Abstract
Decisions result from an interaction between multiple functional systems acting in parallel to process information in very different ways, each with strengths and weaknesses. In this review, the authors address three action-selection components of decision-making: The Pavlovian system releases an action from a limited repertoire of potential actions, such as approaching learned stimuli. Like the Pavlovian system, the habit system is computationally fast but, unlike the Pavlovian system permits arbitrary stimulus-action pairings. These associations are a “forward” mechanism; when a situation is recognized, the action is released. In contrast, the deliberative system is flexible but takes time to process. The deliberative system uses knowledge of the causal structure of the world to search into the future, planning actions to maximize expected rewards. Deliberation depends on the ability to imagine future possibilities, including novel situations, and it allows decisions to be taken without having previously experienced the options. Various anatomical structures have been identified that carry out the information processing of each of these systems: hippocampus constitutes a map of the world that can be used for searching/imagining the future; dorsal striatal neurons represent situation-action associations; and ventral striatum maintains value representations for all three systems. Each system presents vulnerabilities to pathologies that can manifest as psychiatric disorders. Understanding these systems and their relation to neuroanatomy opens up a deeper way to treat the structural problems underlying various disorders.
Keywords: decision making, dorsal striatum, hippocampus, rat, ventral striatum
The brain is an information-processing machine evolved to make decisions: it takes information in, stores it in memory, and uses that knowledge to improve the actions the organism takes. At least three distinct action-selection systems have been identified in the mammalian brain: a Pavlovian action-selection system, a deliberative action-selection system, and a habit action-selection system (see Box 1).1 In this review, we analyze these decision systems from an information-processing standpoint. We consider their similarities, differences, and interactions in contributing to a final decision. The Pavlovian action-selection system learns about stimuli that predict motivationally relevant outcomes such that Pavlovian stimuli come to release actions learned over an evolutionary timescale (Dayan and others 2006). Although diverse stimuli can participate in Pavlovian learning, the available actions remain limited (e.g., salivate, approach, freeze; Bouton 2007). Deliberative action-selection is a complex process that includes a search through the expected consequences of possible actions based on a world model. These consequences can then be evaluated online, taking current goals and/or motivational state into account, before selecting an action (Niv and others 2006). Although deliberation is very flexible, it is computationally expensive and slow. The habit system entails an arbitrary association between a complexly recognized situation and a complex chain of actions (Sutton and Barto 1998). Once learned, such cached actions are fast but can be hard to change.
Box 1. Functional subsystems.
The concept of multiple functional systems should not be taken to imply that there are truly separable “modules”— these systems depend on interactions among multiple brain structures, each of which is providing a different computational component. A useful analogy is that of a hybrid gas/electric car: although there are two separate systems, which depend on dissociable components, both systems share many components. The car, for example, has only one drive train. Similarly, the car requires numerous other support systems between the two components, such as the steering system. We would therefore predict that while there will be dissociations in the information processing and effects of lesions between the systems (van der Meer and others 2010; Yin and Knowlton 2004), individual anatomy structures will also be shared between the systems, although they may provide different computational components to each system. For example, the ventral striatum seems to be involved in all three components, including providing mechanisms to reevaluate changes in Pavlovian value (McDannald and others 2011), covert representations of valuation during deliberative events (van der Meer and Redish 2009), and training up habit systems (Atallah and others 2007).
At least five functional subsystems can be indentified as playing roles in decision-making: Pavlovian action-selection, habit-based action-selection, deliberative action-selection, and the motivational and situation-recognition support systems. Although our knowledge of the anatomical instantiations of these systems is still obviously incomplete and the roles played by each structure in each functional subsystem are still an area of active research, we can make some statements about components known to be important in each subsystem.
