Abstract
Background
Plasmacytoid dendritic cells (pDCs) bridge innate and adaptive immune responses and are important regulators of immuno-inflammatory diseases. However, their role in atherosclerosis remains elusive.
Methods and Results
Here, we used genetic approaches to investigate the role of pDCs in atherosclerosis. Selective pDC deficiency in vivo was achieved using CD11c-Cre × Tcf4−/flox BM transplanted into Ldlr−/− mice. Compared to control Ldlr−/− chimeric mice, CD11c-Cre × Tcf4−/flox mice had reduced atherosclerosis levels. To begin to understand the mechanisms by which pDCs regulate atherosclerosis, we studied chimeric Ldlr−/− mice with selective MHCII deficiency on pDCs. Significantly, these mice also developed reduced atherosclerosis compared to controls without reductions in pDC numbers or changes in conventional DCs. MHCII-deficient pDCs showed defective stimulation of ApoB100-specific CD4+ T cells in response to native LDL, whereas production of IFN-α was not affected. Finally, the athero-protective effect of selective MHCII deficiency in pDCs was associated with significant reductions of pro-atherogenic T cell-derived Ifn-γ and lesional T cell infiltration, and was abrogated in CD4+ T cell-depleted animals.
Conclusions
This study supports a pro-atherogenic role for pDCs in murine atherosclerosis and identifies a critical role for MHCII-restricted antigen presentation by pDCs in driving pro-atherogenic T cell immunity.
Keywords: antigen, atherosclerosis, immune system, low-density lipoprotein, Plasmacytoid Dendritic cell, T cell, Interferon gamma
Introduction
The first suggestion of adaptive immune activity in atherosclerosis came from the observation that HLA-DR was abundantly expressed in both immune and vascular cells of human atherosclerotic lesions 1. In the late 1980s, researchers reported that low-density lipoproteins (LDL) undergo oxidative modification in vivo and incite the generation of autoantibodies to modified LDL 2. This was followed in the mid 1990s by the discovery that CD4+ T lymphocytes from human atherosclerotic lesions recognize LDL-derived antigen in an HLA-DR-dependent manner 3 and by the identification of vascular dendritic cells (DCs) in human aortic intima 4 These seminal studies generated great interest in the immune mechanisms of atherosclerosis and were followed by 2 decades of intensive research into the roles of adaptive immune responses in disease initiation and progression. The studies defined the distinct roles of T lymphocyte subsets in the disease process 5: Thl-biased responses promote atherosclerosis whereas Tregs play a major counter-regulatory role and limit lesion inflammation and development, in part through the anti-atherogenic roles of IL-10 and TGF-β 5-7. Until recently, however, only a few studies addressed the contribution of DCs to the immune responses of atherosclerosis.
DCs are detected in normal vessels 8, 9 preferentially in regions predisposed to atherosclerosis 8 where they accumulate lipid and contribute to the development of early fatty streaks 10. Besides these lipid scavenging properties, investigators have recently interrogated the roles of DCs in shaping atherosclerotic immune responses. DCs from normal and atherosclerotic vessels are able to process and present model antigens to CD4+ T cells in an MHCII-dependent manner 9,11, 12. Adventitial DCs, like spleen and lymph node DCs, engage in sustained interactions with T cells, leading to T cell proliferation and cytokine secretion 11. However, the outcome of these interactions between conventional DCs (cDCs) and T cells on atherosclerosis is still unclear. For example, genetic manipulations to expand or deplete the general pool of cDCs (and CD11c-expressing macrophages) using the CD11c-diptheria toxin receptor mouse did not reduce the development or progression of atherosclerosis 13. This disappointing and unexpected finding could be attributed either to a dominant role of cDCs in the modulation of cholesterol homeostasis or to the critical role of cDCs in the control of steady-state myelogenesis 14, blurring any potential role of DCs in adaptive immune responses to atherogenic stimuli.
A few studies addressed the role of a distinct DC subset, plasmacytoid DCs (pDCs), in atherosclerosis. pDCs originate in the bone marrow, circulate in the blood and home to secondary lymphoid organs as well as sites of inflammation. pDCs are specialized type I interferon (IFNs) producers in response to virus infection and as such are major players in innate immune responses 15, 16. As the name suggests, pDCs are also capable of antigen presentation to T cells 17, 18, a function shown to be critical in some autoimmune disease models 19, although not in viral infection responses 20. pDCs are detected in normal and atherosclerotic vessels, both in humans and mice 21-23. Reduced blood levels of pDCs in humans are suggested to reflect increased plaque infiltration and correlate with coronary artery disease 24, 25. Vascular pDCs are able to present antigen to T cells in vitro 23 and can load a model peptide on MHCII in vivo 26. However, the outcome and relevance to atherosclerosis remains uncertain. While some studies suggested a pro-atherogenic role for pDCs 23, 26, other investigators reported an athero-protective effect 22. The reasons for these discrepancies remain unknown and the mechanisms through which pDCs alter immune responses in atherosclerosis remain elusive. In particular, each of the above-mentioned atherosclerosis studies used antibody-mediated depletion of pDCs targeting PDCA1 (BST-2/CD317), which is not entirely specific for pDCs 27, especially in inflammatory settings. Therefore, alternative approaches are required to definitively address the role of pDCs and the mechanisms through which they modulate immune-mediated diseases. Here, we used selective genetic approaches to interrogate the role of pDCs in the development of murine atherosclerosis. We identify a critical role for MHCII expression on pDCs in driving pro-atherogenic T cell immunity. The results may have broad implications for the understanding of the immune mechanisms of atherosclerosis and other related immune diseases.
