Abstract
Membranoproliferative glomerulonephritis (MPGN) denotes a general pattern of glomerular injury that is easily recognized by light microscopy. With additional studies, MPGN subgrouping is possible. For example, electron microscopy resolves differences in electron-dense deposition that are classically referred to as MPGN type I (MPGN I), MPGN II and MPGN III, while immunofluorescence typically detects immunoglobulins in MPGN I and MPGN III but not MPGN II. All three MPGN types stain positive for complement component 3 (C3). Subgrouping has led to unnecessary confusion primarily because immunoglobulin-negative MPGN I and MPGN III are more common than once recognized. Together with MPGN II, which is now called Dense Deposit Disease, immunoglobulin-negative, C3-positive glomerular diseases fall under the umbrella of C3 Glomerulopathies. The evaluation of immunoglobulin-positive MPGN should focus on identifying the underlying trigger driving the chronic antigenemia or circulating immune complexes in order to begin disease-specific treatment. The evaluation of C3 Glomerulopathies, in contrast, should focus on the complement cascade, as dysregulation of the alternative pathway and terminal complement cascade underlies pathogenesis. Although there are no disease-specific treatments currently available for C3 Glomerulopathies, a better understanding of their pathogenesis would set the stage for the possible use of anti-complement drugs.
INTRODUCTION
Membranoproliferative glomerulonephritis (MPGN) denotes a general pattern of glomerular injury characterized by an increase in mesangial cellularity and matrix with thickening of glomerular capillary walls secondary to subendothelial deposition of immune complexes and/or complement factors, cellular entrapment and new basement membrane formation. This pattern of injury is easily recognized by light microscopy making the diagnosis of MPGN relatively straight forward; however immunofluorescence (IF) and electron microscopy (EM) resolve differences amongst MPGN that have led to the adoption of classification systems to subgroup MPGN types. Subgrouping is driven by an effort to better understand this diverse spectrum of diseases under the presumption that histologically driven subclassification is reflective of pathogenic similarities, which may have bearing on directing clinical care.
Applying electron microscopy (EM) to MPGN resolves electron-dense deposits relative to the glomerular basement membrane (GBM), as subendothelial, intramembranous (within the lamina densa) or both subendothelial and subepithelial. These distinctions are classically referred to as MPGN type I (MPGN I), MPGN II and MPGN III, respectively.1–4 Immunofluorescence (IF) studies to detect proteinaceous deposits in MPGN will typically reveal immunoglobulins (usually IgG or IgM) in MPGN I and MPGN III, while MPGN II is noteworthy because of their absence. While the three MPGN types stain positive for C3 (complement component 3) consistent with complement activation, as early as the 1970s it was observed that C3-positive but immunoglobulin-negative examples of MPGN I and MPGN III exist.5 Together with MPGN II, which is appropriately called Dense Deposit Disease (DDD; reviewed in ref 6), this group of C3-positive Ig-negative glomerular diseases has been labeled C3 Glomerulopathies (C3G) (Figure 1A).7,8
