Abstract
Chronic visceral pain syndromes are important clinical problems with largely unmet medical needs. Based on the common overlap with other chronic disorders of visceral or somatic pain, mood and affect, and their responsiveness to centrally targeted treatments, an important role of central nervous system in their pathophysiology is likely. A growing number of brain imaging studies in irritable bowel syndrome, functional dyspepsia and bladder pain syndrome/interstitial cystitis has identified abnormalities in evoked brain responses, resting state activity and connectivity, as well as in grey and white matter properties. Structural and functional alterations in brain regions of the salience, emotional arousal, and sensorimotor networks, as well as in prefrontal regions, are the most consistently reported findings. Some of these changes show moderate correlations with behavioral and clinical measures. Most recently, data driven machine-learning approaches to larger data sets have been able to classify visceral pain syndromes from healthy control subjects. Future studies need to identify the mechanisms underlying the altered brain signatures of chronic visceral pain and identify targets for therapeutic interventions.
Keywords: irritable bowel syndrome, functional dyspepsia, bladder pain syndrome, interstitial cystitis, multimodal brain imaging, brain networks
INTRODUCTION
Chronic visceral pain syndromes, in particular those related to the gastrointestinal (GI) tract (irritable bowel syndrome [IBS], functional dyspepsia [FD] and functional esophageal [FE] disorders) and the urinary tract (bladder pain syndrome/interstitial cystitis [BPS/IC>]) are important clinical problems with largely unmet medical needs [8; 13]. Based on the common overlap with other chronic disorders of visceral or somatic pain, mood and affect, and the shared responsiveness of these disorders to centrally targeted treatments, there is a growing consensus that alterations of the central nervous system (CNS) play an important role in their pathophysiology and symptom generation [50], and that some of these central abnormalities may be shared between syndromes.
In order to understand and treat the complex human pain experience, it is crucial to keep in mind that there is no linear relationship between the information that is encoded by primary afferents in the GI tract and the complex subjective experience of pain. Multiple factors including cognitive, emotional and reward processes, as well as memories of past experiences, salience appraisal, and prediction of future experiences are integrated with sensory information in specific brain circuits (including the salience and emotional arousal circuits), and this integrated information ultimately determines a patient’s experience and related autonomic and behavioral responses [7; 62; 68; 69]. This multifactorial determination of the subjective pain experience is illustrated by some patients with widespread inflammation of the GI tract reporting minimal pain, and on the other extreme, by patients with no detectable nociceptive signals from the gut reporting severe pain. The only currently available technique to dissect the various components of this subjective human experience into biological substrates is multimodal brain imaging. Functional brain imaging (resting state and evoked responses) allows for the quantification of the viscerosensory input reaching the brain, and of an understanding of how non-sensory factors contribute to the overall phenotype. Together with structural and diffusion tensor imaging (DTI), multimodal brain imaging makes it possible to identify human brain circuits which correlate with various phenotypic and behavioral manifestations of functional gastro-intestinal disorders (FGIDs), such as psychological states, traits, symptoms, and dysregulations of the hypothalamic pituitary adrenal (HPA) axis and the autonomic nervous system (ANS) [15].
In this article, we provide a brief review of functional and structural imaging studies obtained with magnetic resonance imaging (MRI) approaches and published during the past 5 years, which have been performed in healthy subjects and in those reporting chronic visceral pain referred to the stomach, intestine and urinary bladder. The extensive literature on putative peripheral factors contributing to the pathophysiology of these chronic visceral pain conditions was not included in this review due to space limitations. Based on the published literature and the rapid progress made in the understanding of the CNS in the pathophysiology of different chronic pain states, we will provide a conceptual framework which is consistent with current brain imaging and clinical data, and suggests possible future directions for this exciting field of neuroscience.
FUNCTIONAL MRI STUDIES PUBLISHED BETWEEN 1997 AND 2008
A growing number of reports on brain imaging results obtained in patients with chronic abdominal pain have been published during the last decade [51; 66], and have lead to some insights into the understanding of central aspects of visceral pain modulation in humans. A previous review of positron emission tomography (PET) and functional MRI studies published between 1997 and 2008, found that even though there were wide variations in terms of methods of stimulation, study procedures, study populations and number of subjects and image analysis, the most consistently activated brain regions in all reports were the insular (INS) and anterior cingulate cortices (ACC), followed by primary sensory cortex (S1), prefrontal cortex (PFC), posterior parietal cortex (PPC) and thalamus (THAL) [51]. Results from two recent meta analyses of functional magnetic resonance imaging (fMRI) studies in IBS patients and healthy control subjects (HCs) using controlled rectal balloon distension as a stimulus have reported consistent activation of INS, ACC and THAL in both populations [61; 67]. However, IBS patients differed from HCs by showing activation in regions associated with emotional arousal (including the pregenual cingulate cortex [pgACC] and the amygdala) and activation of a midbrain cluster, possibly related to regions involved in endogenous pain inhibition systems. In contrast, HCs showed more activation of the medial and lateral PFC, possibly related to greater engagement of cortico-pontine-spinal pain inhibition systems. Despite wide variations in experimental protocols and analyses used, these findings demonstrated alterations in emotional arousal, cognitive control, and endogenous pain modulation systems involving THAL, INS and ACC subregions [15; 22; 31]. This consistent pattern leads to the erroneous concept of a “pain matrix”, with greater engagement in patient populations. However, it soon became obvious that the great majority of brain imaging studies in HCs and various disease states showed activation of the same brain regions, suggesting that these brain regions are part of a salience network that is engaged in response to any salient stimulus, not limited to pain. The greater responses within these salience regions in patients with chronic visceral pain, is consistent with the concept of greater salience attribution to visceral signals by patients with chronic visceral pain.
MULTIMODAL BRAIN IMAGING RESULTS PUBLISHED BETWEEN 2009 AND 2014
Evoked brain responses
Modulation by emotional factors
Consistent with clinical and epidemiological data, [65], neuroimaging studies have demonstrated an interaction between experimental visceral stimuli (e.g. painful or non-painful esophageal stimulation), emotional state (e.g. neuroticism), subjective perception, and brain activation. For example, non-painful balloon distention of the esophagus during a negative emotional state was perceived more intensely than that in a neutral emotional state, and this enhanced perception was associated with increased activation of the dorsal ACC and the anterior INS, key regions of a salience network [57]. Exposure to both non-painful and painful esophageal distention in negative emotional states confirmed the original results [12]. During pain expectation, a positive correlation between neuroticism and brain activity in regions of the salience network and the parahippocampal gyrus were identified in another study [12]. In contrast, when the noxious stimulus was delivered, neuroticism showed a negative correlation with these regions. Based on these findings, it has been speculated that that individuals with high trait anxiety/neuroticism attribute greater salience and exhibit increased emotional arousal during pain expectation, but inhibit these brain networks during the experience of pain. These results were later expanded with the demonstration that personality traits might effect visceral sensation [20]. Subjects undergoing fMRI underwent real-time ANS testing and had serum cortisol measured at baseline and following painful stimulation. Two distinct patient clusters were identified. Cluster one showed higher neuroticism, had greater baseline sympathetic tone, and higher cortisol release. These subjects had increased parasympathetic response to pain, had lower pain thresholds, and habituated less to pain. The other cluster had the opposite profile at both baseline and during pain [20].
