Abstract
Purpose
To incorporate phospho-ibuprofen (P-I), a lipophilic, water insoluble novel anti-cancer agent, into pegylated liposomes and upon formulation optimization to evaluate its antitumor activity in vitro and in vivo.
Methods
P-I loaded liposomes were prepared using the thin-film hydration method, and characterized for size, zeta potential, drug content and drug release. We examined their physical stability by particle size changes; their lyophilization ability in the presence of cryoprotectants; and their antitumor activity in vitro in human cancer cell lines and in vivo in a xenograft murine model.
Results
P-I was successfully loaded into liposomes consisting of soy-PC and PEG2000-PE. These liposomes were <150 nm in diameter; exhibited prolonged stability in suspension and can be lyophilized using sucrose as cryoprotectant. P-I liposomes inhibited the growth of human cancer cell lines in vitro and in vivo of xenograft in nude mice to a greater extent than free P-I.
Conclusions
High levels of P-I can be incorporated into liposomes which can be lyophilized in the presence of sucrose and showed good stability upon storage. Moreover, these drug-incorporating liposomes were capable of inhibiting the growth of xenografted tumors in mice more effectively than free P-I. These results justify further development of the P-I liposomes.
Keywords: anticancer agent, liposomes, NSAIDs, pegylated phospholipids, phospho-ibuprofen
INTRODUCTION
Inflammation is a critical component of tumor progression and non-steroidal anti-inflammatory drugs (NSAIDs) are efficacious in inhibiting early neoplastic progression and malignant conversion (1). For example, the NSAID ibuprofen reduces the risk of various human cancers, including those of the colon, breast, lung and prostate (2–5). A major obstacle in the long-term use of NSAIDs for the control of cancer is their high toxicity and relatively low efficacy. To overcome these limitations, we developed novel chemical modifications of conventional NSAIDs (6–8). One such modified NSAID is phospho-ibuprofen (P-I), a derivative of ibuprofen, consisting of ibuprofen covalently attached to the diethylphosphate group via a spacer moiety. We have recently reported on its metabolism, pharmacokinetics and pharmacodynamics in mice (8). P-I inhibited the growth of colon cancer xenografts in nude mice, but its effect was suboptimal (68% maximal inhibition under our experimental protocol). We reasoned that an improved method of drug delivery could enhance its antitumor efficacy and/or afford us the opportunity to use lower drug doses.
Liposomes, used extensively as drug carriers, have already found significant clinical applications due to their versatility and biocompatibility (9). They can accommodate poorly soluble lipophilic agents and increase their therapeutic index (10,11 ) as it was demonstrated with pegylated liposomes incorporating various anticancer agents (12). Major contributors to this effect are the prolonged circulation time of these liposomes due to avoidance of the recticuloendothelial system (RES), and their preferential accumulation in solid tumors through the “enhanced permeability and retention” (EPR) effect (13,14 ).
P-I has low water solubility (logP = 5.22) and the use of hydrophobic vehicles is required to dissolve it. In this study, we report on the preparation of P-I loaded liposomes. We studied the effect of lipid compositions and cryoprotectants on drug entrapment efficiency, liposome size, zeta potential and long-term stability. We also assessed the effect of optimized liposome formulations on the growth of several human cancer cell lines in vitro and on the growth of a xenograft tumor in mice.
MATERIALS AND METHODS
Materials
Soy-phosphatidylcholine (soy-PC), egg-phosphatidylcholine (egg-PC), soy-phosphatidylserine (soy-PS), soy-phosphatidylethanolamine (soy-PE) and methoxy-poly (ethylene glycol) 2000-distearoylphosphatidylethanolamine (PEG2000-PE) were obtained from Avanti Polar Lipids (Alabaster, AL). Human insulin, cholesterol, octadecylamine (stearylamine), acetonitrile, chloroform, miscellaneous reagents and solvents, all of analytical grade, were obtained from Sigma (St. Louis, MO).
