Abstract
Aging is associated with impaired learning and memory accompanied by reductions in adult hippocampal neurogenesis and brain expression of neurotrophic factors among other processes. Epigallocatechin-3-gallate (EGCG, a green tea catechin), β-alanine (β-ala, the precursor of carnosine), and exercise have independently been shown to be neuroprotective and to reduce inflammation and oxidative stress in the central nervous system. We hypothesized that EGCG, β-ala supplementation or exercise alone would improve learning and memory and increase neurogenesis in aged mice, and the combined intervention would be better than either treatment alone. Male Balb/cByJ mice (19 mo) were given AIN-93M diet with or without EGCG (182 mg/kg/d) and β-ala (417 mg/kg/d). Half of the mice were given access to a running wheel (VWR). The first 10 days, animals received 50 mg/kg bromodeoxyuridine (BrdU) daily. After 28 days, learning and memory was assessed by Morris water maze (MWM) and contextual fear conditioning (CFC). Brains were collected for immunohistochemical detection of BrdU and quantitative mRNA expression in the hippocampus. VWR increased the number of BrdU cells in the dentate gyrus, increased expression of brain-derived neurotrophic factor, decreased expression of the inflammatory cytokine interleukin-1β, and improved performance in the MWM and CFC tests. The dietary intervention reduced brain oxidative stress as measured by 4-hydroxynonenal in the cerebellum, but had no effect on BrdU labeling or behavioral performance. These results suggest that exercise, but not a diet containing EGCG and β-ala, exhibit pro-cognitive effects in aged mice when given at these doses in this relatively short time frame.
Keywords: exercise, cognition, learning, epigallocatechin gallate, beta-alanine, mice
1. Introduction
The percentage of the population 60 years of age and older is rapidly growing worldwide [1](United Nations 2012) and therefore, research investigating therapeutic interventions to combat aging-related pathological changes in the brain has garnered high interest [2-4]. Deterioration of brain function is a consequence of normal aging leading to cognitive impairments that are independent of disease [5-7]. In particular, the functional integrity of the hippocampus is vulnerable to the aging process impacting learning and memory in various animal models and in humans [8-11]. During aging, pathological changes in the brain include increased production of pro-inflammatory cytokines and higher levels of oxidative stress that, in turn, can precipitate a decrease in adult hippocampal neurogenesis and decline in learning and memory. Down regulation of neurotrophic factors, most notably brain-derived neurotrophic factor (BDNF), has been linked to cognitive decline and the age-related decrease in neurogenesis [12-16]. BDNF plays a critical role in synaptic plasticity, learning and memory [17] and promotes hippocampal neuron survival and differentiation [18-20]. The complex interaction of increased pro-inflammatory cytokines, increased oxidative stress, decreased neurotrophin expression and decreased neurogenesis promotes a microenvironment conducive to age-related cognitive decline.
Green tea, a polyphenol-rich beverage has drawn much attention due to its health benefits in cardiovascular disease, diabetes, inflammatory diseases, and its prevention and treatment of cancer [21]. Green tea is rich in flavonoids and contains many catechins, including (-)-epigallocatechin-3-gallate (EGCG), which is the most abundant (∼60%) catechin in green tea [22]. Green tea consumption is related to lower prevalence of cognitive impairment in aged humans [23] and, in animals, tea catechins increase adult neurogenesis perhaps by reducing neuroinflammation [24-27]. EGCG reduces microglia activation [24, 28], oxidative stress [29, 30], and inflammation [31]. Based on these observations, catechins from green tea are thought to act as a general neuroprotective factor helping to prevent neurodegenerative diseases [29].
β-alanine (β-ala) is the β form of the amino acid alanine and is a precursor molecule for carnosine [32]. β-ala supplementation and subsequent increases in muscle carnosine have been shown to improve muscle function and decrease fatigue in high intensity exercise [33]. β-ala is used as a dietary supplement by athletes for this reason. Less is known about the effect of β-ala on learning or memory. A recent study demonstrated an increase in β-ala in the hippocampus of rats at 5 minutes and 6 hours after a Morris water maze probe trial [34], a finding the authors speculate may indicate a role for β-ala in retrieval of spatial memory. β-ala is thought to act as a neurotransmitter in the hippocampus, is a structural intermediate between established amino acid neurotransmitters glycine and γ-aminobutyric acid (GABA), and is recognized by multiple receptors in the central nervous system (CNS) [32]. However, the exact role of β-ala in the CNS is not presently clear. L-carnosine, a dipeptide of β-ala and L-histidine, has been studied for its effects on cognition. L-carnosine supplementation improved cognitive flexibility and efficiency as well as reaction time in schizophrenic patients [35]. It is hypothesized that the CNS effects of L-carnosine supplementation are mediated, at least in part, by its potent anti-oxidant effects [36, 37]; a theory supported by the protective role of carnosine against cerebral ischemia in animal models [38, 39]. However, as with β-ala, the functional role of L-carnosine in the CNS remains poorly defined.
In contrast, a large body of literature has established that regular exercise increases adult neurogenesis in the hippocampus of mice, and this increase has been related to improved spatial memory in the Morris water maze [40, 41], radial arm maze [42], and y-maze [43]. Our group has shown that adult hippocampal neurogenesis is required for exercise-induced improvements in spatial memory, as irradiation-induced reduction in neurogenesis was sufficient to eliminate the positive wheel running effect on performance in the Morris water maze [44]. BDNF is thought to play a major role in the increase in neurogenesis and learning/memory as a result of physical exercise [45, 46]. Inhibition of the exercise-induced increase in BDNF action in mice abrogates the exercise-related improvements in spatial memory [47]. Importantly, our recent study in older adults demonstrated an exercise training-induced increase in circulating BDNF concomitant with increases in hippocampal volume and performance on memory tasks [48].
Despite the number of studies that have demonstrated that exercise and dietary supplementation with EGCG or β-ala are beneficial for preventing or recovering age-related deficits individually, no research has been conducted that investigates whether additive or synergistic effects between dietary supplementation with EGCG, β-ala and exercise exist for enhancing age-related cognitive loss and reducing oxidative stress and inflammation in the aged brain. Moreover, most studies have utilized long-term (e.g. 6-7 months) administration [49-53] of catechins as a means of preventing age-related cognitive loss. It is unclear whether cognitive loss and age-related hippocampal changes can be reversed with short-term supplementation with or without exercise. We hypothesized that exercise supplemented with dietary EGCG and β-ala would reduce neuroinflammation, increase the formation of new cells in the dentate gyrus of the hippocampus and improve performance in tests of spatial learning and memory when compared to each treatment (e.g. exercise or EGCG/p-ala) individually and that all interventions would significantly improve all outcomes relative to untreated aged mice.
