Abstract
In comparison to other classes of cell surface receptors, the medicinal chemistry at P2X (ligand-gated ion channels) and P2Y (G protein-coupled) nucleotide receptors has been relatively slow to develop. Recent effort to design selective agonists and antagonists based on a combination of library screening, empirical modification of known ligands, and rational design have led to the introduction of potent antagonists of the P2X1 (derivatives of pyridoxal phosphates and suramin), P2X3 (A-317491), P2X7 (derivatives of the isoquinoline KN-62), P2Y1 (nucleotide analogues MRS 2179 and MRS 2279), P2Y2 (thiouracil derivatives such as AR-C126313), and P2Y12 (nucleotide/nucleoside analogues AR-C69931X and AZD6140) receptors. A variety of native agonist ligands (ATP, ADP, UTP, UDP, and UDP-glucose) are currently the subject of structural modification efforts to improve selectivity. MRS2365 is a selective agonist for P2Y1 receptors. The dinucleotide INS 37217 potently activates the P2Y2 receptor. UTP-γ-S and UDP-β-S are selective agonists for P2Y2/P2Y4 and P2Y6 receptors, respectively. The current knowledge of the structures of P2X and P2Y receptors, is derived mainly from mutagenesis studies. Site-directed mutagenesis has shown that ligand recognition in the human P2Y1 receptor involves individual residues of both the TMs (3, 5, 6, and 7), as well as EL 2 and 3. The binding of the negatively-charged phosphate moiety is dependent on positively charged lysine and arginine residues near the exofacial side of TMs 3 and 7.

Introduction
P2 nucleotide receptors include two families of receptors: Ligand-gated ion channels, designated P2X, and metabotropic “7TM” receptors, having seven transmembrane helical domains, designated P2Y [1]. Just as there are both ionotropic and metabotropic receptors that respond to acetylcholine, there are at least fifteen P2 receptors spanning both categories, that respond to extracellular nucleotides [2-4]. The process of cloning and identifying P2 receptors has been taking place over the last decade, and there are still a number of related orphan receptor sequences that may yet prove to be P2 receptors. The occurrence and physiological importance of these receptor systems are vast; nearly every cell type in the body expresses one or more P2 receptors, and they are involved in numerous physiological processes.
The medicinal chemistry at P2 nucleotide receptors has been relatively slow to develop due to a variety of limitations in the study of nucleotide pharmacology. Nucleotides are subject to metabolic processes, leading to both their degradation and production. This results in endogenous levels of receptor-active agents that are subject to wide fluctuations. A large family of ectonucleotidases [5] is associated with the production and degradation of P2 receptor agonists in vivo and consequently with the generation of the biologically-active metabolite adenosine, which can further confound interpretation of pharmacological data. In addition, the synthesis and purification of nucleotide analogues by classical chemical means can often be quite tedious, making extensive studies of structure activity relationships (SAR) difficult [6,7]. The pharmacological evaluation of newly synthesized nucleotide analogues is also complex [8], due to the lack of high affinity radioligands for nearly all of the P2 receptor subtypes, as well as uncertainty in the correspondence between pharmacological effects and genetically-defined sequences.
The putative endogenous ligands [1,3] thought to act under physiological conditions at P2Y receptors include not only the “classical” P2 agonists ATP 1 and ADP 2, but also the uracil nucleotides UTP 3, and UDP 4, dinucleotides (e.g. Ap4A 5), and sugar nucleotides (e.g. UDP-glucose 6 and UDP-galactose 7). Currently, the challenge is to design and synthesize not only selective, stable and bioavailable agonists, but also selective antagonists. The only known agonists are nucleotides, as no nonnucleotide, small molecule mimicks have yet been reported. The existing P2 antagonists also tend to be large and/or polyanionic molecules that display low affinity and selectivity, and are thus unsuitable as pharmaceutical lead molecules. Thus, defining a given receptor subtype pharmacologically using the existing ligand tools is usually a complex challenge. The aim of this review is to summarize major developments over recent years in the medicinal chemistry of P2 receptors, both in the identification of P2X and P2Y receptor agonists and antagonists and in understanding P2Y receptor structure.
P2X Receptor Ligands
There are seven subtypes of subunits of P2X ligand-gated ion channels [9], denoted P2X1 - P2X7, and each functional ion channel consists of oligomers, probably trimers. The current knowledge of the structure of P2X receptors, which is derived mainly from mutagenesis studies, is described in another chapter in this volume [10]. Both homo- and heterooligomerization seem to occur commonly, although the P2X7 subtype exists exclusively as a homomer and the P2X6 subtype seems never to occur as a homomer. The presence of heteromers complicates the interpretation of structure activity relationships, since each heteromeric complex would have its own pharmacological properties, some of which may be shared with either of the component subunits in homomeric form. The cationic selectivity of the P2X receptors is Na+, K+ > Ca2+ [11]. The distribution of the receptors is broad for many of the subtypes, and many cell types express multiple P2X subunits. The P2X3 subunit and its heteromers with the P2X2 subunit are of special interest in pain control, since they occur mainly on the pain pathways, thus selective antagonists may be useful for this envisioned application [12]. Other therapeutic interests related to P2X receptors are: inflammation (P2X7), neuroprotection (P2X7), bladder control (P2X1), cardiac function (P2X4), cancer (P2X4 and P2X7), and the central nervous system (P2X2,4,6) [6].
Agonists
The compound α,β-meATP 8 distinguishes Group I receptors, i.e. P2X1 and P2X3 receptors, which are activated by 8 (EC50 value 1 – 3 μM), from all other P2X subtypes, which are insensitive to this agonist [11]. Another distinguishing feature is the ability of some P2X receptor subtypes (mainly Group I) to desensitize rapidly. Agonist pre-activation of P2X1 receptors leading to desensitization, for example by 8, has been used pharmacologically in the absence of antagonists.
