Abstract
Context
Dyslipidemia is a feature of polycystic ovary syndrome (PCOS), but its pathogenesis remains controversial.
Objective
The objective of this study was to test the hypothesis that dyslipidemia is a heritable trait in sisters of women with PCOS.
Design
A case-control design was used.
Setting
The study took place at General Clinical Research Centers in four academic medical centers in the United States.
Patients
The subjects included 385 sisters of women with PCOS with the following reproductive phenotypes: sisters with PCOS (n = 51), sisters with hyperandrogenemia and regular menses (HA) (n = 38), unaffected sisters (n = 143), and unknown phenotypes (n = 153). One hundred twenty-five control women of comparable age, body mass index, and ethnicity to women with PCOS were included.
Interventions
Fasting blood was obtained for measurements of lipid profile, reproductive hormones, glucose, and insulin levels.
Main Outcome Measures
The main outcome measures included lipid and lipoprotein levels and prevalence of metabolic syndrome.
Results
Sisters with PCOS and HA phenotypes had higher total (P ≤ 0.001) and low-density lipoprotein cholesterol levels (P ≤ 0.01) compared with unaffected sisters and control women. Triglyceride levels were elevated only in sisters with the PCOS phenotype (P < 0.05). The prevalence of metabolic syndrome was increased in sisters with the PCOS (n = 29) and HA (n = 17) phenotypes compared with unaffected sisters (n = 85) (P < 0.001 and P < 0.05, respectively).
Conclusions
Low-density lipoprotein levels are increased in affected sisters of women with PCOS consistent with a heritable trait. The prevalence of metabolic syndrome is increased in affected sisters.
Polycystic ovary syndrome (PCOS) affects approximately 7% of reproductive age women (1, 2). It is characterized by disordered gonadotropin secretion and hyperandrogenism (3). Women with PCOS also have metabolic abnormalities such as insulin resistance (4–6), glucose intolerance (7, 8), and dyslipidemia (9–13). Increased low-density lipoprotein (LDL) levels are independent of obesity (11–13), whereas increased triglyceride and decreased high-density lipoprotein (HDL) levels are found primarily in obese women with PCOS (10, 11, 13, 14). Familial aggregation of PCOS is well documented, suggesting a genetic susceptibility to the disorder (15–21). We have shown previously that there are two affected reproductive phenotypes in the sisters of women with PCOS: 1) chronic anovulation and hyperandrogenemia consistent with the National Institute of Child Health and Human Development diagnostic criteria for PCOS (classical PCOS phenotype) (22), and 2) hyperandrogenemia with regular menses and apparently normal fertility (HA phenotype) (19, 23). Both affected phenotypes have evidence for insulin resistance compared with unaffected sisters and control women (23). This finding is consistent with a genetic susceptibility to insulin resistance that tracks with hyperandrogenemia in families of women with PCOS (23).
It is unknown whether abnormalities in lipids and lipoprotein levels are also inherited in families of women with PCOS. There is some evidence that family members of women with PCOS have dyslipidemia (15, 18). In women with PCOS, hyperandrogenemia plays an important role in the pathogenesis of LDL elevations (24, 25), and, because hyperandrogenemia is present in 50% of sisters, dyslipidemia may also be common in this population. We performed this study to test the hypothesis that there is a genetic susceptibility to dyslipidemia in sisters of women with PCOS and to determine the type of lipid abnormality associated with the various reproductive phenotypes. Furthermore, we examined the prevalence of metabolic syndrome in sisters based on their reproductive phenotype to determine whether PCOS and/or hyperandrogenemia increased the risk for this condition.
Subjects and Methods
Women with PCOS or women with six or fewer menses per year were recruited by advertisements and from the practices of the coauthors. The study was approved by the Institutional Review Boards of Brigham and Woman’s Hospital, Northwestern University Feinberg School of Medicine, Pennsylvania State University College of Medicine, and University of Pennsylvania Medical Center. Written informed consent was obtained from all participants. To qualify as a PCOS index case, a woman had six or fewer menses per year in addition to hyperandrogenemia defined by either total testosterone (T) more than 58 ng/dl (2 nmol/liter) and/or non-SHBG bound T [unbound testosterone (uT)] more than 15 ng/dl (0.5 nmol/liter) levels greater than 2 sd values above the mean value that we have established in reproductively normal women aged 18–40 yr old in the early follicular phase of the menstrual cycle (19, 23). Other causes of anovulation and hyperandrogenemia were excluded by appropriate laboratory tests (19, 23). We limited our analysis to non-Hispanic white women to avoid potential confounding effects of ethnicity on metabolic endpoints (26, 27).