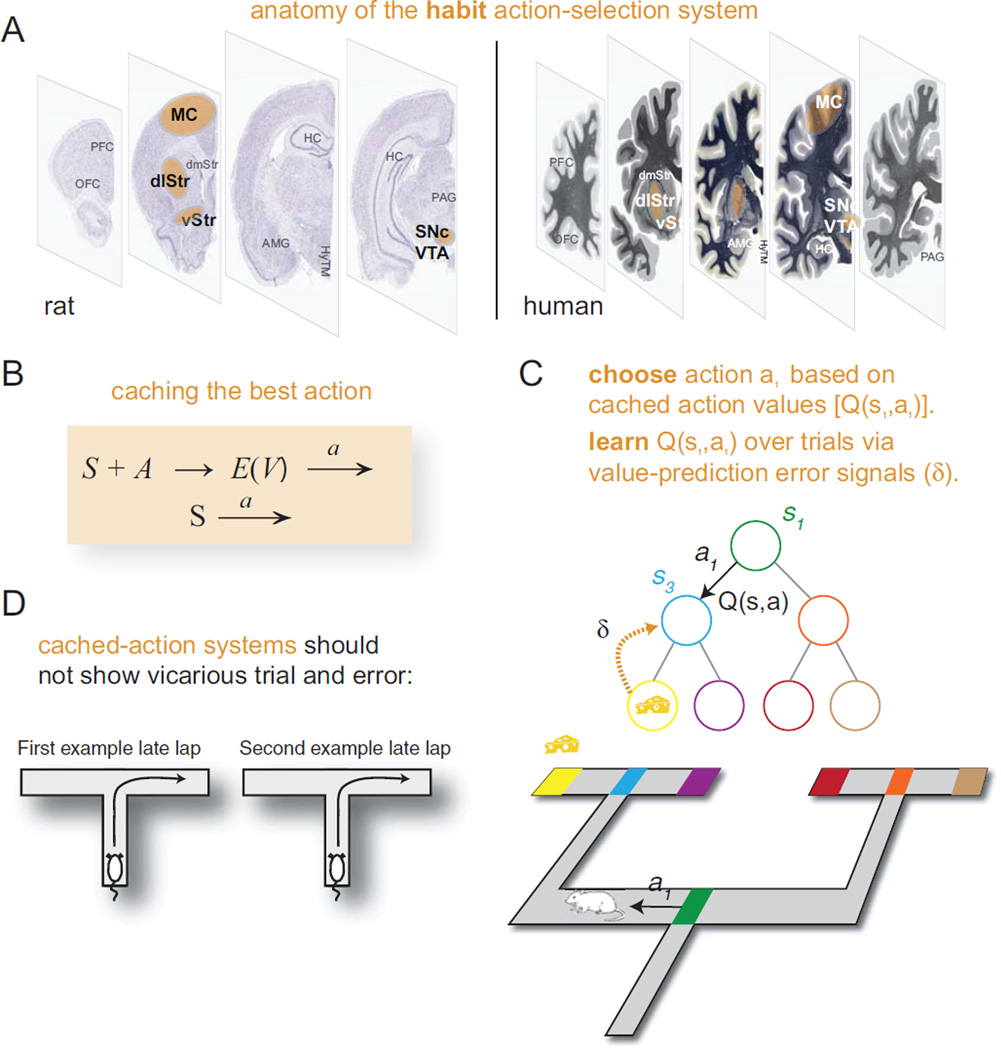
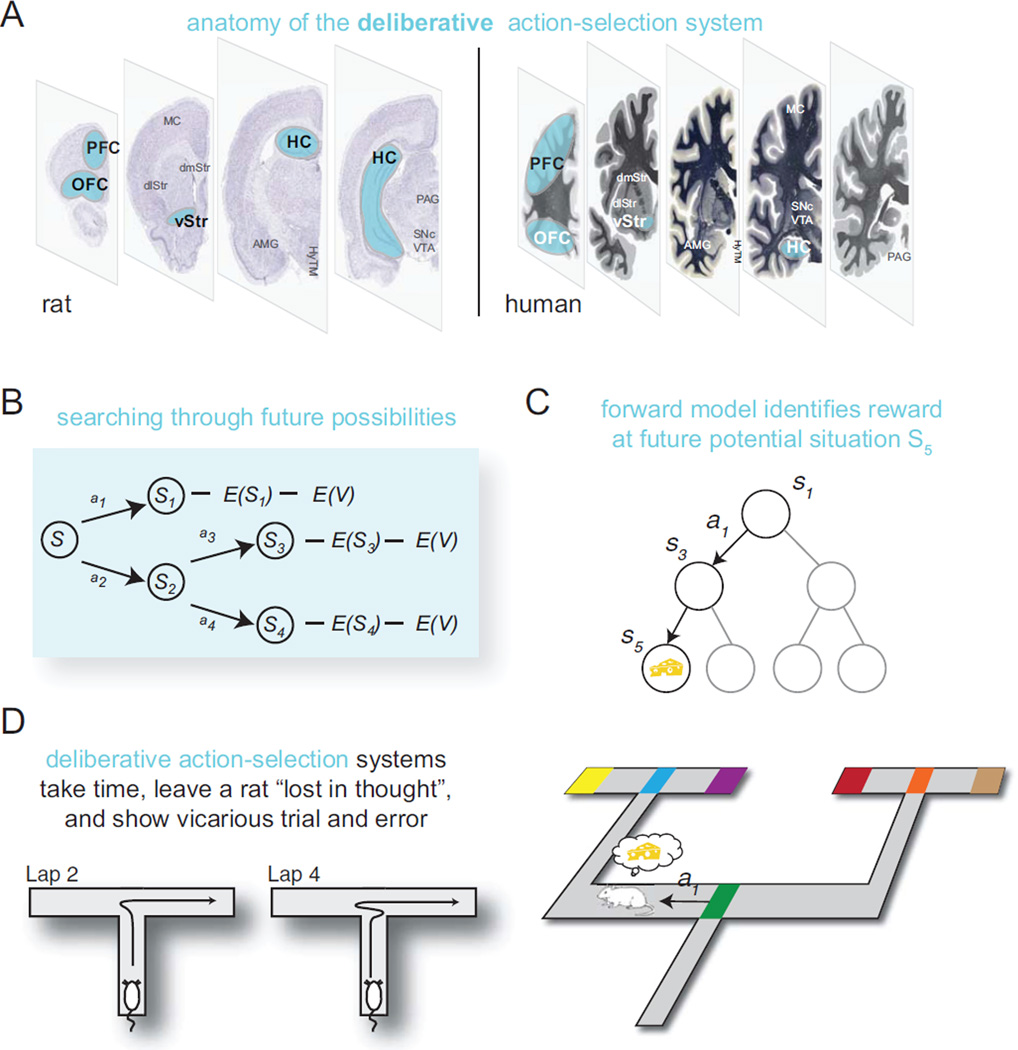
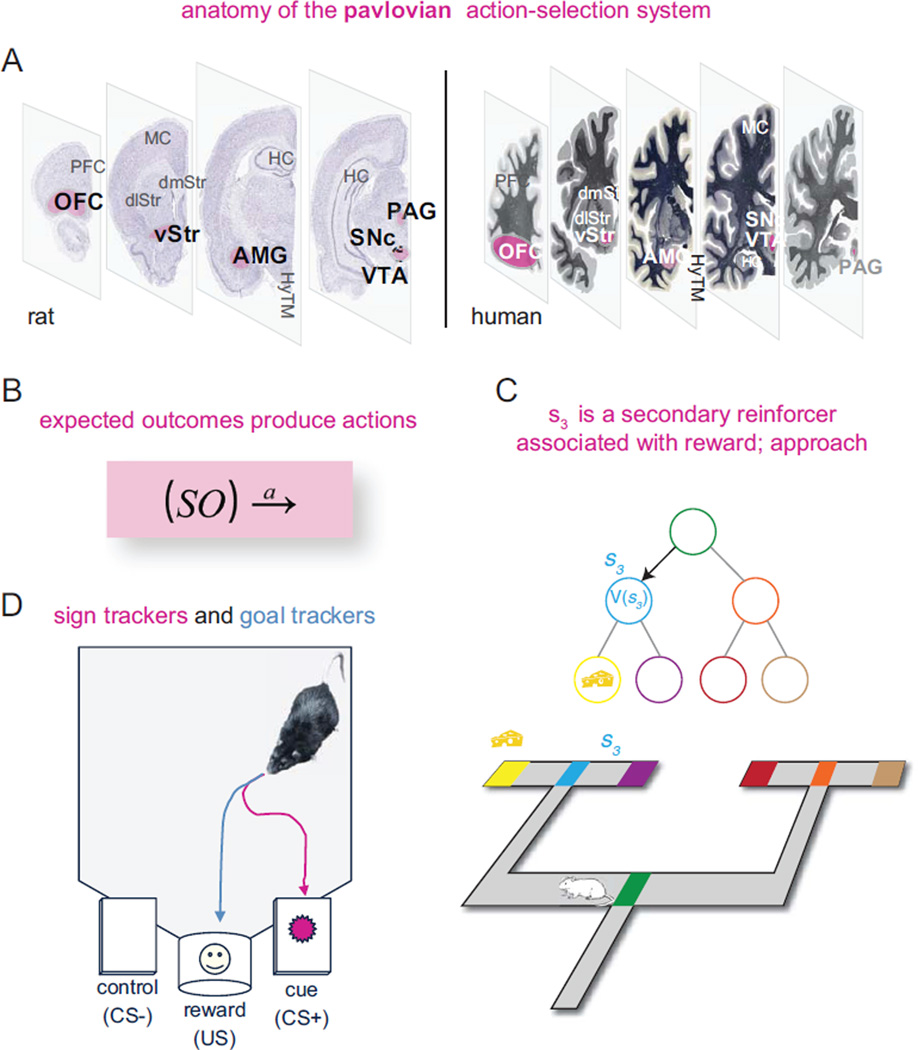
The Pavlovian system (pink, Figure 1) includes the periaqueductal gray (PAG), the ventral tegmental area (VTA), the amygdala (AMG), the ventral striatum (vStr), and the orbitofrontal cortex (OFC) (Ledoux 2002; McDannald and others 2011). The habit system (orange, Figure 2) includes the substantia nigra pars compacta (SNc), the dorsolateral striatum (dlStr), the ventral striatum (vStr), and likely the motor cortex (MC) (Cisek and Kalaska 2010; Yin and Knowlton 2004). The deliberative system (blue, Figure 3) includes the hippocampus (HC), the prefrontal cortex (PFC), the ventral striatum (vStr), and likely the ventral tegmental area (VTA) and the dorsomedial striatum (dmStr) (Johnson and Redish 2007; Schacter and Addis 2011; van der Meer and Redish 2009; Yin and Knowlton 2004). In addition, decision-making involves several support structures, not discussed in depth in this review: a motivation system, likely including the hypothalamus (HyTM), the ventral striatum (vStr), and the insula and cortical visceral areas (Craig 2003; Sanfey and others 2003), as well as a situation categorization system, likely including most of neocortex (Redish and others 2007).