Brain imaging studies in IBS have confirmed the close relationship between increased affect, enhanced visceral stimulus perception and altered brain responses, where increased intensity ratings of rectal distension induced pain and discomfort were correlated with subjective symptoms of anxiety and depression. Anxiety symptoms were significantly correlated with stimulus-induced activation of mid and pregenual cingulate cortices, while depression symptoms were correlated with the cerebellum and PFC regions. Group differences between patients and HCs (in PFC and anterior INS) were no longer seen after correcting for anxiety and depression scores [16; 17; 58]. These findings are also consistent with the increased engagement of an emotional arousal circuit in IBS patients, in particular during pain expectation [20; 40; 41].
Modulation by cognitive factors and expectations
Alterations in cognitive mechanisms, such as attention, salience attribution and prediction play an important role in most types of chronic pain, including FGIDs [31]. Selective attention to threat, hypervigilance, increased salience attribution to somatic sensation and the failure to correct prediction error (“catastrophizing”) have all been demonstrated in chronic pain populations including those with IBS, and some of these behavioral measures have been shown to correlate with symptoms severity [31; 35].
Pain expectation
In a study with IBS patients divided into normo-sensitive and hypersensitive subgroups, it was found that even though the two IBS groups did not differ in reported symptom severity or psychological symptoms, the hypersensitive group had greater activation in the INS, and reduced deactivation in the pregenual cingulate. Brain responses during expectation were similar to those during actual distension, with both IBS groups showing greater responses than the HC group [42]. Schmid et al. [59] studied HCs during controlled aversive rectal distension while subjects received an intravenous application of an inert substance while expectations were modulated by instructions about the nature of the stimulus. These instructions suggested that the infusion was either associated with pain relief (placebo), or with an increase of pain (nocebo effect), and these effects were compared to neutral instructions. Expected and perceived pain intensity was significantly increased in the nocebo group, and significantly decreased in the placebo group. The placebo response was associated with reduced activation of the somatosensory cortex during expectation, and of the INS, somatosensory cortex and amygdala during the aversive distension compared to neutral expectations. Negative expectations were associated with increased INS activation during the aversive stimulus. When brain responses were compared between groups, negative expectation was associated with an increased response in somatosensory cortex. Using a similar design, brain responses in IBS and HCs were studied during aversive rectal distension and its expectation while receiving an inert intravenous infusion while receiving suggestions about the therapeutic effect of the infusion [43]. Even though both groups showed similar subjective placebo analgesia, IBS subjects showed greater activation of INS, mid cingulate cortex (MCC), and ventrolateral PFC during distension under placebo compared to the control group. Ventrolateral PFC and MCC have previously been identified as an important component of a cortico-limbic pontine pain component of the endogenous pain inhibition system [45; 52].
These neuroimaging findings support the well known clinical phenomenon that expected severity of an announced stimulus, either based on a cue preceding the stimulus, or based on verbal instructions can result in marked differences in the subjective experience of visceral pain. These expectations are associated with the engagement of brain regions of prefrontal, affective and sensory brain regions. Interestingly, negative expectations (either induced by a cue, or by negative instructions) were associated with greater activation of somato- and viscero-sensory brain regions. The mechanism(s) by which expectations increase the activity in sensory brain regions is unclear, but may be mediated by greater engagement of cortico-limbic-pontine circuits and related descending facilitatory pathways increasing dorsal horn excitability and activity in ascending viscerosensory pathways.
Prediction error
Several factors may underlie the persistence of prediction errors about the severity of future pain in chronic pain conditions [72], including alterations in the cognitive ability to extinguish aversive memories. In asymptomatic subjects, erroneous expectations about pain are corrected once the individual realizes that the expected outcome is not as severe as predicted and this ability is based on cognitive flexibility and set shifting in response to error feedback. To test these hypotheses about cognitive deficits, Aizawa et al. [2] studied brain responses in IBS and HCs during the Wisconsin Card Sorting Test [56]. IBS subjects had more perseverative errors and set-maintenance difficulties than HCs. This was accompanied by decreased activity of the right dorsolateral PFC and right hippocampus, and increased activity in the left posterior INS at error feedback during set shifting. Dynamic causal modeling showed reduced prefrontal to pre-supplementary motor area connectivity in IBS. These findings are consistent with previously demonstrated alterations in PFC and INS activation and connectivity during aversive visceral stimulation and support the concept that prediction error and the inability to correct such errors plays an important part in chronic visceral pain and IBS pathophysiology.
Another mechanism that may be involved in the persistence of prediction error about pain involves the enhanced learning of a conditioned response (acquisition) and the inability to correct the error if the expected negative outcome does not happen (extinction learning). Kattoor et al. [29; 30] found that fear conditioning resulted in learned unpleasantness of a previously neutral visual stimulus, and this was associated with anticipatory activation of the ACC, somatosensory cortex, cuneus and amygdala. During extinction learning, anticipatory engagement of the dorsolateral PFC and cerebellum was observed, while in the reinstatement phase, a trend for parahippocampal activation was observed. These results demonstrate that in HCs, conditioned responses quickly disappear, and learning of new predictive cue properties is paralleled by prefrontal activation. Labus et al [38] found that the corticotropin releasing factor type one receptor (CRF-R1) antagonist GW876008, compared to placebo, produced greater suppression of brain activity in the patient group during acquisition in a wide range of regions, including the medial PFC, anterior INS, hippocampus and pons, supporting an alteration primarily in the extinction process.
Modulation by heterotopic stimulation
Several psychophysical and brain imaging studies (see above) support the concept that IBS patients have an altered engagement of endogenous pain modulation systems during heterotypic stimulation, e.g. while receiving an aversive stimulus at a site distant from the chronic visceral pain [9; 16; 58; 73]. These findings point towards an important role of alterations in spinal and/or supraspinal endogenous pain modulation mechanisms (in particular cortico-limbic-pontine circuits [52], and involved brain networks in the pathophysiology of chronic visceral pain. It is conceivable that chronically engaged descending pain facilitatory pathways result in persistent amplified ascending input to primary sensory brain regions (posterior INS, primary somatosensory cortex) which in turn results in neuroplastic grey and white matter changes, as well as alterations in intrinsic oscillations in these sensory pathways and brain regions as observed in several studies [27].
Imaging genetics
Imaging genetics is based on the concept that certain gene polymorphisms are associated with brain endophenotypes relevant to chronic pain. A few studies have evaluated the role of gene polymorphisms related to the serotonin (5-HT) signaling system on brain responses. Kilpatrick et al. [33] showed that regardless of IBS diagnosis, the C/C genotype of the c.-42C>T polymorphism in serotonin receptor gene HTR3A compared with T carrier status was associated with greater anxiety and amygdala responsiveness during emotional and non-emotional conditions. Fukudo et al. [23] found that individuals with the s/s genotype of the 5-HTTLPR polymorphism showed greater distension induced increases in regional cerebral blood flow (rCBF) in emotional regulation regions, including the ACC, parahippocampal gyrus and orbitofrontal cortex compared to those with the l allele. In addition, Farmer and colleagues [20] reported that the 5-HTTLPR short allele was over-expressed in a patient cluster with higher neuroticism, cortisol response, baseline sympathetic tone, and frontal cortex response to visceral pain. The results of these studies are consistent with the well-known role of the serotonin signaling system in brain-gut interactions and in the modulation of affective behavior [22].