A total of 385 sisters of women with PCOS participated in the study and were assigned reproductive phenotypes as follows: classical PCOS, six or fewer menses per year and elevated T and/or uT levels (n = 51); HA, regular menses every 27–35 d and elevated T and/or uT levels (n = 38); unaffected, regular menses every 27–35 d and normal T, uT, and dehydroepiandrosterone sulfate (DHEAS) levels (n = 143); and unknown (n = 153). Unknown phenotype was assigned if the sisters were on confounding medications or were premenarchal, pregnant, lactating, menopausal, or had a hysterectomy (23). Confounding medications were oral contraceptive agents (28), hypertensive medications (29), and insulin-sensitizing medications (30, 31). There were 25 families who had more than one sister with the same phenotype. To control for the over-representation of families with multiple sisters, we averaged the data from sisters with the same phenotype to yield one value per phenotype per family. The findings were unchanged when the sisters’ data were averaged; thus, the results are reported using the individual sister data. Data on reproductive hormones, glucose, and insulin levels for 197 sisters have been reported previously (19, 23).
A total of 125 non-Hispanic white reproductively normal control women with 27- to 35-d menstrual cycles between the ages of 18 and 47 yr were studied. The control women were recruited to be of comparable weight with the probands with PCOS and had the following body weight distribution: 16% nonobese [body mass index (BMI) < 25 kg/m2], 32% overweight (25 kg/m2 ≤ BMI < 30 kg/m2), and 52% obese (BMI ≥ 30 kg/m2). Control women were in good health, sedentary, and did not have a history of hypertension or diabetes mellitus, personally or in a first-degree relative. None of the control women were on any of the confounding medications listed above for at least 1 month (3 months for oral contraceptives) before study.
Sisters were evaluated at one of the four study sites (on-site subjects, n = 146) or in a local clinical laboratory with phlebotomy and blood processing capability (off-site subjects, n = 86). All control women were studied on-site and examined by a physician investigator. Control women had no clinical or biochemical evidence of hyperandrogenism. Standardized forms were used to obtain medical history, including information on exercise habits and tobacco use. Sixty-four percent of subjects provided information on tobacco use, and 89% reported information on exercise history. Height, weight, waist, and hip measurements were determined on all on-site subjects. Blood pressure was determined in the seated position in the right arm as the average of three separate readings obtained 2 min apart after a 5-min rest. For the off-site subjects, the same standardized forms were used to obtain medical history, but the height and weight were self-reported. We have previously validated self-reported height and weight (19, 23).
A fasting morning blood sample was obtained from all subjects as described previously (19, 23). Sisters studied on-site and all control women underwent 75-g oral glucose tolerance test after 3 d of 300 g carbohydrate diet and an overnight fast. Blood was drawn for glucose and insulin at baseline and 2 h after the oral administration of glucose. All control women had normal glucose tolerance by World Health Organization criteria (32). The prevalence of metabolic syndrome in the sisters of women with PCOS was determined according to the Adult Treatment Panel III guidelines (33) based on the presence of three or more of the following features: 1) abdominal obesity (waist circumference of >88 cm; 2) high triglyceride level [≥150 mg/dl (≥1.69 mmol/liter)]; 3) low HDL cholesterol level [<50 mg/dl (1.29 mmol/liter)]; 4) high blood pressure (systolic ≥ 130 mm Hg or diastolic ≥ 85 mm Hg); and 5) high fasting plasma glucose concentration [≥110 mg/dl (6.1 mmol/liter)]. Metabolic syndrome could only be assessed in sisters studied on-site who had waist and blood pressure measurements (n = 131); these sisters had the following reproductive phenotypes: PCOS (n = 29), HA (n = 17), and unaffected (n = 85).
Assays
Plasma glucose levels were determined by the glucose oxidase technique. Insulin, T, uT, DHEAS, SHBG (19), total and HDL cholesterol, and triglyceride levels were determined as reported previously (13). The LDL cholesterol level was calculated using the Friedewald equation (34).