2. Methods
2.1 Animals
For all studies, aged (19 month old) male BALB/cByJ mice were utilized. Retired breeder mice (8-10 months old) were purchased from Jackson Labs (Bar Harbor, ME) and aged in our facility. Upon arrival at our facility, all mice were fed 8640 Teklad 22/5 rodent diet (Harlan Teklad, Indianapolis, IN) and autoclaved water ad libitum. Mice were individually housed in polypropylene cages under a reverse 12-h-light/-dark cycle at 24°C. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2 Diets containing EGCG and β-ala
At the start of the intervention (e.g. 18-19 mos. old), mice received either a control diet (AIN-93M, Research Diets, New Brunswick, NJ) or an experimental diet containing 1.5 mg Teavigo (90% EGCG, DSM Nutritional Products, Basel, Switzerland) and 3.43 mg β-ala (NutraBio, Middlesex, NJ) per g of AIN-93M diet. The experimental diet was mixed by Research Diets (New Brunswick, NJ). A sample of each diet was assayed independently by Covance, Inc. (Princeton, NJ), and found to contain 99.3% and 97.4% of expected EGCG and β-ala contents, respectively. The compounds were stable in the diet for at least 4 months. The control diet was found to be free of both EGCG and β-ala. Dietary components are listed in Table 1. Based upon the amount of diet disappearance and mouse body weights, we estimated that mice ingested on average 182 mg/kg/day and 417 mg/kg/day, of EGCG and β-ala, respectively. The rationale for the EGCG dosage was based on previous studies demonstrating beneficial effects of EGCG on cognition in mice [51, 52]. As there are few studies examining the effects of β-ala supplementation on cognition or muscle function in mice, our β-ala dosage was calculated from the effective dose in aged humans of 2.4 g/d that led to improved physical work capacity [54]. For a 70 kg person, this would equate to 34 mg/kg/d. The dose was adjusted for species using the FDA-recommended conversion factor of 12.3 [55] resulting in a target dose of 418 mg/kg/d.
Table 1. Diet (AIN-93M) composition.
| Component | Con | EGCG/β-ala | |
|---|---|---|---|
| Protein | (% by wt.) | 14 | 14 |
| Carbohydrate | “ | 73 | 73 |
| Fat | “ | 4 | 4 |
|
| |||
| Energy | (kcal/g) | 3.8 | 3.8 |
|
| |||
| Casein | (g/kg) | 140 | 140 |
| L-Cystine | 1.8 | 1.8 | |
| Corn Starch | “ | 496 | 496 |
| Maltodextrin 10 | “ | 125 | 125 |
| Sucrose | “ | 100 | 100 |
| Cellulose, BW 200 | “ | 50 | 50 |
| Soybean Oil | “ | 40 | 40 |
| tButylhydroquinone | “ | 0.008 | 0.008 |
| Mineral Mix S10022M | “ | 35 | 35 |
| Vitamin Mix V10037 | “ | 10 | 10 |
| Choline Bitartrate | “ | 2.5 | 2.5 |
| Blue Dye #1 | “ | — | 0.05 |
|
| |||
| Teavigo (EGCG) | (mg/g) | 0 | 1.5 |
| β-ala | “ | 0 | 3.43 |
2.3 Voluntary Wheel Running (VWR)
VWR mice were housed in cages with access to a running wheel (Respironics, Bend, OR). Sedentary (SED) mice were housed without a running wheel in standard shoebox cages. Wheel distance was continuously monitored and recorded every hour by a computerized system (VitalView software, Respironics, Bend, OR) and analyzed as wheel distance per 24 hour period. Mice remained in wheel or shoebox cages throughout the duration of the study, including during the behavioral testing period. Mice were euthanized prior to the onset of the dark cycle on the final day of the study, thus they had remained sedentary (i.e. without significant wheel activity) for ∼11 hours prior to tissue collection to control for any acute effects of wheel running.
2.4 Study Design
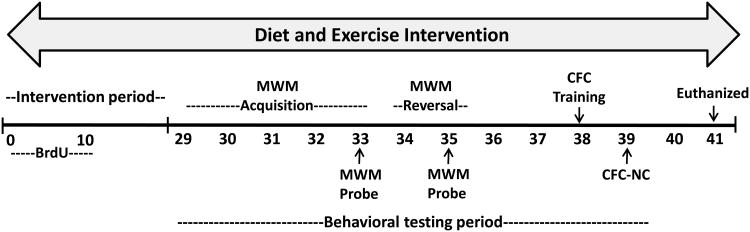
Mice were provided AIN-93M diet or the experimental diet containing EGCG and β-ala for 28 days prior to and during an 11 day test period for spatial learning and memory (Figure 1). Food and water disappearance and body weight were measured twice-weekly during the study. Bromodeoxyuridine (BrdU) was injected at a dose of 50 mg/kg i.p. for 10 consecutive days at the start of the experiment to label proliferating cells. After 28 days, the Morris water maze was used to determine hippocampal-dependent learning and memory over a 7 day period. Muscle function and coordination testing including grip strength, endurance treadmill run and rotarod was performed in all mice on days 8 and 9 and will be reported in another publication. Mice then underwent the hippocampal-dependent contextual fear conditioning task on days 38-39 (Figure 1). All behavior testing occurred during the dark phase of the light/dark cycle. Animals were euthanized by CO2 asphyxiation 24 hours after the final test on day 41.
Figure 1. Experimental timeline.
Experimental intervention (dietary and exercise) continued through duration of study. Abbreviations: Morris water maze (MWM), contextual fear conditioning (CFC), novel context (NC), bromodeoxyuridine (Brdu).
2.5 Morris Water Maze (MWM)
Mice were handled daily for 7 days prior to assessment of learning and memory to habituate them to being manipulated during the testing period. In this test the animals use distinctive visuospatial cues surrounding the pool to navigate a direct path to the hidden platform [56-58]. A circular pool (100 cm diameter, 23-26°C) with a round platform (10 cm diameter) hidden 0.5 cm below the surface of opaque water was positioned in one of the four quadrants. The platform remained in the same quadrant during the 5 day acquisition testing. Trials were conducted using a pseudorandom protocol in which mice were placed in the water in one of the three quadrants not containing the platform. Animals were placed in the water and allowed to swim freely for 60 s or until the platform was reached. If mice did not locate the platform in 60 s, they were guided to the platform and allowed to remain on it for 20 s. Animals were removed from the pool and returned to their home cage for 10 s before repeating the trial. After completion of four consecutive trials, mice were placed in their home cage under a heat lamp until dry. On day 5, the platform was removed and mice received a 60 s probe trial to assess memory for the platform location. On the sixth day, mice were subjected to two days of reversal testing in which the hidden platform was moved to the opposite quadrant of the pool, but all the distal visual cues remained constant. Reversal learning measures how quickly an animal is able to extinguish memory of the initial position of the platform and learn the new location. After the last mouse completed reversal testing, the platform was removed and mice received a 60 s reversal probe trial to assess learning of the platforms new location. A video camera mounted to the ceiling directly above the center of the maze was used in conjunction with a computerized animal tracking system (EthoVision; Noldus Information Technologies, Netherlands) to calculate swim speed (cm/s), latency to platform (s), and distance swam (pathlength to platform) (cm).