Figure 1.
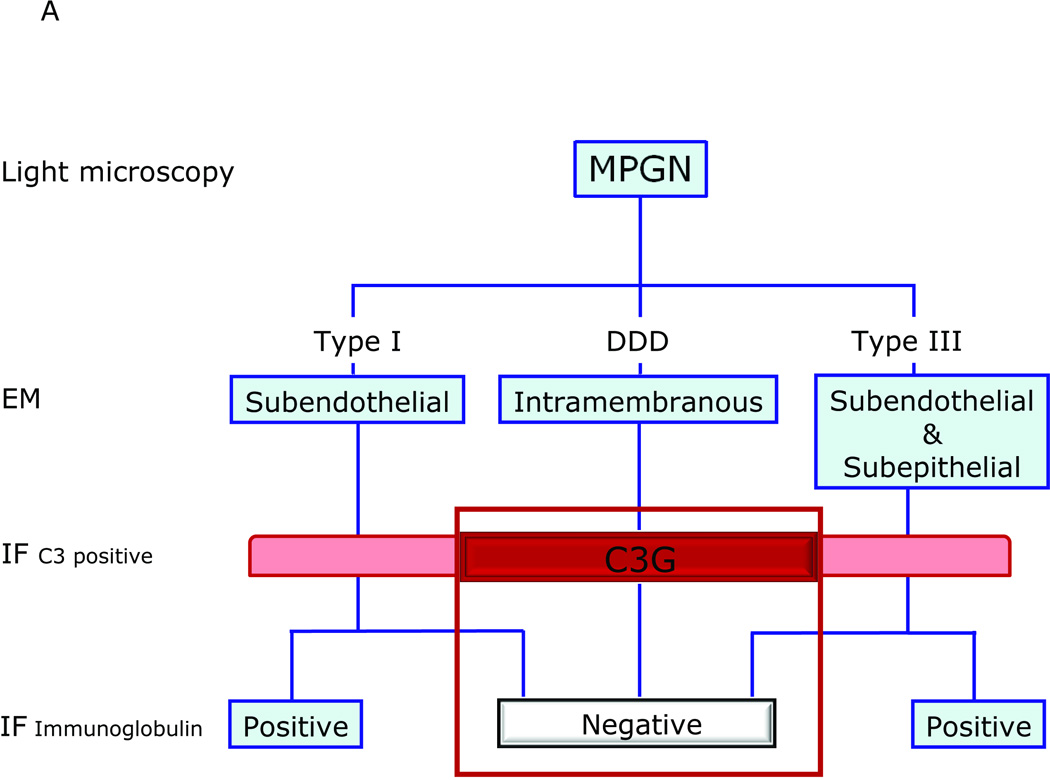
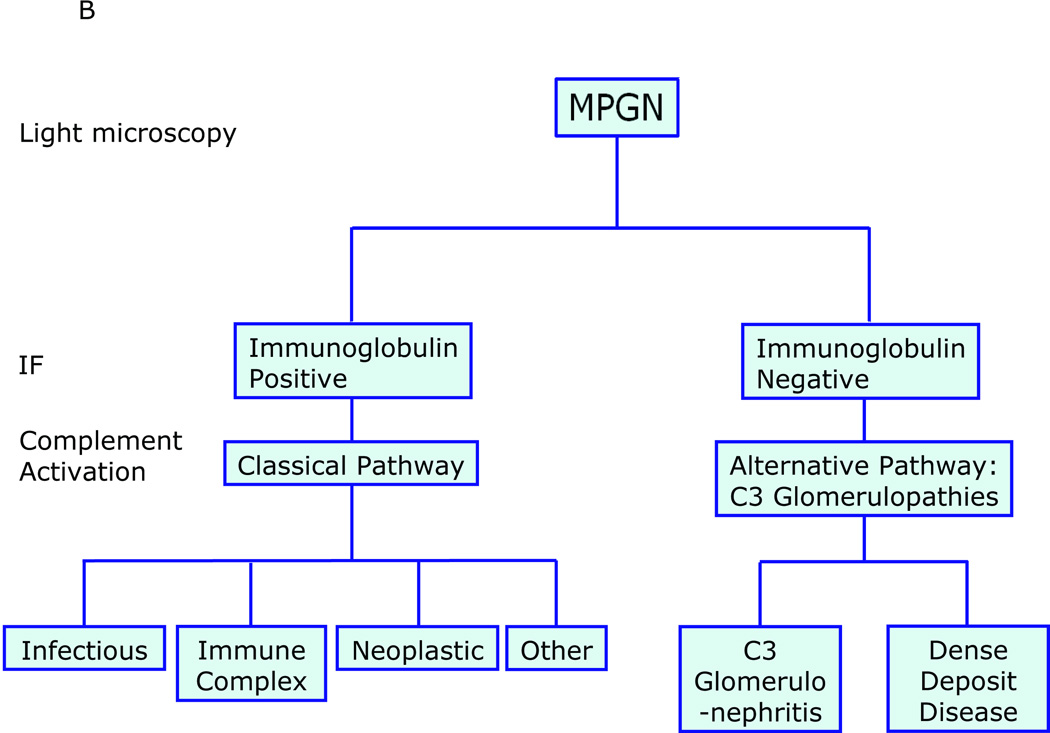
A: MPGN-based and C3G-based classifications of glomerular disease overlap and are confusing because these two classifications are driven by different starting points - findings on EM for MPGN and on IF for C3G. As a general rule, MPGN I and MPGN III are immune-complex diseases characterized by subendothelial or subendothelial/subepithelial densities resolved by EM, however examples of immunoglobulin-negative ‘MPGN I’ and ‘MPGN III’ have been recognized for decades. These types of pathology fall under the umbrella of C3G and are often called C3 Glomerulonephritis (C3-GN). MPGN II or DDD (the preferred name) is another type of C3G. B: A simpler classification is driven by findings on IF, and classifies MPGN as immunoglobulin positive or negative. The terms MPGNI and MPGNIII are not used, thereby avoiding unnecessary confusion. Immunoglobulin-positive MPGN suggests CP activation and a concerted effort should be made to identify the underlying cause of antigenemia. Immunoglobulin-negative, C3-positive MPGN is due to dysregulation of the AP and TCC. Depending of the relative degree of dysregulation, the EM picture can resemble DDD or C3GN. Non-MPGN C3 Glomerulopathies are also seen (see Table 2, Light Microscopy).
MPGN-based and C3G-based classifications overlap because their fundamental perspectives differ: the MPGN classification is based on EM whilst the C3G classification is based on IF microscopy. This overlap is the source of unnecessary confusion, which we will address in this review by focusing on the pathophysiology, evaluation, and treatment of these diseases in the context of a simplified classification (Figure 1B). Four illustrative cases are also described. Because the complement cascade is integral to both MPGN and C3G, we will begin by briefly reviewing this aspect of innate immunity.
THE COMPLEMENT CASCADE
The complement system is the cornerstone of innate immunity and its linchpin is C3. The three initiating pathways of complement activation - the classical (CP), lectin (LP) and alternative (AP) - all converge in a cascade fashion on this molecule to generate an enzyme complex called C3 convertase that cleaves C3 into C3a and C3b. C3a is a potent pro-inflammatory mediator, while C3b is a potent opsonin and a metastable building block from which additional C3 convertase is made, creating a powerful amplification loop. Newly generated C3b interacts with nearby organisms and basement membranes leading to C3 convertase formation on these surfaces. The association of C3b with C3 convertase also generates C5 convertase, initiating the terminal complement complex (TCC) which terminates in the assembly of the membrane attack complex (MAC) on cell surfaces leading to cell lysis (Figure 2).
Figure 2.