Data analysis
The homeostatic index of insulin resistance (HOMA IR) was calculated according to the following formula: [fasting glucose (millimoles per liter) × fasting insulin (microunits per milliliter)] ÷ 22.5 (35). Because there were significant differences in age and BMI after the sisters were grouped by phenotype, data were analyzed using analysis of covariance (ANCOVA) adjusted for age and BMI for each continuous variable. Post hoc analysis using least significance difference was applied to determine differences among the groups. Data were log transformed to achieve homogeneity of variance when necessary.
Categorical variables were compared using χ2 analysis. The prevalence of increased LDL (LDL ≥ 130 and ≥ 100 mg/dl) and of metabolic syndrome was compared between the various sister phenotypes and control women. The prevalence of each feature of metabolic syndrome was also assessed for affected (PCOS and HA phenotypes) and unaffected sisters. For this analysis, data for sisters with PCOS and HA phenotypes were combined because the number of subjects in the HA group was too small after stratification. The prevalence of metabolic syndrome in the overweight and in obese body weight categories was compared between the sisters with the affected phenotype (n = 14 for overweight and n = 25 for obese) and the unaffected sisters (n = 23 for overweight and n = 18 for obese). Control women were not included in these analyses because we specifically excluded control women with features of the metabolic syndrome, e.g. hypertension.
Linear correlations were assessed by Pearson’s correlation coefficients between affected sisters (PCOS or HA phenotypes) and their proband sisters for total, LDL, and HDL cholesterol and triglyceride levels. Multivariate regression analyses were performed to assess the predictors of total and LDL cholesterol, as well as triglyceride levels in affected sisters, with age, BMI, uT levels, and HOMA IR as independent variables. The regression analysis was repeated with addition of tobacco and exercise history as independent variables because the sample size was smaller for this analysis. Statistical analyses were performed using SAS 8.2 (SAS Institute, Inc., Cary, NC) and SPSS 12.0 (SPSS, Inc., Chicago, IL) data analysis softwares. Data are presented as the untransformed mean ± 1 sd.
Results
Sisters with the PCOS and HA phenotypes were younger than unaffected sisters and control women (Table 1). Sisters with the PCOS phenotype were heavier than sisters with HA phenotype and unaffected sisters but were similar in BMI to control women by design (Table 1). Levels of total T and uT as well as DHEAS were significantly higher in sisters with HA and PCOS phenotypes compared with unaffected sisters and control women, as we reported previously (Table 1) (19). Insulin and glucose/insulin were significantly higher in sisters with PCOS and HA phenotypes compared with unaffected sisters and control women, as reported previously (Table 1) (23). HOMA IR was significantly higher in sisters with the PCOS phenotype compared with both control women and unaffected sisters (Table 1) and was also significantly higher in sisters with the HA phenotype compared with control women (Table 1). HOMA IR tended to be higher (P = 0.06) in sisters with the PCOS phenotype compared with sisters with the HA phenotype.
TABLE 1.
Clinical and biochemical features of sisters and control women
| Variable | PCOS (n = 51) | HA (n = 38) | UA (n = 143) | Controls (n = 125) | P |
|---|---|---|---|---|---|
| Age (yr) | 28 ± 7a | 26 ± 6a | 31 ± 9 | 33 ± 8 | <0.001 |
| BMI (kg/m2) | 32.9 ± 7.0b | 29.4 ± 5.9c | 26.6 ± 6.4d | 30.8 ± 8.2 | <0.001 |
| Waist (cm) | 96 ± 16 | 87 ± 15 | 82 ± 13 | 91 ± 16 | 0.13 |
| Systolic (mm Hg) | 119 ± 15 | 118 ± 12 | 114 ± 14 | 115 ± 11 | 0.06 |
| Diastolic (mm Hg) | 72 ± 10 | 71 ± 7 | 70 ± 9 | 73 ± 10 | 0.67 |
| T (ng/dl) | 82 ± 37a | 74 ± 20a | 30 ± 12 | 28 ± 10 | <0.001 |
| uT (ng/dl) | 26 ± 14a | 23 ± 11a | 6 ± 3 | 6 ± 3 | <0.001 |
| Glucose (mg/dl) | 89 ± 10e | 84 ± 8 | 87 ± 8e | 84 ± 8 | <0.001 |
| Insulin (µU/ml) | 25 ± 12a | 22 ± 20a | 14 ± 8 | 15 ± 8 | <0.001 |
| Glucose/insulin (ratio) | 4.4 ± 2.4a | 5.6 ± 3.2a | 7.3 ± 2.8 | 6.6 ± 2.9 | <0.001 |
| HOMA IR | 5.6 ± 3.2a | 4.8 ± 4.8e | 3.0 ± 1.8 | 3.2 ± 1.7 | <0.001 |
HA, HA phenotype; UA, unaffected sisters. P value, ANOVA adjusted for age and BMI with least significant difference post hoc test.
