Abstract
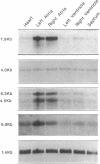
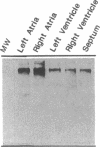
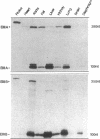
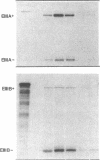
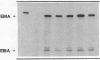
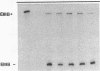
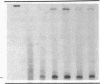
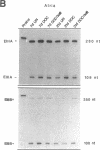
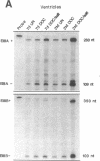
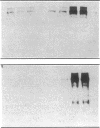
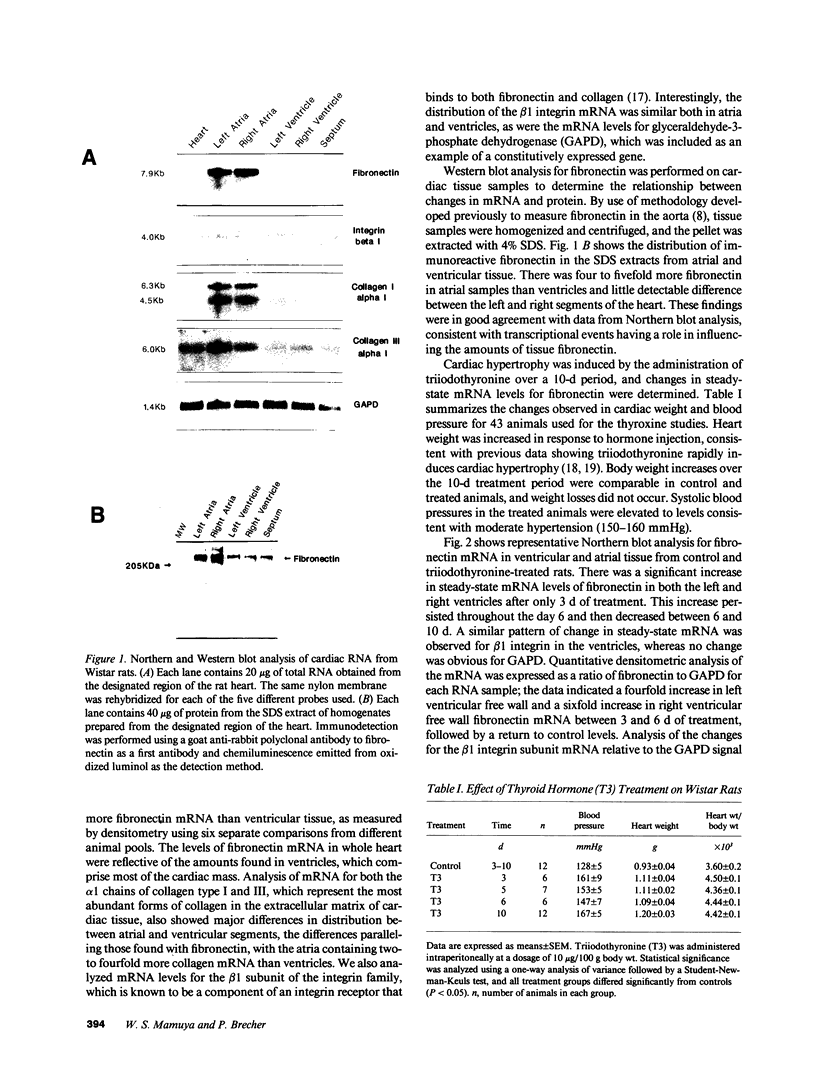
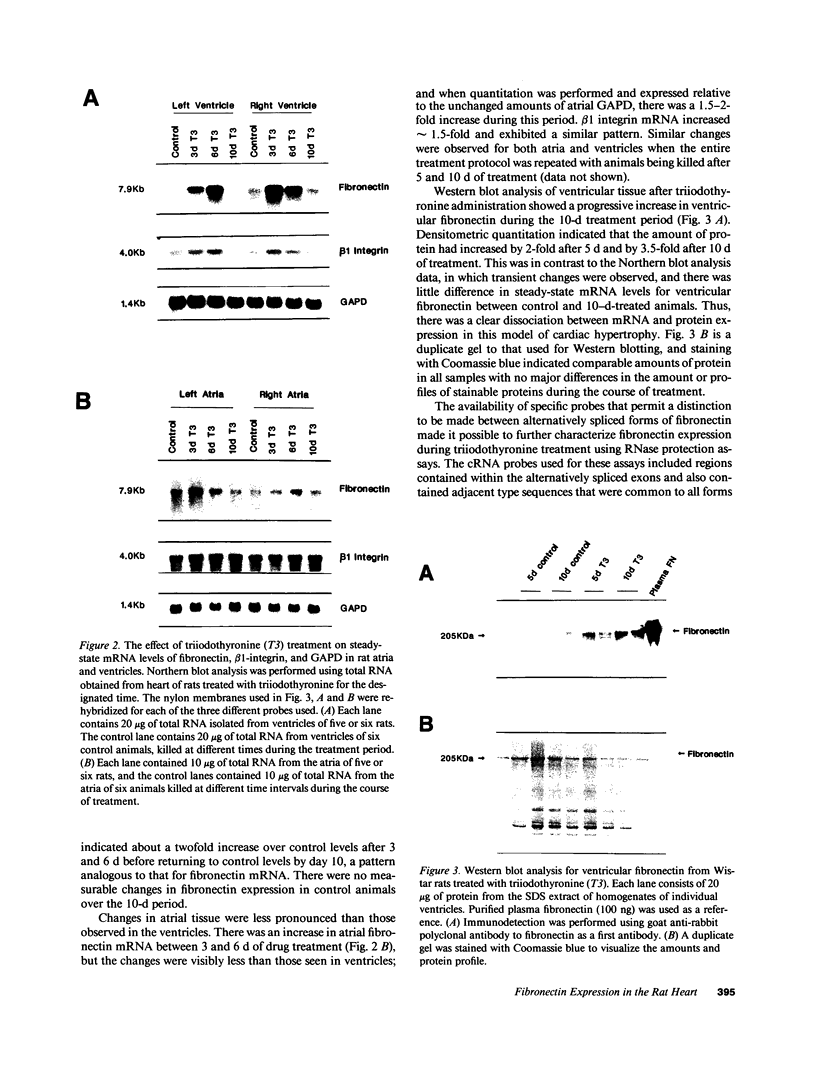
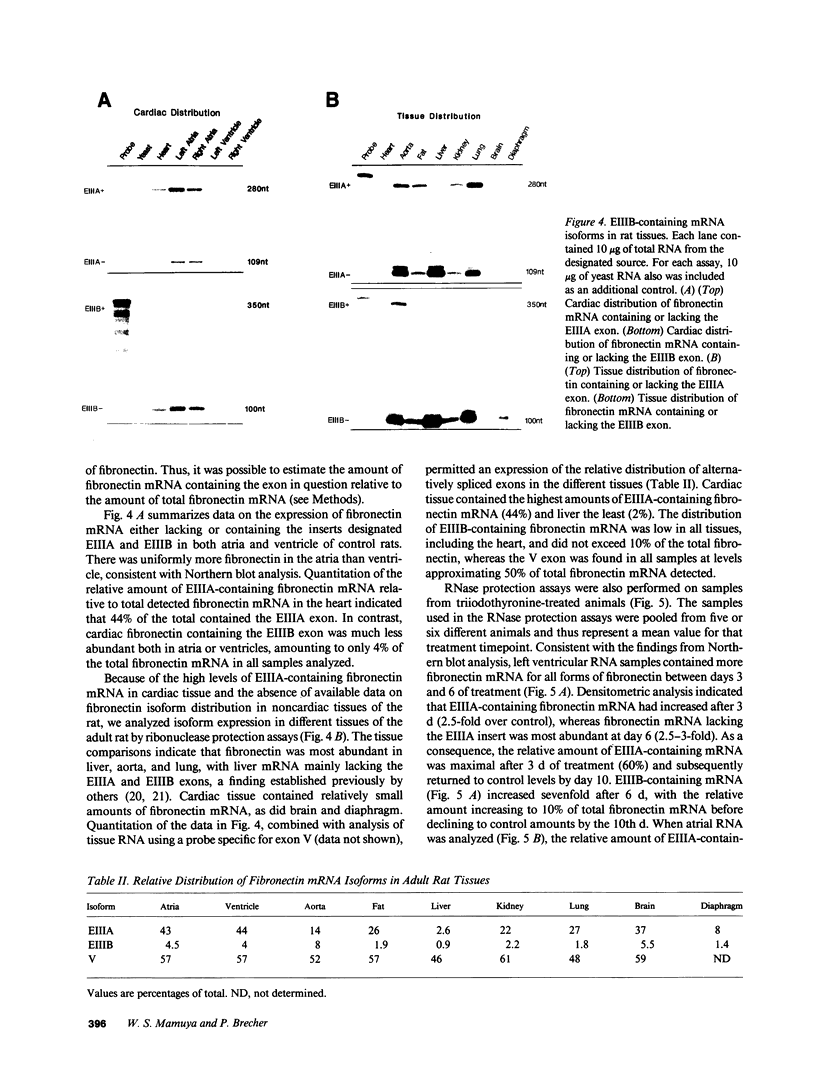
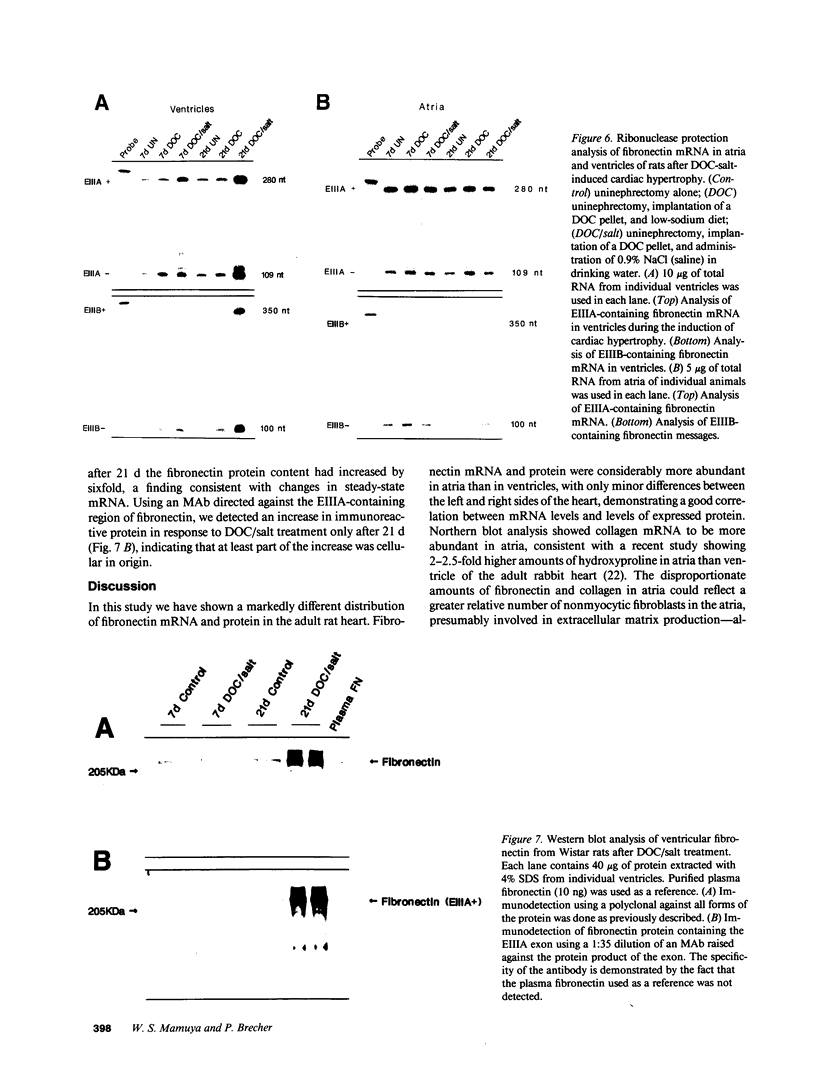
We examined changes in the expression of fibronectin during the induction of cardiac hypertrophy by L-triiodothyronine administration and by mineralocorticoid- and salt-induced experimental hypertension. By use of Northern and Western blotting procedures, fibronectin was localized mainly in the atria of normal rat hearts. Atria contained 10- and 5-fold higher relative concentrations of fibronectin mRNA and protein, respectively, compared with ventricles. During the progression of cardiac hypertrophy induced by L-triiodothyronine over a 10-d period, there was a progressive increase in fibronectin mRNA for the first 6 d followed by a return to control levels. The major change could be accounted for by changes in ventricular mRNA, which increased about four- to sixfold. In contrast, protein levels in ventricles increased progressively over the 10-d treatment period. Ribonuclease protection analysis indicated that the relative amounts of fibronectin isoforms containing exons designated EIIIA and EIIIB increased during the progression of hypertrophy. When cardiac hypertrophy was induced by mineralocorticoid and salt treatment, increases in ventricular fibronectin mRNA and protein and the induction of alternatively spliced forms of fibronectin were also observed. However, the extent and temporal pattern of fibronectin expression differed between the two experimental models.