Pharmacological and non-pharmacological modulation of brain activity
Pharmacological modulation
Several candidate central signaling alterations have been implicated in the pathophysiology of IBS, including those involving 5-HT, norepinephrine, and corticotropin releasing factor [22; 53]. Acute tryptophan depletion (ATD) was associated with an increased response of an extensive brain network to rectal distension, including nodes of the emotional arousal and salience network [39]. The effect was greater during high inflation pressures, suggesting greater engagement of the central 5-HT system with more aversive visceral stimuli. ATD was also associated with a loss of negative feedback inhibition of the amygdala by prefrontal regions. When viewed together with earlier 5-HT related brain imaging studies [6] and those reviewed in this article [23; 33], there is strong evidence supporting a role of the central 5-HT signaling system in the inhibition of the emotional arousal system in IBS, and this inhibition is likely to play a central role in the therapeutic benefits of 5-HT modulating drugs in IBS symptoms [10]. Another monoaminergic system that has been implicated in the pathophysiology of chronic visceral pain is the noradrenergic system. Berman et al. [5] found that IBS patients showed higher plasma norepinephrine levels and reduced yohimbine-mediated activity in a central arousal circuit, consistent with fewer functional presynaptic alpha2 adrenergic receptors. Yohimbine-mediated reduction of brainstem and amygdala activity was inversely correlated with early life trauma, an event which has been linked to increased responsiveness of central stress circuits. These findings demonstrate increased noradrenergic activity in IBS subjects, which may be related to a downregulation of presynaptic inhibitory alpha2 adrenergic receptors in the brain. Potential therapeutic effects of intrarectal lidocaine on IBS visceral hypersensitivity have previously been reported [70]. The same group assessed how functional connectivity of the default mode network (DMN) and its temporal association with 3 pain-related networks was modulated by rectal lidocaine treatment in IBS patients [44]. During lidocaine, increased within-network connectivity of DMN structures was observed, suggesting that DMN plasticity is sensitive to analgesic effects, and that reduced pain ratings via analgesia reflect DMN connectivity more similar to pain-free individuals.
Non-pharmacological modulation
Several studies reported on the effects of non-pharmacologic interventions (hypnosis and acupuncture) with possible therapeutic value for IBS patients [71]. Lowen et al. [48] found that clinically successful treatment (combined responders from hypnotherapy and the educational intervention) was associated with significant attenuation of the dorsal and ventral anterior INS during rectal distension. Hypnotherapy responders showed a reduction in posterior INS activation during distension, and significant reductions in brain activation during the expectation condition. Following treatment, the IBS brain response to distension was similar to that observed in the HCs.
Non-evoked brain alterations – resting state fMRI studies
Rather than evaluate task- or stimulus dependent brain activity, resting state fMRI (rsfMRI) evaluates brain activity independent of visceral stimulation. rsfMRI parameters have been found to correlate with observed activity during stimulus evoked brain responses [49] Several studies have employed RS analysis to study HCs and those with IBS, FD and BPS/IC, and these are summarized in table 1.
Table 1.
Resting state fMRI
| Reference # |
Sample | Analysis Strategy | Main Findings |
|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | |||
| Hong et al., 2013 | Female HC=76 Male HC=42 Female IBS=31 Male IBS=29 |
Measured power spectrum intensity using spontaneous brain oscillations |
Contrast: Female IBS vs. Female HCs The frequency power distribution was skewed
|
| Hong et al., 2014 | Female HC=24 Male HC=24 Female IBS=24 Male IBS=24 |
Measured intrinsic FC of the aINS |
Contrast: Female IBS vs. Female HCs
|
| Functional Dyspepsia (FD) | |||
| Liu et al., 2013 | Female HC=19 Male HC=11 Female FD=20 Male FD=10 |
Used support vector machine (SVM) multivariate pattern analysis (MVPA) based on regional homogeneity to classify FD subjects from HCs |
Classification Algorithm:
|
| Liu et al., 2013 | Female HC=25 Male HC=14 Female FD=31 Male FD=18 |
Independent component analysis (ICA) was used to determine disease related resting state spatial differences within the DMN |
Contrast: FD vs. HC Significant spatial differences within the DMN were observed in the following regions
|
| Nan et al., 2013 | Female HC=20 Male HC=20 Female FD=20 Male FD=20 |
Multivariate pattern analysis (MVPA) based on functional connectivity between regions of interest was used to classify FD subjects from HCs. Training dataset used a leave one out approach, which was then validated in a replication test dataset. |
Classification Algorithm:
Abnormal functional connections measured using a connectivity severity index (CSI) was
|
| Zhou et al., 2013 | Female HC=9 Male HC=7 Female FD=19 Male FD=10 |
After controlling for sex and age, group differences in amplitude low-frequency fluctuations (ALFF) and fractional (fALFF) changes were investigated using permutation-based nonparametric tests |
Contrast: FD vs. HC Significant differences in fALFF (but not ALFF) were found in:
|
| Babaei et al., 2013 | HC=14 | Intrinsic functional connectivity was measured across 3 conditions:
|
|
| Bladder Pain Syndrome/Interstitial Cystitis (PBS/IC) | |||
| Kilpatrick et al., 2014 | Female HC=85 Female PBS/IC=82 |
Group differences in intrinsic brain oscillations were determined by investigating alterations in frequency power distribution and functional connectivity patterns |
Contrast: PBS/IC vs. HC Investigating frequency power, PBS/IC subjects indicated
For PBS/IC subjects who reported pain during bladder filling indicated increased functional connectivity between:
|
Abbreviations:
Groups: IBS, irritable bowel syndrome; FD, functional dyspepsia; BPS/IC, bladder pain syndrome/interstitial cystitis; HC, healthy control FC, functional connectivity; SVM, support vector machine; MVPA, multivariate pattern analysis; ICA, independent component analysis; ALFF, amplitude low-frequency fluctuations; fALFF, fractional amplitude low-frequency fluctuations; HF, high frequency; LF, low frequency; CSI, connectivity severity index
Regions/Networks: DMN, default mode network; aINS, anterior insula; pINS, posterior insula; mPFC, medial prefrontal cortex; dmPFC, dorsal medial prefrontal cortex; vmPFC, ventromedial prefrontal cortex; dlPFC, dorsal lateral prefrontal cortex; OFC, orbital frontal cortex; mSMA, medial supplementary motor area; pgACC, pregenual anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; MCC, mid cingulate cortex
Irritable bowel syndrome
Female IBS subjects (compared to female HCs) had a power spectrum intensity of spontaneous brain oscillation frequencies skewed towards high frequency to a greater extent in the anterior INS, amygdala and hippocampus, and towards low frequency in the sensorimotor cortex to a greater extent [25]. The findings suggest that the frequency power distribution may play roles in salience detection and sensorimotor integration in female IBS patients. In a follow up study, it was reported that female IBS patients (compared to female HCs) had greater negative functional connectivity of the dorsal anterior INS with medial PFC and precuneus, both regions of the DMN [24; 69].