2.6 Contextual Fear Conditioning (CFC)
Mice were individually placed into a square chamber (32 cm L × 28 cm W × 30 cm H, dark grey walls) with a metal bar grid floor connected to a shock scrambler controlled by a digital timer (Med Associates, St. Albans, VT, USA). On the first day, mice were placed into the chamber and allowed to acclimate for two minutes. After the acclimation period, the mice were presented with a tone for 20 seconds. During the last two seconds of the tone, a 0.75 mA shock was delivered to the feet. The same tone and shock delivery pattern was repeated 60 seconds later (at 200 seconds). The mouse remained in the chamber for an additional 30 seconds before being returned to their home cage. On the second day, mice were tested for freezing (immobility) in either the original or novel contexts. The novel context was an octagon shaped chamber with white and black striped walls and a smooth floor. The presentation of the contexts was counterbalanced with half of the mice being first placed in the novel context and then the original context or vice versa. When placed in the original context, square box with the grid floor, mice remained in the chamber for a total of 250 seconds in absence of tone or shock. When placed in the novel context for 250 seconds, mice were presented a tone at 120 and 200 seconds. There was no shock delivered in either context during the testing phase. Freezing and distance traveled was recorded by TopScan video tracking software (CleverSystems, Reston, VA, USA). Freezing is the total number of seconds when the animal's center of mass did not register horizontal movement (< 1 mm). Freezing data were converted into percent time spent freezing by dividing the total number of seconds a mouse spent freezing by the total number of seconds of testing (250 seconds) multiplied by 100. Dependent variables are distance traveled (cm) and percent time frozen.
2.7 Tissue Collection
Mice were euthanized by CO2 asphyxiation then intra-cardially perfused with saline. Brains were removed and longitudinally cut into hemispheric sections. One half of the brain was dedicated to immunohistochemistry and was immediately placed in 4% paraformaldehyde to fix overnight. The other half was dedicated to dissection of brain regions (hippocampus and cerebellum) before snap frozen in liquid nitrogen and stored at -80°C until processing.
2.8 Immunohistochemistry
After overnight fixation in 4% paraformaldehyde, the tissue was transferred into 30% sucrose solution. Brains were sectioned at 40 μm using a cryostat. A one-in-six series was stained for bromodeoxyuridine (BrdU) to identify newly divided cells. Immunohistochemistry was performed according to Kohman et al. [59] using rat anti-BrdU (1:100; AbD Serotec, Raleigh, NC) as the primary antibody and biotinylated goat anti-rat (1:250; Vector Laboratories, Burlingame, CA) as the secondary antibody. After incubation in the secondary antibody, sections were then treated with the avidin/biotinylated enzyme complex ABC system (Vector, Burlingame, CA) and stained using a diaminobenzidine kit (DAB; Sigma, St. Louis, MO).
2.9 BrdU positive cell identification
Estimates of total number of BrdU positive cell counts have been previously described [59]. Briefly, the entire granule layer (bilateral) of the dentate gyrus was outlined, and BrdU positive nuclei were automatically counted by a validated fixed threshold to remove the background for each image. The fraction of cells predicted to cross the boundary were removed to produce unbiased estimates.
2.10 Hippocampal Gene Expression
Total RNA from homogenized hippocampal tissue was isolated using the Tri Reagent protocol (Sigma, St. Louis, MO, USA). Synthesis of cDNA was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). A custom Taqman® Low Density Array (TLDA) card (Applied Biosystems, Foster City, CA, USA) was designed for use with hippocampal cDNA, which contained reference genes (18S rRNA and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) and genes of interest (Table 2). Plates were loaded with cDNA, converted from 1000 ng of total RNA, mixed with an equal volume of Taqman® Universal PCR Master Mix (2×). Amplification was run according to the manufacturer's instructions on a Prism 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Data analysis was conducted using RQ Manager (Applied Biosystems, Foster City, CA, USA). Data was analyzed using the comparative threshold cycle (Ct) method [60] and results are expressed as relative change fold to the standardized relative quantification (RQ) baseline (RQ =1). Control-sedentary (Con-SED) was set as the calibrator/control group. A value higher than “1” would represent a fold increase in mRNA expression relative to control for a particular gene, while a value between “0” and “1” would represent a fold decrease in mRNA expression relative to control.
Table 2. Quantitative real-time PCR primer information.
| Gene | Classification | Assay Identificationa |
|---|---|---|
| Bdnf | Neurotrophin | Mm01334042_m1 |
| Ngf | Neurotrophin | Mm00443039_m1 |
| Igf1 | Neurotrophin | Mm00439560_m1 |
| Vegfa | Neurotrophin | Mm01281449_m1 |
| Tgfb1 | Neurotrophin | Mm01178820_m1 |
| Tnf | Pro-inflammatory | Mm00443258_m1 |
| mp | Pro-inflammatory | Mm00434228_m1 |
| Il6 | Pro-Inflammatory | Mm00446190_m1 |
| Itgam (Cd11b) | Pro-Inflammatory | Mm00434455_m1 |
| Ccl2 | Chemokine | Mm00441242_m1 |
| Cx3cl1 | Chemokine | Mm00436454_m1 |
| Cxcl12 | Chemokine | Mm00445553_m1 |
Applied Biosystems TaqMan Gene Expression Assay identification number.
2.11 Oxidative Stress
4-hydroxynonenal (4-HNE) was analyzed in cerebellar extracts by enzyme-linked immunosorbant assay (OxiSelect HNE Adduct ELISA Kit, Cell Biolabs, San Diego, CA) according to manufacturer's instructions. Briefly, cerebellums were homogenized in sterile PBS supplemented with 5 μl of protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO). Samples were assayed for protein content by DC Protein Assay (Bio-Rad, Hercules, CA) and diluted to 10 μg total protein∙ml-1 in PBS for use in the kit then immediately used for 4-HNE assays. All samples were run in duplicate.
2.12 Statistics
Descriptive (Table 3) and MWM acquisition data were analyzed by 2-way (exercise × diet) ANOVA with repeated-measures. CFC, hippocampal gene expression, neurogenesis and 4-HNE data were analyzed by ANOVA using a 2 × 2 arrangement of treatments. MWM probe and reversal trials were analyzed using chi square analyses. Appropriatepost-hoc analyses were used in the event of a significant interaction or main effect of exercise or diet. Significance was set at p < 0.05. Statistical analyses were performed using SPSS software version 22 (IBM Corp., Armonk, NY). All values are means ± sem with an n=8-15/treatment combination dependent on the variable.