This review consists of three parts. In part 1, we discuss each of the three decision-making systems, with an emphasis on the underlying information-processing steps that differentiate them. In part 2, we discuss specific brain structures and what is known about their individual roles in each of the systems. In part 3, we discuss some of the implications of the multiple decision-making systems theory. Evidence suggests that all three decision-making systems are competing and interacting to produce actions in any given task. We address the question of how they interact in the discussion.
Throughout this review, we concentrate on data from the rat because (1) concentrating on a consistent organism allows better comparisons between systems and (2) it is the best studied in terms of detailed neural mechanisms for each of the systems. However, these same systems exist in humans and other primates, and we connect the rat data to primate (human and monkey) homologies when the data are available.
Action-Selection Systems in the Mammalian Brain
Pavlovian Action-Selection
Pavlovian action-selection arises because hardwired species-specific actions can be governed by associative learning processes (Bouton 2007). Classically, unconditioned responses (URs) are physiological responses, such as salivation when smelling a lemon or a galvanic skin response following a shock, but they also include responses more recognizable as actions, such as approach to a sound, freezing in anticipation of shock, or fleeing from a predator. As organisms learn associative relationships between different events (stimuli including contexts) in the world, originally neutral stimuli (i.e., not capable of evoking an UR) can come to release conditioned responses: a bell that predicts food delivery triggers salivation. The bell becomes a conditioned stimulus (CS), to which the organism emits a conditioned response (CR). The action-releasing component of this association depends on a circuit involving the ventral striatum, the amygdala, and their connections to motor circuits (Cardinal and others 2002; Ledoux 2002).
A distinguishing feature of Pavlovian responses is that they occur in the absence of any relationship between the response and subsequent reinforcement. For instance, pigeons typically peck at a cue light predictive of food delivery (CS), even though there is no reward for doing so. Moreover, this so-called autoshaping behavior can persist even if the experiment is arranged such that pecking the CS actually reduces reward obtained (Breland and Breland 1961; Dayan and others 2006). Thus, Pavlovian actions are selected on the basis of an associative relationship with a particular outcome rather than on the basis of the action being reinforced.
In rats, Pavlovian action-selection is illustrated by comparing sign-tracking and goal-tracking behavior: when a light signals the availability of food at a separate port, some rats learn to approach the light and chew on it (sign tracking), as if the light itself has gained some food-related concept in the rat’s mind. Obviously, the better decision would be to approach the food port when the light turns on (goal tracking). Which rats show sign tracking and which rats show goal tracking correlates with and depends on dopamine signals in the ventral striatum (Flagel and others 2011).
A convenient way of thinking about the mechanism underlying Pavlovian action-selection is that the relationship between conditioned and unconditioned stimuli (CS and US) gives rise to a neural representation of (aspects of) the US, known as an expectancy, when the CS is presented; for instance, when the bell is rung, an expectancy of food is produced. To the extent that this expectancy resembles a representation of the US itself, the CR can resemble the UR, but Pavlovian CRs are not restricted to simply replicating the UR. For instance, if the US is devalued (e.g., pairing food with illness), then the CR is strongly attenuated, and different CRs can be produced depending on the properties of different CSs associated with the same US (Bouton 2007). Expectancies can have outcome identity– specific properties (e.g., food vs. water) as well as more general properties (appetitive vs. aversive). These properties interact with current motivational state and the identity of the CS to produce particular CRs.
Furthermore, Pavlovian expectancies can modulate instrumental action-selection, an effect termed Pavlovian-instrumental transfer, which entails an interaction between motivational components driven by Pavlovian valuation and other action-selection systems (Talmi and others 2008).
In summary, purely Pavlovian action-selection is characterized by a limited, hardwired “repertoire” of possible actions, arising from the interplay of an expectancy generated by the CS-US association, motivational state, and actions afforded by the environment (Huys 2007). Critically, Pavlovian actions can be acquired in the absence of instrumental contingencies and can therefore be irrelevant or even detrimental to instrumental performance (Breland and Breland 1961; Dayan and others 2006). However, expectancies generated through Pavlovian relationships can powerfully modulate instrumental action-selection (Talmi and others 2008).