To convert total T and uT to nanomoles per liter, multiply by 0.0345. To convert glucose to millimoles per liter, multiply by 0.05551. To convert insulin to picomoles per liter, multiply by 6.
P < 0.05 vs. unaffected sisters and control.
P < 0.05 vs. HA and unaffected sisters.
P < 0.05 vs. PCOS and unaffected sisters.
P < 0.05 vs. PCOS, HA, and control.
P < 0.05 vs. control.
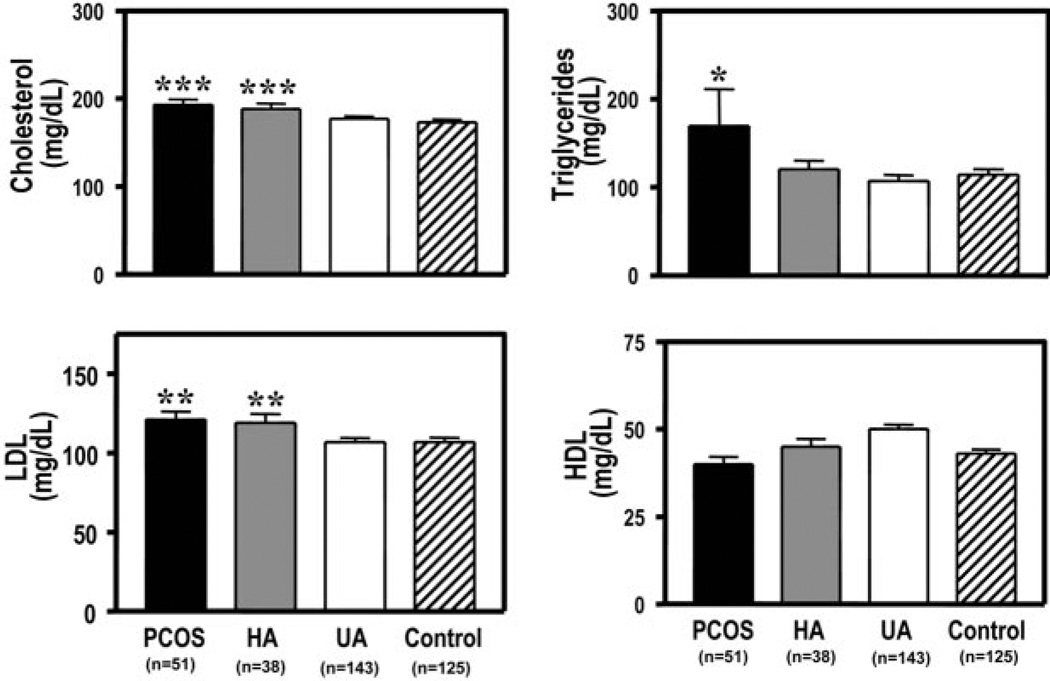
Total and LDL cholesterol levels were significantly higher (P ≤ 0.001 and P ≤ 0.01, respectively) in sisters with the PCOS and HA phenotypes compared with unaffected sisters and control women (Fig. 1). There were no differences in the prevalence of LDL ≥130 mg/dl between the various sister phenotypes and control women. However, the prevalence of LDL ≥100 mg/dl was significantly higher (P < 0.05) in sisters with PCOS phenotype (79%) compared with unaffected sisters (61%) and control women (57%) (P < 0.01). The prevalence of LDL of ≥100 mg/dl for sisters with HA phenotype (72%) was not different from that of sisters with PCOS phenotype, unaffected sisters, or control women.
Fig. 1.