Table 3. Descriptive data.
| Variable | Con-Sed | EGCG/β-ala-Sed | Con-VWR | EGCG/β-ala-VWR |
|---|---|---|---|---|
| day 0 Body Weight (g) | 31.1 ± 0.5 | 30.3 ± 0.6 | 30.5 ± 0.6 | 30.7 ± 0.5 |
| day 41 Body Weight (g) | 30.0 ± 0.6 | 29.1 ± 0.6 | 27.0 ± 0.5 * | 27.5 ± 0.5 * |
| Food Disappearance (g/day) | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 |
| Water Disappearance (g/day) | 2.7 ± 0.2 | 3.4 ± 0.2 Λ | 3.1 ± 0.2 | 3.3 ± 0.2 Λ |
| Spleen Weight (mg) | 94 ± 5 | 88 ± 7 | 89 ± 9 | 81 ± 7 |
| Running Distance (km/day) | 4.8 ± 0.8 | 4.5 ± 0.4 |
p < 0.05 for the main effect of VWR;
p < 0.05 for the main effect of diet. n= 10-15/treatment.
3. Results
3.1 Descriptive Data
At the initiation of the diet and exercise intervention, body weight between treatment groups was not different (Table 3). As expected, VWR resulted in a significant (F1,52=37; p<0.001 for time × VWR) reduction in body weight. There was no effect of the diet on body weight over time. Food disappearance was not significantly different between groups. Water disappearance was significantly (F1,52=6.0; p=0.02) increased (∼15%) in response to consumption of a diet containing EGCG and β-ala when compared to the control diet. There were no significant treatment-induced differences in spleen or adrenal weights. Mice in the VWR group ran ∼4.6 km/day and there were no differences between those on control or EGCG/β-ala supplemented diets (Table 3).
3.2 Morris Water Maze
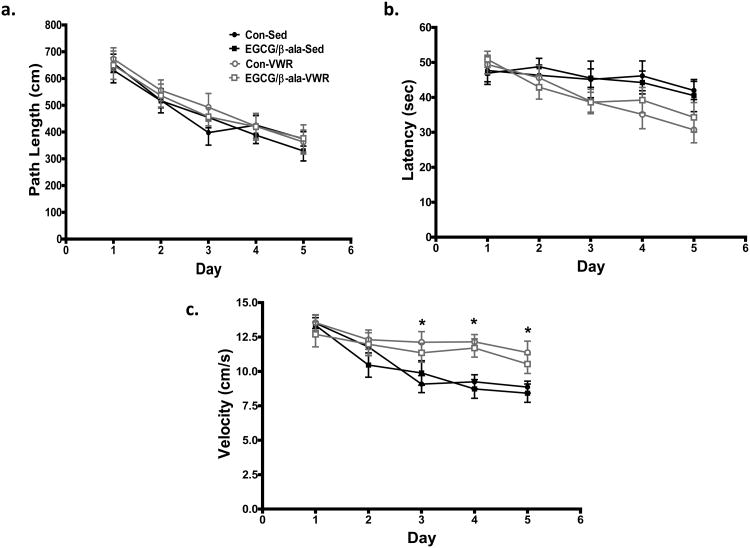
Analysis of path length to the hidden platform revealed that all of the mice acquired the task as path length decreased across the five days of testing (F4,208 = 34.3, p<0.001; Figure 2a). There was a significant VWR × day interaction (F4,208 = 3.0, p=0.02) in latency (e.g. time to reach hidden platform) as VWR mice found the platform quicker on days 3-5 of testing (Figure 2b). The decrease in latency over the 5 day period corresponded to significantly (F4,208 = 5.6, p<0.001, for time × VWR) faster swim speeds in the VWR mice at Days 3-5 of testing (Figure 2c). There was no effect of VWR on path length, and no effect of any experimental diet on path length, latency or swim speed relative to sedentary mice on the control diet.
Figure 2. Exercise, but not EGCG/β-ala, decreases latency during a test of spatial learning of aged mice in the Morris water maze.
All mice learned the task as evidenced by a significantly shorter path length to the hidden platform over time (1b). VWR mice found the platform faster later in the acquisition period (1b), this effect was most probably due to significantly faster swim speeds (1c). There was no main effect of the diet or diet × VWR or diet × VWR × time interactions for any of the variables. Data are shown as the mean ± SEM. *p < 0.05 for the main effect of exercise. n = 12, 14, 15 and 15 for Con-Sed, EGCG/β-ala-Sed, Con-VWR and EGCG/β-ala-VWR, respectively.
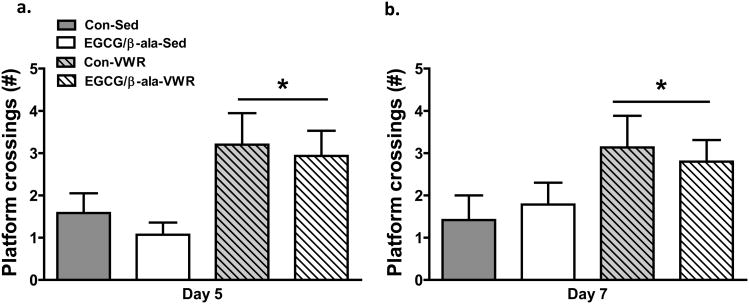
On Day 5 of training, the platform was removed for a 60 s probe test 1 h after the last trial to test recall. Analysis of time spent in the target zone did not reveal any significant main effects (data not shown). However, the number of platform location crossings was greater (F1,52 = 9.3, p=0.004) in the VWR mice (Figure 3a).
Figure 3. Exercise, but not EGCG/β-ala, enhances reference memory on probe trials in the Morris water maze.
On both Days 5 (2a) and 7 (2b), VWR mice crossed the original location of the platform more often than sedentary mice. Data are shown as the mean ± SEM. *p < 0.05 for the main effect of exercise. n = 12, 13, 14 and 15 for Con-Sed, EGCG/β-ala-Sed, Con-VWR and EGCG/β-ala-VWR, respectively.
To assess spatial working memory, the hidden platform was moved to the opposite quadrant for two days of reversal training (e.g. days 34 & 35, Figure 1). Animals must be able to incorporate new information (i.e. new platform location) with existing memories (spatial locations of visual cues) in order to complete this task. Two days of reversal training did not reveal any significant main effects for diet or VWR (or their interaction) in path length or latency, despite faster swim speeds for VWR (data not shown).
A 60 s probe test was performed on Day 7 of testing after two days of reversal testing 1 h after the last reversal test. As was the case for the Day 5 probe test, we found a significant (F1,52 = 5.1, p=0.03) main effect for VWR for the number of platform location crossings (Figure 3b). The experimental diet containing EGCG and β-ala was without effect.
3.3 Contextual Fear Conditioning
As expected, animals subjected to shock during training (day 38, Figure 1) demonstrated a significant (F1,48=16.8, p<0.001) increase in freezing time during the subsequent exposure to the original context compared to those that did not receive a shock (data not shown). Analysis of treatment differences in shocked mice revealed that mice in the VWR groups exhibited significantly (F1,48=8.1, p=0.007) elevated freezing behavior (Figure 4a). There was no effect of diet. In the novel context test on Day 11 of testing, VWR mice displayed a significantly (F1,38=4.9, p=0.03) elevated freezing behavior in the absence of a tone (Figure 4b). Freezing behavior was elevated in response to the tone compared to pre-tone values, but there were no effect of exercise or diet. In the post-tone period, VWR again exhibited significantly (F1,38=5.1, p=0.03) higher freezing when compared to sedentary mice. Again, there was no effect of a diet supplemented with EGCG and β-ala.