Functional dyspepsia
Similar to IBS, FD is defined by symptom criteria, with symptoms of recurrent abdominal pain and discomfort referred to the upper abdomen [64]. FD overlaps with IBS diagnosis in up to 50% of patients [74] and transitions between the two syndromes over time in the same patient have been reported [63], suggesting similar brain mechanisms involved in the two conditions. Liu et al. [46] used a multivariate pattern analysis (MVPA) approach of resting state data to classify subjects according to diagnosis. The mean classifier accuracy was almost 90%, with a number of highly discriminative brain regions including the regions of the “pain matrix”, prefrontal regions, the supplementary motor area and cerebellum. Correlations between some of these brain regions and clinical parameters were identified. Nan et al. [55] also studied functional connectivity differences between FD and HCs, and used multivariate pattern analysis to classify patients from HCs. FD related differences in functional connectivity were mainly observed in regions of the limbic/paralimbic system (including amygdala, THAL, INS, ACC, MCC, putamen, and parahippocampus), prefrontal and temporoparietal areas. About 96% of the subjects among the original dataset were correctly classified and 88% accuracy was also validated in a replication dataset. The classification features were significantly correlated with some of the clinical parameters. In another study Liu et al. [47] used independent component analysis to investigate FD related alterations in the default mode network. In the subjects, spatial differences were mainly found in prefrontal regions, pgACC, THAL, parahippocampal gyrus, precuneus, parietal cortex, and temporal pole. Dyspepsia symptom scores showed positive correlations with the pgACC, and negative correlations with the orbitofrontal cortex. Zhou et al. [76] reported a FD related alteration in the fractional amplitude of low-frequency fluctuations in resting state activity in multiple brain regions including the right INS, brainstem and cerebellum. Seed-based resting state functional connectivity analysis revealed that FD patients have increased correlations between the right cerebellum and multiple brain regions including the bilateral brainstem, THAL, left para-/hippocampus and basal ganglia. Values for frequency amplitudes in the right INS were positively correlated with symptom severity. Babaei reported augmented overall functional connectivity of the right aINS during esophageal acid stimulation in HCs, demonstrating that the INS is not only responsive to noxious, but also to subliminal esophageal chemical stimulation [3].
Bladder Pain Syndrome/Interstitial Cystitis
BPS/IC is one of the Urological Pelvic Pain Syndromes. Like other chronic pain syndromes, the pathophysiology of BPS/IC remains incompletely understood but is thought to involve central disturbance in the processing of pain and viscerosensory signals [11]. Kilpatrick et al. [32] reported differences in the regional frequency power of intrinsic brain oscillations between BPS/IC and HCs in in viscerosensory (posterior INS), somatosensory (postcentral gyrus) and motor regions (anterior paracentral lobule, and medial and ventral supplementary motor areas). Increased functional connectivities between motor cortical regions and the cerebellum were correlated with subjective reports of pain during bladder filling.
In summary, a growing number of rsfMRI studies performed in 3 different, but often overlapping visceral pain syndromes have been reported. Even though these studies have been performed in larger samples as earlier studies, the fact that they use a variety of different analysis techniques makes it difficult to directly compare results between these studies, and with earlier studies showing disease related evoked brain response differences. Brain regions consistently identified include the anterior INS, hippocampus/parahippocampus, as well as prefrontal and sensorimotor regions. Moderate correlations between clinical parameters and some of these regions have been reported.
Non-evoked brain alterations – Grey matter
A growing number of studies in patients with chronic visceral pain have reported both increases and decreases of grey matter volume (GMV) and cortical thickness (CT) in different brain regions (summarized in table 2), even though the molecular mechanisms underlying GM changes in various chronic pain populations remain incompletely understood.
Table 2.
Grey matter MRI
| Reference # |
Sample | Analysis Strategy | Main Findings |
|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | |||
| Seminowicz et al., 2010 | Female HC=48 Female IBS=55 |
Group differences in grey matter (GM) were investigated using voxel-based morphometry (VBM) and cortical thickness analyses |
Contrast: IBS vs. HC
|
| Jiang et al., 2013 | Female HC=155 Male HC=21 Female IBS=70 Male IBS=20 |
Group differences in GM were investigated using cortical thickness (CT) analyses |
Contrast: IBS vs. HC
|
| Labus et al., 2014 | Female HC=119 Female IBS=82 |
|
Contrast: Female IBS vs. Female HC
Group differences in regional network organization were observed in the following:
|
| Elsenbruch et al., 2014 | Female HC=52 Male HC=40 |
Correlations between GM VBM in regions in pain relevant regions and visceral sensitivity were conducted using linear regression models | Using rectal sensory pain thresholds to measure visceral sensitivity:
|
| Functional Dyspepsia (FD) | |||
| Zeng et al., 2013 | Female HC=26 Male HC=14 Female FD=32 Male FD=18 |
Group differences in GM density were examined using VBM in meal related FD subjects compared to HCs |
Contrast: FD vs. HC
The structural ↓ in the ACC were significantly negatively correlated with dyspepsia symptom severity and dyspepsia symptom duration |
| Bladder Pain Syndrome/Interstitial Cystitis (PBS/IC) | |||
| Kairys et al., 2014 | Female HC=33 Female PBD/IC=33 |
Group differences in GM density were examined using VBM |
Contrast PBS/IC vs. HC
GM volume in the right S1 was significantly positively correlated with pain, anxiety, and urological symptoms |
Abbreviations:
Groups: IBS, irritable bowel syndrome; FD, functional dyspepsia; BPS/IC, bladder pain syndrome/interstitial cystitis; HC, healthy control GM, grey matter; CT, cortical thickness; VBM, voxel based morphometry; Regions/Networks: PFC, prefrontal cortex; mPFC, medial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; PPC, posterior parietal cortex;
pgACC, pregenual anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; MCC, mid cingulate cortex; OFC, orbital frontal cortex; mOFG, medial orbital frontal gyrus; aINS, anterior insula; mINS, mid insula; pINS, posterior insula; SFG, superior frontal gyrus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SMA, supplementary motor area
Irritable bowel syndrome
Seminowicz et al. [60] reported regional GM density reductions in prefrontal and posterior parietal cortices, ventral striatum and THAL, while regional increases were observed in pgACC and posterior INS/secondary sensory cortex. Jiang et al. [27] reported results from a CT analysis showing that female IBS patients showed both regional increases (somatosensory and primary motor cortices) and decreases (bilateral INS and subgenual cingulate cortex) compared to female HCs [27]. In a larger follow up study, group differences in GM volume were identified in a large number of brain regions, including IBS related GM reductions in frontal, prefrontal and anterior cingulate regions, basal ganglia, affective regions (amygdala and hippocampus) and INS [36]. Similar to the CT findings by Jiang et al. [27], GM increases were seen in the left post central gyrus [36]. Using graph theoretical analysis of the structural data, it was shown that the regional alterations were associated with altered global and regional properties of large-scale brain volumetric networks [36]. Elsenbruch et al. [18] reported results from a study in young HCs which had undergone structural brain imaging and determination of perception thresholds of controlled rectal distension. Lower rectal sensory threshold (i.e., increased sensitivity) correlated significantly with reduced GM volume in the THAL, INS, posterior cingulate cortex, ventrolateral and orbitofrontal prefrontal cortices, amygdala, and basal ganglia (all PFWE < .05). In contrast to a similar study performed in healthy subjects using a somatic pain stimulus, no differences in sensory cortices were reported [19].
Functional dyspepsia
Zeng et al. [75] reported regional gray matter density differences between a subgroup of FD subjects with postprandial pain (“post prandial distress syndrome”) and HCs, using voxel based morphometry. The patient group showed GM reductions the bilateral precentral gyrus, medial PFC, INS, anterior cingulate subregions, and orbitofrontal cortex. Some of these differences were related to anxiety and depression symptoms, and some were correlated with clinical symptoms.