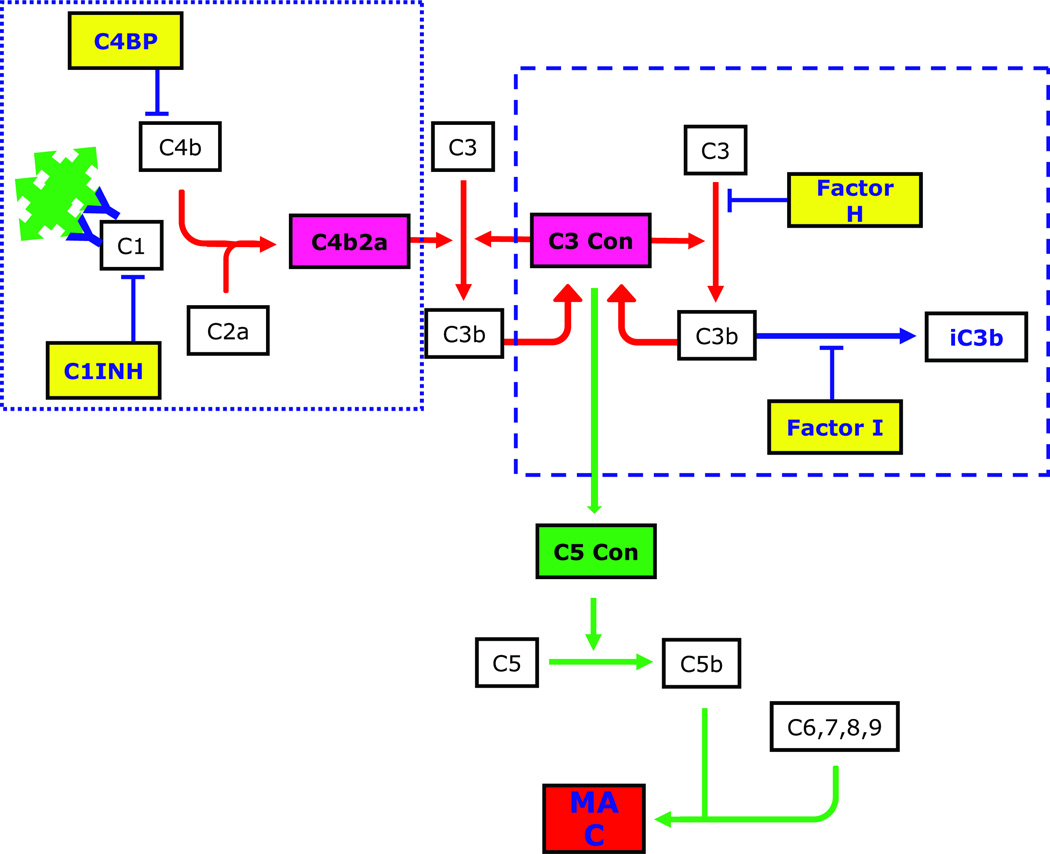
The complement cascade is initiated by the CP (dotted rectangle), AP (dashed rectangle) or LP (not shown). The principle trigger of the CP, immunoglobulin-complexed antigen, is the driving force for C3 deposition in immunoglobulin-positive MPGN (see Figure 1B). Two or more adjacent IgG antibodies provide the structural framework for activation of C1, which cleaves C2 and C4 to generate C2a and C4b, respectively. These two proteins form the CP C3 convertase. The AP is constitutively active, a process that is referred to as ‘tick over’. Binding of CFB, CFD and properdin to hydrolyzed C3 or to C3b leads to formation of the AP C3 convertase C3bBb. This process also generates C3a, a potent anaphylatoxin. When an additional C3b molecule associates with C3 convertase, C5 convertase is formed, initiating the TCC and leading to the generation of MAC. Fluid-phase regulators of the CP include C4BP and C1INH, and of the AP, CFH and CFI (shown in yellow rectangles).
Numerous proteins regulate complement activity to prevent untoward host damage. Broadly divided into fluid-phase (proteins in the circulation) and surface-phase (proteins bound to cell membranes) regulators, these proteins generally modulate activity of the C3 and C5 convertases. Examples of fluid-phase regulators include C1INH (C1 inhibitor) and C4BP (C4 binding protein), which regulate the CP, and CFH (complement Factor H) and CFI, which regulate the AP. Examples of surface-phase complement regulators include membrane-bound proteins like CR1 (complement receptor 1, CD35), CD55 (decay-accelerating factor, DAF), CD46 (membrane co-factor protein, MCP), CD59 and CRIg (complement receptor of the immunoglobulin superfamily, also known as VSIG4 (V-set and Ig domain-containing 4)). Clusterin, vitronectin and CFHR1 (complement Factor H-related 1) regulate the TCC. Several fluid-phase regulators including CFH, clusterin, vitronectin, CFHR1 and C4BP can also attach to cells and biomembranes adding an additional protective layer in these surfaces to limit active complement products.9
The interaction between complement activators and regulators is complex yet subtle. Perturbation of this balance provides the molecular foundation for understanding C3G. How dysregulation of the C3 and C5 convertases and uncontrolled activity of the AP and TCC lead to C3G is discussed next.
PATHOPHYSIOLOGY
Immune-Complex Associated MPGN
Immune-complex associated MPGN (MPGN I and III in Figure 1A) is driven by chronic antigenemia or circulating immune complexes (Figure 1B). In ‘idiopathic’ or ‘primary’ MPGN, a causal etiology cannot be found, while in ‘secondary’ cases the associated systemic disease or infection is known. As a general rule, the term ‘idiopathic’ MPGN should be used infrequently as the underlying reason for antigenemia can usually be found in most cases.8
Immune-complex associated MPGN in the adult population is most commonly due to antecedent hepatitis B or C viral infection that drives persistent antigenemia and immune-complex formation. In addition to other viruses, infectious causes of MPGN also include those of bacterial origin like shunt nephritis and infective endocarditis, and protozoal diseases like malaria and schistosomiasis. MPGN can also result as a complication of circulating immune-complexes associated with autoimmune diseases such as mixed cryoglobulinemia, systemic lupus erythematosus, Sjögren’s syndrome and scleroderma. Finally, deposition of monoclonal immunoglobulins in the mesangium and along the glomerular capillary walls is another important cause of immune-complex MPGN, and can occur in the setting of monoclonal gammopathy of undetermined significance (MGUS), chronic lymphocytic leukemia, low grade B-cell lymphomas, and multiple myeloma (Table 1).8,10,11
Table 1.
Immunoglobulin-positive MPGN
| Antigenic Stimulus | Associated Systemic Disease |
|---|---|
| Infectious | Viral: Hepatitis B and C; HIV Bacterial: endocarditis; shunt nephritis; abscesses Protozoal: malaria; schistosomiasis Others: mycoplasma; mycobacterial |
| Autoimmune diseases | Systemic lupus erythematosus Scleroderma Sjögren’s syndrome Mixed cryoglobulinemia |
| Monoclonal immunoglobulins and paraproteinemias | MGUS Leukemias Lymphomas Myeloma |
| Miscellaneous | Liver disease - hepatitis; cirrhosis Carcinoma Sarcoidosis Drugs “Idiopathic” |
Immune complexes activate the CP triggering an acute injury in the glomerular capillaries and mesangium with influx of inflammatory cells that gives rise to proliferative glomerular changes. The reparative glomerular response is characterized by endothelial and mesangial cell generation of neo-basement membranes, which trap immune complexes thus forming double contours. Mesangial expansion also occurs due to an increase in mesangial cells, infiltration of mononuclear cells, and increase in mesangial matrix. In concert, these changes make the glomerular tufts appear hyper-lobulated. The proportion of cells-to-matrix varies with disease duration and with time. Chronicity is associated with development of nodular mesangial sclerosis and well-formed double contours with a concomitant decrease in cellularity.