Habit-Based Action-Selection
Purely Pavlovian decisions can only release of a limited set of actions. In contrast, the habit, or “cached-action,” system forms arbitrary associations between situations and actions, which are learned from experience (Figure 2). Computationally, cached-action system performance entails two deceptively simple steps: recognize the situation and release the associated action. The complexity in cached-action systems arises in the learning process, which must both learn a categorization to recognize situations and which action to take in that situation to maximize one’s reward.
Figure 2. Habit-based action-selection.
(A) Anatomy of the habit action-selection system in rat (left) and human (right). (B) We can write habit-based action-selection in terms of cached value, as an association between a situation (S), a potential action (A), and an expected value (E[V]) leading to a choice of action, or as cached-action, as an association between a situation (S) and an action (A). (C) Current theories suggest that habit action-selection occurs by learning action-values, Q(S,A) = E(V), given situation S and potential action A, which are learned through a comparison between observed and expected values—the value prediction error (δ). (D) Because cached-action selection is fast, it should not require time to process. As shown in the video (Supplemental Video S1), behavior becomes extremely stereotyped as the habit system takes over. Diagrams correspond to the late laps shown in the video.
There are models of both of these components that have been well integrated with neurophysiology. First, the situation-recognition likely happens through content-addressable mechanisms in cortical systems (Redish and others 2007). These systems are dependent on the presence of dopamine, particularly for stability of representations. (In the presence of dopamine, situation representations are stable. In the absence of dopamine, situation representations become less stable.)
Second, the association between situation and action is well described by temporal-difference reinforcement learning (TDRL) algorithms (Sutton and Barto 1998) driven by dopaminergic influences on dorsal (especially dosolateral) striatal systems (Box 2)—the association is trained up by the dopaminergic value-prediction error signal (Schultz and others 1997). When the value-prediction error is greater than zero, the system should increase its likelihood of taking an action, and when the value-prediction error is less than zero, the system should decrease its likelihood of taking an action. Thus, unlike Pavlovian systems, cached-value system decisions are dependent on a history of reinforcement; that is, they are instrumentally learned. Anatomically, these striatal systems include both go (increase likelihood of taking an action) and no-go (decrease likelihood of taking an action) systems, each of which is influenced by the presence or absence of a dopaminergic signal (Frank 2011).
Box 2. Temporal-difference reinforcement learning (TDRL) in three systems.
Current theories of reinforcement learning are based on the concept of the temporal-difference rule. The basic concept of this system is that an agent (a person, animal, or computer simulation) traverses a state-space of situations. In many simulations, this state-space is provided to the simulation, but real agents (animals or humans) need to determine the situations and their relationships. (What is the important cue in the room you are in right now?) Differences in the interpretation of that state-space can produce dramatic differences in decision-making (Kurth-Nelson and Redish 2012). Different forms of TDRL have been applied to each of the decision-making systems.
Pavlovian
“Blocking” experiments demonstrated that if an animal has learned that CS1 predicts a certain US, then pairing CS1+CS2 with the US does not result in a CR to CS2 subsequently presented alone (Bouton 2007). (However, if aspects of the US change, then the second CS will gain associations related to the observed changes; Bouton 2007; McDannald and others 2011.) Rescorla and Wagner (1972) proposed that Pavlovian learning requires a prediction error: a mismatch between what is expected and what occurs. Since in the blocking experiment, the US is fully predicted by CS1, no CS2-US association develops. In the 1990s, Sutton and Barto showed that this is a special case of the temporal-difference learning rule, in which one associates value with situations through a value-prediction-error signal (Sutton and Barto 1998). The temporal-difference rule maintains an estimated future reward value for each recognized situation such that prediction errors can be computed for any transition between situations, not just for those resulting in reward. Neurophysiological recordings of the firing of dopamine neurons and fMRI BOLD signals of dopamine-projection areas have been shown to track the value-prediction-error signal in Pavlovian conditions remarkably accurately (D’Ardenne and others 2008). Flagel and colleagues have found that dopamine release in the core of the nucleus accumbens (the ventral striatum) of sign-tracking rats (but not goal-tracking rats) matches this value-prediction-error signal and that only sign-tracking rats (not goal-tracking rats) can use the CS as a subsequent CR for secondary conditioning (Flagel and others 2011).
Habit
In the TDRL literature, “habit” learning corresponds to the original temporal-difference rule proposed by Bellman in 1958 and introduced into the literature by Sutton and Barto (Bellman 1958; Sutton and Barto 1998). In the most likely formulation (known as the actor-critic architecture), one component learns to predict the value of actions taken in certain situations based on differences between observed value and expected value. That difference signal is also used to train up situation-action associations. It can be shown that under the right conditions of exploration and stationarity, this architecture will converge (eventually) on the decision-policy that maximizes the total reward available in the task (Sutton and Barto 1998); however, this can take many trials and is inflexible in nonstationary worlds (Dayan and Niv 2008).