Cell Culture
MCF-7 and MDA-MB-231 human breast cancer and SW840 human colon cancer cell lines were purchased from American Type Culture Collection (ATTC, Manassas, VA) and grown as monolayers. Cell culture media (MEM for MCF-7 cells; L-15 for MDA-MB-231 cells; and RPMI 1640 for SW480 cells) were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% antibiotics (50 U/ml penicillin and 0.05 mg/ml streptomycin) and 0.01 mg/ml human insulin (insulin used only for MCF-7). Cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Preparation and Characterization of Liposomes Containing P-I
A chloroform solution of lipids and P-I was slowly evaporated in a rotary evaporator (Buchi R11, Switzerland) to prepare a thin lipid film in the inner wall of a 100-ml round bottom flask. For every liposome formulation a standard initial molar ratio of lipids and drug was used consisting of lipids:PEG-PE:drug 4.8:0.5:4.7. The lipid film was hydrated with 3 ml of phosphate buffered saline (PBS, pH 7.4) and vortexed for 10 min. The resultant suspensions of multilamellar vesicles were subjected to sonication for 10 min in a 5510 Branson bath type sonicator (Danbury, CT) followed by extrusion 5 times through a 0.4-μm-polycarbonate membrane and 20 times through a 0.2-μm-polycarbonate membrane using an extruder device (Lipex Northern Lipids Inc., BC, Canada). Non-entrapped drug was removed by overnight dialysis with excess PBS.
Entrapped P-I was quantified with high-performance-liquid-chromatography (HPLC) Waters Alliance 2695 equipped with a Waters 2998 photodiode array detector (220 nm) (Milford, MA) and a Thermo BDS Hypersil C18 column (150×4,6 mm, particle size 3 μm) (Thermo Firsher Scientific, Waltham, MA) by dissolving a small volume of the liposome suspension into 1 ml of acetonitrile. The mobile phase followed a gradient between buffer A (H2O, acetonitrile, trifluoroacetic acid 94.9:5:0.1 v/v/v) and buffer B (acetonitrile). The retention time for P-I was previously determined to be 7.42 min (8).
The size and zeta potential of the liposomes were determined using dynamic light scattering (DLS) and microelectrophoresis, respectively, 10 min after diluting the samples in PBS and housing them at 25°C using the Zeta-Plus Brookhaven instrument (Holtsville, NY).
The morphology of the liposomes was determined by transmission electron microscopy (TEM). For negative staining, liposomes were diluted with distilled water and dropped on a copper grid and air-dried for 1 min at room temperature. After adhesion of liposomes, 10 μl of 2% uranyl acetate solution, filtered through a 0.2 μm filter prior to use, were dropped onto the grid as a staining solution. The excess staining solution was removed with filter paper after 30 s and the sample was air-dried for 10 min at room temperature, followed by observation under TEM.
Nuclear magnetic resonance (NMR) was performed using a Varian Instrument (Santa Clara, CA) at 500 Mhz by dissolving lyophilized liposomes with entrapped drug in deuterated chloroform to determine the PEG content of the dispersion.
The entrapment efficiency of the drug (EE) is defined as the ratio of the amount of drug entrapped in liposomes to the total amount of drug initially introduced in the formulation.
Lipid Substitution
We used various lipid mixtures to obtain liposomal dispersions of P-I and determined their EE, size and zeta potential. The lipid mixtures included either partial substitution of soy-PC with other lipids as soy-PS and soy-PE or complete substitution of soy-PC with egg-PC. We also evaluated the addition of cholesterol or stearylamine to the liposome formulations. (Table I lists all tested formulations). All experiments were performed at least in duplicate.
Table I.
Liposome Formulation Groups
| Group | Soy-PC | Egg-PC 100% of total lipids mol:mol | Soy-PS 10% of total lipids mol:mol | Soy-PE 10% of total lipids mol:mol | Cholesterol % of total l ipid mol:mol | Stearylamine % of total lipids mol:mol |
|---|---|---|---|---|---|---|
| A | + | − | − | − | − | − |
| B | + | − | + | − | − | − |
| C | + | − | − | + | − | − |
| D | − | + | − | − | − | − |
| E | + | − | − | − | + 1% | − |
| F | + | − | − | − | + 5% | − |
| G | + | − | − | − | + 10% | − |
| H | + | − | − | − | − | + 5% |
| I | + | − | − | − | − | + 10% |
| J | + | − | − | − | − | + 15% |
In Vitro Release of P-I from Liposomes and Liposome Stability
Release of P-I from P-I loaded liposomes was studied using the dialysis method at room temperature. Liposome samples of 0.5 ml (7 mg/ml) were placed in dialysis bags (MWCO 3.500, Thermo Scienctific, Rockford, IL) and tightly sealed. The bag was immersed in 100 ml of PBS under mild stirring. Samples of the release medium (0.5 ml) were taken in predetermined time intervals and replaced with the same volume of fresh medium. The concentration of P-I was determined by HPLC.
Liposome dispersions were kept at room temperature and at predetermined time intervals their size and zeta potential were assessed to evaluate the stability of the liposome formulations.