C3 Glomerulopathy
C3G is caused by dysregulation of the AP and TCC (Figures 2 and 3). Over the past few years, our understanding of this group of diseases has rapidly advanced largely through detailed genetic studies of animal models and a few human families that segregate this type of renal injury. We highlight three of these familial studies as they provide unique insights into C3G.
Figure 3.
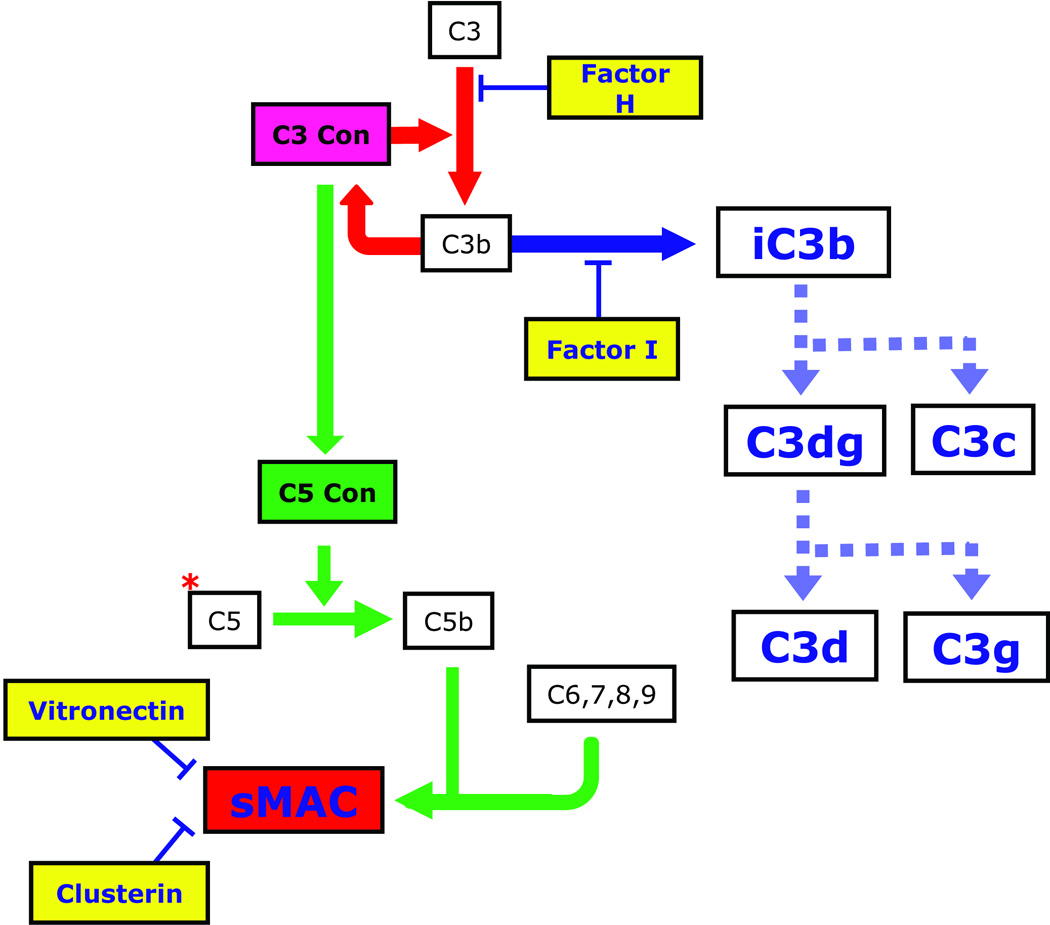
C3G is caused by dysregulation of the AP and TCC. The prototypic example is DDD, in which there must be fluid-phase dysregulation of the C3 convertase, often with dysregulation of the C5 convertase. Dysregulation of the C3 convertase results in increased serum levels of CFI-generated iC3b, which accumulates in the GBM as the characteristic sausage-shaped dense deposits. C3b breakdown products are also detectable in plasma as C3c and C3d. Some patients with C3G also have elevated serum levels of sMAC. Why dysregulation of the AP and TCC leads to DDD in some patients and to C3GN in others is NOT understood at this time. However genetic evidence suggests that a contributing factor may be the relative degree of dysregulation of the C3 and C5 convertases. For example, in the family described by Martínez-Barricarte and colleagues, there is dysregulation of C3 convertase but not C5 convertase. Clarifying the differences between DDD and C3GN is likely to be germane to the treatment of these diseases as the availability of anti-complement therapies is currently limited to eculizumab, an anti-C5 monoclonal antibody (shown as a red *) that prevents cleavage of C5 to C5a and C5b, thereby preventing propagation of the TCC.
In 2010, Martínez-Barricarte and colleagues identified a small nuclear family in which a mother and her two identical twin boys segregated a two amino-acid deletion in MG7 of C3(Δ923–924AspGly).12 This mutant form of C3 was resistant to cleavage by C3 convertase and therefore could not form activated C3b. However, through the normal ‘tickover’ process which forms a hydrolyzed C3 convertase, the mutant C3 protein did form C3Δ923–924DG convertase. This convertase was capable of cleaving circulating wild-type C3 translated from the normal (non-mutated) allele but was resistant to CFH-mediated regulation. As a consequence, the phenotype in these three people was one of persistent fluid-phase mutant C3 convertase activity, and at the level of the renal glomerulus, the picture was one of classic DDD. The mother and one twin progressed to end-stage renal disease (ESRD), which was treated in the mother with three transplants, the last still functioning after three years although in the face of microhematuria, proteinuria and progressing renal insufficiency. The second twin has not yet progressed to ESRD. Importantly, both accelerated decay of the mutant C3 convertase by CD55 and CD46 cofactor activity for CFI are NOT affected by the two amino acid deletion, providing conclusive evidence that DDD in this family results EXCLUSIVELY from fluid-phase AP dysregulation (Figure 3).
A second genetic cause of C3G was recently reported by Gale et al.13 These investigators identified two families segregating autosomal dominant microscopic hematuria. Both families originated from the Troodos mountain region of Cyprus suggesting an ancestral founder effect. Renal biopsy was remarkable for variable glomerular inflammation in association with subendothelial and mesangial electron-dense deposits positive by IF for C3. Genome-wide linkage analysis localized the genetic abnormality to chromosome 1q31–32, a region that includes CFH and the CFHR genes. Using multiplex ligation-dependent amplification, the authors identified a non-homologous allelic recombination (NHAR) event that results in the duplication of CFHR5 to create a novel CFHR5 fusion gene.