Figure 4. Exercise but not EGCG/β-ala improves learning in the contextual fear conditioning test.
In pre-conditioned animals, VWR resulted in a significant increase in time spent frozen when re-introduced into the original context 24 hrs later when compared to sedentary aged mice (5a). There was no effect of the diet or a diet × VWR interaction. In the novel context (5b), VWR increased freezing in the pre- and post-tone conditions with no diet or diet × VWR interaction. There were no significant group differences in the tone condition. Data are shown as the mean ± SEM. *p < 0.05 for the main effect of exercise. n = 10, 11, 11 and 10 for Con-Sed, EGCG/β-ala-Sed, Con-VWR and EGCG/β-ala-VWR, respectively.
3.4 Hippocampal Cell Proliferation
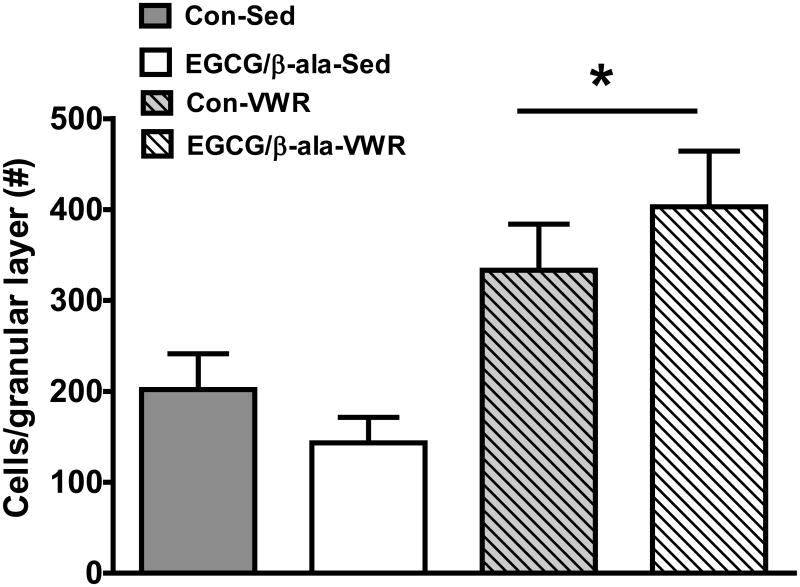
Analysis of BrdU+ cells in the hippocampal granular cell layer revealed that while the experimental diet had no effect (F1,50=0.01, p=0.91), VWR significantly (F1,50=16.2, p=0.001) increased (about 2-fold) the total number of BrdU+ cells (Figure 5).
Figure 5. VWR, but not EGCG and β-ala, increases hippocampal cell proliferation.
Data are shown as the mean ± SEM. *p < 0.05 for the main effect of exercise. n = 12, 13, 14 and 15 for Con-Sed, EGCG/β-ala-Sed, Con-VWR and EGCG/β-ala-VWR, respectively.
3.5 Hippocampal Gene Expression
The expression of mRNA for a number of neurotrophins, cytokines and chemokines known to be either related to learning/memory, neurogenesis or age-related dysfunction (Table 4) was measured. With respect to neurotrophins, VWR significantly (F1,36=7.1; p=0.01) increased hippocampal Bdnf gene expression. There was no diet or VWR effect on hippocampal Igf1, Ngf, Vegfa or Tgfb1gene expression. VWR significantly reduced hippocampal expression of two cytokines, Il1β (F1,35=6.8; p=0.01) and Itgam/CD11b (F1,37=12.4; p=0.001), while the experimental diet tended (F1,38=3.2; p=0.08) to reduce Tnf. Il6 mRNA was unchanged in response to any treatment. Of the chemokines, the EGCG/β-ala reduced Cx3Cl1 (F1,38=4.1; p=0.05), however there were no treatment effects or interactions for Ccl2 or Cxcl12 expression.
Table 4. Hippocampal gene expression following 40 days of dietary and VWR treatment.
| Gene | Group | Statistics (p values) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Con-SED | EGCG/β-ala-SED | Con-VWR | EGCG/β-ala-VWR | Diet | VWR | Diet × VWR | |
| Neurotrophins | |||||||
| Bdnf | 1.01 ± 0.04 | 0.88 ± 0.03 | 1.11 ± 0.06 | 1.16 ± 0.09 | 0.89 | 0.01 | 0.45 |
| Ngf | 1.03 ± 0.08 | 0.98 ± 0.08 | 1.05 ± 0.04 | 0.96 ± 0.05 | 0.29 | 0.94 | 0.73 |
| Igf1 | 1.03 ± 0.07 | 1.12 ± 0.08 | 1.13 ± 0.07 | 1.12 ± 0.08 | 0.62 | 0.52 | 0.54 |
| Vegfa | 1.00 ± 0.04 | 0.92 ± 0.09 | 1.00 ± 0.09 | 0.86 ± 0.07 | 0.14 | 0.74 | 0.19 |
| Tgfb1 | 1.05 ± 0.07 | 1.00 ± 0.10 | 1.12 ± 0.07 | 1.01 ± 0.07 | 0.33 | 0.62 | 0.64 |
| Pro-inflammatory | |||||||
| Tnf | 1.00 ± 0.17 | 0.83 ± 0.14 | 0.99 ± 0.10 | 0.71 ± 0.10 | 0.08 | 0.59 | 0.63 |
| Il1b | 1.00 ± 0.11 | 0.82 ± 0.11 | 0.68 ± 0.08 | 0.65 ± 0.06 | 0.54 | 0.01 | 0.24 |
| Il6 | 1.10 ± 0.17 | 1.09 ± 0.14 | 1.33 ± 0.17 | 1.23 ± 0.20 | 0.78 | 0.30 | 0.79 |
| Itgam (Cd11b) | 1.02 ± 0.06 | 0.96 ± 0.07 | 0.82 ± 0.04 | 0.79 ± 0.04 | 0.35 | 0.001 | 0.81 |
| Chemokines | |||||||
| Ccl2 | 1.00 ± 0.14 | 0.90 ± 0.14 | 0.96 ± 0.14 | 0.93 ± 0.20 | 0.68 | 0.98 | 0.82 |
| Cx3cl1 | 0.99 ± 0.03 | 0.88 ± 0.06 | 1.01 ± 0.04 | 0.94 ± 0.04 | 0.05 | 0.35 | 0.66 |
| Cxcl12 | 1.00 ± 0.04 | 1.00 ± 0.11 | 1.03 ± 0.06 | 0.86 ± 0.05 | 0.18 | 0.42 | 0.23 |
Values are reported relative to Con-SED using ∆∆CT method and GAPDH as the control gene. n=8-12/treatment.