Methods
An expanded methods section is available in the online supplementary material.
Mice
All experiments were approved by the Home Office, UK. pIII+IV−/− mice were previously described28.
Flow cytometry
Single cell suspensions were stained with fluorophore-conjugated antibodies (Supplemental Table 1) and analyzed using LSRII Fortessa (BD) or CyAN ADP (Beckman Coulter) flow cytometers using the gatings shown in Supplemental Figure 1.
Analysis of in vivo antigen presentation
The Eα-GFP/Y-Ae system was described previously 26. To determine the anatomical location of the antigen processing pDCs, Ldlr−/− mice were injected with DQ-OVA. After 1 h the aortic sinus was harvested for immunohistochemical analysis. For in vivo OT-II stimulation, C57BL6 mice were injected with CFSE –labeled OT-II T cells, then injected with ovalbumin-loaded pDCs. OT-II T cell proliferation was assessed after 3 days by flow cytometry.
In vitro dendritic cell culture
Bone marrow (BM) pDCs or spleen CD11c+ cells were isolated using an AutoMACS Pro separator. Supplemental Figure 2 shows pDC purity. For cytokine production, purified pDCs were treated with CpG oligonucleotides or control GpC. For antigen presentation assays, DCs were preincubated with antigen (OVA or native LDL) then with antigen-specific T cells. T cell activation was measured by proliferation or Il-2 production.
Statistics
Results were presented as mean ± S.E. They were analyzed in GraphPad Prism (La Jolla, CA, USA) using unpaired t-test, non-parametric Mann-Whitney U test, one way analysis of variance or repeated measures two-way analysis of variance, as appropriate. P value (two-sided) of <0.05 was considered significant.
Results
Selective blockade of pDC development limits atherosclerosis in Ldlr−/− mice
Basic helix-loop-helix transcription factor E2-2/Tcf4 is an essential regulator of the pDC lineage, and CD11c-restricted deletion of Tcf4 (as in CD11c-Cre × Tcf4−/flox mice) selectively blocks pDC development and maintenance 20, 29. The phenotype of these ‘pDC-less’ mice has been previously reported, demonstrating for example that they display selective defects in pDC responses and are susceptible to MHV infection 20. We therefore reconstituted lethally irradiated Ldlr−/− mice with either CD11c-Cre × Tcf4−/flox (conditional Tcf4 deletion in CD11c+ cells, designated Tcf4-cKO thereafter) or CD11c-Cre × Tcf4+/flox (control, designated Tcf4-WT thereafter) BM 20. After recovery, mice were put on high fat diet (HFD) for 8 weeks. Ldlr−/− Tcf4-cKO mice displayed marked reduction of pDC (CD11c+ B220+ PDCA1+ cells; see Supplemental Figure 1 for an example of gating strategy) numbers in blood, spleen, lymph nodes (Figure 1A) and aortas (Supplemental Figure 3A) compared with control Ldlr−/− Tcf4-WT mice. The depletion was selective for pDCs, as we found no difference in other cell populations (T cells, B cells, monocytes and neutrophils) in blood or lymphoid organs (Supplemental Figure 3B-3D). Of note, contrary to the phenotype of cDC-less mice 14, blockade of pDC development did not alter myelogenesis, despite chronic feeding with a HFD (Supplemental Figure 3D). We also assessed the numbers of other DC subtypes. As previously reported for Tcf4-cKO mice 29, a B220lo cDC-like (CD11chi MHCII+) population that derives from converted Tcf4−/− pDCs was increased in the spleen and lymph nodes (Figure 1B), consistent with the role of Tcf4 in maintaining the cell fate of mature pDC through active opposition of a cDC ‘default’ program. We also found increased numbers of CD11chi MHCII+ B220− DCs in spleens and lymph nodes of Ldlr−/− Tcf4-cKO mice compared with controls (Figure 1C). The proportions of CD11b+ and CD8α+ cells within this population were not significantly changed (data not shown). Loss of pDCs and increase of cDCs may have effects on regulatory T cells. However we found no differences in the levels of spleen regulatory T cells between groups (Supplemental Figure 3E).