The nucleoside 5′-di- and 5′-triphosphate derivatives generally diverge in activity at P2X subtypes [11]. ADP 2 elicits agonist action at the P2X1 receptor but not at other P2X subtypes. None of the P2X receptors are activated by either AMP or adenosine, although 2-thioether substitution of the 5′-monophosphate may result in considerable activity at some P2X subtypes [13]. Both α,β–methylene, and β,γ–methylene modifications in 8 and 9a, respectively, as well as the ω-thiophosphate group present in ATP-γ-S 10a generally increase stability of the triphosphate group towards enzymatic hydrolysis while maintaining potency at P2X1 receptors [11]. The L-enantiomer of β,γ-methylene-ATP 9b activates rat P2X1 receptors with an EC50 value of 2 μM [11]. Compound 10a activates all P2X subtypes, except P2X7 receptors, in the concentration range of 3 – 16 μM. 2-Thioether substitution of the adenine moiety of ATP is known to enhance potency at various P2 receptor subtypes [13]. Thus, the p-aminophenylethylthio analogue (PAPET-ATP) 11 is the most potent agonist yet reported [6] of the rat P2X3 receptor (EC50 17 nM). The effects of ribose substitution in ATP analogues on potency at P2X receptors has not been fully explored. The derivative 2′-&3′-O-(4-benzoyl-benzoyl)-ATP (BzATP) 12, which is a mixture of ester species, is of nanomolar potency in activating the P2X1 receptor [14] and, although less potent at the P2X7 receptor, it is nevertheless the most potent reported activator of that subtype. Dinucleotides 13 of varying numbers of linked phosphate groups have been studied as agonists and partial agonists of P2X receptors [15]. Other nucleobases have been tested for activation of P2X receptors. Among the few non-adenine derivatives found to activate, 5′-CTP is an agonist of the rat P2X3 receptor with an EC50 value of 18 μM [11].
Antagonists
Many of the “classical” antagonists of P2X receptors are highly charged polycyclic compounds. The trypanocidal drug suramin 14 and its derivatives were found to be P2X antagonists, and suramin antagonism of P2 responses is readily reversible upon washout [11]. This antagonist action was unrelated to the clinical use of suramin. The potency order of 14 at P2X receptors is (IC50 in μM): P2X1, P2X5 (1-4) > P2X2, P2X3 (10-15) > P2X7 (78) > P2X4, P2X6 (>500). Lambrecht and coworkers have studied related, highly potent antagonists, selective for the P2X1 receptor, such as NF279 15 and NF449 16, which have roughly nanomolar IC50 values at the rat P2X1 receptor [16]. Newer and more potent receptor P2X receptor antagonists in this series include NF110 17, which shows some selectivity for the P2X3 receptor in the guinea pig ileum (IC50 = 1.7 μM) [17].
Some antagonists derived from dyes, such as Reactive-blue 2 18, antagonize both P2X and P2Y receptors [18]. Reactive-blue 2 potently inhibits P2X2 responses with an IC50 value of 0.36 μM [11]. Frahm and coworkers have introduced novel analogues of Reactive blue 2, including compounds such as 19 [18].
The SAR of antagonists derived from pyridoxal phosphate, which were also introduced by Lambrecht and coworkers, have been explored at P2X receptors [17]. The 2′,4′-disulfonate derivative PPADS 20 and its 2′,5′-disulfonate isomer “isoPPADS” 21 are somewhat more potent at P2X than at P2Y receptors. The pyridoxal phosphate derivative PPNDS 23 is a highly potent antagonist at the P2X1 receptor [17]. The phosphate linkage of PPADS analogues may be replaced with more stable phosphonates [19]. For example, the phosphonate analogue MRS 2257 22 is a highly potent antagonist at both rat P2X1 (IC50 5.1 nM) and P2X3 (IC50 21.8 nM) receptors (with the receptors expressed in the Xenopus oocyte). Analogues in the PPADS series in which the diazo linkage has been replaced with carbon bridges have been synthesized [20]. One such analogue, MRS 2335 24, was roughly equipotent to the corresponding diazo derivative at the P2X1 receptor. While high potency at P2X receptors has been achieved for PPADS derivatives, a disadvantage is the noncompetitive binding they display, accompanied by slow on- and off-rates [20].
Various nucleotide derivatives have also been found to antagonize P2X receptors. Such nucleotide antagonists are advantageous as they display more favorable binding kinetic properties than the above antagonists discussed previously. Faster on- and off-rates of binding faster than those of PPADS have been demonstrated for the antagonist TNP-ATP 25, which binds potently to P2X1, P2X3, and P2X2/3 (heteromeric) receptors [21]. A dinucleotide derivative, Ip5I 26, potently antagonizes the P2X1 receptor [22].
A novel antagonist A-317491 27, selective for P2X2/3 and P2X3 receptors, was reported by Jarvis and colleagues [23]. The compound 27, the S-enantiomer, contains three carboxylic acid groups, and is consequently of limited bioavailability. This compound reduces chronic inflammatory and neuropathic pain in the rat.
At the P2X4 receptor, none of the commonly used antagonists bind appreciably [11]. At P2X5 and P2X6 receptors, antagonists have not yet been identified; this due in part to the difficulty of expressing these receptors in Xenopus oocytes [11]. The P2X7 receptor occurs naturally in many mast cell cultures, and its prolonged activation induces the formation of a large pore which passes solutes up to MW ∼900. Calmidazolium 28, a calmodulin inhibitor, blocks some P2X7 receptor responses with an IC50 value of ∼10 nM [11]. P2X7 receptor antagonists have been identified among a variety of tyrosine isoquinoline derivatives [24], e.g. KN-62 29a, which was originally introduced as an inhibitor of CaM kinase II and thus is not receptor-selective. A closely related derivative, KN-04 29b, inhibits the human P2X7 receptor but is inactive at CaM kinase II. KN-62 antagonizes the human P2X7 receptor more potently than mouse or rat homologues. The tyrosine derivative MRS 2409 30a (IC50 of 200 nM at hP2X7) and its derivatives [25] act as potent antagonists of both mouse and human P2X7 receptors. A functionalized congener approach to the design of P2X7 receptor antagonists [25] has resulted in the amine-functionalized antagonist MRS 2483 30b. Baraldi and coworkers have explored the SAR in this series of P2X7 receptor antagonists, identifying 31 as a particularly potent congener with an IC50 value of 1.3 nM at hP2X7 receptors [26].