3.6 Oxidative Stress in the Brain
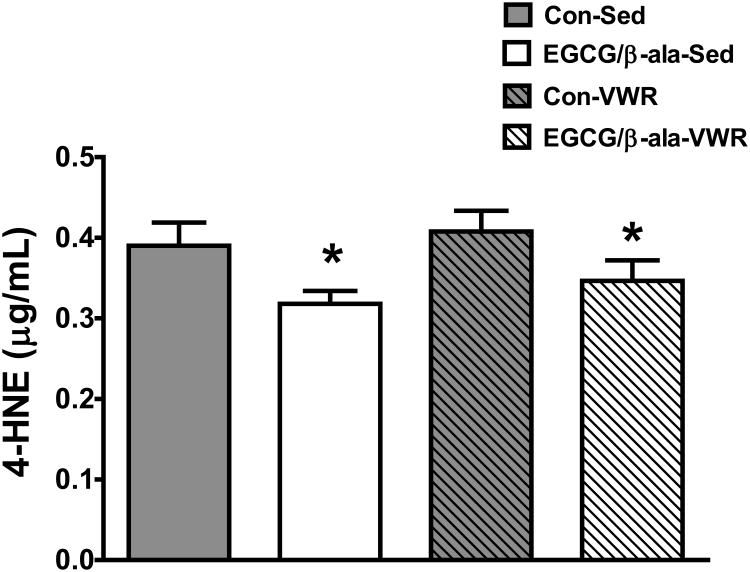
4-HNE was measured as a marker for oxidative stress in the cerebellum. Interestingly, diet containing EGCG and β-ala, but not VWR, significantly (F1,35=7.5, p=0.01 for diet main effect) reduced oxidative stress (Figure 6).
Figure 6. A diet containing EGCG and β-ala reduces 4-HNE in the cerebellum of aged mice.
Data are represented as means ± SEM. *p < 0.05 for the main effect of diet. n = 9, 10, 9, 11 for Con-Sed, Con-VWR, EGCG/β-ala-Sed and EGCG/β-ala-VWR, respectively.
4. Discussion
We hypothesized that a 4 week administration of a diet containing EGCG and β-ala would lead to additive or synergistic improvements in learning and memory when combined with exercise in aged mice. Thus, our goal was to improve age-related cognitive decline in old mice using exercise and targeted dietary supplementation. The rationale for this hypothesis was based upon findings that similar duration interventions of either VWR or EGCG administration have been found to improve learning and memory either in normal aging or in models of cognitive loss in rodents. For example, van Praag et al [61] found that 35 days of VWR (∼3.9km/d) significantly improved MWM performance and hippocampal neurogenesis in 19 month old male C57Bl/6 mice. Moreover, EGCG administered i.p. at a dose of 100 mg/kg/d decreased the impairment in learning and memory in spontaneously hypertensive rats [62]. β-ala was included because it has been shown to improve muscle function and decrease fatigue [33] and thus might indirectly (via increasing exercise capacity) improve cognition. In addition, a more direct role for β-ala has been suggested in the retrieval of spatial memory because hippocampal β-ala increases after a MWM probe trial [34].
Our results clearly support a role for exercise in improving age-related reductions in behavioral performance in a one month time frame. We found that VWR mice swam faster in the water maze and therefore were able to get to the hidden platform faster. However, it is not certain that VWR aged mice in our study displayed better spatial abilities on the water maze, as the path length to the platform was similar to sedentary animals (Figure 2a). Although the greater number of crossings through the platform could be interpreted as better spatial memory (Figure 3a and b), it also is consistent with faster swimming. It is notable that C57BL/6J do not behave this way [61]. More work with BALB/cByJ using other measures of spatial learning such as the Barnes maze, radial-arm maze, T-maze will be necessary to confirm spatial cognition benefits from exercise in BALB/cByJ. Pro-cognitive effects of exercise were much clearer in contextual and cued fear conditioning. VWR mice exhibited elevated behavioral freezing in the original context test of the CFC (Figure 4a) and in a novel context (Figure 4b). These findings support a growing literature demonstrating that either long-term (e.g. months) or short-term (e.g. weeks) exercise can improve associative learning in adult and aged animals [63].
The pro-cognitive effects of exercise are likely mediated by a complex microenvironment that includes increased hippocampal neurogenesis, increased BDNF, IGF-1, FGF-2, EGF, increased vasculature, glial status, inflammation, neurotransmitter levels, energy metabolism, biochemistry, among many other factors [64]. Consistent with this, VWR mice in our study exhibited significantly higher hippocampal BDNF mRNA expression (Table 4) and BrdU+ cell number in the granular layer of the dentate gyrus (Figure 5). VWR also led to a reduction in hippocampal IL-1β (a powerful pro-inflammatory cytokine) and ITGAM (CD11b, a microglial inflammatory marker) mRNA expression (Table 4) indicating an anti-inflammatory effect. In this study, we measured a number or pro-inflammatory cytokines and chemokines because aging is associated with a chronic inflammatory state (e.g. ‘inflammaging’) [65] and exercise and EGCG have documented anti-inflammatory effects [66, 67]. In the context of the brain, IL-1β expression down-regulates BDNF in the hippocampus causing learning and memory disturbances and exercise appears able to counteract these negative effects of inflammation [68].
Our results do not support a role for short-term (e.g. 4wk) feeding of EGCG and β-ala with or without exercise as a means of improving age-related cognitive loss. However, we do not want to suggest that these two nutrients would have no effect on cognition if administered over longer durations, at different doses, levels of bioavailability, or as a preventative strategy starting at an earlier age. We observed no independent or additive/synergistic effects of the EGCG/β-ala diet on performance in the MWM (Figures 2-3) or CFC (Figure 4) tests. We also found no effect of the experimental diet on hippocampal BDNF mRNA (or other neurotrophins), inflammatory cytokines or chemokines (Table 4), or number of BrdU cells as marker for hippocampal cell proliferation (Figure 5). Taken together, these findings provide strong evidence of a lack of effect of EGCG and β-ala given over the course of a 4 week period on age-related behavioral learning or hippocampal cell proliferation in mice. Interestingly, the diet was effective at reducing 4-HNE (a by-product of lipid peroxidation) in the cerebellum (Figure 6), but there was no diet × exercise interaction. EGCG has been reported to have direct anti-oxidant properties as strong radical scavengers and metal chelators in vitro and indirect anti-oxidant properties by inducing antioxidant enzymes (perhaps by acting as a low level oxidant) such as superoxide dismutase, catalase, and enzymes related to glutathione metabolism in both animal models and humans [69]. While we did not see any treatment-induced changes in gene expression for superoxide dismutase 2 (SOD2; mitochondrial manganese SOD) in the hippocampus (data not shown), we did not assess other endogenous antioxidant genes or their activity. While there is some controversy as to whether β-ala acts as an anti-oxidant, carnosine (which increases in tissues after β-ala supplementation) scavenges both reactive oxygen and nitrogen species [70]. These properties could explain the reduction seen in 4-HNE in this study.