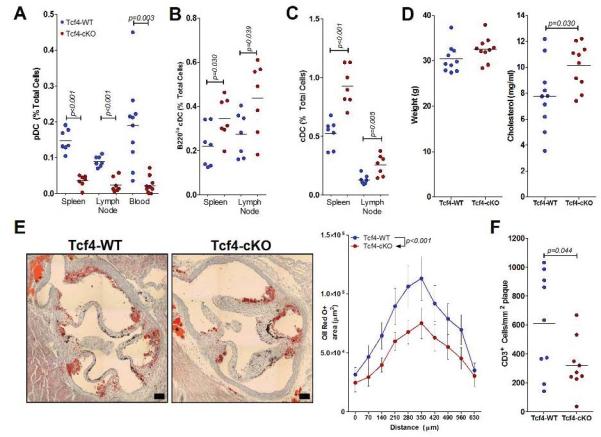
Figure 1.
Conditional Tcf4 deficiency in CD11c+ cells reduces tissue pDC levels and decreases atherosclerosis. Ldlr−/− mice transplanted with Tcf4-WT or Tcf4-cKO BM were fed a HFD for 8 weeks. A-C. Lymphoid tissue and blood levels of pDCs (A), B220lo cDCs (B) and cDCs (C). See methods for gating strategy. Results representative of 2 separate experiments with similar results. D. Terminal weights and serum total cholesterol levels. E. Representative images of oil red O-stained aortic root lesions (bar represents 100 μm). Quantification of total plaque area at the aortic root in 10 serial sections beginning at the start of the aortic valves (0 μm). Data represents mean ± S.E. at each position (n=10/group). Statistical significance determined by two-way ANOVA. F. Quantification of plaque CD3+ cells/mm2 in the aortic root. For each figure, significant differences between groups are indicated by p values.
Animal weight (Figure 1D), plasma HDL-cholesterol (2.46 ± 0.36 mM vs 2.81 ± 0.22 mM; p=0.64) and triglycerides (5.51 ± 0.73 mM vs 5.29 ± 0.54 mM; p=0.45) were similar between the 2 groups of mice. However, Ldlr−/− Tcf4-cKO mice showed a significant, although relatively small, increase of plasma total cholesterol levels in comparison with Ldlr−/− Tcf4-WT animals (Figure 1D). A similar phenotype has previously been reported in cDC-depleted Ldlr−/− or Apoe−/− mice 13, suggesting a similar potential role for pDCs in cholesterol metabolism. Previously, increased cholesterol in cDC-depleted mice was proposed to explain the lack of effect of cDC depletion on atherosclerosis 13. It is therefore remarkable that despite higher plasma cholesterol levels, pDC-less Ldlr−/− Tcf4-cKO mice showed significantly reduced atherosclerosis compared with Ldlr−/− Tcf4-WT controls (Figure 1E). Reduced lesion development was associated with a substantial reduction in plaque T cell accumulation (Figure 1F). Thus, blockade of pDC development substantially limits pro-atherogenic adaptive immunity, indicating a prominent role in disease development.
MHCII-restricted antigen presentation to T cells by pDCs
We next addressed the potential functions of pDCs that may be influencing atherosclerosis. In general, pDCs have so far been found to be less potent stimulators of CD4+ T cells in the presence of cognate antigen than cDCs or inflammatory/bone-marrow derived DCs. Nevertheless, pDCs are capable of antigen presentation in a number of conditions 19, 26, 30. We therefore addressed the role of MHCII-dependent functions of pDCs. Aortic pDCs from Apoe−/− mice have already been shown to take up injected Eα antigen and present it in the context of MHCII 26. Aortic pDCs from Ldlr−/− mice are also capable of Eα antigen presentation (Figure 2A). Staining with the Y-Ae antibody (which recognizes the Eα peptide specifically in the context of MHCII I-Ab) was readily detectable on aortic pDCs from chow and HFD-fed Ldlr−/− mice injected with Eα-GFP but not those injected with PBS (Figure 2A). In addition, after injection of DQ-OVA (a self-quenched conjugate of ovalbumin that exhibits bright green fluorescence upon proteolytic degradation), cells in the aortic root plaques of Ldlr−/− mice staining positive for the pDC marker Siglec-H were also positive for processed DQ-OVA (Figure 2B).
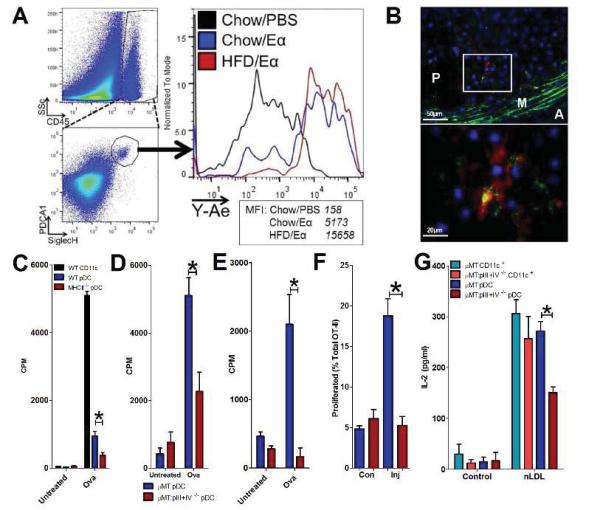
Figure 2.