CFHR5 is a 65kDa plasma protein that consists of 9 short consensus repeat (SCR) domains. The NHAR event in these Cypriotic families creates a larger CFHR5 protein in which its first two SCRs are duplicated to generate a genetic rearrangement that is translated into an 11-SCR novel CFHR5 protein (CFHR51,2,1,2,3–9). The plasma concentration of CFHR5 is approximately 3–6 ug/ml or ~1% that of CFH, and while its precise physiological role is not known, it does co-localize with complement in diseased glomeruli and can associate with surface-bound C3 (C3b) to inhibit C3 convertase activity.14–16 Functional studies with the mutant CFHR51,2,1,2,3–9 protein showed that it was less effective than wild-type CFHR5 in associating with surface-bound C3b suggesting a dominant-negative mechanism of action.13 This finding also highlights the importance of CFHR5 in complement processing within the kidney, a role that is further supported by the identification of CFHR5 mutations in some patients with DDD.17
The third family was reported by Habbig et al, who described two siblings of consanguineous parentage with childhood-onset hematuria and proteinuria.18 Detailed complement studies showed serum decreases in C3 and CFB, although C4 was normal and C3d was increased. Renal biopsy was remarkable for prominent mesangial deposition of C3 and C5b-9, and by EM there were numerous osmiophilic mesangial deposits with intramembranous and subendothelial roundish GBM deposits. Mutation screening of CFH showed that both children were homozygous for the deletion of a single amino acid, lysine, at position 224 (CFH K244). This deletion affects SCR4 of CFH and leads to defective complement control by severely reducing cofactor, decay accelerating activity and C3b binding.19 Cell-binding activity, however, remains normal. Because the typical linear hyperosmiophilia of DDD was absent, the authors proposed calling this pattern of injury C3 deposition glomerulopathy (C3DG).
In aggregate these familial cases suggest that C3G is a disease spectrum that is dependent on the level and degree of dysregulation of the AP and TCC. In most cases of DDD and C3GN, however, the family history is negative, making the identification of risk factors, whether genetic or acquired, a challenge. It is possible, for example, that allele variants of membrane-bound complement regulators may affect the disease phenotype, a hypothesis that warrants testing. In addition, more in depth studies are needed to determine whether specific biomarkers of complement activity can be used to differentiate DDD from C3GN and predict response to therapy. Lastly, efforts should be made to clarify the biological role of CFHR5 and the other CFHR proteins in normal and disease renal physiology.
EVALUATION AND TREATMENT
Immune-Complex Associated MPGN
The evaluation of immune-complex associated MPGN should focus on identifying the underlying triggering disease in order to begin appropriate disease-specific treatment (Table 1). The cornerstone of therapy in this setting is often an anti-cell proliferation agent such as a calcineurin inhibitor, mycophenolate mofetil, cyclophosphamide or rituximab.20 In many patients, evaluation of the complement system will often show evidence for activation of the CP, with decreased serum levels of C3 and C4, and an abnormal CH50. Whether anti-complement drugs will be complementary to anti-proliferative agents in this setting has not been explored. In a small subgroup of immune-complex associated MPGN, it may be impossible to determine the origin of the deposited Ig. These cases should be labeled ‘idiopathic’ MPGN. There may also be a small subset of immune-complex associated MPGN which demonstrates concurrent AP abnormalities. It is important to point out that in such cases, the deposition of immune-complexes in the glomeruli drives the disease process, and it is imperative to perform investigations to determine the etiology of the deposited immunoglobulins.
C3 Glomerulopathy
Little data exist on the long-term consequences of C3GN. Servais and colleagues reported on a series of 19 patients with C3GN.21 Median age-at-onset was 29.9 years (range 7–70 years) and median follow-up was 12.3 years (range 0.4–34.0 years). At the time of the study, three patients had progressed to ESRD and required dialysis, while six additional patients had substantial renal dysfunction, with creatinine clearances of less than 60 ml/min. While these outcomes are based on limited data they do suggest that while the prognosis for C3GN may be poor, it carries a better prognosis than DDD. Over half of DDD patients progress to ESRD within 10 years of diagnosis and when transplanted, face the dismal reality of near universal disease recurrence leading to a 5-year allograft failure rate of 50%.22
The outlook for either C3GN or DDD is unlikely to change until disease-specific treatments become available. Following a biopsy consistent with C3G, most patients should be placed on angiotensin II type 1 receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors to improve renal dynamics, decrease proteinuria, control blood pressure and limit glomerular leukocyte infiltration.22,23 All patients should undergo targeted studies to evaluate the AP by measuring serum complement levels (C3, C4, CH50, AH50), complement breakdown products (C3c and sMAC) and disease-associated autoantibodies like C3 nephritic factors (C3Nefs) and autoantibodies to CFH and CFB. Genetic testing is also indicated and at a minimum should include CFH although several other genes have also been implicated in C3GN and DDD6,24 Mutations in CFH are also associated with atypical hemolytic uremic syndrome, where they tend to cluster in the last two SCRs of the protein and affect its ability to regulate cell membrane-bound C3 convertase.25
The pathophysiology of C3G suggests that a number of different treatments should be considered. For example, in the family described by Habbig and colleagues, CFH replacement is likely to be beneficial.18 It is possible that additional CFH may also help in the Cypriotic form of C3GN. Importantly, while CFH replacement is currently available only through plasma exchange, pharmaceutical preparations may be available for therapeutic use in the near future.26,27 It is equally clear, however, that exogenous CFH would be of no benefit to DDD patients with the C3Δ923DG mutation reported by Martínez-Barricarte and colleagues since the mutant C3 convertase is CFH resistant. For these patients, specific treatment options that restore C3 convertase control, impair C3 convertase activity or remove C3 breakdown products from the circulation are required but remain to be developed.