A family of adamantane derivatives including 32 have been shown to be antagonists of the P2X7 receptor [27,28]. Piperidine and piperazine derivatives such as 33 and 34 have been proposed as non-nucleotide P2X7 receptor antagonists [29,30].
P2Y Receptor Ligands
P2Y receptors are 7TM receptors, which typically couple preferentially, via Gq, to phospholipase C, and lead to a rise in intracellular calcium [31]. Subclasses of P2Y receptors have been defined based on: clustering of sequence (although, in general there exists a low homology among subtypes), ligand preference, secondary messengers, and receptor sequence analysis. The numbering system for P2Y subtypes is currently discontinuous; P2Y1 - P2Y14 receptors have been defined [3], however six of the intermediate numbered cloned receptor sequences (P2Y3,5,7-10) do not correspond to distinct, mammalian subtypes of functional nucleotide receptors. A putative dinucleotide receptor [32] has not yet been cloned and in fact may not represent a molecularly distinct species. The distribution of P2Y receptors is broad, and the therapeutic interests include antithrombotic therapy, modulation of the immune system, diabetes, and treatment of cystic fibrosis and other pulmonary diseases.
P2Y12, P2Y13, and P2Y14 receptors couple via Gi proteins and belong to a structurally distinct cluster of P2Y receptor sequences [3,31]. Stimulation of P2Y12 receptors, cloned in 2002 [33], is an important pro-aggregatory signal in platelets. The most recently cloned receptor, P2Y14, responds to UDP-glucose 6 and has a sequence more similar to the P2Y12 and P2Y13 receptors [34] than to the other P2Y subtypes [3]. The P2Y14 receptor is also activated by UDP-galactose 7. Some recently reported orphan receptors, such as GPR87 and GPR99 [35], have been noted to contain ligand recognition elements previously identified [36] for P2Y1 receptors.
Adenine Nucleotide-Preferring P2Y Receptors: P2Y1, P2Y11, P2Y12, and P2Y13
Agonists
P2Y1 and P2Y13 receptors are more potently activated by ADP 2 than by ATP 1, while at P2Y11 receptors, ATP is preferred [31,34,37]. At P2Y12 receptors, adenosine 5′-diphosphate derivatives activate and 5′-triphosphate derivatives antagonize. Terminal thiophosphate groups appear in many useful P2Y nucleotide agonists. For example, at P2Y11 receptors, which occur primarily on hematopoietic cells, ATP-γ-S 10a is a more potent agonist than ATP [31]. The corresponding diphosphate, ADP-β-S 10b is a potent agonist of the P2Y1 receptor (EC50 96 nM), although it also activates the P2Y12 subtype (EC50 82 nM) [6]. The 2-position of the adenine ring has also proven to be amenable to derivatization for P2Y agonists. Most notably, the 2-alkylthio ethers [38] appear to provide high potency at the P2Y1 receptor, which are present on platelets and on vascular endothelial cells. The 2-methylthio derivative of ADP 35 is a potent agonist (EC50 in nM) at P2Y1 (6), P2Y12 (1), and P2Y13 (1) receptors [6,34]. It is weak or inactive at P2Y2,4,6,11 receptors. The corresponding triphosphate, 2-MeS-ATP 36, is a less potent and selective P2Y receptor agonist, since it also activates P2X receptors [11]. At P2Y13 receptors, under optimal experimental conditions, ATP 1 and 2-MeSATP 36 are equipotent. The potency order of 36 at rat P2X receptors expressed in Xenopus oocytes is (EC50 in μM): P2X3, P2X1 (0.2-0.4) > P2X2, P2X6 (7-9) > P2X5 (20) > P2X4 (74) > P2X7 (300).
A number of adenosine 5′-monophosphate analogues, such as HT-AMP 37 (EC50 59 nM at the turkey P2Y1 receptor) have been found to potently activate P2Y1 receptors [39]. The α-thio modification of AMP analogues (e.g. 38), increases potency at the P2Y1 receptor [40]. Such monophosphate derivatives have also been reported to inhibit ectonucleotidases, which complicates their use as P2Y receptor agonists. 5′-(1-Boranotriphosphate) derivatives such as the 2-methylthio derivative 39 have been found to potently activate the P2Y1 receptor [41]. Two isomers of the boranophosphates were isolated. The more potent isomer of 39 displayed an EC50 value of 2.6 nM at the rat P2Y1 receptor.
Non-glycosidic substitution of the ribose moiety has also met with success in the design of ligands for P2Y1 and other P2Y receptors. For example, a dehydroanhydrohexitol (six-membered ring) analogue 40 activates P2Y1 receptors with an EC50 value of 3.0 μM [42]. Carbocyclic and constrained carbocyclic rings have also been substituted for the ribose moiety. Some of the more useful examples of this approach are methanocarba analogues [42,43], which have conformationally constrained bicyclic carbon rings in place of the ribose moiety that may adopt one of two conformations ((N) =Northern, or (S) =Southern) depending on position of fusion of the two rings. Correlation of ring geometry with the biological activities has helped define the conformational requirements of the ribose moiety in receptor binding. The rigid methanocarba ring systems are present in the P2Y1 receptor agonists the (N)-isomer MRS 2340 41 and its less potent (S)-isomer MRS 2312 42, which have EC50 values at the human P2Y1 receptor of 52 nM and 7.2 μM, respectively [43]. The binding site of the P2Y1 receptor displays a preference for the (N) conformation of the ribose-like ring over the corresponding Southern conformation as represented in an isomeric (S)-methanocarba series of ATP derivatives. The enhancement of P2Y1 agonist potency upon freezing the preferred conformation in a pseudoribose ring may approach 300-fold. An example of such an enhancement is (N)-methanocarba-β,γ–methylene-ATP 43, which is a full agonist with an EC50 value at the human P2Y1 receptor of 158 nM, while the corresponding riboside 9a is a weak partial agonist [44]. MRS 2365 44 is the most potent known agonist of the P2Y1 receptor, with EC50 values at the human P2Y1 and P2Y2 receptors of 0.4 nM and ∼10 μM, respectively.
