-quality images, which facilitated the identification of retinal boundaries. In a clinical setting, factors may decrease the ability to identify layer boundaries. For example, media opacity or patient eye movement could degrade images and compromise the automatic segmentation accuracy.5,13,14 The relative ability of our automatic software and the commercially available software to segment boundaries under these adverse conditions remains to be determined. Another limitation is that different patients were imaged on different systems. We are now collecting data whereby the same subject is imaged on both systems during the same imaging session so that we may directly compare thickness measurements obtained by the two different systems.
Analysis of retina/RPE and retinal thickness was determined for eyes with DME using images obtained by two commonly used OCT systems, Spectralis and Cirrus. The performance of our DOCTRAP software on other SD-OCT systems is not yet known. Regardless, the results which show a high degree of agreement for thickness determined by commercial software and by DOCTRAP software, when applied to different common outer layer boundary lines, are encouraging; these data suggest that DOCTRAP software may be useful to compare retinal thickness in eyes with DME across OCT platforms in the clinic and in interventional and natural history trials.
Acknowledgments
Supported in part by National Institutes of Health Grant R01 EY022691.
Disclosure: J.Y. Lee, None; S.J. Chiu, P; P.P. Srinivasan, None; J.A. Izatt, P; C.A. Toth, P; S. Farsiu, P; G.J. Jaffe, None
Footnotes
JYL and SJC are joint first authors.
References
- 1. Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010; 26: 1587–1597. [DOI] [PubMed] [Google Scholar]
- 2. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103: 1796–1806. [PubMed] [Google Scholar]
- 3. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010; 117: 1064–1077 e1035. Available at: http://www.sciencedirect.com/science/article/pii/S0161642010002435. Accessed November 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao W, Tátrai E, Ölvedy V, et al. Investigation of changes in thickness and reflectivity from layered retinal structures of healthy and diabetic eyes with optical coherence tomography. J Biomed Sci Eng. 2011; 4: 657–665. [Google Scholar]
- 5. Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral-domain optical coherence tomography. Ophthalmology. 2010; 117: 1177–1189 e1174. Available at: http://www.sciencedirect.com/science/article/pii/S016164200901224X. Accessed November 6, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Krebs I, Smretschnig E, Moussa S, Brannath W, Womastek I, Binder S. Quality and reproducibility of retinal thickness measurements in two spectral-domain optical coherence tomography machines. Invest Ophthalmol Vis Sci. 2011; 52: 6925–6933. [DOI] [PubMed] [Google Scholar]
- 7. Kakinoki M, Miyake T, Sawada O, Sawada T, Kawamura H, Ohji M. Comparison of macular thickness in diabetic macular edema using spectral-domain optical coherence tomography and time-domain optical coherence tomography. J Ophthalmol. 2012; 2012: 959721 Available at: http://www.hindawi.com/journals/joph/2012/959721/. Accessed November 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heussen FM, Ouyang Y, McDonnell EC, et al. Comparison of manually corrected retinal thickness measurements from multiple spectral-domain optical coherence tomography instruments. Br J Ophthalmol. 2012; 96: 380–385. [DOI] [PubMed] [Google Scholar]
- 9. Chiu SJ, Izatt JA, O'Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2012; 53: 53–61. [DOI] [PubMed] [Google Scholar]
- 10. Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010; 18: 19413–19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995; 113: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 12. Park HY, Jeon SH, Park CK. Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primary open-angle glaucoma. Ophthalmology. 2012; 119: 10–20. [DOI] [PubMed] [Google Scholar]
- 13. Lammer J, Scholda C, Prunte C, Benesch T, Schmidt-Erfurth U, Bolz M. Retinal thickness and volume measurements in diabetic macular edema: a comparison of four optical coherence tomography systems. Retina. 2011; 31: 48–55. [DOI] [PubMed] [Google Scholar]
- 14. Ibrahim MA, Sepah YJ, Symons RC, et al. Spectral- and time-domain optical coherence tomography measurements of macular thickness in normal eyes and in eyes with diabetic macular edema. Eye (Lond). 2012; 26: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]