We can offer several possible explanations as to why the diet containing EGCG and β-ala failed to improve existing age-related cognitive deficits as hypothesized. First, our dosages of EGCG and β-ala may have been too low. Our strategy for dosing was to administer a high oral dose, but with enough of a safety factor that adverse effects would not be exhibited. It has been demonstrated that high oral doses of EGCG >500 mg/kg/d cause hepatotoxicity and mortality [69] and doses >1000 mg/kg/d increase ex vivo pro-inflammatory cytokine production [71] in mice. Moreover, high (>800 mg) oral doses of β-ala can cause paresthesia in humans [72] and doses >∼5000 mg/kg/day can cause taurine depletion in rodents [73]. We chose our dosages of EGCG and β-ala based upon prior published studies in rodents and humans that have documented beneficial effects on brain and muscle function [51, 52, 74]. For example, Li et al [52] found that 6 months of 160 mg/kg/d of green tea catechins could prevent age-related spatial learning and memory decline in C57BL/6 mice. As such, mice in our study consumed on average 182.4 mg/kg/d of EGCG and 417 mg/kg/d of β-ala. For EGCG, the human dose-equivalent for a 70 kg person would be about 60-70 cups of green tea/d (150-180 mg of EGCG per cup of green tea); a dose that could be achievable in people taking EGCG supplements. We feel that our dosages were high enough to see an effect if one existed and low enough to be confident that detrimental side effects did not occur.
We observed no signs or symptoms of illness or altered behavior in our EGCG/β-ala-treated mice. There were no significant differences in food disappearance, body or spleen weight, or running distance between the experimental and control diet groups (Table 3). The treated animals also displayed no observable behavioral sickness phenotype (e.g. ruffled fur, hunched posture, lethargy) and no difference in swim velocity on the MWM when compared to control-fed mice. Interestingly, water disappearance was statistically greater (∼15%) in the EGCG/β-ala-treated mice compared to controls (Table 3). To our knowledge, this phenomena has not been reported in the EGCG or β-ala literature. In fact, higher dose 3% β-ala intake in drinking water has been reported to reduce fluid and sodium excretion and reduce fluid intake [75]. Lastly, it could be that the length of our feeding regimen was too short to cause beneficial changes in behavioral learning in these aged mice. Indeed, Li et al [52] demonstrated that long-term (6 months from 14-20 months of age) feeding (in drinking water) of green tea catechins dose-dependently prevented age-related loss of spatial learning by increasing CREB phosphorylation and BDNF and Bcl-2 expression in C57BL/6J mice. Their high dose corresponded to ∼160 mg/kg/d which was similar to our dosage (182 mg/kg/d). It was encouraging in our study that the experimental diet reduced oxidative stress in the brain suggesting that a longer period of oxidative protection may be needed to affect learning, memory and hippocampal neurogenesis. Future studies should examine the effect of long-term EGCG supplementation with exercise to determine if there is an additive or synergistic effect on prevention of memory loss; clearly in our study this short-term regimen was unable to alter age-related cognitive loss.
Studies on the bioavailability of green tea catechins like EGCG yield different results. Catechins are conjugated in enterocytes and methylated, sulfated and glucuronidated in the liver [76]. Fractions of catechins not absorbed in the small intestine are acted upon by the gut microbiota forming smaller molecules [77]. Nonetheless, a fraction of ingested catechins do reach the systemic circulation in humans [78] and mice [79]. Access of catechins to the brain is also a matter of debate. One in vivo study demonstrated that, while flavonols or their metabolites appear in the circulation, they do not cross the blood brain barrier [80]. This is in contrast to in vitro experiments demonstrating that such molecules can cross endothelial cell barriers [81] and to a study in rats that found that low levels of EGCG and its metabolites could be detected in several brain regions following oral administration of 100 mg/kg of EGCG. β-ala is much more bioavailable and ingestion has been robustly found to increase β-ala and, importantly, carnosine in muscle and brain [73, 82].
5. Conclusions
In conclusion, we have demonstrated that 4 weeks of voluntary wheel running, but not a diet containing EGCG and β-ala enhanced associative memory, increased hippocampal cell proliferation and BDNF expression while reducing inflammation in aged Balb/cByJ mice. Future research is needed to examine whether the dosages of EGCG and β-ala were sufficient to increase brain EGCG and/or carnosine levels. Likewise, the impact of long-term feeding in conjunction with exercise should be explored as a strategy to prevent age-related cognitive loss. Our data suggest that short-term feeding of EGCG and β-ala, at fairly high oral dosages, do not independently or synergistically act with exercise to reverse age-related cognitive loss.
Acknowledgments
We would like to thank and acknowledge Drs. Neile Edens and Sean Garvey (Abbott) for their assistance with determining dietary dosages of EGCG and β-alanine and in editing the manuscript.
Support: Funded by Center for Nutrition, Learning and Memory, a partnership between the University of Illinois and Abbott.