Antagonists
The introduction of potent nucleotide-derived antagonists of the P2Y1 receptor was made possible by the observation that naturally occurring adenosine bisphosphate derivatives such as A3P5P 45 are either partial agonists or antagonists of the receptor [45]. A later generation derivative, MRS 2179 46, the corresponding 2-chloro analogue MRS 2216 47, and its (N)-methanocarbo equivalent MRS 2279 48a were demonstrated to be high affinity competitive and selective antagonists at this subtype [42,46,47]. [33P]MRS 2179 and [3H]MRS 2279 have been introduced as radioligands for the P2Y1 receptor in platelets and in other tissue [47,48]. Weak antagonism by 46 of the rat P2X1 receptor expressed in Xenopus oocytes was observed, however many of the known P2 receptor antagonists is magnified the potency of in this assay. Thus, for most applications, 46 and its congeners are highly selective for the P2Y1 receptor, with inactivity demonstrated at P2Y2,4,6,11,12,13 and P2X2,3,4,7 receptors [34,46]. Unlike 46, MRS 2216 47 was inactive at Group 1 P2X receptors [13]. Recently, a highly potent P2Y1 receptor antagonist in the (N)-methanocarba series, MRS 2500 48b, was reported (Ki = 0.79 nM at hP2Y1). A dipeptide conjugate of adenosine 49a was found to antagonize hP2Y1 receptor responses with a KB value of 4.0 μM [49]. Metabolites of hypolipidemic drugs, such as the coenzyme A conjugate of nafenopin 49b, were found to act as P2Y1 receptor antagonists [55]. 49b displayed a KB value of 58 nM at the human P2Y1 receptor. Raboission et al. have synthesized a C-nucleotide 50 that antagonized P2Y1 receptors [50].
In addition to the approach of rigidifying the ribose moiety in a conformation that approximates the conformation preferred in receptor binding, the opposite approach – using a flexible ribose equivalent – has met with some success. Acyclic nucleotide analogues of bisphosphate antagonists, such as MRS 2298 51, were found to be moderately potent P2Y1 receptor antagonists without residual agonism [51]. A related analogue containing the metabolically-stable phosphonate linkage, MRS 2496 52, displayed an IC50 at the rat platelet P2Y1 receptor of 0.68 μM.
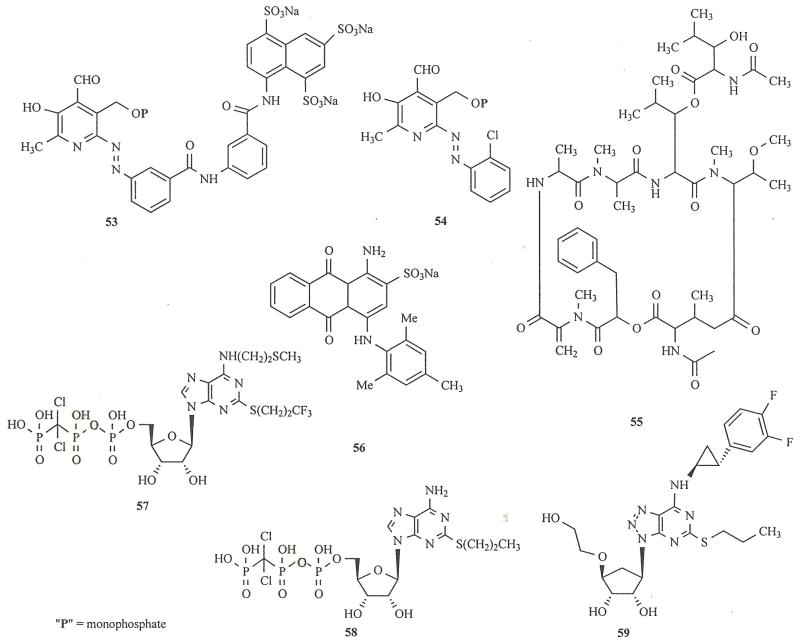
A hybrid molecule SB9 53, consisting of PPADS-like and suramin-like components was found to potently antagonize P2Y1 receptors [53]. Certain PPADS analogues, such as MRS 2210 54, display selectivity for P2Y1 vs. P2X subtypes [54]. Recently, a cyclic depsipeptide YM-254890 55, a fermentation product of a Chromobacterium isolated from soil, was found to potently antagonize P2Y1 receptors [64]. 55 inhibited the aggregation of platelets with an IC50 of 31 nM and was inactive at P2Y12 receptors. Derivatives of Acid blue 129, such as 56, were also found to act as P2Y1 receptor antagonists [56].
Nucleotide antagonists of high affinity for the platelet P2Y12 receptor were reported, including AR-C69931MX 57 [57], which was in clinical trials as an antithrombotic agent. A nucleotide in this series (AR-C67085MX 58) has been shown to potently activate the P2Y12 receptor [58]. In this series it has been possible to substitute the unwieldy triphosphate group in this series with uncharged moieties such as short alcohols, esters, etc., thus proving that a highly anionic moiety is not needed for recognition by the P2Y12 receptor. This discovery led to compounds such as AZD6140 59, which is an orally active P2Y12 receptor antagonist of nM affinity that inhibits platelet aggregation up to 8 h after administration [59]. The presence of the 3,4-difluorophenyl group limits the metabolism of 59.
The action of the successful antithrombotic drug clopidogrel 60 is dependent on the P2Y12 receptor present on platelets. 60 produces a metabolite 61, which acts as an irreversible P2Y12 receptor antagonist [60]. Other nonnucleotide, nonhighly charged P2Y12 antagonists, such as CS-747 62, which also acts through a metabolite, and the sulfonamide analog CT50547 63 have been reported [61,62].