Liposome Lyophilization
Aliquots of freshly prepared liposomes were lyophilized in the presence or absence of cryoprotectant. Briefly, 0.2 ml of liposome suspension were placed inside Eppendorf tubes and mixed with a small volume of concentrated cryoprotectant solution. The mixture was vortexed for 1 min followed by rapid freezing with liquid nitrogen and 24-h lyophilization. The lyophilized liposome powder was re-suspended in the original volume of water and vortexed for 1 min. The size of the liposomes in the suspension was determined as above. Sucrose, mannitol and trehalose were studied as cryoprotectant agents in different molecular ratios with the lipids.
In Vitro Cytotoxicity Assay
The cytotoxicity of P-I loaded liposomes against MDA-MB-231 and MCF-7 breast and SW480 colon cancer cells was determined using the MTT assay and compared to that of free P-I (15). Briefly, 104 cells/well were plated in 96-well flat-bottom tissue-culture plates in 100 μl culture medium, and incubated for 24 h, when the test compounds were added. Twenty four hours later MTT solution (5 mg/ml in PBS pH 7.4) was added into each well and the plates were incubated at 37°C for 4 h. A volume of 100 μl of 10% SDS in 0.01 M HCl was added into each well and the plates were incubated at 37°C overnight to solubilize the formazan crystals. Absorbance was determined at 570 nm using a microplate reader (Molecular Devices, USA). The experiments were performed in hexaplicates and repeated twice.
Treatment of Nude Mice with Colon Cancer Xenografts
SW480 cells (2×106, suspended in 100 μl Matrigel (BD Matrigel Matrix, BD Biosciences)/PBS (1:1)) were xenografted subcutaneously in both flanks of 5–6 weeks old female SCID mice (Harlan Sprague–Dawley, Indianapolis, IN). When the average xenograft tumor volume reached ~70 mm3, animals were divided into three treatment groups: a) vehicle (n=8); b) free P-I 100 mg/kg (n=10); c) liposome P-I 100 mg/kg(n=10); and d) liposome P-I 300 mg/kg(n=10), all administered by intraperitoneal injection once a day 5 d/week for 17 d. Tumor volume was calculated by measuring with a caliper its length (L) and width (W) according to the formula, L×W×(L+W/2)×0.56. Xenograft growth inhibition in response to treatment was calculated by comparing the difference in percent increase of tumor growth from their 0 time volume to their volume at sacrifice between drug- and vehicle-treated groups.
RESULTS
Liposome Formulations
P-I liposomes were prepared by extrusion of multi-lamellar liposomes using various phospholipid mixtures (Table I). To generate homogenous unilamellar vesicles, hydration of the drug-lipid film was employed, followed by extrusion through polycarbonate membranes. Liposomes were characterized in terms of their size, zeta potential, drug loading and long-term stability. Table II summarizes these results.
Table II.
Characteristics of Liposome Formulations
| Group | Hydrodynamic diameter, nm | PDI | Zeta potential mV | EE % | Drug content mg/ml |
|---|---|---|---|---|---|
| A | 140±5 | 0.122±0.03 | −28.7±4 | 57±3 | 15±3 |
| B | 132±6 | 0.120±0.04 | −31.1±1 | 54±6 | 15±4 |
| C | 133±8 | 0.117±0.04 | −30.4±4 | 60±4 | 17±2 |
| D | 125±4 | 0.181±0.03 | −40±4 | 49±2 | 13±1 |
| E | 150±3 | 0.112±0.03 | −34.7±1 | 60±1 | 16±1 |
| F | 148±6 | 0.131±0.04 | −32±2 | 61±1 | 16±1 |
| G | 170±5 | 0.179±0.03 | −32.7±4 | 60±4 | 16±2 |
| H | 131±8 | 0.082±0.08 | −24±4 | 48±6 | 13±2 |
| I | 127±2 | 0.098±0.01 | −9.7±2 | 51±4 | 13±3 |
| J | 123±3 | 0.112±0.01 | 10.3±4 | 52±7 | 14±2 |
All determinations were performed at least in duplicate; values, mean±SEM
Liposomes composed of phosphatidylcholine (PC) and equimolar quantities of P-I showed satisfactory entrapment efficiency, (around 60%), with drug content of about 15 mg/ml, which is considered sufficiently high for a hydrophobic molecule like P-I (10). The average hydrodynamic diameter of these liposomes was 140 nm and their zeta potential −28.7 mV. Images from negative-staining TEM revealed liposomes of discrete and round structure, ranging in diameter from 100 to 150 nm (Fig. 1); these results are in close agreement with their hydrodynamic diameter (Table II).
Fig. 1.
Soy-PC liposomes with incorporated P-I. Upper panel: TEM microphotographs of liposomes. Bar = 100 nm. Lower panel: 1H NMR spectrum of lyophilized liposomes with incorporated P-I at 500 MHz after dissolving them in deuterated chloroform.