Deliberative
In the TDRL literature, “deliberative” decision-making is based on the concept of model-based TDRL (Sutton and Barto 1998). Here, the agent is assumed to have a model of the causal structure of the world, which it can use to predict the consequences of its actions. From these predictions, the agent can evaluate those expected consequences at the time of the decision, taking into account its current needs and desires (Niv and others 2006). This hypothesis predicts that deliberative decision-making will be slow (because it includes search, prediction, and evaluation steps; van der Meer and others 2010) and that representations of hypothesized outcomes and covert representations of reward expectation will be detectable in structures critical for deliberative decision-making. As is discussed in the main text, such predictive and covert representations have been found in the hippocampus, ventral striatum, and orbitofrontal cortex (Johnson and Redish 2007; van der Meer and others 2010; van der Meer and Redish 2009; Steiner and Redish, Society for Neuroscience Abstracts 2010).
The cached-action system can be seen as a means of shifting the complexity of decision-making from action-selection to situation-recognition. Particularly vivid examples arise in sports. A batter has to decide whether to swing a bat; a quarterback has to decide which receiver to throw to. The action itself is habitual and fast. The hard part is knowing whether this is the right moment to take the action. This arrangement offloads the hard computational work to situation-categorization, which the human brain is extraordinarily good at.
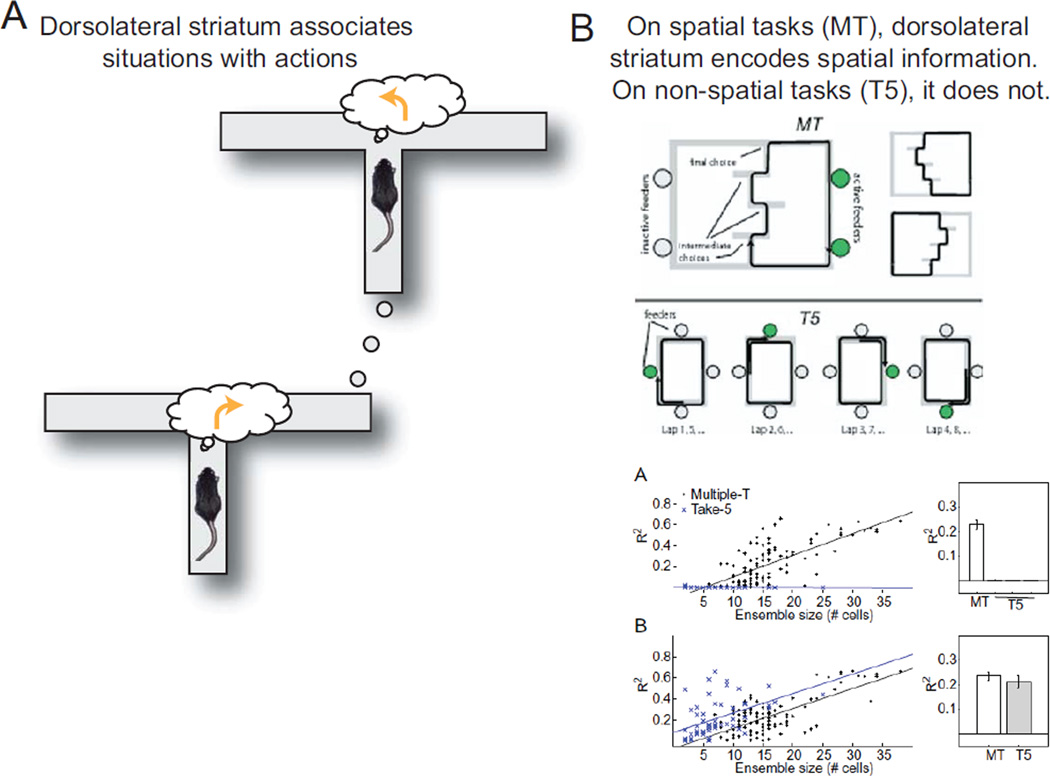
An important prediction of this cached-action learned association is that the dorsolateral association neurons should represent situation-action pairs but only those pairs that are useful to the animal. From these descriptions, we can make several predictions about these neural representations. (1) They should develop slowly. (2) They should only reflect the current situation. (3) They should only represent information about the world if that information is informative about reward delivery. In the discussion of dorsal striatum (below), we see that all three of these predictions are correct descriptions of dorsolateral neural ensembles in the rat.
The limitations of cached-action systems reside in their inflexibility (Niv and others 2006). Although a cached-action system can react quickly to a recognized situation, modifying the association takes time and requires extensive experience.2 Furthermore, the cached-action system is not aware of outcomes (for example, it is insensitive to devaluation); instead, a stimulus or situation leads directly to an action without consideration of the consequences. The deliberative system addresses this limitation.