The acyclic template used in MRS 2298 51 has been adapted to antagonists of the P2Y12 receptor [52]. Upon replacement of the two phosphate groups with hydrophobic esters, such as in the dipivaloate MRS 2395 64, the selectivity shifted entirely from the P2Y1 receptor to the P2Y12 receptor. MRS 2395 displayed an IC50 of 3.6 μM in the inhibition of ADP-induced aggregation of rat platelets. Since both receptor subtypes are integral to ADP-induced platelet aggregation, the inhibition of either P2Y1 or P2Y12 receptors impedes platelet aggregation. Analogues of ADP having neutral, hydrophobic substitutions at the ribose 2′-and 3′-hydroxyl groups and the adenine NH2 position were found to antagonize the P2Y2 receptor. One such analogue is INS 49266 65, which displayed a KB of 361 nM in the inhibition of platelet aggregation [63]. The agonist potencies of 65 at P2Y1 and P2Y2 receptors were >10 and 14 μM, respectively.
Selective antagonists of the P2Y11 or P2Y13 receptors are still unknown. The P2Y12 antagonist AR-C67085MX 58 acts as a potent agonist at the P2Y11 receptor. The following compounds were found to antagonize action at the P2Y13 receptor (IC50 in μM): suramin 14 (2.3), PPADS 20 (11.7), Ap4A 5 (0.216), and AR-C69931MX 57 (0.004) [34].
Uracil Nucleotide-Recognizing P2Y Receptors: P2Y2, P2Y4, P2Y6, and P2Y14
Agonists
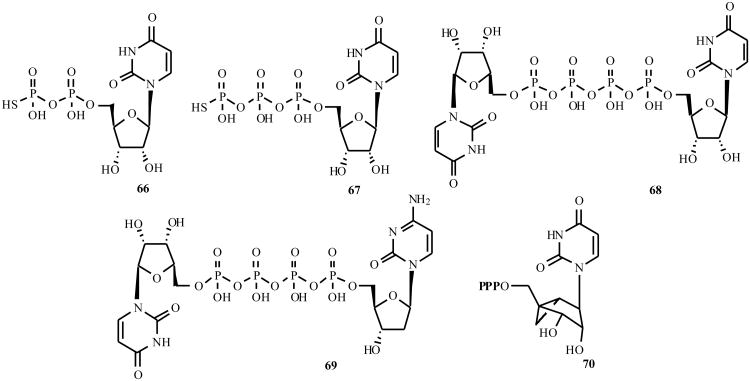
The P2Y2 receptor is activated nearly equipotently by UTP and ATP, but is not activated by the corresponding 5′-diphosphates, i.e. UDP and ADP. The P2Y4, P2Y6, and P2Y14 receptor are primarily activated by uracil nucleotides. The P2Y6 receptor is activated by uridine 5′-di- but not 5′-triphosphates, while the human P2Y4 receptor is activated by uridine 5′-triphosphates and inhibited by adenosine 5′-triphosphates [4,65]. Uridine β-thiodiphosphate (UDP-β-S) 66 and the γ-thiophosphate (UTP-γ-S) 67 are selective agonists for P2Y6 and P2Y2/P2Y4 receptors, respectively [66]. Numerous substitutions of the uracil ring of UTP have been reported to reduce potency at the P2Y2 receptor [67]. The 5-bromo modification of UDP preserves potency at the P2Y6 receptor [8]. The adenine dinucleotide Ap4A 5 is a potent agonist at the rat P2Y4 receptor and is less potent than ATP at P2Y2 receptors [8]. Some uracil dinucleotides, such as INS 365 68, potently activate the P2Y2 receptor. Newer generation P2Y2 receptor agonists such as INS 37217 69 have been reported [68,69]. P2Y2 receptor agonists are of clinical interest for the treatment of pulmonary and ophthalmic diseases, and possibly cancer. Ribose substitution with the (N)-methanocarba ring system has been shown to preserve the potency of both adenine and uracil nucleotides at the P2Y2 receptor, and UTP (e.g. MRS2341 70) at the P2Y4 receptor [43]. However, inclusion of the same (N)-methanocarba ring system in the corresponding 5′-diphosphate prevented activation of the P2Y6 receptor.
Antagonists
Suramin 14 is a weak antagonist at the P2Y2 receptor with an IC50 value of 48 μM [4]. A family of selective, heterocyclic antagonists of the P2Y2 receptor based on thiouracil, including AR-C126313 71a and AR-C118925 71b, has been reported [70,71]. Reactive blue-2 18 at a concentration of 100 μM effectively blocks rat P2Y4 receptors, but only partially blocks human P2Y4 receptors. ATP antagonizes the human but not rat P2Y4 receptor [69]. The isothiocyanate DIDS 72 has been shown to weakly antagonize the P2Y6 receptor, although it is not selective since it also acts at P2X receptors [72].
Flavonoids have been identified as a new lead for the design of P2Y2 receptor antagonists [73]. Tangeretin 73, is a potent, noncompetitive, antagonist with an IC50 value of 12 μM. Antagonists of the P2Y14 receptor are still unknown.
P2Y Receptor Structure
The structures of P2X receptors [10] are not yet amenable to molecular modeling due to the lack of a protein template. However, P2Y receptors have been successfully modeled using the high resolution structure of bovine rhodopsin as a template [36,51,74-77] by means of the homology modeling technique. Our models descend from a multiple sequence alignment based on a combined manual and automatic approach which take into account not only the primary structure of the proteins but also the 3D information deducible from the secondary and tertiary structure of the template.