Bladder Pain Syndrome/Interstititial Cystitis
Kairys et al. [28]used voxelbased morphometry to study structural brain differences between female patients with BPS/IC and HCs. IC patients displayed significant increased GM volume in several sensorimotor regions, including the right (S1), the superior parietal lobule bilaterally, and the right supplementary motor area. GM volume in the right S1 was associated with greater pain, mood (anxiety), and urological symptoms.
In summary, despite differences in disease populations, sex, and analysis techniques, there was consistency in these reports for disease related differences in a small number of brain regions. These included GM increases (in the form of CT, and density) in sensorimotor cortices (4 out of 5 studies), and GM decreases in the INS (3 out of 5 studies). Disease related differences for other brain regions (including prefrontal, anterior cingulate and basal ganglia were inconsistent between reports.
Non-evoked brain alterations – White matter abnormalities
A few studies have studied white matter abnormalities in IBS, FD and BPS/IC compared to HCs (summarized in table 3).
Table 3.
White matter fMRI
| Reference # |
Sample | Analysis Strategy | Main Findings |
|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | |||
| Ellingson et al., 2013 | Female HC=72 Male HC=21 Female IBS=21 Male IBS=12 |
Group differences in:
|
Fractional Anisotropy (FA):
|
| Functional Dyspepsia (FD) | |||
| Zhou et al., 2013 | Female HC=23 Male FD=13 Female FD=22 Male FD=14 |
Group differences were measured using fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) measures in a subset of FD subjects (postprandial distress syndrome) compared with HC subjects |
Contrast: FD vs. HC
No significant correlations were found between measures of the white matter tracts and symptom severity or disease duration |
Abbreviations:
Groups: IBS, irritable bowel syndrome; FD, functional dyspepsia; BPS/IC, bladder pain syndrome/interstitial cystitis; HC, healthy control GM, grey matter; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD mean diffusivity; RD, radial diffusivity
Regions/Networks: PFC, prefrontal cortex; ACC, anterior cingulate cortex
Irritable bowel syndrome
Ellingson et al. [14] studied 126 subjects (33 IBS) using DTI and probabilistic tractography and reported microstructural white matter abnormalities in the IBS group in somatosensory brain regions (THAL, basal ganglia) and sensory-motor integration areas. In addition, they reported altered connectivity between the THAL and prefrontal cortex, and between thalamic subnuclei and the ACC. Surprisingly, the observed changes showed no significant correlations with subjective symptoms.
Chronic inflammatory GI conditions
Structural imaging approaches have also been used to identify abnormal central pain processing in patients with of the viscera (inflammatory bowel disorders) [1; 26; 78], e.g. disorders that differ from functional GI disorders by the presence of a documented peripheral pathology. Chronic pancreatitis patients had microstructural changes in the somatosensory (SII), prefrontal, frontal and mid cingulate cortical regions, and some of these abnormalities correlated with patients’ clinical pain scores [21]. Another study in patients with Crohn’s disease in remission and HCs found GMV reductions in frontal and midcingulate cortices, and a negative correlation between GMV of several brain regions and disease duration [1]. Greater CT in ACC and in primary somatosensory cortex was identified in patients with ulcerative colitis (UC) compared with both IBS and HCs [26]. Compared with HCs, UC subjects showed lower CT in orbitofrontal cortex and in primary interoceptive regions (mid and posterior INS), while IBS subjects showed lower CT in the anterior INS. Strong correlations between thickness in the orbitofrontal cortex and postcentral gyrus and with symptom duration were only observed in UC subjects.
Functional dyspepsia
Zhou et al. [77] used DTI to study white matter abnormalities in 36 HCs and 36 patients with postprandial distress syndrome, a subcategory of functional dyspepsia. They reported widespread changes in white matter integrity, even though the majority of group differences disappeared when correcting for depression and anxiety. No significant correlations between white matter changes and subjective symptom measures were observed.
In summary, the majority of studies looking at brain structure report both increases and decreases in GM, and evidence for microstructural remodeling of the brain in subjects with functional and organic GI disorders. Many of the differences are no longer present after controlling for symptoms of anxiety and depression, and strong correlations of these structural changes with subjective symptoms are only present in subjects with chronic inflammatory pain. In general, structural abnormalities (both in terms of grey and white matter) have been observed in regions involved in sensory processing, integration and modulation (THAL, basal ganglia, somatosensory cortex), in prefrontal modulatory regions and in regions of the salience network (ACC, INS) (Fig. 1). Furthermore, there is evidence that some regional GM differences correlate with perceptual sensitivity to visceral and to somatic experimental stimuli in HCs [18], suggesting that such changes may not result from nociceptive input to the brain, but may be genetically or epigenetically determined differences in regional brain structure, which may predispose to chronic pain disorders.
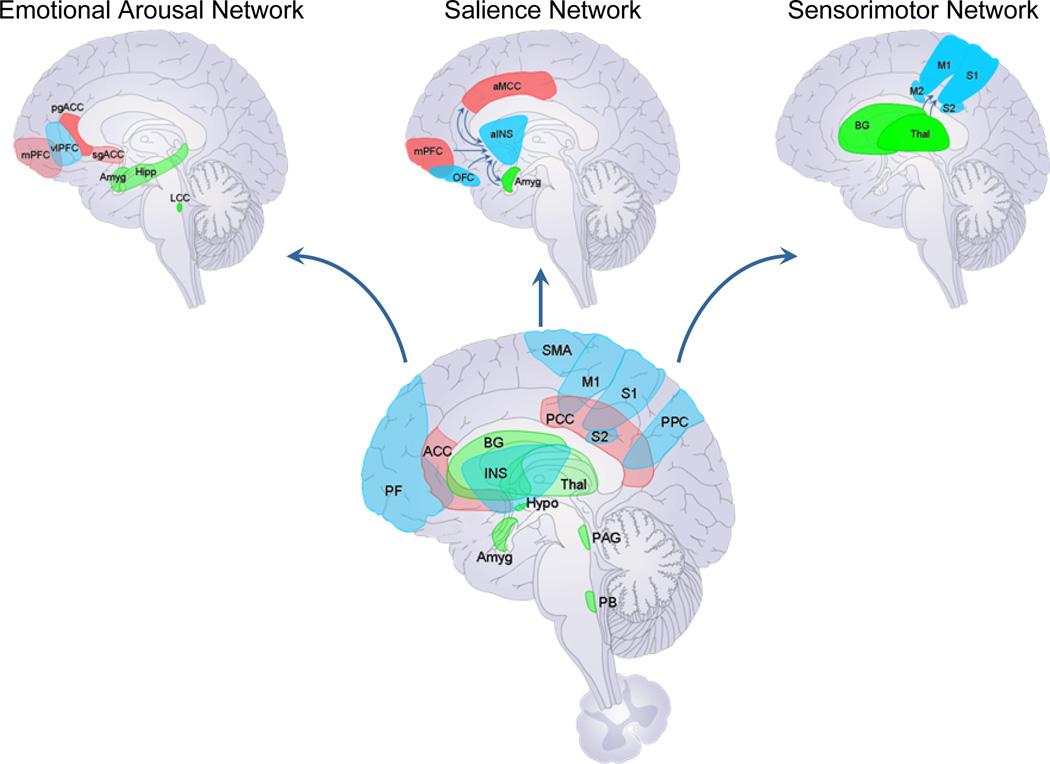
Fig. 1. Brain networks contributing to chronic visceral pain.