Sisters with the PCOS and HA phenotypes had significantly higher (***, P ≤ 0.001) total cholesterol levels compared with unaffected sisters (UA) and control women (top left). Total triglyceride levels were significantly higher (*, P < 0.05) in sisters with the PCOS phenotype compared with sisters with the HA phenotype, unaffected sisters, and control women (top right). Sisters with the PCOS and HA phenotypes had significantly higher (**, P ≤ 0.01) LDL levels compared with unaffected sisters and control women (bottom left). HDL cholesterol levels did not differ significantly among the groups (bottom right). Black bars, Sisters with PCOS phenotype; gray bars, sisters with HA phenotype; white bars, unaffected sisters; and hatched bars, control women. To convert total, LDL, and HDL cholesterol to millimoles per liter, multiply by 0.02586; to convert total triglyceride to millimoles per liter, multiply by 0.01138.
Total triglyceride levels were significantly higher (P < 0.05) in sisters with the PCOS phenotype compared with sisters with the HA phenotype, unaffected sisters, and control women (Fig. 1). HDL levels tended to be higher (P = 0.07) in unaffected sisters compared with sisters with the PCOS and HA phenotypes, as well as control women. There were significant (P < 0.01) positive linear correlations between probands with the PCOS and their affected sisters (PCOS and HA phenotypes) for all lipid parameters. In a multivariate regression analysis, only age was a predictor of total cholesterol (r2 = 0.213; P < 0.01) and LDL (r2 = 0.123; P < 0.05) levels in affected sisters. Neither BMI, uT levels, HOMA IR, smoking, nor exercise history predicted total or LDL cholesterol levels. The only predictor of triglyceride levels in affected sisters was HOMA IR (r2 = 0.170; P < 0.05). Neither age, BMI, uT levels, smoking, nor exercise history predicted triglyceride levels in the affected sisters.
Prevalence of metabolic syndrome
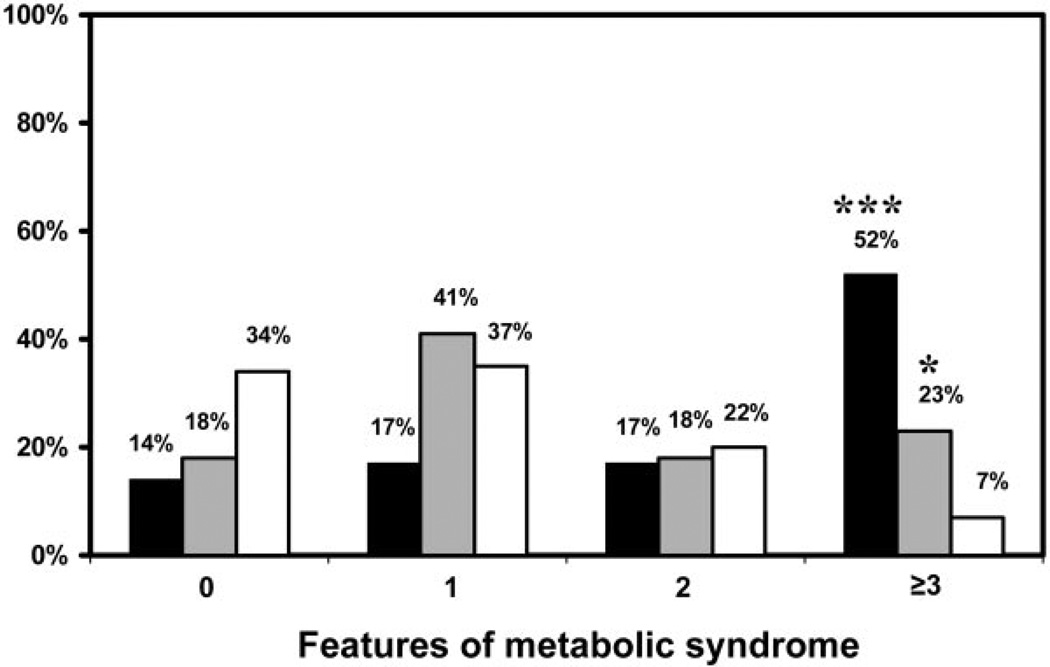
The prevalence of metabolic syndrome was significantly increased in sisters with the PCOS (52%) and the HA (23%) phenotypes compared with the unaffected sisters (7%) (P < 0.001 and P < 0.05, respectively) (Fig. 2). There was a trend (P = 0.07) toward an increase in prevalence of metabolic syndrome in sisters with the PCOS phenotype compared with sisters with the HA phenotype (Fig. 2). Low HDL (<50 mg/dl) was the most common abnormality in affected sisters, followed by high waist circumference (>88 cm) (Table 2).
Fig. 2.