Antigen presentation by pDCs in vivo and in vitro. A. Chow or HFD-fed Ldlr−/− mice injected with Eα-GFP (or PBS) and whole aortas were digested and analysed by flow cytometry for pDC uptake of Eα-GFP using Y-Ae antibody. Data are from 4 pooled aortas/group. B. pDCs detected by staining for Siglec-H (red) in the aortic sinus of Ldlr−/− mice were able to uptake and process DQ-OVA [green or yellow (green + red)]. Cell nuclei are stained by DAPI (blue). A: Adventitia, M: Media, P: Plaque. Representative images from analysis of 5 mice. C. Proliferation of OT-II CD4 T cells after incubation with spleen CD11c+ from WT mice or BM pDCs from WT and MHCII−/− mice incubated with or without 100 μg/ml ovalbumin. Data representative of 2 separate experiments. *p<0.05. D. OT-II CD4 T cell proliferation after incubation with pDCs from μMT or μMT.pIII+IV−/− mice with or without 100 μg/ml ovalbumin continuously. *p<0.05. E. OT-II CD4 T cell proliferation after incubation with pDCs from μMT or μMT:pIII+IV−/− mice with pre-incubated with or without 100 μg/ml ovalbumin and/or CpG-B (5 μg/ml) prior to addition of OT-II T cells only.*p<0.05. F. OT-II T cell proliferation in vivo (% of total OT-II) after injection of ova-loaded μMT or μMT:pIII+IV−/− pDCs in popliteal lymph nodes from the uninjected control (Con) or injected (Inj) hindlimbs. *p<0.05. G. Activation of human ApoB100-specific T cell hybridoma (48-5T), measured by Il-2 secretion, after 15h co-culture with spleen CD11c+ cells or BM pDCs from μMT or μMT:pIII+IV−/− mice with or without native human LDL (25 μg/ml). Data representative of at least 2 experiments in D and E, and 4 experiments in G with similar results. *p<0.05.
We then investigated the ability of pDCs to present the model antigen ovalbumin to purified OVA-specific OT-II CD4+ T cells, using Mhcii−/− mice to confirm the antigen dependency. Both WT splenic cDCs and BM pDCs induced T cell proliferation in the presence of OVA (Figure 2C). As expected, pDCs stimulated OT-II T cells to a lesser extent, but the majority of their effect was dependent on MHCII, since there was significantly less T cell proliferation in the presence of MHCII-deficient pDCs (Figure 2C).
To target MHCII selectively in pDCs, we took advantage of the cell and tissue-specific promoters of the MHCII transactivator (CIITA), pI, pIII, and pIV, which specifically controls expression of MHCII genes and a handful of antigen-presentation related genes19. The pI promoter controls MHCII expression in cDCs, macrophages and microglia, pIII selectively controls MHCII expression in pDCs and B cells, whereas pIV controls MHCII expression by thymic epithelial cells and immune-stimulated non-hematopoietic cells 28. Therefore, mice receiving BM cells lacking pIII and pIV (pIII+IV−/−) allow the study of the role of MHCII-restricted antigen presentation by pDCs and B cells. We backcrossed pIII+IV−/− mice with B cell deficient μMT mice in order to generate (“μMT:pIII+IV−/−”) mice lacking MHCII-restricted antigen presentation only by pDCs. Compared to μMT controls, μMT:pIII+IV−/− pDCs did not express detectable MHCII above isotype control staining in flow cytometry analysis (Supplemental Figure 4A). Firstly, we studied the role of selective MHCII deficiency in pDCs on antigen-specific T cell activation in culture. Importantly, μMT:pIII+IV−/− pDCs secreted inflammatory cytokines at normal levels in response to CpG activation (Supplemental Figure 4B), confirming that their innate functions were intact 19.
To confirm a defect in antigen presentation by pDCs in the absence of pIII+IV, we repeated the OT-II stimulation experiments in the presence of either μMT:pIII+IV−/− or μMT:pIII+IV+/+ control BM pDCs. T cell proliferation in the presence of OVA was significantly attenuated in μMT:pIII+IV−/− BM pDCs compared to μMT pDCs (Figure 2D). These experiments were conducted in the continuing presence of ovalbumin protein. When ovalbumin was removed before addition of OT-II T cells (OVA pulse), the effect of pIII+IV deficiency on pDCs was much greater (Figure 2E). To confirm an antigen presentation defect in vivo, we adoptively transferred CFSE-labeled OT-II T cells into C57/BL6 mice and, after 24h, injected μMT or μMT:pIII+IV−/− pDCs pre-incubated with ovalbumin into the footpad. OT-II T cell proliferation was detectable in the popliteal lymph node of the injected hindlimb of μMT pDC recipients, but no proliferation was detected in μMT:pIII+IV−/− pDC recipients above that seen in the uninjected hindlimb popliteal lymph nodes (Figure 2F).