The benefit of targeting MAC is more difficult to assess as the role of C5 activation and C5 convertase dysregulation in the pathogenesis of C3G requires further investigation. There are, however, C3GN patients with elevated plasma levels of sMAC who would be predicted to benefit from eculizumab, a monoclonal antibody that blocks C5 activation (and therefore MAC production) and is approved for the treatment of paroxysmal nocturnal hemoglobinuria (Figure 3).28 Although eculizumab also appears to be a very effective therapy for atypical hemolytic uremic syndrome,29 its use in C3GN patients needs careful consideration. Certainly, it seems unlikely that patients such as those reported by Martínez-Barricarte and colleagues would gain much benefit from C5 inhibiting strategies except perhaps during secondary triggers of complement activation (such as infections) that may lead to acute disease exacerbation.
ILLUSTRATIVE CASES
Case 1 (Figure 4)
Figure 4.
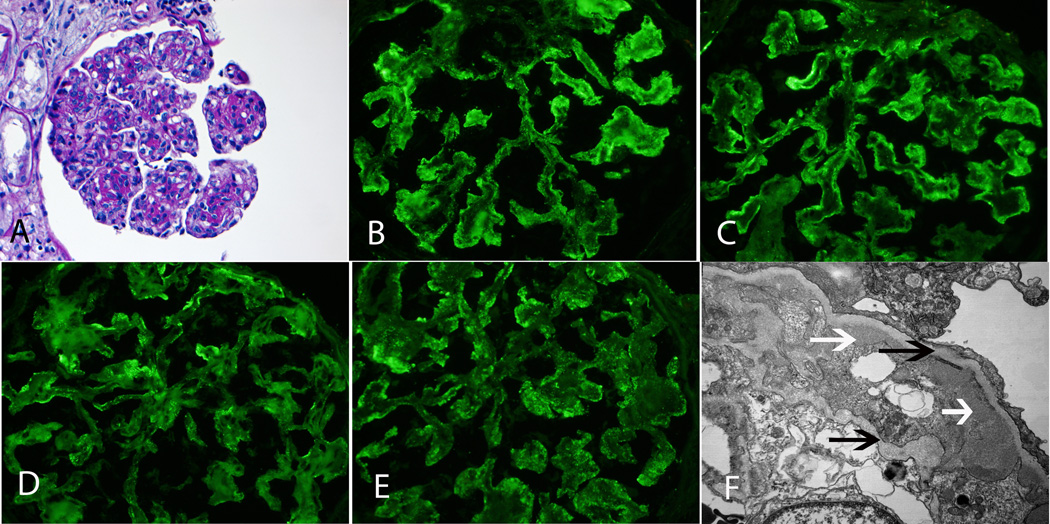
MPGN secondary to Hepatitis C. A: LM shows MPGN with mesangial and endocapillary proliferation and thickened capillary walls with double contour formation (PAS ×20). B, C, D, E: IF microscopy shows mesangial and capillary wall staining for IgM, C3, kappa and lambda light chains (×40). F: EM shows subendothelial deposits (white arrows) with double contour formation (black arrows) (×13500).
Case 1 shows a MPGN pattern on LM. By IF, there is predominant IgM and C3 staining in the mesangium and capillary walls. There is also staining for both kappa and lambda light chains. EM studies show subendothelial deposits with double contour formation. Further evaluation confirmed hepatitis C infection. Diagnosis: Membranoproliferative glomerulonephritis secondary to hepatitis C.
Case 2 (Figure 5)
Figure 5.
MPGN secondary to monoclonal immunoglobulins. A: LM shows an MPGN with mesangial and endocapillary proliferation and thickened capillary walls with double contour formation. A small cellular crescent is also noted (PAS ×40). B, C, D, E: IF microscopy shows mesangial and capillary wall staining for IgG, C3 and kappa light chains but is negative for lambda light chains (×40). F: EM shows small subendothelial deposits (white arrow) with double contour formation (black arrows) (×3400).
Case 2 shows a MPGN pattern on LM. A small cellular crescent is also noted. IF studies show staining for IgG, C3, and kappa light chains along the capillary walls; lambda light chains are negative. EM studies show subendothelial deposits with double contour formation. Further evaluation showed monoclonal IgG kappa on immunofixation studies and 5% plasma cells on bone marrow biopsy. Diagnosis: Membranoproliferative glomerulonephritis secondary to monoclonal IgG kappa.
Case 3 (Figure 6)
Figure 6.
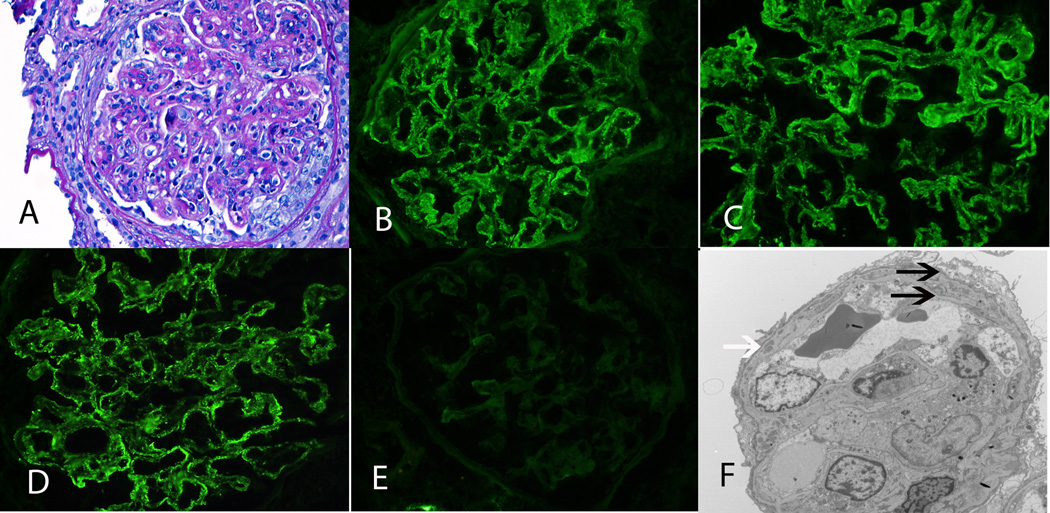
C3GN. A: LM shows MPGN with thickened capillary walls and double contour formation (PAS ×20). B: IF microscopy shows strong capillary wall staining for C3 (×40). C, D: EM shows large subendothelial deposits (white arrows), occasional subepithelial deposit (thick black arrow) with double contour formation (thin black arrows) (N, neutrophil) (C ×1650, D ×4200).