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahumada G. G., Rennard S. I., Figueroa A. A., Silver M. H. Cardiac fibronectin: developmental distribution and quantitative comparison of possible sites of synthesis. J Mol Cell Cardiol. 1981 Jul;13(7):667–678. doi: 10.1016/0022-2828(81)90274-1. [DOI] [PubMed] [Google Scholar]
- Ahumada G. G., Saffitz J. E. Fibronectin in rat heart: a link between cardiac myocytes and collagen. J Histochem Cytochem. 1984 Apr;32(4):383–388. doi: 10.1177/32.4.6707462. [DOI] [PubMed] [Google Scholar]
- Bartosová D., Chvapil M., Korecký B., Poupa O., Rakusan K., Turek Z., Vízek M. The growth of the muscular and collagenous parts of the rat heart in various forms of cardiomegaly. J Physiol. 1969 Feb;200(2):285–295. doi: 10.1113/jphysiol.1969.sp008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S. P., Melsen L. R. Myocardial necrosis, fibrosis, and DNA synthesis in experimental cardiac hypertrophy induced by sudden pressure overload. Circ Res. 1976 Aug;39(2):238–245. doi: 10.1161/01.res.39.2.238. [DOI] [PubMed] [Google Scholar]
- Blatti S. P., Foster D. N., Ranganathan G., Moses H. L., Getz M. J. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1119–1123. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casscells W., Kimura H., Sanchez J. A., Yu Z. X., Ferrans V. J. Immunohistochemical study of fibronectin in experimental myocardial infarction. Am J Pathol. 1990 Oct;137(4):801–810. [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Dean D. C., Newby R. F., Bourgeois S. Regulation of fibronectin biosynthesis by dexamethasone, transforming growth factor beta, and cAMP in human cell lines. J Cell Biol. 1988 Jun;106(6):2159–2170. doi: 10.1083/jcb.106.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A. W., Clark W. A., Chizzonite R. A., Zak R. Change in synthesis rates of alpha- and beta-myosin heavy chains in rabbit heart after treatment with thyroid hormone. J Biol Chem. 1983 Feb 25;258(4):2421–2425. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Retroviral expression of alternatively spliced forms of rat fibronectin. J Cell Biol. 1990 Mar;110(3):833–847. doi: 10.1083/jcb.110.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Bahl J. J., Morkin E. Thyroid hormone regulates expression of a transfected alpha-myosin heavy-chain fusion gene in fetal heart cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3122–3126. doi: 10.1073/pnas.84.10.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Kornblihtt A. R. Identification of a third region of cell-specific alternative splicing in human fibronectin mRNA. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7179–7182. doi: 10.1073/pnas.84.20.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild C. C., Prescott M. F., Chobanian A. V. Effects of hypertension and its reversal on aortic intima lesions of the rat. Hypertension. 1980 Jan-Feb;2(1):33–44. doi: 10.1161/01.hyp.2.1.33. [DOI] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Forsberg E., Johansson S., Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988 Jul;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka K., Naito S., Seko Y., Fujii J. Hydroxyproline in all parts of the rabbit heart in hypertension and in its reversal. J Mol Cell Cardiol. 1989 Dec;21 (Suppl 5):133–139. doi: 10.1016/0022-2828(89)90779-7. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardami E., Fandrich R. R. Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol. 1989 Oct;109(4 Pt 1):1865–1875. doi: 10.1083/jcb.109.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara E., Mukai A., Oda Y., Nakanishi I., Iwa T. Left ventriculotomy of the heart: tissue repair and localization of collagen types I, II, III, IV, V, VI and fibronectin. Virchows Arch A Pathol Anat Histopathol. 1990;417(3):229–236. doi: 10.1007/BF01600138. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kaida T., Shibazaki Y., Kurabayashi M., Katoh Y., Hoh E., Takaku F., Yazaki Y. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990 Mar 5;265(7):3595–3598. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Bloch K. D., Pfeffer J. M., Pfeffer M. A., Neer E. J., Seidman C. E. Atrial natriuretic factor gene expression in ventricles of rats with spontaneous biventricular hypertrophy. J Clin Invest. 1988 Feb;81(2):431–434. doi: 10.1172/JCI113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev Biol. 1988 Oct;129(2):315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P., Simpson P. C. Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. J Clin Invest. 1989 Mar;83(3):1078–1082. doi: 10.1172/JCI113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Reidy M. A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab Invest. 1989 Jun;60(6):755–765. [PubMed] [Google Scholar]
- Mjaatvedt C. H., Lepera R. C., Markwald R. R. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987 Jan;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Morkin E., Flink I. L., Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Prog Cardiovasc Dis. 1983 Mar-Apr;25(5):435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Murata Y., Seo H., Sekiguchi K., Imai T., Lee J., Matsui N. Specific induction of fibronectin gene in rat liver by thyroid hormone. Mol Endocrinol. 1990 May;4(5):693–699. doi: 10.1210/mend-4-5-693. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B., Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989 Dec;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R., Zarain-Herzberg A., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Alpert N. R., Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P. A. A low dose of a calcium antagonist (nitrendipine) ameliorates cardiac and renal lesions induced by DOC in the rat. Exp Mol Pathol. 