Sisters with PCOS (n = 29) and HA (n = 17) phenotypes had significantly higher (***, P < 0.001 for PCOS vs. unaffected sisters; *, P < 0.05 HA vs. unaffected sisters) prevalence of metabolic syndrome compared with unaffected sisters (n = 85). There was a trend toward higher (P = 0.07) prevalence of metabolic syndrome in sisters with PCOS phenotype compared with sisters with HA phenotype. Black bars, Sisters with PCOS phenotype; gray bars, sisters with HA phenotype; white bars, unaffected sisters.
TABLE 2.
Prevalence of features of metabolic syndrome by Adult Treatment Panel III criteria
| Metabolic syndrome features | Affecteda (n = 46) |
UA (n = 85) |
Pb |
|---|---|---|---|
| Waist circumference > 88 cm | 50 (23) | 21 (18) | <0.001 |
| Triglyceride ≥ 150 mg/dl | 37 (17) | 15 (13) | <0.01 |
| HDL < 50 mg/dl | 78 (36) | 58 (49) | <0.05 |
| SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg | 26 (12) | 12 (10) | <0.05 |
| Fasting glucose ≥ 110 mg/dl | 2 (1) | 0 | c |
Data are presented as prevalence percentage (n). UA, Unaffected sisters; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Affected sisters, PCOS and HA phenotypes.
By χ2 test.
Insufficient samples for analysis.
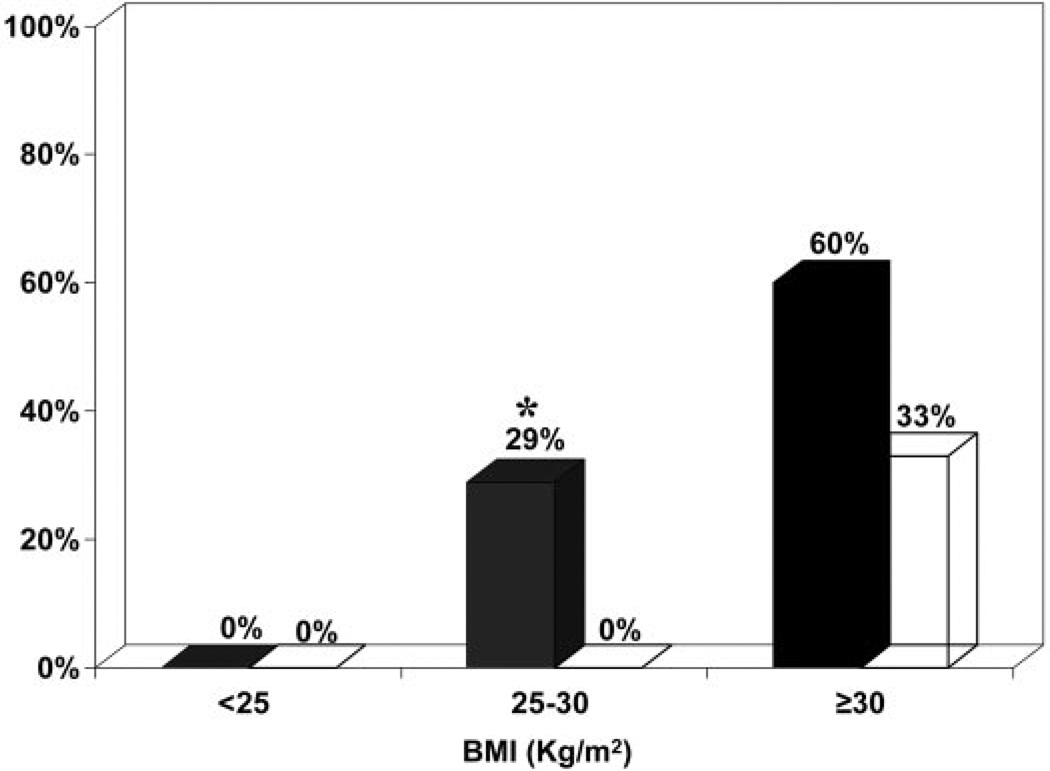
The prevalence of metabolic syndrome in overweight affected sisters (n = 14) was significantly increased (P < 0.05) compared with unaffected sisters (n = 23) in the same body weight category (Fig. 3). In obese sisters, the prevalence of metabolic syndrome tended to be higher (P = 0.07) in affected sisters (n = 25) compared with unaffected sisters (n = 18) (Fig. 3).