Deliberative Action-Selection
Sometimes, one has to make decisions without having the opportunity to try them out multiple times. Take, for example, a postdoc with two faculty offers, at very different universities in very different locations. That postdoc does not get the opportunity to try each of those two jobs and use any errors in value-prediction to learn the value of each offer. Instead, our intrepid postdoc must imagine him or herself in each of those two jobs, evaluate the likely rewards and costs associated with those offers, and then make a decision. This is the process of deliberation (Figure 3).
Figure 3. Deliberative action-selection.
(A) Anatomy of the deliberative action-selection system in rat (left) and human (right). (B) Deliberation can be conceptualized as a serial search through future possibilities, including expectations of potential situations (E[S]) and valuations performed online of those expecations (E[V]). (C) Computationally, this requires a forward model to search over. (D) In practice, this computation takes time and produces pausing and VTE behavior. As shown in the video (Supplemental Video S1), deliberative behavior is visible as pausing and head swings. Diagrams correspond to laps 2 and 4 shown in the video.
Deliberation requires knowledge of the consequences of one’s potential actions: a world model. Computational models have thus termed deliberative processes “model-based” to differentiate them from cached-action processes (“model-free”; Niv and others 2006). Historically, the idea that rats and other animals could deliberate was first proposed by Tolman in the 1930s (Tolman 1932), but without the available mathematical understanding of information processing, algorithm, or computational complexity, it was impossible to understand how a deliberation system might work. Tolman’s hypothesis that rats deliberated over options came from observations originally made by Meunzinger and Gentry in 1931 that under certain conditions, rats would pause at a choice point and turn back and forth, alternately toward the multiple options, before making a decision (Muenzinger and Gentry 1931). This process was termed vicarious trial and error (VTE). VTE events occur after an animal has become familiar with an environment but when animals are still learning, when they must be flexible about their choices, and when they have to change from a learned habit (Tolman 1932). Tolman explicitly hypothesized that animals were imagining themselves in the future during VTE.
Imagining oneself in a future is a process called episodic future thinking (Atance and O’Neill 2001; Buckner and Carroll 2007) and requires an interaction between the hippocampus and the prefrontal cortex (Hassabis and Maguire 2011; Schacter and Addis 2011). It entails pulling together concepts from multiple past experiences to create an imagined future (Schacter and Addis 2011). Because this imagined future is constructed as a coherent whole, only one future tends to be constructed at a time (Atance and O’Neill 2001). That is, deliberation entails a serial search between options. Also, because this imagined future is constructed, it depends greatly on what aspects of that future event are attended to (Hill 2008). Attention appears again in the evaluation step because deliberative decisions tend to occur between options with very different advantages and disadvantages. However, this makes the deliberation process flexible—by changing his or her attention to compare teaching and research opportunities or to compare lifestyles in the two cities, our postdoc could change the valuation of the two options, before having to make the decision to take one of the two jobs.
As discussed below, we now know that during VTE events, hippocampal representations sweep forward serially through the possibilities (Johnson and Redish 2007) and both ventral striatal and orbitofrontal reward-related representations covertly signal reward expectations (Steiner and Redish, Society for Neuroscience Abstracts 2010; van der Meer and Redish 2009). Interestingly, dorsolateral striatal neurons (thought to be involved in cached-action systems) do not show any of these effects (van der Meer and others 2010).
Structures Involved in Decision-Making
In a sense, the agent itself is a decision-making machine; thus, the entire brain (and the entire body) is involved in decision-making. However, some of the specific aspects of the action-selection systems detailed above map onto distinct computational roles, mediated by dissociable decision-making circuits in the brain.
Hippocampus
Tolman suggested that the brain uses a “cognitive map” to support decision-making. In his original conception, this map was a representation of both spatial relationships (“If I turn left from here, I will be over there … ”) and causal relationships (“If I push this lever, good food will appear … ”) (Johnson and Crowe 2009; Tolman 1932). The key to deliberative decision-making is the ability to create a representation of other places and other times (in the case of a coherent, rich representation; this is sometimes called mental time travel; Buckner and Carroll 2007; Schacter and Addis 2011). In humans, this ability depends on the hippocampus, whether those other places and other times are in the past (episodic memory; Cohen and Eichenbaum 1993) or the future (episodic future thinking; Hassabis and Maguire 2011).