MHCII-restricted presentation of native LDL to T cells by pDCs
To investigate an antigen with relevance to atherosclerosis, we utilized a T cell hybridoma specific for human native LDL (nLDL) cloned from hApoB100tgx Ldlr−/− mice, originally described by Hermansson et al. (see online-only Supplemental Methods). Antigen-specific stimulation of the T cell hybridoma was measured by enhanced production of IL-2 after 15h co-culture with cDCs or pDCs pre-incubated with nLDL. We confirmed a defect in nLDL-specific T cell activation in μMT:pIII+IV−/− BM pDCs compared to μMT pDCs, but no differences between cDCs from the two genotypes (Figure 2G). Importantly, pDCs stimulated nLDL-specific T cells to the same extent as cDCs (Figure 2G), suggesting an enhanced ability to present this type of antigen compared to the model antigen OVA (Figure 2C). The results suggest a prominent and previously unsuspected role for pDCs in MHCII-restricted presentation of LDL-derived epitopes to CD4+ T cells.
MHCII expression on pDCs promotes atherogenesis
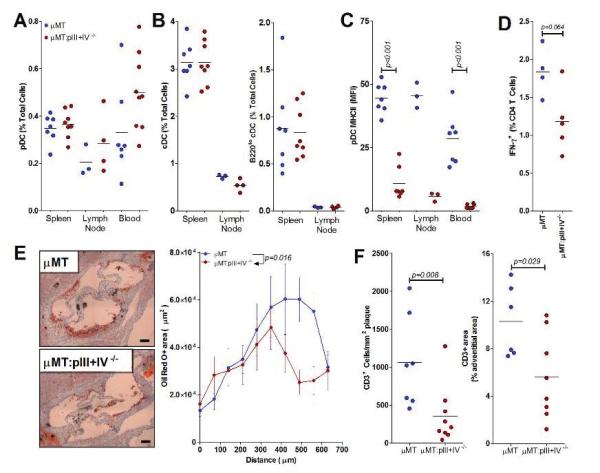
We therefore examined the role of selective deletion of MHCII in pDCs on the adaptive immune response to HFD and its consequence on the development of atherosclerosis. Lethally irradiated Ldlr−/− mice reconstituted with either μMT:pIII+IV−/− or control μMT BM were analyzed after 4 weeks recovery followed by a HFD for 6 weeks. Animal weights (29.69 g ± 0.94 vs 30.77 g ± 0.97) and total plasma cholesterol (7.49 ± 0.79 g/l vs 7.71 ± 0.52 g/l, in μMT:pIII+IV+/+ → Ldlr−/− and μMT:pIII+IV−/− → Ldlr−/− mice, respectively, p=0.82) were similar between groups. Numbers of blood monocytes and neutrophils were also comparable between the 2 groups of mice (Supplemental Figure 5A). Unlike Tcf4-cKO mice, pIII+IV deletion had no effect on the distribution of pDCs (Figure 3A) or cDCs (Figure 3B). However, μMT:pIII+IV−/− → Ldlr−/− mice displayed a selective abrogation of MHCII expression on pDCs (Figure 3C). MHCII expression on cDCs was unaltered (Supplemental Figure 5B) and there were no differences in cDC activation markers including CD40, CD80 and CD86 between the 2 groups of mice (data not shown). Interestingly, aortic root lesion size was significantly reduced in μMT:pIII+IV−/− → Ldlr−/− mice (Figure 3E). We therefore assessed the effect of this pDC-restricted MHCII deficiency on T cell responses. pIII+IV deletion had no impact on Tregs levels in the spleen and did not alter their suppressive potential (Supplemental Figures 5C and 5D). However, we found a significant reduction of pro-atherogenic Ifn-γ producing CD4+ T cells (but no differences in Il-17+ T cells) in μMT:pIII+IV−/− → Ldlr−/− compared with μMT:pIII+IV+/+ → Ldlr−/− mice, using intracellular flow cytometry staining on freshly isolated spleen T cells (Figure 3D and Supplemental Figures 5E and 5F). Importantly, there was a substantial decrease of vascular T cell infiltration in lesions of μMT:pIII+IV−/− → Ldlr−/− mice (Figure 3F). Thus, MHCII expression by pDCs is required to drive a pro-atherogenic T cell immunity.
Figure 3.
Conditional MHCII deficiency in pDCs decreases atherosclerosis in B cell-deficient mice. Ldlr−/− mice transplanted with μMT or μMT: pIII+pIV−/− BM were fed a HFD for 6 weeks. Results representative of 3 separate experiments with similar results. A. Lymphoid tissue and blood levels of pDCs. See methods for gating strategy. B. Lymphoid tissue levels of cDCs and B2201o cDCs. See methods for gating strategy. C. Mean fluorescence intensity (MFI) of MHCII staining on pDCs. D. Percentage of spleen CD4+ T cells positive for IFN-γ by intracellular flow cytometry (see methods). N=5/group. See also Supplemental Figure 4D. E. Representative images of oil red O stained lesions (bar represents 100 μm). Quantification of total plaque area at the aortic root in 10 serial sections beginning at the start of the aortic valves (0 μm). Data represents mean at each position ± S.E. (n=7 μMT, 9 μMT:pIII+IV−/−). Statistical significance determined by two-way ANOVA. F. Quantification of plaque and adventitial CD3+ cells in the aortic root. For each figure, significant differences between groups are indicated by p values.