1984 Dec;41(3):309–320. doi: 10.1016/0014-4800(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Nickerson P. A., Conran R. M. Parathyroidectomy ameliorates vascular lesions induced by deoxycorticosterone in the rat. Am J Pathol. 1981 Nov;105(2):185–190. [PMC free article] [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Deregulation of alternative splicing of fibronectin pre-mRNA in malignant human liver tumors. J Biol Chem. 1989 Jun 25;264(18):10331–10334. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Brecher P., Chobanian A. V. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989 Apr;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Guriev S. B., Irgashev S. B., Koteliansky V. E. Immunofluorescent identification of fibronectin and fibrinogen/fibrin in experimental myocardial infarction. J Mol Cell Cardiol. 1990 May;22(5):533–541. doi: 10.1016/0022-2828(90)90955-2. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Takasaki I., Chobanian A. V., Sarzani R., Brecher P. Effect of hypertension on fibronectin expression in the rat aorta. J Biol Chem. 1990 Dec 15;265(35):21935–21939. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzke H., Schwarz H. P., Weissel M. Fibronectin during thyroid hormone replacement therapy. Thromb Res. 1987 Apr 15;46(2):347–353. doi: 10.1016/0049-3848(87)90296-9. [DOI] [PubMed] [Google Scholar]
- Weber K. T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989 Jun;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- Winegrad S., Wisnewsky C., Schwartz K. Effect of thyroid hormone on the accumulation of mRNA for skeletal and cardiac alpha-actin in hearts from normal and hypophysectomized rats. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2456–2460. doi: 10.1073/pnas.87.7.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer J., Klaubert A. The effect of triiodothyronine on the cardiac mRNA. J Mol Cell Cardiol. 1982 Oct;14(10):559–571. doi: 10.1016/0022-2828(82)90143-2. [DOI] [PubMed] [Google Scholar]