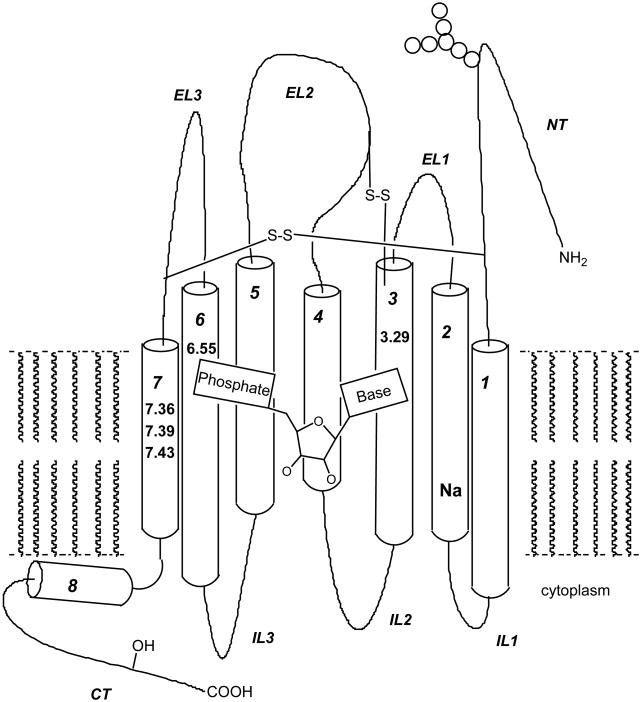
As all other GPCRs, P2Y receptors are constituted by a single polypeptide chain, which crosses the cell membrane seven times, forming seven TMs connected by three extracellular (ELs) and three intracellular loops (ILs). The amino terminal region (NT) is located outside the cell, while the carboxyl terminal region (CT) is located in the cytoplasm (Figure 1). The great majority of the TM residues are arranged in α-helical structures, while in EL2 an antiparallel β-sheet is present. Especially in the regions with defined secondary structure there is a great overall similarity between all P2Y models and the template, the only significant deviation being present in very flexible NT, EL1, El3, IL1, IL2 and IL3. As in the crystal structure of rhodopsin the alpha-helical structure of TM3, TM4 and TM6 extends into the cytoplasm for all the P2Y subtypes. At the cytoplasmic end of TM7 in all subtypes, with the exception of P2Y2 and P2Y11, the protein folds at an angle of ∼90° to form a helical segment (H8) that runs parallel to the plane of the cell membrane.
Fig. (1).
Schematic structure of showing general features of P2Y receptors, including seven TMs (indicated with Arabic numerals) and a proposed internal helix 8. The putative nucleotide binding site, based on mutagensis and molecular modeling, experiments is indicated. Key residues found to interact with the ligand in the human P2Y1 and P2Y2 receptors are indicated on the helix by identifiers of the form X.YZ, where X is the helix number and YZ is the amino acid residue numbered in relation to the residue of each helix, which is most highly conserved among Group 1 GPCRs [78]. A table at the bottom gives the identity of these key residues within the family of P2Y receptors.
Ligand affinity SAR, sequence analysis of cloned P2Y receptors and site-directed mutagenesis studies [54,74,77,78] have led to the refinement of computer-based models for ligand binding to P2Y receptors. Structural insights have been gained using molecular modeling based on a rhodopsin template in conjunction with mutagenesis to suggest recognition elements important for nucleotide binding in TMs 3, 5, 6, and 7 and in EL2 [75].
Most of the mutagenesis work in the P2Y family has been carried out on the human P2Y1 receptor. In order to ascertain which residues of this receptor were involved in ligand recognition, individual residues of both the TMs (3, 5, 6, and 7), as well as EL 2 and 3 were mutated to alanine and various charged residues [36,77], a cluster of positively charged lysine and arginine residues near the exofacial side of TMs 3 and 7 and, to a lesser extent TM6, putatively coordinated the phosphate moieties of nucleotide agonists and antagonists. Agonists were inactive at R128A (TM3) and R310A and S314A (TM7) mutant receptors and had a markedly reduced potency at K280A (TM6) and Q307A (TM7) mutant receptors. Positively charged residues of the human P2Y2 receptor (H262, R265, and R292 in TM6 and TM7) were similarly found to be critical for activation [76] suggesting that residues on the exofacial side of TM3 and TM7 were critical determinants of the ATP binding pocket. In contrast, there was no change in the potency or efficacy of agonists in the S317A mutant receptor, and alanine replacement of F131, H132, Y136, F226, or H277 resulted in mutant receptors that exhibited a 7- to 18-fold reduction in potency compared to that observed with the wild type receptor. These residues thus appear to serve a less important modulatory role in ligand binding to the P2Y1 receptor.
Several charged residues in EL2 (E209) and EL3 (R287) were critical for receptor activation, suggesting that the role of the ELs in ligand recognition was as important as that of the TMs [36,77]. Moreover, energetically favorable “meta-binding sites” in the P2Y1 receptor have been defined, involving the critical residues of the ELs. At these docking sites for nucleotides that are postulated to lie distal to the principal TM site, a ligand may bind en route to the principal TM binding site. Such secondary binding sites may then serve to guide the ligand in its approach to the TM binding site and reduce the energy barrier to ligand/receptor complex formation.
Two essential disulfide bridges in the extracellular domains of the human P2Y1 receptor were also identified: one conserved among GPCRs and another, between the N-terminal domain and EL3, characteristic of P2Y receptors.
Since changes in the potency of 2MeSADP 35 and HT-AMP 37 paralleled the changes in potency of 2MeSATP 36 at the various mutant receptors, it appeared that the β- and γ-phosphates of the adenine nucleotides were less important than the α-phosphate in ligand/P2Y1 receptor interactions [75]. However, T221A and T222A mutant receptors exhibited much larger reductions in triphosphate (89- and 33-fold versus wild type receptors, respectively) versus dior mono-phosphate potency, a result indicating a greater role of these TM5 residues in γ-phosphate recognition.
Taken together, the results suggest that the adenosine and α-phosphate moieties of ATP bind to the P2Y1 receptor in the exofacial side of the cavity delimited by TM3, TM6 and TM7 and capped with EL2.
Recently, a detailed model of antagonist binding to the P2Y1 receptor binding was presented [75]. Ligand docking in the P2Y1 receptor model provided a hypothesis for the coordination of nucleotide antagonists in the TM regions, consistent with site-directed mutagenesis results, with a binding mode very similar to the one of the agonist. Molecular recognition in the P2Y1 receptor of nonnucleotide antagonists such as derivatives of PPADS 20, has also been studied [54]. The structural similarity between the potent nucleotide antagonist, MRS 2179 46, and nucleotide agonists suggests that receptor activation resulting in a specific conformational change depends on subtle differences between ligands [78].