Case 3 shows a MPGN pattern on LM. IF studies were strongly positive for C3 in the mesangium and along capillary walls, but negative for IgG, IgM, IgA, C1q, kappa and lambda light chains. EM studies showed numerous subendothelial and mesangial deposits with double contour formation. A few intramembranous and occasional subepithelial deposits were also noted. Evaluation of the AP identified antibodies to Factor H. Diagnosis: C3GN: membranoproliferative glomerulonephritis secondary to AP dysfunction caused by anti-Factor H antibodies.
This case is noteworthy because the differential diagnosis of C3GN includes post-infectious GN, particularly the so-called resolving/healing post-infectious GN characterized by the presence of subepithelial humps (Table 2). The absence of any history of infection anteceding the development of GN, the bright C3 staining and the absence of immunoglobulins on IF should raise the possibility of C3GN. EM is also helpful in that C3GN typically shows large subendothelial and mesangial deposits; intramembranous and subepithelial deposits are also often noted. The deposits are lighter and have a fuzzy lobular appearance as compared to the darker and sharper immunoglobulin-containing deposits of post-infectious GN.
Table 2.
Renal biopsy: Post-infectious Glomerulonephritis and C3 GN
| Light Microscopy | Immunofluorescence* | Electron Microscopy | |
|---|---|---|---|
| Post-infectious GN | Exudative, with endocapillary proliferation | Immunoglobulin, typically IgG, kappa and lambda light chains, with C3 | Distinct subepithelial humps, few subendothelial deposits, rare double contours, few mesangial deposits |
| C3GN | MPGN pattern (sometimes a mesangial, proliferative or sclerosing pattern) | Bright C3, with almost complete absence of immunoglobulin and light chains | Mostly large subendothelial and mesangial deposits, few intramembranous deposits and subepithelial hump-like deposits, double contours common, deposits have a fuzzy lighter staining pattern than immunoglobulin-containing deposits |
mesangial and capillary wall
Case 4 (Figure 7)
Figure 7.
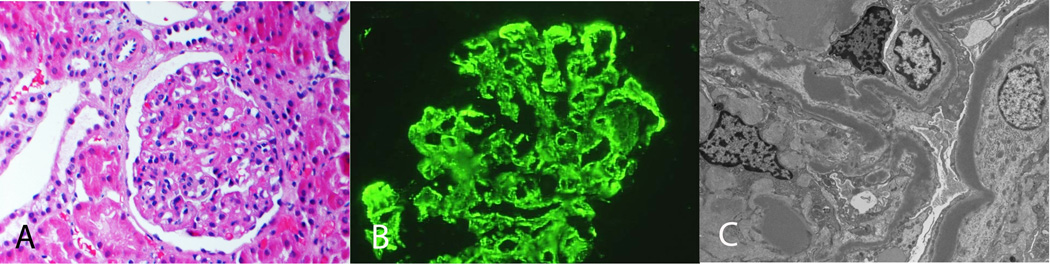
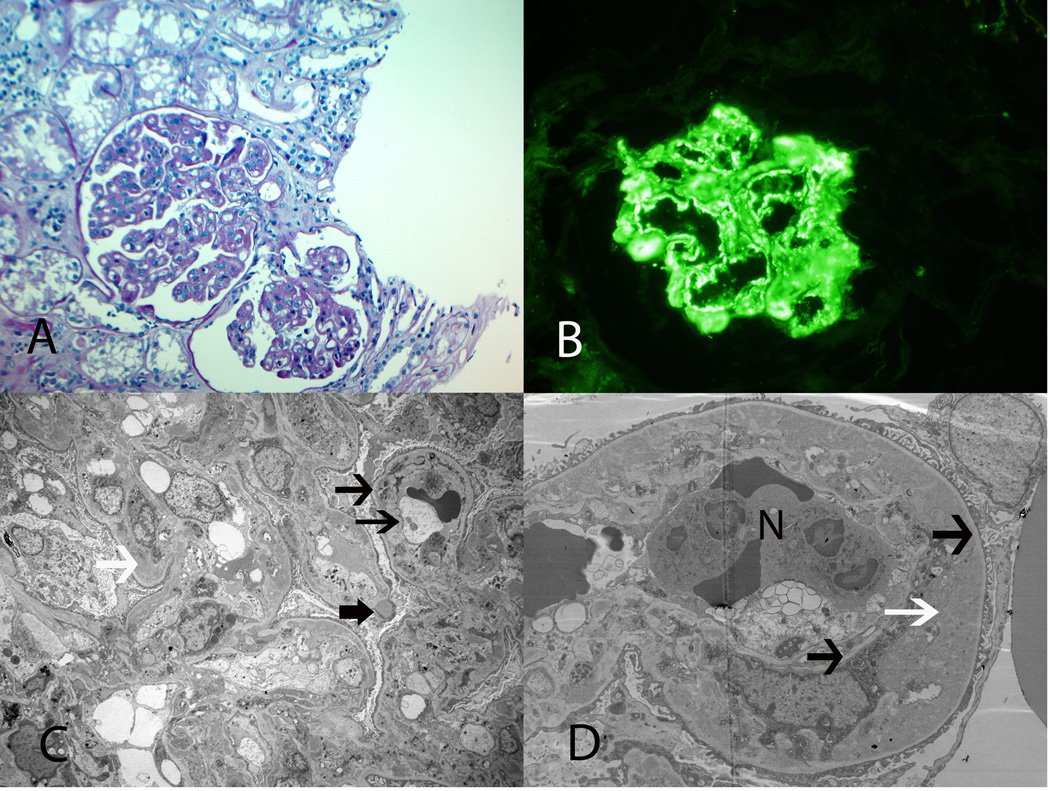
Dense Deposit Disease. A: LM shows MPGN (H and E, ×20). B: IF microscopy showing mesangial and capillary wall staining for C3. C: EM shows abundant dense deposits along the glomerular basement membranes and mesangium (×3400).
Case 4 shows a MPGN pattern on LM. IF studies were positive for C3 in the mesangium and along capillary walls, but negative for IgG, IgM, IgA, C1q, kappa and lambda light chains. EM studies showed dense deposits along the glomerular basement membranes and in the mesangium, and complement studies identified high titers of C3Nefs. Diagnosis: Dense Deposit Disease.