Fig. 3.
The prevalence of metabolic syndrome was significantly higher (*, P < 0.05) in overweight affected sisters (PCOS and HA phenotypes, n = 14) compared with unaffected sisters (n = 23). There was a trend toward higher (P = 0.07) prevalence of metabolic syndrome in obese affected sisters (n = 25) compared with unaffected sisters (n = 18). Black bars, Affected sisters; white bars, unaffected sisters.
Discussion
Sisters with the PCOS phenotype and sisters with the HA phenotype had similar and elevated total and LDL cholesterol levels compared with unaffected sisters and control women. Triglyceride levels were increased only in sisters with the PCOS phenotype compared with other groups, and markers of insulin resistance (HOMA IR) were the sole predictors of triglyceride levels in affected sisters. LDL levels in affected sisters were correlated with LDL levels in their proband sisters with PCOS, consistent with a heritable trait. There was an increase in the prevalence of the metabolic syndrome in sisters with the PCOS and HA phenotypes compared with unaffected sisters. Furthermore, this prevalence began to increase in the overweight affected sisters (PCOS and HA phenotypes) compared with unaffected sisters (36).
Elevations in LDL levels have been found in PCOS independent of obesity (12, 13). The androgen receptor antagonist flutamide significantly decreased LDL levels in women with PCOS without weight loss or improvements in insulin sensitivity (24, 25). In contrast, insulin sensitizing agents did not decrease LDL levels in affected women (25, 30). These observations suggest that hyperandrogenemia rather than insulin resistance is important in the pathogenesis of LDL elevations in women with PCOS (12, 24, 25). Our finding that LDL levels were similarly elevated in both groups of hyperandrogenemic sisters is consistent with an important role for androgens in the pathogenesis of this defect.
Triglyceride levels were elevated only in sisters with the PCOS phenotype. Sisters with the PCOS phenotype tended to be more insulin resistant than sisters with the HA phenotype by HOMA IR. Furthermore, HOMA IR was the only predictor of triglyceride levels in affected sisters, whereas BMI was not a predictor. These findings suggest that insulin resistance may have contributed to elevated triglyceride levels in sisters with the PCOS phenotype. These findings are similar to our previous cross-sectional studies in which only obese women with PCOS, who are the most profoundly insulin resistant (37), had elevated triglyceride levels compared with both nonobese women with PCOS and weight-comparable control women (11, 13). It remains likely that additional factors associated with PCOS contributed to triglyceride elevations because HOMA IR accounted for only 17% of the variance in triglyceride levels in sisters with the PCOS phenotype.
Metabolic syndrome was more prevalent in sisters with the PCOS and HA phenotypes than in unaffected sisters, and it tended to be more prevalent in sisters with the PCOS phenotype compared with those with the HA phenotype. Unaffected sisters are an ideal control group for the prevalence of metabolic syndrome because they share the same genetic and environmental background as their affected sisters but do not have elevated androgen levels. In affected sisters of women with PCOS, the prevalence of metabolic syndrome began to increase in overweight sisters (25 kg/m2 ≤ BMI < 30 kg/m2) compared with unaffected sisters. However, these findings should be interpreted with caution because samples sizes were relatively small when the sisters were stratified by both affected status and body weight.
The most common feature of metabolic syndrome in affected sisters was low HDL levels. However, affected sisters were significantly more obese than unaffected sisters, which may account for the differences in prevalence of low HDL levels. In our previous cross-sectional studies of women with PCOS, we have found that low HDL levels were associated with obesity rather than PCOS (11, 13). Fifty percent of the control women in our study were obese, which most likely accounted for the low mean HDL levels in this population because similar levels of HDL have been reported in obese reproductively normal non-Hispanic white women (38).
In summary, we have shown that the affected sisters of women with PCOS have elevated LDL levels. This abnormality is associated with hyperandrogenemia rather than irregular menses. These findings are similar to those reported in their proband sisters consistent with a heritable trait. However, because hyperandrogenemia is a heritable trait and LDL elevations track with this abnormality, it is not possible to determine whether these findings reflect a causal association or are closely linked genetic traits. In affected sisters, the prevalence of metabolic syndrome is increased at lower body weights compared with unaffected sisters. This finding suggests that hyperandrogenemia and adiposity have a synergistic effect to increase the risk for metabolic syndrome. Insofar as the affected sisters are more obese, they are also at risk for low HDL levels in addition to increased LDL levels. Affected sisters of women with PCOS have two risk factors for cardiovascular disease: increased LDL levels and an increased prevalence of features of the metabolic syndrome, in particular low HDL levels. Whether these risk factors translate into an actual increase in cardiovascular events requires prospective studies. Nevertheless, screening the sisters of women with PCOS for metabolic abnormalities should be considered.