Using phophatidylserine (PS) and, to a lesser extent, phosphatidylethanolamine (PE) in liposome preparation has been shown to reduce their size without significantly altering their zeta-potential at physiological pH (16,17 ). Thus, we substituted, partially or completely, egg-PC, soy-PE and soy-PS for soy-PC and determined the effect of each substitution on drug entrapment and liposome size and other physicochemical characteristics. The goal of these substitutions was to reduce the liposomal size or increase the drug EE without significantly altering the zeta potential of the liposome suspension. Complete substitution, however, of egg-PC for soy-PC failed to meaningfully improve these two parameters. Although the average size of the liposomes was reduced on average by 15 nm, drug EE was also significantly reduced by 8%. Similarly, partially substituting soy-PS and soy-PE for soy-PC provided no substantial improvement.
To ensure that the ratios of the component lipids were not significantly altered during liposome preparation, the drug:lipid ratio was monitored throughout their preparation process using 1H NMR spectroscopy. Figure 1 lower ( panel) shows a 1H NMR spectrum of a liposome suspension at its final stage. Assignments of the 1H resonances reveal that the molar ratio of the soy-PC to the PEG-PE is 10:1 and the molar ratio of drug:soy-PC is 1:1. Both results are identical to the input ratios, indicating that the drug:lipid ratio is maintained throughout the formation of the liposomes. Similar results we obtained with egg-PC liposomes (data not shown).
Liposome Stability and Release
Liposome stability is a property that can critically impact their intended clinical application (9). Instability of a liposome suspension leads to aggregation or fusion of liposomes during storage, which in turn increases particle size. Particles of greater size are generally taken up rapidly by the RES resulting in their rapid clearance and shortened half-life. Additionally, the instability of the liposomes accelerates drug release during storage.
The development of liposomes as effective drug delivery systems was aided by the incorporation of membrane rigidifying agents such as cholesterol. Early studies demonstrated that cholesterol enhanced the retention of entrapped agents by modulating the fluidity of the liposomal membrane (18,19). Indeed, cholesterol in the phospholipid membrane bilayer can increase the stability of liposomes (19 ). We therefore assessed the effect of cholesterol on liposome stability. To this end, only soy-PC liposomes was used since, as shown above, soy-PE, soy-PS and egg-PC had no favorable effects on the characteristics of the liposomes.
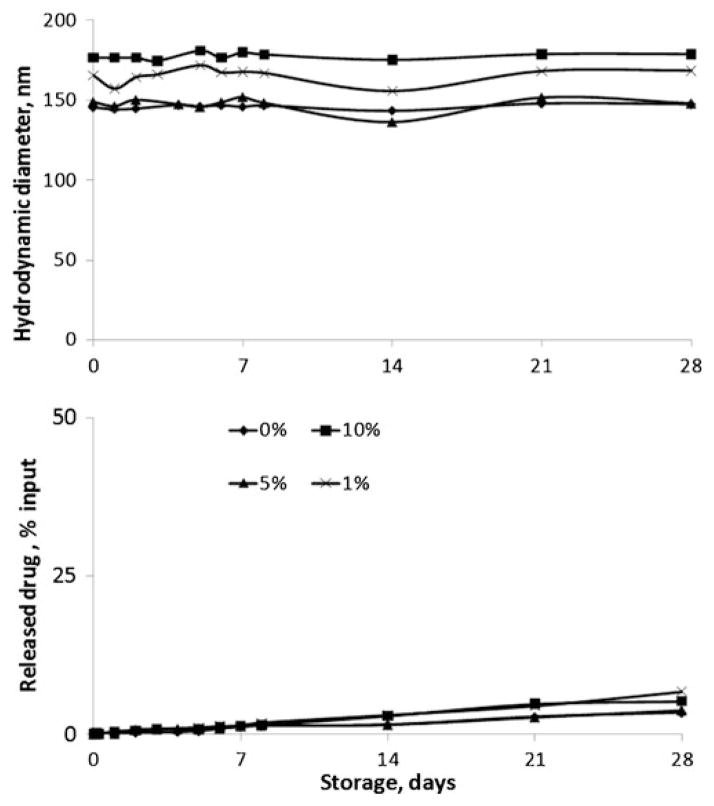
As shown in Table II (groups E–G), cholesterol 1–10% did not influence drug EE but increased the diameter of the liposomes (from 150 to 170 nm), a change that paralleled cholesterol content. Figure 2 demonstrates that stability of the liposomes, evaluated by liposome size and the rate of drug release, was independent of cholesterol content under the employed experimental conditions. Based on these results, cholesterol was not included in further studies. Of interest, this study also established the prolonged stability of liposomes with little or no aggregation or precipitation for at least up to 1 month of storage.