The pro-atherogenic effect of pDC-selective MHCII expression requires the presence of CD4+ T cells
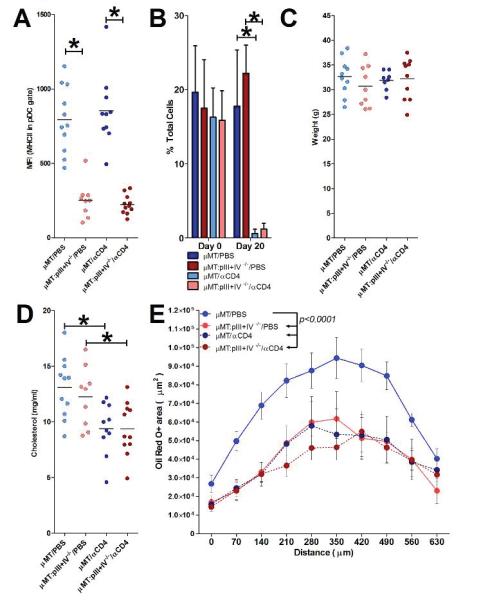
To further substantiate the T cell-dependent effects of pDC MHCII, we repeated the experiment with additional groups of μMT:pIII+IV+/+ → Ldlr−/− and μMT:pIII+IV−/− → Ldlr−/− receiving a depleting anti-CD4 antibody (see Supplemental Methods) during 8 weeks of HFD. As expected, μMT:pIII+IV−/− → Ldlr−/− mice displayed a selective abrogation of MHCII expression on pDCs (Figure 4A), and T cell depletion was substantial in anti-CD4-treated mice (Figure 4B) and maintained throughout the experiment (data not shown). Animal weights were similar between groups (Figure 4C). pIII+IV deficiency had no effect on serum cholesterol, whereas CD4+ T cell depletion led to a 25% decrease (Figure 4D), as previously reported in Apoe−/−/Ragl−/− and Ldlr−/−/Ragl−/− mice 31. CD4 depletion led to a 50% decrease in atherosclerosis in μMT:pIII+IV+/+ → Ldlr−/− mice (Figure 4E), which is consistent with the phenotype of Rag1-deficient animals 31, 32 and the pro-atherogenic role of CD4+ T cells 33. Remarkably, CD4 depletion did not reduce lesion development in μMT:pIII+IVA−/− → Ldlr−/− mice (despite reduced cholesterol), indicating that pDC MHCII deficiency had abrogated the pro-atherogenic properties of CD4+ T cells (Figure 4E). The results strongly support an MHCII-CD4+ T cell dependent pathway for the pro-atherogenic effect of pDCs.
Figure 4.
The protective effect of MHCII deficiency in pDCs is dependent on CD4+ T cells. Ldlr−/− mice transplanted with μMT or μMT: pIII+pIV−/− BM were fed a HFD for 8 weeks and injected every 10 days with either PBS or a CD4 T cell depleting antibody (αCD4). A. Mean fluorescence intensity (MFI) of MHCII staining on pDCs. *p<0.05. B. Blood CD4+ T cell levels before (Day 0) and at Day 20. *p<0.05. C+D. Final body weights and serum total cholesterol. *p<0.05. E. Quantification of total plaque area at the aortic root in 10 serial sections beginning at the start of the aortic valves (0 μm). Data represents mean at each position ± S.E. (n=11 μMT/PBS, 9 μMT:pIII+IV−/−/PBS, 10 μMT/αCD4, 11 μMT:pIII+IV−/−/αCD4). Statistical significance determined by two-way ANOVA.