Schematic depiction of main brain networks identified in chronic visceral pain based on functional and structural brain imaging studies. Other brain networks including attentional and central autonomic networks are likely to be involved in the pathophysiology of chronic visceral pain syndromes, but have not been studied in detail. Reported brain abnormalities show moderate correlations with behavioral and clinical measures, even though their role in generating the subjective spontaneous pain experience remain to be determined.
Sex related differences in multimodal imaging studies
While symptoms and subjective responses to experimental visceral distension are similar in healthy men and women, female IBS patients are more sensitive to such stimuli, and show greater sensitization by repeated delivery of such stimuli than both male patients and male and female controls [41]. A limited number of studies included sufficient male and female subjects in both disease and control groups (summarized in table 4) to make it possible to speculate about possible neurobiological mechanisms underlying these behavioral sex related differences.
Table 4.
Sex differences
| Reference # |
Sample | Analysis Strategy | Main Findings |
|---|---|---|---|
| Labus et al., 2008 | Female IBS=24 Male IBS=22 |
Sex differences in effective connectivity during rectal balloon distention (INF) and during undelivered distention (EXP) were identified using a multivariate task partial least square analysis (PLS) and structural equation modeling (SEM) |
Emotional-arousal network:
|
| Labus et al., 2013 | Female HC=38 Male HC=29 Female IBS=27 Male IBS=20 |
Group differences in effective connectivity of affective and modulatory cortical circuits using SEM were measured in response to non-painful emotional stimuli |
Contrast: Male (IBS, HC) vs. Female (IBS, HC)
|
| Kilpatrick et al., 2010 | Female HC=36 Male HC=25 Female IBS=62 Male IBS=24 |
Group differences in prepulse inhibition (PPI) of the acoustic startle response, a measure of sensorimotor gating was examined | Compared to HC:
|
| Hong et al., 2013 | Female HC=76 Male HC=42 Female IBS=31 Male IBS=29 |
Measured power spectrum intensity using spontaneous brain oscillations |
Contrast: Female (IBS, HC) vs. Male (IBS, HC) The frequency power distribution was skewed
|
| Hong et al., 2014 | Female HC=24 Male HC=24 Female IBS=24 Male IBS=24 |
Measured intrinsic functional connectivity (FC) of the aINS |
Contrast: Male (IBS, HC) vs. Female (IBS, HC)
|
| Jiang et al., 2013 | Female HC=155 Male HC=21 Female IBS=70 Male IBS=20 |
Group differences in grey matter (GM) were investigated using cortical thickness (CT) analyses |
Contrast: Female IBS vs. Male IBS
|
| Ellingson et al., 2013 | Female HC=72 Male HC=21 Female IBS=21 Male IBS=12 |
Group differences in:
|
Contrast: Female IBS vs. Male IBS
|
Abbreviations:
Groups: IBS, irritable bowel syndrome; FD, functional dyspepsia; BPS/IC, bladder pain syndrome/interstitial cystitis; HC, healthy control INF, inflation during rectal balloon distention; EXP, expectation during undelivered distention; PLS, partial least squares; SEM, structural equation modeling; PPI, prepulse inhibition; FC, functional connectivity; GM, grey matter; CT, cortical thickness; DTI, diffusion tensor imaging; HF, high frequency; LF, low frequency; FA, fractional anisotropy; MD mean diffusivity
Regions/Networks: iACC, infragenual cingulate cortex; ACC, anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; INS, insula; pINS, posterior insula; aINS, anterior insula; mOFC, medial orbital frontal cortex; PFC, prefrontal cortex; mPFC, medial prefrontal cortex; dlPFC, dorsal lateral prefrontal cortex
An early functional brain imaging study using visceral stimuli suggested that male and female IBS patients showed largely similar brain activation, however, male IBS patients demonstrated stronger responses of the INS [4]. Subsequent studies have demonstrated stronger coupling among emotional-arousal regions in female (compared to male) IBS patients, primarily during expectation of visceral pain [41]. In contrast, male (compared to female) IBS patients demonstrated stronger coupling in cortical modulatory regions during expectation of pain. Sex differences in IBS patient responses have also been demonstrated for non-painful emotional stimuli with male IBS subjects demonstrating greater engagement of cortical and affect-related brain circuitry compared to male HCs, when viewing faces depicting emotions previously shown to elicit greater behavioral and brain responses in male subjects [37]. Using prepulse inhibition (PPI) of the acoustic startle response (a measure of sensorimotor gating) [34], male IBS subjects had significantly reduced PPI compared to male HCs, suggesting compromised filtering of visceral signals which may be related to the previously mentioned alterations in cortical control regions expressed in male IBS patients. In contrast, female IBS subjects had significantly enhanced PPI responses, suggesting hypervigilance may be related to altered emotional arousal circuitry expressed in female IBS patients.
Sex differences have also been observed in structural and rsfMRI studies of IBS patients. Female but not male IBS subjects were found to have increased CT of sensorimotor cortex and decreased CT in subgenual ACC compared to same-sex HCs [27]. Female IBS subjects were also found to show reduced integrity of white matter tracts subserving sensorimotor functions compared to male IBS, while these sex differences were not seen in HCs [14]. In addition, in a rsfMRI study, female compared to male IBS subjects demonstrated frequency power distribution skewed towards low frequency in sensorimotor cortex and towards high frequency in the INS [25]. A recent rsfMRI study demonstrated positive dorsal anterior INS connectivity with medial PFC (a key region in both the DMN and executive control network) in male IBS patients while female IBS patients demonstrated more negative dorsal anterior INS connectivity with medial PFC and precuneus (a key region of the DMN) [24]. In view of the role of the anterior INS in switching medial PFC engagement with either DMN or executive control network depending on conditions [54], these results suggest the medial PFC may be more engaged in cognitive function for male IBS patients during rest and more engaged in self-referential mental activity for female IBS patients.
In summary, a limited number of brain imaging studies support sex related differences in evoked brain responses in IBS patients, as well as in brain resting state, grey and white matter findings. Some of the results of these studies are consistent with earlier reports of greater engagement of emotional brain circuits in female patients, and greater engagement of prefrontal circuits in male patients. However, when using stimuli which elicit greater behavioral responses in males (emotions of fear and anger), male IBS subjects showed increased connectivity of cortical and emotional arousal circuits. In addition to these differences seen in evoked brain responses, structural and resting state analyses indicate sex related differences in brain systems involved in sensory integration and motor responses.