Abbreviations
- 4-HNE
4-hydroxynonenal
- β-ala
Beta-alanine
- CFC
Contextual fear conditioning
- EGCG
Epigallocatechin gallate
- MWM
Morris water maze
- TLDA
Taqman low density array
- VWR
Voluntary wheel running
References
- 1.Population Ageing and Development 2012 Chart. United Nations; 2012. [Google Scholar]
- 2.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–53. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–8. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3:130–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–31. [PMC free article] [PubMed] [Google Scholar]
- 6.Wynne AM, Henry CJ, Godbout JP. Immune and behavioral consequences of microglial reactivity in the aged brain. Integr Comp Biol. 2009;49:254–66. doi: 10.1093/icb/icp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–7. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 8.Amrein I, Isler K, Lipp HP. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci. 2011;34:978–87. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Porte Y, Buhot MC, Mons NE. Spatial memory in the Morris water maze and activation of cyclic AMPresponse element-binding (CREB) protein within the mouse hippocampus. Learn Mem. 2008;15:885–94. doi: 10.1101/lm.1094208. [DOI] [PubMed] [Google Scholar]
- 12.Barnes P, Thomas KL. Proteolysis of proBDNF is a key regulator in the formation of memory. PLoSOne. 2008;3:e3248. doi: 10.1371/journal.pone.0003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez MM, Das D, Taylor JL, Noda A, Yesavage JA, Salehi A. BDNF polymorphism predicts the rate of decline in skilled task performance and hippocampal volume in healthy individuals. Transl Psychiatry. 2011;1:e51. doi: 10.1038/tp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulte-Herbruggen O, Eckart S, Deicke U, Kuhl A, Otten U, Danker-Hopfe H, et al. Age-dependent time course of cerebral brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 in APP23 transgenic mice. J Neurosci Res. 2008;86:2774–83. doi: 10.1002/jnr.21704. [DOI] [PubMed] [Google Scholar]
- 15.Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–24. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, et al. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–45. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 18.Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 20.Lindholm D, Carroll P, Tzimagiogis G, Thoenen H. Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur J Neurosci. 1996;8:1452–60. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneider C, Segre T. Green tea: potential health benefits. Am Fam Physician. 2009;79:591–4. [PubMed] [Google Scholar]
- 22.Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. The American journal of clinical nutrition. 2006;83:355–61. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–31. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 25.van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:5869–78. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res. 2012;56:1292–303. doi: 10.1002/mnfr.201200035. [DOI] [PubMed] [Google Scholar]
- 27.Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM, et al. (-)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res. 2010;24:1065–70. doi: 10.1002/ptr.3083. [DOI] [PubMed] [Google Scholar]
- 28.Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid Based Complement Alternat Med. 2012;2012:163106. doi: 10.1155/2012/163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, Weinreb O, Amit T, et al. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland BA, Rahman RM, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. The Journal of nutritional biochemistry. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Jeong HJ, Lee KM, Myung NY, An NH, Yang WM, et al. Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. The Journal of nutritional biochemistry. 2007;18:587–96. doi: 10.1016/j.jnutbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Tiedje KE, Stevens K, Barnes S, Weaver DF. Beta-alanine as a small molecule neurotransmitter. Neurochem Int. 2010;57:177–88. doi: 10.1016/j.neuint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Artioli GG, Gualano B, Smith A, Stout J, Lancha AH., Jr Role of beta-alanine supplementation on muscle carnosine and exercise performance. Medicine and science in sports and exercise. 2010;42:1162–73. doi: 10.1249/MSS.0b013e3181c74e38. [DOI] [PubMed] [Google Scholar]
- 34.Sase A, Dahanayaka S, Hoger H, Wu G, Lubec G. Changes of hippocampal beta-alanine and citrulline levels are paralleling early and late phase of retrieval in the Morris Water Maze. Behav Brain Res. 2013;249:104–8. doi: 10.1016/j.bbr.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 35.Chengappa KN, Turkin SR, DeSanti S, Bowie CR, Brar JS, Schlicht PJ, et al. A preliminary, randomized, double-blind, placebo-controlled trial of L-carnosine to improve cognition in schizophrenia. Schizophr Res. 2012;142:145–52. doi: 10.1016/j.schres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Boldyrev AA, Stvolinsky SL, Tyulina OV, Koshelev VB, Hori N, Carpenter DO. Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol. 1997;17:259–71. doi: 10.1023/A:1026374114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hipkiss AR, Preston JE, Himsworth DT, Worthington VC, Keown M, Michaelis J, et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann N Y Acad Sci. 1998;854:37–53. doi: 10.1111/j.1749-6632.1998.tb09890.x. [DOI] [PubMed] [Google Scholar]
- 38.Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, et al. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30:1283–8. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- 39.Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, et al. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–31. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- 40.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 42.Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–9. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- 43.Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121:324–34. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 44.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 45.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 46.Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–81. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 47.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 48.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assuncao M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free radical biology & medicine. 2010;48:831–8. doi: 10.1016/j.freeradbiomed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Assuncao M, Santos-Marques MJ, Carvalho F, Lukoyanov NV, Andrade JP. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. Neurobiol Aging. 2011;32:707–17. doi: 10.1016/j.neurobiolaging.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Li Q, Zhao H, Zhao M, Zhang Z, Li Y. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6J mice. Brain Res. 2010;1353:28–35. doi: 10.1016/j.brainres.2010.07.074. [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, et al. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–15. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues J, Assuncao M, Lukoyanov N, Cardoso A, Carvalho F, Andrade JP. Protective effects of a catechin-rich extract on the hippocampal formation and spatial memory in aging rats. Behav Brain Res. 2013;246:94–102. doi: 10.1016/j.bbr.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 54.Stout JR, Graves BS, Smith AE, Hartman MJ, Cramer JT, Beck TW, et al. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55-92 Years): a double-blind randomized study. J Int Soc Sports Nutr. 2008;5:21. doi: 10.1186/1550-2783-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guidance for industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2005. [Google Scholar]
- 56.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 57.Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3958–68. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 59.Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain, behavior, and immunity. 2012;26:803–10. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 61.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang MH, Chang WJ, Soung HS, Chang KC. (-)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behav Pharmacol. 2012;23:771–80. doi: 10.1097/FBP.0b013e32835a3bc8. [DOI] [PubMed] [Google Scholar]
- 63.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331–62. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 68.Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, et al. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:11578–86. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budzen S, Rymaszewska J. The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med. 2013;22:739–44. [PubMed] [Google Scholar]
- 71.Pae M, Ren Z, Meydani M, Shang F, Smith D, Meydani SN, et al. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. The Journal of nutritional biochemistry. 2012;23:526–31. doi: 10.1016/j.jnutbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Decombaz J, Beaumont M, Vuichoud J, Bouisset F, Stellingwerff T. Effect of slow-release beta-alanine tablets on absorption kinetics and paresthesia. Amino Acids. 2012;43:67–76. doi: 10.1007/s00726-011-1169-7. [DOI] [PubMed] [Google Scholar]
- 73.Murakami T, Furuse M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: antidepressant versus anxiolytic-like effects. Amino Acids. 2010;39:427–34. doi: 10.1007/s00726-009-0458-x. [DOI] [PubMed] [Google Scholar]
- 74.Everaert I, Stegen S, Vanheel B, Taes Y, Derave W. Effect of beta-alanine and carnosine supplementation on muscle contractility in mice. Medicine and science in sports and exercise. 2013;45:43–51. doi: 10.1249/MSS.0b013e31826cdb68. [DOI] [PubMed] [Google Scholar]
- 75.Mozaffari MS, Azuma J, Patel C, Schaffer SW. Renal excretory responses to saline load in the taurine-depleted and the taurine-supplemented rat. Biochem Pharmacol. 1997;54:619–24. doi: 10.1016/s0006-2952(97)00213-x. [DOI] [PubMed] [Google Scholar]
- 76.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. The American journal of clinical nutrition. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 77.Del Rio D, Calani L, Cordero C, Salvatore S, Pellegrini N, Brighenti F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition. 2010;26:1110–6. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 78.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 79.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 80.Zini A, Del Rio D, Stewart AJ, Mandrioli J, Merelli E, Sola P, et al. Do flavan-3-ols from green tea reach the human brain? Nutr Neurosci. 2006;9:57–61. doi: 10.1080/10284150600637739. [DOI] [PubMed] [Google Scholar]
- 81.Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, Weksler B, et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food & function. 2011;2:39–44. doi: 10.1039/c0fo00100g. [DOI] [PubMed] [Google Scholar]
- 82.Derave W, Everaert I, Beeckman S, Baguet A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports medicine. 2010;40:247–63. doi: 10.2165/11530310-000000000-00000. [DOI] [PubMed] [Google Scholar]