Selective MHCII expression on pDCs promotes atherogenesis in the presence of B cells
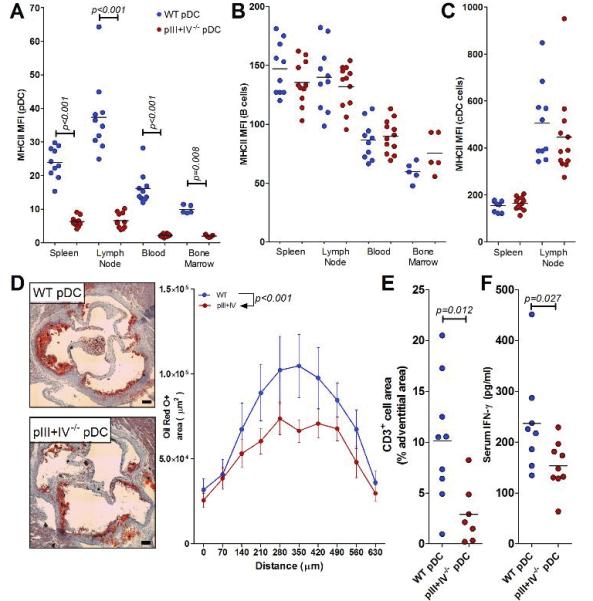
B cells are known to significantly regulate atherosclerosis 34-36, and pDCs might influence B cell responses. Since the above pIII+IV−/− experiments were performed in B cell-deficient animals, we generated B cell-sufficient mice with selective abrogation of MHCII in pDCs. To this aim, lethally irradiated Ldlr−/− mice were reconstituted with a mixture of 80% BM from μMT:pIII+IV−/− mice and 20% BM from WT mice. In this case, B cells only develop from the 20% WT BM and are MHCII+. However, 80% of pDCs will be generated from the μMT:pIII+IV−/− BM and should therefore be deficient in MHCII. Control Ldlr−/− mice were reconstituted with a mixture of 80% BM from μMT mice and 20% BM from WT (all B cells and pDCs are MHCII+). After recovery, mice were fed a HFD for 8 weeks. Proportions of pDCs, cDCs, T and B cells were similar between the 2 groups of mice (Supplemental Figure 6) and only pDCs were defective in MHCII expression (Figure 5A-C). This pDC-specific MHCII deficiency again resulted in a significant reduction of lesion size (Figure 5D) and 70% reduction of vascular T cell infiltration (Figure 5E) along with reduced systemic levels of Ifn-γ (Figure 5F) despite no change of plasma cholesterol levels (μMT/WT: 10.96 ± 0.61 g/l, μMT:pIII+IV−/−/WT: 9.91 ± 0.58 g/l, p=0.22).
Figure 5.
Conditional MHCII deficiency in pDCs decreases atherosclerosis in B cell- sufficient mice. A-C. Mean fluorescence intensity (MFI) of MHCII staining on pDCs (A), B cells (B) or cDCs (C). D. Representative images of plaque area. Bar represents 100 μm. Quantification of total plaque area at the aortic root in 10 serial sections beginning at the start of the aortic valves (0 μm). Data represents mean ± S.E at each position (n=10 WT pDC group, 12 pIII+IV−/− pDC group). E. Quantification of CD3+ cells in the vascular lesions. F. Serum IFN-γ quantified by luminex assay (see methods). For each figure, significant differences between groups are indicated by p values.
Discussion
Atherosclerosis development is driven by both innate and adaptive immune responses. Recent studies further highlighted the role played by LDL in driving antigen-specific pro-atherogenic T cell immunity 37. T cell-mediated responses and disease severity were shown to be highly dependent on cDC subtype. CCL17-expressing DCs restrain Treg responses and promote atherosclerosis 38 whereas Flt3-dependent CD103+ DCs and CD11c-restricted MyD88 signaling sustain athero-protective Tregs 39,40, as do DCs that were manipulated to exert tolerogenic activity 41. However, whether these distinct effects require antigen presentation by DC subsets remains elusive. Reduction of atherosclerosis in mice lacking MHCII-associated invariant chain CD74 42 is frequently cited as evidence for a potential role of antigen presentation in atherosclerosis. However, CD74-deficient mice display defective CD4+ T cell selection and massive reduction of thymic and spleen CD4+ T cells already in the absence of atherosclerosis 43, precluding any direct conclusion regarding the distinct role of antigen presentation in disease development. Therefore, the in vivo role of MHCII-restricted antigen presentation by cDCs in the development of atherogenic immunity remains unknown. In addition, as mentioned above, sustained total cDC depletion did not result in athero-protection.
Recent studies therefore focused on the pDC subset and its potential role in atherosclerosis, but discrepant results and mechanisms were reported 22, 23, 26. As an alternative to the antibody depletion strategy, used in all 3 previous studies that addressed the role of pDCs in atherosclerosis, we used genetically-modified mice with selective deficiency in pDCs. Our results clearly show that the development of atherosclerosis is reduced in pDC-less mice, which strongly argues in favor of a major role of pDC-mediated immunity in driving the atherogenic process.
A limitation of the depleting strategies mentioned above and the use of pDC-less mice is that they allow no conclusion about innate versus adaptive functions of pDCs in atherosclerosis. Indeed, besides their major role in shaping innate immune responses, pDCs have also been suggested to function as antigen presenting cells (APCs). They are capable of antigen cross-presentation to CD8+ T cells 44,45, express MHCII molecules and acquire a mature phenotype to internalize, process and present antigen to CD4+ T cells 17,18. However, such APC function could not be observed in vivo using models of virus infection and antibody-mediated pDC depletion 46. It appears that under conditions of acute viral infection, pDCs mainly act via type IIFN production 20, whereas the contributions of innate versus adaptive immune functions of pDCs to chronic immune diseases require more investigation. An APC function was recently demonstrated in a model of experimental autoimmune encephalomyelitis (EAE), where pDCs inhibited T cell-mediated autoimmunity 19. Whether this result could be translated to other (auto)immune-mediated diseases was still unknown. Here, we addressed this question in the context of atherosclerosis by generating mice with selective abrogation of MHCII expression in pDCs and provided strong evidence for a critical role of MHCII-restricted antigen presentation by pDCs in driving pro-atherogenic T cell responses. The results are of high importance and should prompt a re-assessment of the differential roles of pDCs and cDCs in shaping adaptive immune responses during atherogenesis.