Summary and conclusions
The growing number of multimodal brain imaging studies reported during the past decade in patients with different chronic visceral pain conditions, support earlier results from smaller studies looking at evoked brain responses. Structural and functional alterations in brain regions of the salience, emotional arousal and sensorimotor networks, as well as in prefrontal regions, are the most consistently reported findings (Fig. 1). Machine learning approaches to larger data sets have shown the possibility of using multimodal brain imaging results to accurately classify subjects into disease and control groups. Limitations of the reported cross sectional studies include the often reported poor correlation of brain changes with behavioral and clinical measures, the correlational nature of all brain alterations with symptoms, which do not allow to separate cause from effect, and the fact that different analysis approaches used in the various studies prevent the combination of multiple data sets. Furthermore, none of the reported findings has been confirmed in a validation set of experiments. However, based on these findings, one can hypothesize that chronic visceral pain conditions are characterized by changes in brain systems and pathways involved in the processing of sensory information, from the THAL to the sensorimotor cortex. These changes could be a central abnormality preceding the development of chronic pain, or they could be the consequence of chronic viscerosensory input to the brain from the viscera. Such input could come from abnormal motor activity (tonic contractions) of the gut, urinary bladder or pelvic floor muscles, from altered signals of the gut or bladder microbiome, or from activated immune cells in the viscera. An alternative source of such chronically enhanced ascending signals to the sensory cortex may be increased excitability of dorsal horn neurons secondary to tonically enhanced descending facilitatory influences from the brain. The observed alteration in an emotional arousal circuit could play a causative role in this descending pain facilitation. The consistently observed changes in regions of the salience network, in particular the anterior INS could be a genetically and epigenetically determined vulnerability factor for the development of chronic pain conditions, or it could be the consequence of chronic increased somato and viscerosensory input to the brain.
Even though the field has seen a significant increase in our understanding of brain visceral interactions in chronic visceral pain during the past decade, several steps will be necessary to come to a more mechanistic understanding of these syndromes. These include: 1) Studies confirming initial brain findings from data driven analyses in large validation sets, as well as longitudinal studies to better understand the role of brain changes as moderators and mediators of clinical symptoms. 2) Identification of correlation of plausible biological correlates (including gene expression profiles in immune cells and microbial metabolites. 3) Developmental studies in children before the onset of longstanding pain symptoms to address the causality of the brain changes. 4) Interventional phenotyping studies in large patient samples to identify neurobiological subgroups of patients, which can be identified by their brain signatures. 5) Mechanistic studies performed by multidisciplinary teams of investigators using reverse translational approaches. The majority of these approaches will require standardized neuroimaging approaches to large well phenotyped patient populations. It is likely that the sample sizes required for such studies will only be possible through the use of brain imaging repositories, such as the recently established Pain Repository (PainRepository.org).
Acknowledgements
Supported in parts by National Institutes of Health grants R01 DK048351 (EAM), P50 DK064539 (EAM), P30 DK041301 (CURE) and K01 DK085133 (LK). The authors thank Ms Cathy Liu for invaluable editoral and graphic design assistance.
REFERENCES
- 1.Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, Farinelli V, Marchetta C, Calabrese C, Rizzello F, Gionchetti P, Ercolani M, Campieri M, Nichelli P. New insights into the brain involvement in patients with Crohn's disease: a voxel-based morphometry study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(2):e147–e182. doi: 10.1111/nmo.12017. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, Kanazawa M, Shima K, Mushiake H, Hongo M, Fukudo S. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143(5):1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 3.Babaei A, Siwiec RM, Kern M, Douglas Ward B, Li SJ, Shaker R. Intrinsic functional connectivity of the brain swallowing network during subliminal esophageal acid stimulation. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(12) doi: 10.1111/nmo.12238. 992-e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain-London. 2000;4(2):157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 5.Berman S, Suyenobu B, Naliboff BD, Bueller J, Stains J, Wong H, Mandelkern M, Fitzgerald L, Ohning G, Gupta A, Labus JS, Tillisch K, Mayer EA. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. NeuroImage. 2012;63(4):1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD, Mayer EA. Condition-specific deactivation of brain regions by 5-HT(3) receptor antagonist Alosetron. Gastroenterology. 2002;123(4):969–977. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- 7.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch PC, Bosch DC. Treating interstitial cystitis/bladder pain syndrome as a chronic disease. Reviews in urology. 2014;16(2):83–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Bouhassira D, Moisset X, Jouet P, Duboc H, Coffin B, Sabate JM. Changes in the modulation of spinal pain processing are related to severity in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(7) doi: 10.1111/nmo.12123. 623-e468. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135(6):1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ Group MRNS. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC urology. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coen SJ, Yaguez L, Aziz Q, Mitterschiffthaler MT, Brammer M, Williams SC, Gregory LJ. Negative mood affects brain processing of visceral sensation. Gastroenterology. 2009;137(1):253–261. 261, e251–e252. doi: 10.1053/j.gastro.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15(3):237–241. [PubMed] [Google Scholar]
- 14.Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013 doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain, behavior, and immunity. 2011;25(3):386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139(4):1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59(4):489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 18.Elsenbruch S, Schmid J, Kullmann JS, Kattoor J, Theysohn N, Forsting M, Kotsis V. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: A voxel-based morphometry study. Pain. 2014;155(2):244–249. doi: 10.1016/j.pain.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153(8):1602–1609. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Farmer AD, Coen SJ, Kano M, Paine PA, Shwahdi M, Jafari J, Kishor J, Worthen SF, Rossiter HE, Kumari V, Williams SC, Brammer M, Giampietro VP, Droney J, Riley J, Furlong PL, Knowles CH, Lightman SL, Aziz Q. Psychophysiological responses to pain identify reproducible human clusters. Pain. 2013;154(11):2266–2276. doi: 10.1016/j.pain.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Frokjaer JB, Olesen SS, Gram M, Yavarian Y, Bouwense SAW, Wilder-Smith OHG, Drewes AM. Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut. 2011;60(11):1554–1562. doi: 10.1136/gut.2010.236620. [DOI] [PubMed] [Google Scholar]
- 22.Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154(Suppl 1):S63–S70. doi: 10.1016/j.pain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Fukudo S, Kanazawa M, Mizuno T, Hamaguchi T, Kano M, Watanabe S, Sagami Y, Shoji T, Endo Y, Hongo M, Itoyama Y, Yanai K, Tashiro M, Aoki M. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. NeuroImage. 2009;47(3):946–951. doi: 10.1016/j.neuroimage.2009.04.083. [DOI] [PubMed] [Google Scholar]