Acknowledgments
This work was supported by National Institutes of Health Grants U54 HD34449, P50 HD44405, K12 RR017707, M01 RR00048, M01 RR10732, and M01 RR02635.
Abbreviations
- BMI
Body mass index
- DHEAS
dehydroepiandrosterone sulfate
- HA
hyperandrogenemia phenotype
- HDL
high-density lipoprotein
- HOMA IR
homeostatic index of insulin resistance
- LDL
low-density lipoprotein
- PCOS
polycystic ovary syndrome
- T
testosterone
- uT
unbound T
Contributor Information
Susan Sam, Division of Endocrinology, Metabolism, and Molecular Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611.
Richard S. Legro, Department of Obstetrics and Gynecology, Pennsylvania State University College of Medicine, Hershey, Pennsylvania 17033.
Rhonda Bentley-Lewis, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women’s Hospital, Boston, Massachusetts 02115.
Andrea Dunaif, Division of Endocrinology, Metabolism, and Molecular Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 3.Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–370. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65:499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 6.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 9.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–951. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 10.Wild RA, Bartholomew MJ. The influence of body weight on lipoprotein lipids in patients with polycystic ovary syndrome. Am J Obstet Gynecol. 1988;159:423–427. doi: 10.1016/s0002-9378(88)80099-1. [DOI] [PubMed] [Google Scholar]
- 11.Graf MJ, Richards CJ, Brown V, Meissner L, Dunaif A. The independent effects of hyperandrogenaemia, hyperinsulinaemia, and obesity on lipid and lipoprotein profiles in women. Clin Endocrinol (Oxf) 1990;33:119–131. doi: 10.1111/j.1365-2265.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 12.Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, Daniels T, Engberg RA. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998;51:415–422. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 13.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 14.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;37:119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 15.Givens JR. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am. 1988;17:771–783. [PubMed] [Google Scholar]
- 16.Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28:23–30. doi: 10.1159/000293493. [DOI] [PubMed] [Google Scholar]
- 17.Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol (Oxf) 1993;38:653–658. doi: 10.1111/j.1365-2265.1993.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 18.Norman RJ, Masters S, Hague W. Hyperinsulinemia is common in family members of women with polycystic ovary syndrome. Fertil Steril. 1996;66:942–947. doi: 10.1016/s0015-0282(16)58687-7. [DOI] [PubMed] [Google Scholar]
- 19.Legro RS, Driscoll D, Strauss JF, III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 22.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam G, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 23.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2699–2705. doi: 10.1210/jcem.83.8.5041. [DOI] [PubMed] [Google Scholar]
- 25.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, Pasquali R. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:241–249. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 26.Saad MF, Lillioja S, Nyomba BL, Castillo C, Ferraro R, De Gregorio M, Ravussin E, Knowler WC, Bennett PH, Howard BV. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med. 1991;324:733–739. doi: 10.1056/NEJM199103143241105. [DOI] [PubMed] [Google Scholar]
- 27.Dunaif A, Sorbara L, Delson R, Green G. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes. 1993;42:1462–1468. doi: 10.2337/diab.42.10.1462. [DOI] [PubMed] [Google Scholar]
- 28.Godsland IF, Walton C, Felton C, Proudler A, Patel A, Wynn V. Insulin resistance, secretion, and metabolism in users of oral contraceptives. J Clin Endocrinol Metab. 1992;74:64–70. doi: 10.1210/jcem.74.1.1530790. [DOI] [PubMed] [Google Scholar]
- 29.Pandit MK, Burke J, Gustafson AB, Minocha A, Peiris AN. Drug-induced disorders of glucose tolerance. Ann Intern Med. 1993;118:529–539. doi: 10.7326/0003-4819-118-7-199304010-00008. [DOI] [PubMed] [Google Scholar]
- 30.Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 31.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 α activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 32.Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38:1630–1635. doi: 10.2337/diab.38.12.1630. [DOI] [PubMed] [Google Scholar]
- 33.Anonymous. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 35.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 38.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]