Fig. 2.
Liposome size and drug release during storage. Changes in the size of liposomes (upper) and release of PI from the liposomes (lower) upon storage as a function of storage time for 0%, 1%, 5% and 10% molecular ratios of incorporated cholesterol.
Surface Charge
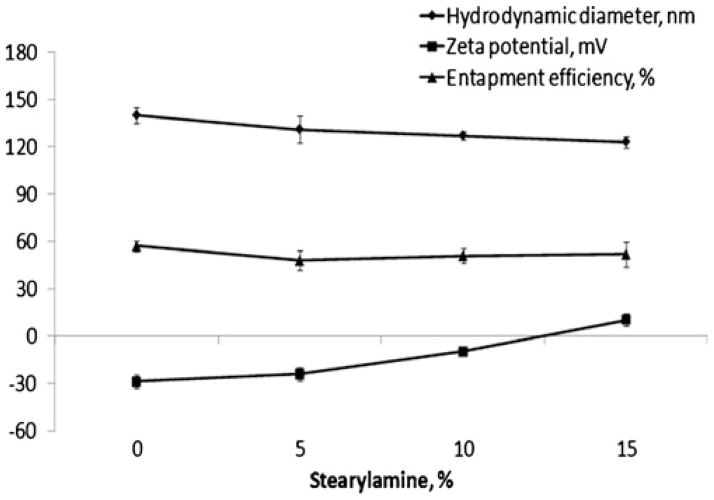
The surface charge of nanocarriers affects the mechanism and extent of their interactions with cells and tissues and may accelerate their plasma clearance after systemic administration (20). For example, the combination of neutral surface charge with a hydrophilic polymer like PEG significantly prolongs the circulation time of nanocarriers in vivo, enhancing their therapeutic effect (13). The use of stearylamine in liposomes has been demonstrated to alter the positive surface charge of the liposomes, facilitating their interaction with negatively charged targets (21). Consequently, we prepared liposomes with stearylamine content between 5% and 15% and determined their size, zeta potential and drug EE (Table 2, groups H–J). While size and drug EE of the liposomes were not significantly altered by stearylamine, their zeta potential changed significantly from −28 mV for liposomes without stearylamine (controls) to +10 mV for 15% of stearylamine (Fig. 3).
Fig. 3.
The effect of stearylamine on liposome characteristics. Particle size, zeta potential and drug EE as a function of the stearylamine content of the liposomes.
Liposome Behavior upon Lyophilization
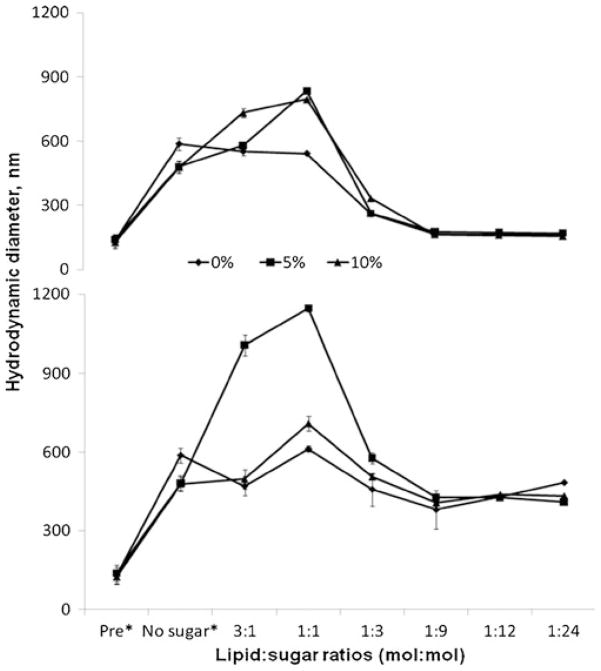
Loss of liposome stability during storage can be overcome by lyophilization during which liposomes are freeze-dried in the presence of cryoprotectants. Lyophilized liposomes exhibit prolonged shelf-life and are reconstituted with vehicle immediately prior to their use. (20). Three potential cryoprotectants were evaluated, sucrose, mannitol and trehalose, by determining their effect on liposome size upon reconstitution. Figure 4 shows the effect of various molar ratios of total lipids:sugar (mol:mol; range 3:1–1:24) on the hydrodynamic radius of liposomes after they were resuspended following lyophilization. Sucrose at lipid: sucrose ratios ≥1:9 protected the liposomes from aggregating during storage. In contrast, mannitol was unable to protect the liposomes from aggregating even at molecular ratios of lipid:mannitol 1:24. Trehalose, a natural α-linked disaccharide often successful in such applications (22), also failed to protect the liposomes in the same molecular ratios (data not shown).