- 24.Hong JH, Kilpatrick L, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith S, Tillisch K, Naliboff B, Mayer EA. Sex and Disease-Related Alterations of Anterior Insula Functional Connectivity in Chronic Abdominal Pain. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.1683-14.2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang ZG, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. Journal of Neuroscience. 2013;33(29):11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JY, Labus JS, Jiang Z, Ashe-Mcnalley C, Dinov I, Gupta A, Shi Y, Stains J, Heendeniya N, Smith SR, Tillisch K, Mayer EA. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. Plos One. 2014;9(1):e84564. doi: 10.1371/journal.pone.0084564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-related differences of cortical thickness in patients with chronic abdominal pain. Plos One. 2013;8(9):e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Interstitial Cystitis/Painful Bladder Syndrome Patients. J Urol. 2014 doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kattoor J, Gizewski ER, Kotsis V, Benson S, Gramsch C, Theysohn N, Maderwald S, Forsting M, Schedlowski M, Elsenbruch S. Fear Conditioning in an Abdominal Pain Model: Neural Responses during Associative Learning and Extinction in Healthy Subjects. Plos One. 2013;8(2) doi: 10.1371/journal.pone.0051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kattoor J, Thurling M, Gizewski ER, Forsting M, Timmann D, Elsenbruch S. Cerebellar contributions to different phases of visceral aversive extinction learning. Cerebellum. 2014;13(1):1–8. doi: 10.1007/s12311-013-0512-9. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy PJ, Clarke G, Quigley EMM, Groeger JA, Dinan TG, Cryan JF. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav R. 2012;36(1):310–340. doi: 10.1016/j.neubiorev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, Bueller JA, Suyenobu B, Jarcho JM, McRoberts JA, Niesler B, Mayer EA. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140(7):1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, Naliboff BD. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biological psychology. 2010;84(2):272–278. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharm Ther. 2004;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 36.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155(1):137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154(10):2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labus JS, Hubbard CS, Fanselow MS, Chen MP, Ebrat B, Bueller JA, Tillisch K, Stains J, Dukes GE, Kelleher D, Naliboff BD, Mayer EA. The Corticotropin Releasing Factor 1 Receptor (CRF-R1) Antagonist Gw876008 Differentially Modulates Brain Response During Acquisition and Extinction of Conditioned Fear in Irritable Bowel Syndrome (IBS) Patients and Healthy Control Subjects (HCS) Gastroenterology. 2012;142(5):S71–S72. [Google Scholar]
- 39.Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TO, Evers EA, Backes WH, Brummer RJ, van Nieuwenhoven MA. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60(9):1196–1203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. NeuroImage. 2009;47(3):952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson MBO, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, Mayer EA, Walter SA. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients With Irritable Bowel Syndrome. Gastroenterology. 2012;142(3) doi: 10.1053/j.gastro.2011.11.022. 463-U111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HF, Hsieh JC, Lu CL, Yeh TC, Tu CH, Cheng CM, Niddam DM, Lin FY, Chang FY. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153(6):1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Letzen JE, Craggs JG, Perlstein WM, Price DD, Robinson ME. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J Pain. 2013;14(10):1077–1087. doi: 10.1016/j.jpain.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. NeuroImage. 2004;22(1):447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 46.Liu P, Qin W, Wang J, Zeng F, Zhou G, Wen H, von Deneen KM, Liang F, Gong Q, Tian J. Identifying neural patterns of functional dyspepsia using multivariate pattern analysis: a resting-state FMRI study. Plos One. 2013;8(7):e68205. doi: 10.1371/journal.pone.0068205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu P, Zeng F, Zhou G, Wang J, Wen H, von Deneen KM, Qin W, Liang F, Tian J. Alterations of the default mode network in functional dyspepsia patients: a resting-state fmri study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(6):e382–e388. doi: 10.1111/nmo.12131. [DOI] [PubMed] [Google Scholar]
- 48.Lowen MBO, Mayer EA, Sjoberg M, Tillisch K, Naliboff B, Labus J, Lundberg P, Strom M, Engstrom M, Walter SA. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment Pharm Ther. 2013;37(12):1184–1197. doi: 10.1111/apt.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastrovito D. Interactions between resting-state and task-evoked brain activity suggest a different approach to fMRI analysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(32):12912–12914. doi: 10.1523/JNEUROSCI.2580-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer E, Bushnell M, editors. Title|, Vol. Volume|. City|: Publisher|, Year|. [Google Scholar]
- 51.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(6):579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Mayer EA, Collins SM. Evolving Pathophysiological Models of Functional GI Disorders: Implications for New Drug Development. New York: Health Education Alliance; 2000. [Google Scholar]
- 54.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nan J, Liu J, Li G, Xiong S, Yan X, Yin Q, Zeng F, von Deneen KM, Liang F, Gong Q, Qin W, Tian J. Whole-brain functional connectivity identification of functional dyspepsia. Plos One. 2013;8(6):e65870. doi: 10.1371/journal.pone.0065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12(4):313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 57.Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, Thompson DG, Hobson AR, Williams SC, Brammer M, Aziz Q. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126(Pt 3):669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberger C, Thurling M, Forsting M, Elsenbruch S, Timmann D, Gizewski ER. Contributions of the Cerebellum to Disturbed Central Processing of Visceral Stimuli in Irritable Bowel Syndrome. Cerebellum. 2013;12(2):194–198. doi: 10.1007/s12311-012-0413-3. [DOI] [PubMed] [Google Scholar]
- 59.Schmid J, Theysohn N, Gass F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. 2013;154(11):2372–2380. doi: 10.1016/j.pain.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology. 2010;139(1) doi: 10.1053/j.gastro.2010.03.049. 48-U82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheehan J, Gaman A, Vangel M, Kuo B. Pooled analysis of brain activity in irritable bowel syndrome and controls during rectal balloon distension. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23(4):336–346. e158. doi: 10.1111/j.1365-2982.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav R. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki H, Hibi T. Overlap syndrome of functional dyspepsia and irritable bowel syndrome - are both diseases mutually exclusive? J Neurogastroenterol Motil. 2011;17(4):360–365. doi: 10.5056/jnm.2011.17.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tack J, Masaoka T, Janssen P. Functional dyspepsia. Curr Opin Gastroen. 2011;27(6):549–557. doi: 10.1097/MOG.0b013e32834b7ca8. [DOI] [PubMed] [Google Scholar]
- 65.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20(Suppl 1):121–129. doi: 10.1111/j.1365-2982.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 66.Tillisch K, Labus JS. Advances in Imaging the Brain-Gut Axis: Functional Gastrointestinal Disorders. Gastroenterology. 2011;140(2) doi: 10.1053/j.gastro.2010.12.014. 407-U499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140(1):91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tracey I, Bushnell MC. How Neuroimaging Studies Have Challenged Us to Rethink: Is Chronic Pain a Disease? Journal of Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105(1–2):223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 71.Whorwell PJ. IBS Hypnotherapy-a wasted resource? Nat Rev Gastro Hepat. 2012;9(1):12–13. doi: 10.1038/nrgastro.2011.235. [DOI] [PubMed] [Google Scholar]
- 72.Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Frontiers in neuroscience. 2013;7:46. doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011;60(11):1589–1599. doi: 10.1136/gutjnl-2011-300253. [DOI] [PubMed] [Google Scholar]
- 74.Yarandi SS, Christie J. Functional Dyspepsia in Review: Pathophysiology and Challenges in the Diagnosis and Management due to Coexisting Gastroesophageal Reflux Disease and Irritable Bowel Syndrome. Gastroenterol Res Pract. 2013;2013:351086. doi: 10.1155/2013/351086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng F, Qin W, Yang Y, Zhang D, Liu J, Zhou G, Sun J, Lu S, Tang Y, Chen Y, Lan L, Yu S, Li Y, Gao X, Gong Q, Tian J, Liang F. Regional brain structural abnormality in meal-related functional dyspepsia patients: a voxel-based morphometry study. Plos One. 2013;8(7):e68383. doi: 10.1371/journal.pone.0068383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou G, Liu P, Wang J, Wen H, Zhu M, Zhao R, von Deneen KM, Zeng F, Liang F, Gong Q, Qin W, Tian J. Fractional amplitude of low-frequency fluctuation changes in functional dyspepsia: a resting-state fMRI study. Magn Reson Imaging. 2013;31(6):996–1000. doi: 10.1016/j.mri.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Zhou G, Qin W, Zeng F, Liu P, Yang X, von Deneen KM, Gong Q, Liang F, Tian J. White-matter microstructural changes in functional dyspepsia: a diffusion tensor imaging study. Am J Gastroenterol. 2013;108(2):260–269. doi: 10.1038/ajg.2012.405. [DOI] [PubMed] [Google Scholar]
- 78.Zikou AK, Kosmidou M, Astrakas LG, Tzarouchi LC, Tsianos E, Argyropoulou MI. Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. Eur Radiol. 2014;24(10):2499–2506. doi: 10.1007/s00330-014-3242-6. [DOI] [PubMed] [Google Scholar]