Chart 1.
Chart 2.
Chart 3.
Chart 4.
Chart 5.
Chart 6.
Chart 7.
Chart 8.
Chart 9.
Chart 10.
Chart 11.
References
- 1.Burnstock G. Introduction: P2 Receptors. doi: 10.2174/1568026043451014. This volume. [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. The UDP-glucose receptor renamed the P2Y14 receptor. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller C. P2-Pyrimidinergic receptors and their ligands. Curr Pharm Des. 2002;8:2353–69. doi: 10.2174/1381612023392937. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann H, Braun N. Ecto-nucleotidases—molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res. 1999;120:371–385. [PubMed] [Google Scholar]
- 6.Jacobson KA, Jarvis MF, Williams M. Perspective: Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht G. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362:340–50. doi: 10.1007/s002100000312. [DOI] [PubMed] [Google Scholar]
- 8.Von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362:310–23. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 9.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 10.Egan TM, Cox JA, Voigt MM. Molecular structure of P2X receptors. doi: 10.2174/1568026043451005. This volume. [DOI] [PubMed] [Google Scholar]
- 11.King BF. Molecular biology of P2X purinoreceptors. In: Burnstock G, Dobson JG Jr, Liang BT, Linden J, editors. Cardiovascular Biology of Purines. Ch.10. Kluwer Academic Publishers; Massachusetts: 1998. pp. 159–186. [Google Scholar]
- 12.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2003 doi: 10.1113/jphysiol.2003.048587. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown SG, King BF, Kim YC, Burnstock G, Jacobson KA. Drug Dev Res. 2000;49:253–259. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 15.Cinkilic O, King BF, van der Giet M, Schluter H, Zidek W, Burnstock G. Selective agonism of group I P2X receptors by dinucleotides dependent on a single adenine moiety. J Pharmacol Exp Ther. 2001;299:131–136. [PubMed] [Google Scholar]
- 16.Braun K, Rettinger J, Ganso, Kassack MM, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. A subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn Schmiedeberg's Arch Pharmacol. 2001;34:285–290. doi: 10.1007/s002100100463. [DOI] [PubMed] [Google Scholar]
- 17.Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure-activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8:2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- 18.Glänzel M, Bültmann R, Starke K, Frahm AW. Proceedings, XVIth International Symposium on Medicinal Chemistry; Bologna, Italy. 2000. [Google Scholar]
- 19.Kim YC, Brown SG, Harden TK, Boyer JL, Dubyak G, King BF, Burnstock G, Jacobson KA. Structure-activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J Med Chem. 2001;44:340–349. doi: 10.1021/jm9904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown SG, Kim YC, Kim SA, Jacobson KA, Burnstock G, King BF. Actions of a series of PPADS analogs at P2X1 and P2X3 receptors. Drug Devel Res. 2001;53:281–291. doi: 10.1002/ddr.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spelta V, Jiang LH, Surprenant A, North RA. Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol. 2002;135:1524–1530. doi: 10.1038/sj.bjp.0704591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol. 1999;128:981–988. doi: 10.1038/sj.bjp.0702876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, A novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as Antagonists of the P2X7 nucleotide receptor: High selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Ravi RG, Kertesy SB, Dubyak GR, Jacobson KA. Functionalized congeners of tyrosine-based P2X7 receptor antagonists: Probing multiple sites for linking and dimerization. Bioconj Chem. 2002;13:1100–1111. doi: 10.1021/bc020025i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcaraz L, Furber M, Mortimore M. WO0061569. Adamantane derivatives. 2000
- 27.Alcaraz L, Furber M, Luker T, Mortimore M, Thorne P. WO0142194. Adamantane derivatives. 2001
- 28.Alcaraz L, Furber M. WO0194338. Adamantane derivatives. 2001
- 29.Baxter A, Kindon N, Pairaudeau G, Roberts B, Thom S. WO0144213. P2x7 receptor antagonists for use in the treatment of inflammatory, immune or cardiovascular diseases. 2001
- 30.Meghani P, Bennion C. WO0146200. Novel piperidine and piperazine derivatives. 2001
- 31.Boeynaems Jean-Marie, Wilkin F, Marteau F, Duhant X, Savi P, Gonzalez NS, Robaye B, Communi D. P2Y Receptors: New subtypes, new functions. Drug Devel Res. 2003;59:30–35. [Google Scholar]
- 32.Diaz-Hernandez M, Pintor J, Miras-Portugal MT. Modulation of the dinucleotide receptor present in rat midbrain synaptosomes by adenosine and ATP. Br J Pharmacol. 2000;130:434–440. doi: 10.1038/sj.bjp.0703300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 34.Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 35.Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O'Dowd BF. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene. 2001;275:83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- 36.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: Molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer RK, Boyer JL, Schacter JB, Nicolas RA, Harden TK. Discovery and mapping of ten novel G protein-coupled receptor genes. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- 38.Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5′-triphosphate. J Med Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyer JL, Siddiqi S, Fischer B, Romera-Avila T, Jacobson KA, Harden TK. Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Brit J Pharmacol. 1996;118:1959–1964. doi: 10.1111/j.1476-5381.1996.tb15630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer B, Chulkin A, Boyer JL, Harden TK, Gendron FP, Beaudoin AR, Chapal J, Hillaire-Buys D, Petit P. 2-Thioether 5′-O-(1-thiotriphosphate)adenosine derivatives as new insulin secretagogues acting through P2Y-receptors. J Med Chem. 1999;42:3636–3646. doi: 10.1021/jm990158y. [DOI] [PubMed] [Google Scholar]