CONCLUSION
MPGN refers to a pattern of glomerular injury that is easily recognized by light microscopy. With EM, differences in electron-dense deposition can be resolved that are classically referred to as MPGN I, MPGN II (more appropriately called DDD) and MPGN III. IF typically detects immunoglobulins in MPGN I and MPGN III but not DDD, although all three MPGN types stain positive for C3. Recently, immunoglobulin-negative MPGN I and MPGN III has become increasingly recognized. Together with DDD, immunoglobulin-negative C3-positive glomerular diseases fall more appropriately under the umbrella of C3 Glomerulopathies.
Immunoglobulin-positive MPGN is driven by chronic antigenemia or circulating immune complexes and evaluation should focus on identifying the underlying trigger in order to begin disease-specific treatment. The cornerstone of therapy is often an anti-cell proliferation agent, though in many patients, evaluation of the complement system will show evidence for activation of the CP, with decreased serum levels of C3 and C4, and an abnormal CH50. The evaluation of C3 Glomerulopathies, in contrast, should focus on the alternative complement cascade. Dysregulation of the AP and TCC underlies pathogenesis, although the differences that lead to either a DDD or C3GN pattern of injury are not understood. The concept of anticomplement therapy as disease-specific treatment for the C3 Glomerulopathies will require better characterization of this group of diseases.
ACKNOWLEDGMENTS
This research was supported in part by NIH grant DK074409 to SS and RJHS.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Anders D, Thoenes W. Basement membrane changes in membranoproliferative glomerulonephritis: a light and electron microscopic study. Virchows Arch Pathol Anat. 1975;369:87–109. doi: 10.1007/BF00433236. [DOI] [PubMed] [Google Scholar]
- 2.Habib R, Kleinknecht C, Gubler MC, Levy M. Idiopathic membranoproliferative glomerulonephritis in children. Report of 105 cases. Clin Nephrol. 1973;1:194–214. [PubMed] [Google Scholar]
- 3.Jackson EC, McAdams AJ, Strife CF, et al. Differences between membranoproliferative glomerulonephritis types I and III in clinical presentation, glomerular morphology and complement pertubation. Am J Kidney Dis. 1987;9:115–120. doi: 10.1016/s0272-6386(87)80088-4. [DOI] [PubMed] [Google Scholar]
- 4.Strife CF, McEnery PT, McAdams AJ, et al. Membranoproliferative glomerulonephritis with disruption of the glomerular basement membrane. Clin Nephrol. 1977;7:65–72. [PubMed] [Google Scholar]
- 5.Levy M, Gubler M-C, Sich M, et al. Immunopathology of membranoproliferative glomerulonephritis with subendothelial deposits (Type I MPGN) Clin Immunol Immunopathol. 1978;10:477–492. doi: 10.1016/0090-1229(78)90160-5. [DOI] [PubMed] [Google Scholar]
- 6.Smith RJH, Harris CL, Pickering MC. Dense deposit disease. Mol Immunol. 2011;48:1604–1610. doi: 10.1016/j.molimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhouri F, Frémeaux-Bacchi V, Noël L-H, et al. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Fervenza F, Zhang Y, et al. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6:1009–1017. doi: 10.2215/CJN.07110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 10.Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409–1418. doi: 10.1007/s00467-009-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Zand L, Leung N, et al. Membranoproliferative Glomerulonephritis Secondary to Monoclonal Gammopathy. Clin J Am Soc Nephrol. 2010;5:770–782. doi: 10.2215/CJN.06760909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–780. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRae JL, Duthy TG, Griggs KM, et al. Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J. Immunol. 2005;174:6250–6256. doi: 10.4049/jimmunol.174.10.6250. [DOI] [PubMed] [Google Scholar]
- 15.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144:1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy B, Georgio T, Machet D, et al. Factor H-related protein 5: a novel component of human glomerular immune deposits . Am J Kidney Dis. 2002;39:24–27. doi: 10.1053/ajkd.2002.29873. [DOI] [PubMed] [Google Scholar]
- 17.Abrera-Abeleda MA, Nishimura C, Smith RJH, et al. Variations in the complement regulator genes factor H (CFH) and factor H related 5 (CFHRB) are associated with membranoproliferative glomerulonephritis type II (Dense Deposit Disease) J Med Genet. 2006;43:582–589. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habbig S, Mihatsch MJ, Heinen S, et al. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75:1230–1234. doi: 10.1038/ki.2008.354. [DOI] [PubMed] [Google Scholar]
- 19.Licht C, Heinen S, Jozsi M, et al. Deletion of Lys224 in regulatory domain 4 of factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kid Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- 20.Hewins P, Smith RJH, Savage COS. Idiopathic Membranoproliferative Glomerulonephritis. In: Berl, Himmelfarb, Mitch, Murphy, Pioli, Wilcox, Salant, Yu, editors. Therapy in Nephrology and Hypertension. 3rd Edition. 2007. pp. 249–256. [Google Scholar]
- 21.Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grünfeld JP, Lesavre P, Noël LH, Fakhouri F. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44:193–199. doi: 10.1136/jmg.2006.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RJH, Alexander J, Barlow PN, et al. New approaches to the treatment of Dense Deposit Disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (Dense Deposit Disease): An update. J Am. Nephrol Soc. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 24.Abrera-Abeleda MA, Nishimura C, Frees K, et al. Allele variants of complement genes associated with Dense Deposit Disease. J Am Soc Nephrol. 2011;22:1581–1589. doi: 10.1681/ASN.2010080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maga TK, Nishimura CJ, Weaver AE, et al. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mut. 2010;31:E1445–E1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- 26.Büttner-Mainik A, Parsons J, Jérôme H, et al. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol J. 2011;9:373–383. doi: 10.1111/j.1467-7652.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt Cq, Slingsby FC, Richards A, et al. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expr Purif. 2011;76:254–263. doi: 10.1016/j.pep.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 29.Kose O, Zimmerhackl LB, Jungraithmayr T, et al. New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Semin Thromb Hemost. 2010;36:669–672. doi: 10.1055/s-0030-1262889. [DOI] [PubMed] [Google Scholar]