Fig. 4.
Changes of the hydrodynamic diameter for liposomes during lyophilization. Liposomes with 0%, 5% and 10% of incorporated stearylamine were studied in the presence and absence of sucrose (upper) or mannitol (lower) used as cryoprotectants. *Pre: liposomal size prior to lyophilization; No sugar: liposomal size after lyophilization without cryoprotectants.
The Effect of Liposomal P-I on the Growth of Cancer Cells In Vitro and In Vivo
Initially, we used the MTT assay to determine the cytotoxicity of P-I incorporated in liposomes (liposomal P-I) in cancer cell lines in vitro. We studied two breast cancer cell lines, MDA-MB-231 and MCF-7, and the SW480 colon cancer cell line and compared the effect of liposomal P-I to that of free P-I. The values of 24-h IC50 (concentration that inhibits cell growth by 50% at 24 h) were as follows: MDA-MB-231 cells: P-I=18 μM, liposome P-I=40 μM; MCF-7 cells: P-I=27 μM, liposome P-I=56 μM; and SW480 cells: P-I=32 μM, liposome P-I=52 μM. Empty liposomes had no effect on cell growth (data not shown).
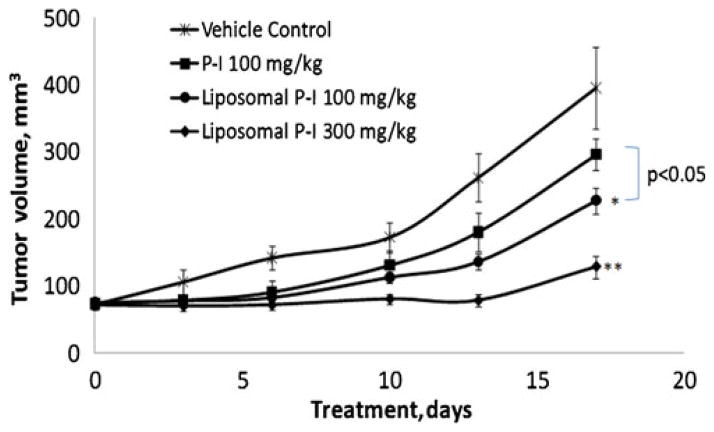
We also evaluated the effect of liposomal P-I on the growth of SW480 subcutaneous xenografts in SCID mice. As shown in Fig. 5, liposomal P-I inhibited in a dose-dependent manner the growth of these xenografts, reducing at the end of the study the growth of xenografts by 42% p ( <0.05) at its lower concentration (100 mg/kg/d) and 67% (p<0.01) at its higher concentration (300 mg/kg/d), compared to control. Free P-I (100 mg/kg/d) had only a modest (25%) and statistically not significant inhibitory effect on tumor growth. Interestingly, the difference in efficacy between free P-I and liposome P-I at the same dose (100 mg/kg/d) is statistically significant (p<0.05). Liposomal P-I was well tolerated by the mice, which showed no weight loss or other signs of toxicity.
Fig. 5.
Effect of free P-I and P-I loaded liposomes on the growth of SW480 xenografts. Each formulation of P-I was administered daily i.p. Values: Mean±SEM. *, p<0.05; **, p<0.01; compared to control.
DISCUSSION
Facilitated drug delivery emerges as a key contributor to the efficacy of novel anticancer agents. Nanocarriers are being increasingly appreciated as useful drug delivery vehicles and their initial promise has now been realized in the clinic (12). For example, liposomal formulations of paclitaxel and doxorubicin have already been approved for the treatment of cancer patients. We have responded to the current paucity of anti-cancer agents by synthesizing the novel modified NSAIDs, represented here by P-I (8). All modified NSAIDs that we have synthesized to date, including P-I, display significant lipophilicity and poor aqueous solubility requiring lipid vehicles for solubilization and delivery (23). Through a systematic process we sought to optimize the liposomal formulation of P-I using PEGylated liposomes and have now demonstrated the feasibility of this approach.
We used a series of lipid mixtures to prepare liposomes and assessed their potential relevance using standard criteria for their intended clinical application. Two of the crucial investigated parameters were liposome size and zeta potential, as they can affect the therapeutic efficacy of the incorporated P-I. Size and charge are determinants of the efficacy and biodistribution of liposomes, especially through the EPR effect (20–22,24 ). Based on these considerations, we sought to generate liposomes less than 200 nm in diameter with zeta potential close to neutrality (13), and optimize drug loading, and stability that makes their use practical by balancing these parameters.