Our results might appear in contradiction with the tolerogenic role assigned to pDCs in other settings. However, previous studies on the role of pDCs in antigen-specific CD4+ T cell responses in vivo used a disease-unrelated model antigen, i.e. OVA 30, which might not faithfully reproduce the regulation of disease-specific immune responses. In other studies, Irla et al. reported an inhibitory role of MHCII-restricted antigen presentation by pDCs in a mouse model of EAE 19. However in the EAE model, the disease process is initiated after active immunization with antigen in association with adjuvants, which is different from the spontaneous development of adaptive immune responses to endogenous LDL-derived antigens in the atherosclerosis model. APC function of pDCs might differ between these 2 different ways of induction of adaptive immunity. Finally, the outcome of antigen presentation by pDCs might depend on the nature of the presented antigen and the local microenvironment where presentation occurs. For example, exposure to oxidized LDL selectively enhanced the surface expression of the scavenger receptor CD36, with enhanced phagocytic function of pDCs and increased capacity to prime antigen-specific T-cell responses 23. It is conceivable that under basal non-inflammatory conditions, LDL presentation by pDCs induces tolerogenic adaptive immune responses, which then gradually switches towards effector responses with the progressive high load of cholesterol and environmental inflammatory stimuli. This hypothesis merits further investigation.
It should be noted that the present work addressed the role of pDCs in early atherosclerosis, at which point pro-atherogenic T cell immunity greatly influences atherosclerosis development in mice 32. Additional studies are needed to determine the contribution of pDC-mediated immunity at later stages of disease development. Since pDCs and T cells infiltrate both early and advanced atherosclerotic lesions in humans 21-25,47, 48, we speculate that our results will also bear relevance to the human disease. However, direct testing of this hypothesis is still required.
In conclusion, we present new evidence that MHCII-restricted antigen presentation by pDCs drives pro-atherogenic T cell immunity. The results shed new light on the role of adaptive immune responses in atherosclerosis and may have implications for the design of specific therapeutic strategies.
Supplementary Material
Acknowledgments
We are indebted to Sonja Firner (Institute of Immunobiology, Kantonal Hospital St. Gallen, Switzerland) for her technical support. HDL-cholesterol and triglycerides were measured by Keith Burling, at the Department of Clinical Biochemistry, University of Cambridge, UK. We thank Paul Garside and James M. Brewer (Institute of Infection, Immunity and Inflammation, University of Glasgow, UK) for stimulating discussions.
Funding Sources: This work was supported by the British Heart Foundation (PG/12/81/29897). G K Hansson is supported by the Swedish Research Council.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985;76:125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobryshev YV, Lord RS. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of Vascular Dendritic Cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol. 1995;58:307–322. doi: 10.1679/aohc.58.307. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 7.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 8.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 11.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 14.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 16.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O’Keeffe M, Ohmura-Hoshino M, Ishido S, Stoorvogel W, Heath WR, Shortman K, Villadangos JA. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 19.Irla M, Kupfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci USA. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–9. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 22.Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doring Y, Manthey HD, Drechsler M, Li evens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zemecke A. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 24.Van Vre EA, Van Brussel I, de Beeck KO, Hoymans VY, Vrints CJ, Bult H, Bosmans JM. Changes in blood dendritic cell counts in relation to type of coronary artery disease and brachial endothelial cell function. Coron Artery Dis. 2010;21:87–96. doi: 10.1097/MCA.0b013e3283368c0e. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz A, Schaller T, Cicha I, Altendorf R, Stumpf C, Klinghammer L, Ludwig J, Daniel WG, Garlichs CD. Predictive value of the decrease in circulating dendritic cell precursors in stable coronary artery disease. Clin Sci (Lond) 2009;116:353–363. doi: 10.1042/CS20080392. [DOI] [PubMed] [Google Scholar]
- 26.Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2569–2579. doi: 10.1161/ATVBAHA.112.251314. [DOI] [PubMed] [Google Scholar]
- 27.Grassia G, MacRitchie N, Platt AM, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells: biomarkers or potential therapeutic targets in atherosclerosis? Pharmacol Ther. 2013;137:172–182. doi: 10.1016/j.pharmthera.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 28.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, Otsuka H, Hijikata A, Watanabe T, Ohara O, Kaisho T, Malissen B. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vase Biol. 2003;23:1449–1454. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- 32.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 34.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vre E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 36.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 37.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MA, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Forster I, Zenke M, Hieronymus T, Junt T, Zernecke A. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 42.Sun J, Hartvigsen K, Chou MY, Zhang Y, Sukhova GK, Zhang J, Lopez-Ilasaca M, Diehl CJ, Yakov N, Harats D, George J, Witztum JL, Libby P, Ploegh H, Shi GP. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122:808–820. doi: 10.1161/CIRCULATIONAHA.109.891887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet JG, Gannage M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Maranon C. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 47.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson G. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Atherosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 48.Xu QB, Oberhuber G, Gruschwitz M, Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990;56:344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.