- 41.Nahum V, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y(1) receptor agonists. J Med Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- 42.Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. JMed Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: High potency of northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1-receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 46.Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldo GL, Corbitt J, Boyer JL, Ravi RG, Kim HS, Ji Xd, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 49.Sak K, Uri A, Enkvist E, Raidaru G, Subbi J, Kelve M, Jarv J. Adenosine-derived non-phosphate antagonists for P2Y(1) purinoceptors. Biochem Biophys Res Commun. 2000;272:327–31. doi: 10.1006/bbrc.2000.2775. [DOI] [PubMed] [Google Scholar]
- 50.Raboisson P, Baurand A, Cazenave JP, Gachet C, Schultz D, Spiess B, Bourguignon JJ. A general approach toward the synthesis of C-nucleoside pyrazolo[1,5-a]-1,3,5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y(1)-receptor antagonists. J Org Chem. 2002;67:8063–8071. doi: 10.1021/jo026268l. [DOI] [PubMed] [Google Scholar]
- 51.Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: Structure activity relationships and receptor docking. J Med Chem. 2001;44:3092–3108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, Kim HS, Bischofberger N, Cook G, Jacobson KA. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: Phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambrecht G, Ganso M, Baumert HG, Spatz-Kumbel G, Hildebrandt C, Braun K, Mutschler E. The novel heteromeric bivalent ligand SB9 potently antagonizes P2Y1 receptor-mediated responses. J Auton Nerv Syst. 2000;81:171–177. doi: 10.1016/s0165-1838(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 54.Guo D, Von Kügelegen I, Moro S, Kim YC, Jacobson KA. Evidence for the recognition of non-nucleotide antagonists within the transmembrane domains of the human P2Y1 receptor. Drug Devel Res. 2002;57:173–181. doi: 10.1002/ddr.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coddou C, Loyola G, Boyer JL, Bronfman M, Huidobro-Toro JP. The hypolipidemic drug metabolites nafenopin-CoA and ciprofibroyl-CoA are competitive P2Y1 receptor antagonists. FEBS Lett. 2003;536:145–150. doi: 10.1016/s0014-5793(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 56.Glänzel M, Bültmann R, Starke K, Frahm AW. Members of the acid blue 129 family as potent and selective P2Y-receptor antagonists. Drug Devel Res. 2003;59:64–71. [Google Scholar]
- 57.Ingall AH, Dixon J, Bailey A, Coombs ME, McInally JI, Hunt SF, Kindon ND, Theobald BJ, Willis PA, Humphries R, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: A novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 58.Bonnert RV, Ingall AH, Springthorpe B, Willis PA. WO9828300. Triazolo[4,5-D]pyrimidinyl derivatives and their use as medicaments. 1998
- 59.Springthorpe B. From ATP to AZD6140: Design of an orally active P2Y12 (P2T) receptor antagonist for the treatment of thrombosis. 225th ACS national meeting, Abstracts, Division of Medicinal Chemistry. 2003:16. [Google Scholar]
- 60.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of Clopidogrel. Thromb Haemost. 2002;84:891–896. [PubMed] [Google Scholar]
- 61.Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type Gi-linked P2T antagonist, CS-747. Br J Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scarborough RM, Laibelman AM, Clizbe LA, Fretto LJ, Conley PB, Reynolds EE, Sedlock DM, Jantzen HM. Novel tricyclic benzothiazolo[2,3-c]thiadiazine antagonists of the platelet ADP receptor (P2Y12) Bioorg Med Chem Lett. 2001;11:1805–1808. doi: 10.1016/s0960-894x(01)00313-4. [DOI] [PubMed] [Google Scholar]
- 63.Douglass J, Patel RI, Redick C, Brubaker K, Jones AC, Shaver SR, Yerxa B, Baurand A, Gachet C, Boyer JL. Ribose and nucleobase modifications to nucleotides that confer antagonist properties against the P2Y12 platelet receptor. Haematologica. 2002;87S1:22. [Google Scholar]
- 64.Taniguchi M, Nagai K, Arao N, Kawasaki T, Saito T, Moritani Y, Takasaki J, Hayashi K, Fujita S, Suzuki KI, Tsukamoto SI. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J Antibiot. 2003;56:358–363. doi: 10.7164/antibiotics.56.358. [DOI] [PubMed] [Google Scholar]
- 65.Harden TK. The G-protein-coupled P2Y receptors. In: Burnstock G, Dobson JG Jr, Liang BT, Linden J, editors. Cardiovascular Biology of Purines. Ch.11. Kluwer Academic Publishers; Massachusetts: 1998. pp. 187–205. [Google Scholar]
- 66.Malmsjö M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPß;S and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pendergast W, Yerxa BR, Douglass JG, III, Shaver SR, Dougherty RW, Redick CC, Sims IF, Rideout J. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg Med Chem Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- 68.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC. Pharmacology of INS37217[P(1)-(uridine 5′)-P(4)-(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- 70.Meghani P. 11th RSC-SCI Medicinal Chemistry Symposium; Sept. 9-12, 2001; Cambridge, UK. [Google Scholar]
- 71.Meghani P. The design of P2Y2 antagonists for the treatment of inflammatory diseases. 224th ACS National meeting, Abstracts, Division of Medicinal Chemistry. 2002:12. [Google Scholar]
- 72.Von Kügelgen I, Jacobson KA. Abstr German Pharmacol Soc. Mainz, Germany: 2001. Blockade of P2Y6 receptors by diisothiocyanatostilbene-disulphonic acid (DIDS) and analogues. [Google Scholar]
- 73.Kaulich M, Streicher F, Mayer R, Müller I, Müller CE. Flavonoids - novel lead compounds for the development of P2Y2 receptor antagonists. Drug Devel Res. 2003;59:72–81. [Google Scholar]
- 74.Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA. Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J Biol Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- 75.Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: A molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38:3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costanzi S, Jacobson KA. ACS National Meeting. New York, NY: Sep 9, 2003. Structural comparison of P2Y receptors based on homology modeling. Abstract MEDI157. In press. [Google Scholar]
- 77.Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274:14639–14647. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang Q, Guo D, Lee BX, van Rhee AM, Kim YC, Nicholas RA, Schachter J, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]