Of the evaluated lipids, soy-PC generated optimum results as judged by the above criteria. All other phospholipids generated liposomes with unfavorable size or drug EE, whereas high cost was an additional factor in rejecting soy-PS and soy-PE. Despite strong evidence in the literature that cholesterol can enhance the stability of liposomes, in the present studies cholesterol made no additional contribution to liposome stability. Stearylamine, known to add cationic charges to the surface of liposomes (21), was of no added benefit to liposome formulation and thus it was not included in the final formulation.
Successful long-term storage of liposomes, as already mentioned, facilitates their clinical use. Sucrose was shown to protect liposomes during lyophilization, a widely employed method to prolong the shelf-life of liposomes. It was of interest that neither mannitol nor trehalose, two commonly used cryoprotectants that have been useful in various systems (25), had no appreciable effect on the investigated liposomes. The reason for these differences in not readily apparent but perhaps it reflects subtle structural differences between these compounds (sucrose and mannitol are disaccharides) and the structural orientation of P-I within the lipid bilayer.
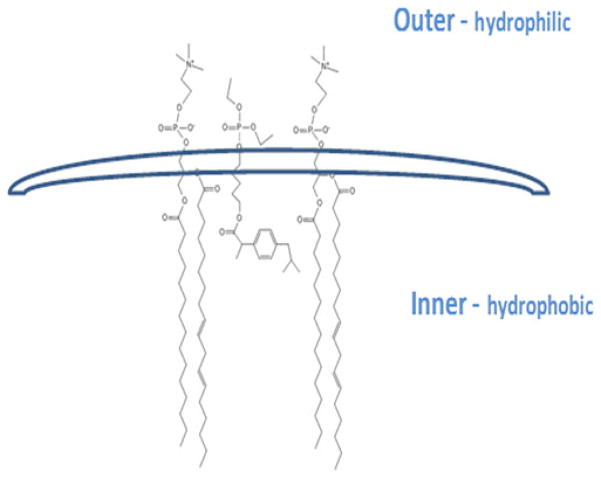
Previous studies suggest that when incorporated inside the liposomal bilayer lipophilic drugs can assume various orientations (26). We propose that P-I is incorporated in liposomes as shown in Fig. 6. This orientation is consistent with the strongly negative zeta potential of P-I loaded liposomes, the high molecular ratio between the lipophilic P-I and lipids in the final stage of the formulation (high drug EE), and the increased stability of the liposomes without the need for cholesterol. The ibuprofen part of the molecule may in fact displace cholesterol from the lipid bilayer.
Fig. 6.
Proposed relative position of P-I in the liposomal bilayer. In this diagram, P-I is positioned between the lipids; two PC molecules are shown. The hydrophilic part of the P-I molecule protrudes from the outer margin of the bilayer.
Liposomal P-I was effective in inhibiting the growth of human cancer cell lines, both in vitro and in vivo. In vitro, however, liposomal P-I has a somewhat higher IC50 compared to unincorporated P-I. The increased IC50 values (decreased cytotoxicity) of liposomal P-I may be related to the steric hindrance from the PEG molecules on the surface of the liposomes, which may retard the release of P-I when the liposomes come into contact with cells (10). In contrast to the in vitro data, when liposomal P-I was administered to nude mice bearing xenografts it was much more effective than plain P-I in inhibiting the growth of human cancer xenografts. Compared at the same dose, liposomal P-I nearly doubled the tumor growth inhibitory effect of P-I. This finding confirms the notion that incorporating P-I in nano-sized liposomes can enhance its therapeutic efficacy (9), which is further supported by the dose-dependence of the effect. Of particular interest, liposomal P-I was well tolerated by the animals based on clinical signs.
CONCLUSIONS
High drug loading liposomes containing the lipophilic drug phospho-ibuprofen (P-I) were obtained by including PEG2000-PE into a formulation composed of soy-PC. The sterically stabilized liposomal formulation exhibited increased stability for an extended period of time and could be lyophilized in the presence of sucrose as a cryoprotectant. Improved antitumor activity was observed with liposomal P-I in xenograft tumor model in nude mice compared to free P-I. These results justify further evaluation of P-I loaded liposomes in cancer treatment.
Acknowledgments
Financial support was from NIH grant HHSN26120 1000109C.
ABBREVIATIONS
- EE
entrapment efficiency
- egg-PC
egg-phosphatidylcholine
- P-I
phospho-ibuprofen
- RES
recticuloendothelial system
- soy-PC
soy-phosphatidylcholine
- soy-PE
soy-phosphatidylethanolamine
- soy-PS
soy-phosphatidylserine
Contributor Information
George Mattheolabakis, Division of Cancer Prevention, Department of Medicine, Stony Brook University Stony, Brook HSC, T-17 Room 080, Stony Brook, New York 11794-8173, USA.
Ting Nie, Division of Cancer Prevention, Department of Medicine, Stony Brook University Stony, Brook HSC, T-17 Room 080, Stony Brook, New York 11794-8173, USA.
Panayiotis P. Constantinides, Medicon Pharmaceuticals, Inc., Stony Brook, New York 11790-3350, USA
Basil Rigas, Email: basil.rigas@stonybrook.edu, Division of Cancer Prevention, Department of Medicine, Stony Brook University Stony, Brook HSC, T-17 Room 080, Stony Brook, New York 11794-8173, USA.
References
- 1.Coussensand LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–5. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Marnett LJ. Aspirin and related nonsteroidal anti-inflammatory drugs as chemopreventive agents against colon cancer. Prev Med. 1995;24:103–6. doi: 10.1006/pmed.1995.1017. [DOI] [PubMed] [Google Scholar]
- 4.Meier CR, Schmitz S, Jick H. Association between acetaminophen or nonsteroidal antiinflammatory drugs and risk of developing ovarian, breast, or colon cancer. Pharmacotherapy. 2002;22:303–9. doi: 10.1592/phco.22.5.303.33189. [DOI] [PubMed] [Google Scholar]
- 5.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–83. [PubMed] [Google Scholar]
- 6.Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, et al. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis. 2010;31:1982–90. doi: 10.1093/carcin/bgq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, et al. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139:1320–32. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie G, Sun Y, Nie T, Mackenzie GG, Huang L, Kopelovich L, et al. Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse models. J Pharmacol Exp Ther. 2011;337:876–86. doi: 10.1124/jpet.111.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 10.Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J Control Release. 2000;63:19–30. doi: 10.1016/s0168-3659(99)00166-2. [DOI] [PubMed] [Google Scholar]
- 11.Markman M. Pegylated liposomal doxorubicin: appraisal of its current role in the management of epithelial ovarian cancer. Cancer Manag Res. 2011;3:219–25. doi: 10.2147/CMR.S15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 13.Gabizon A, Goren D, Horowitz AT, Tzemach D, Lossos A, Siegal T. Long-circulating liposomes for drug delivery in cancer therapy: a review of biodistribution studies in tumor-bearing animals. Adv Drug Deliver Rev. 1997;24:337–44. [Google Scholar]
- 14.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–6. [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Hwang TL, Lee WR, Hua SC, Fang JY. Cisplatin encapsulated in phosphatidylethanolamine liposomes enhances the in vitro cytotoxicity and in vivo intratumor drug accumulation against melanomas. J Dermatol Sci. 2007;46:11–20. doi: 10.1016/j.jdermsci.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Roy MT, Gallardo M, Estelrich J. Influence of size on electrokinetic behavior of phosphatidylserine and phosphatidylethanolamine lipid vesicles. J Colloid Interface Sci. 1998;206:512–7. doi: 10.1006/jcis.1998.5715. [DOI] [PubMed] [Google Scholar]
- 18.Bittmanand R, Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972;11:4831–9. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- 19.Papahadjopoulos D, Cowden M, Kimelberg H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim Biophys Acta. 1973;330:8–26. doi: 10.1016/0005-2736(73)90280-0. [DOI] [PubMed] [Google Scholar]
- 20.Sharmaand A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–40. [Google Scholar]
- 21.Yoshiharaand E, Nakae T. Cytolytic activity of liposomes containing stearylamine. Biochim Biophys Acta. 1986;854:93–101. doi: 10.1016/0005-2736(86)90068-4. [DOI] [PubMed] [Google Scholar]
- 22.Crowe LM, Crowe JH, Rudolph A, Womersley C, Appel L. Preservation of freeze-dried liposomes by trehalose. Arch Biochem Biophys. 1985;242:240–7. doi: 10.1016/0003-9861(85)90498-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, et al. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162:1521–33. doi: 10.1111/j.1476-5381.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumuraand Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 25.Sarisuta N, Benjakul R, Panyarachun B. Preparation of dry reconstituted liposomal powder by freeze-drying at room temperature. J Liposome Res. 2011;21:28–37. doi: 10.3109/08982101003735970. [DOI] [PubMed] [Google Scholar]
- 26.Fahr A, van Hoogevest P, May S, Bergstrand N, Leigh MLS. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery. Eur J Pharm Sci. 2005;26:251–65. doi: 10.1016/j.ejps.2005.05.012. [DOI] [PubMed] [Google Scholar]