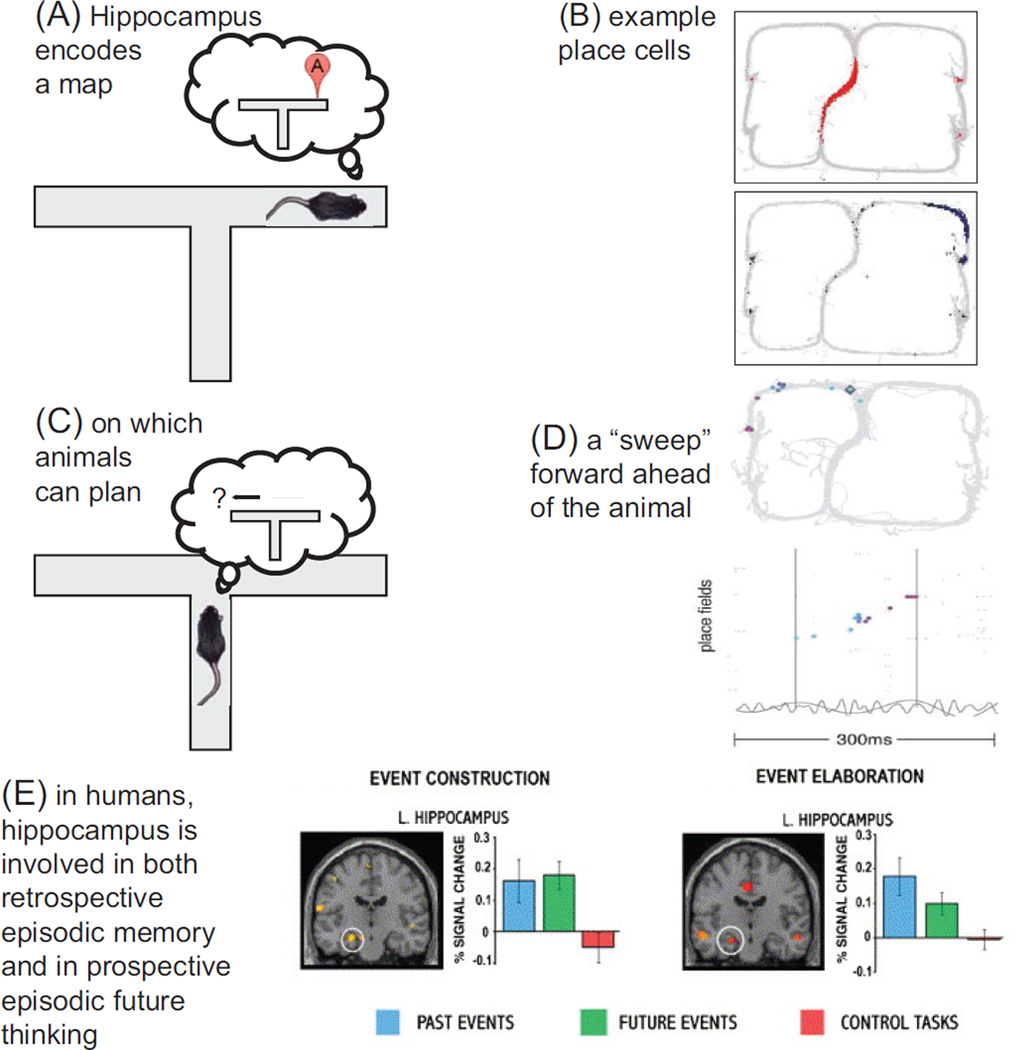
In rats, the primary information encoded by the primary output cells of the hippocampus (excitatory pyramidal cells in CA3 and CA1) is the spatial location of the animal—these are the famous “place” cells (O’Keefe and Nadel 1978; Redish 1999; see Figure 4). Hippocampal cells are also sensitive to nonspatial information, but this nonspatial information (such as the presence of a certain object, the color of the walls) modulates the place representation (Redish 1999).
Figure 4. Hippocampal contributions to decision-making.
(A) The hippocampus encodes a map of the environment through the activity across the place cells. (B) Tw o sample place cells from a choice-task. The animal runs north through the central stem, turns right or left at the top of the maze, and receives food on the right or left return rails depending on a complex decision-making criterion. (C) The existence of this map allows imagination and planning through the firing of cells with place fields away from the animal. (D) A sample planning sequence. The top panel shows the same maze as in panel B, with each spike from each cell that fires within a single 150 ms theta cycle plotted at the center of that cell’s place field. Colors indicate time in the single theta cycle. The bottom panel shows the firing of the same cells, ordered by their place fields around the maze, with the theta cycle in the local field potential beneath. (E) Remembering the past and imagining the future activates the hippocampus in humans. Subjects were instructed to initially imagine or remember an event (construction) and then to bring to mind as many details about that event as possible (elaboration). Compared to a control task, hippocampus was differentially active during both of these processes. Data in panels B and D from Gupta (2011), used with permission. Data in panel E from Addis and others (2007), used with permission of author and publisher.
We see below that dorsolateral striatal cells encode the information needed to get reward. Dorsolateral cells do respond to spatial information on spatial tasks but not on tasks in which the spatial location of the rat is not predictive of reward (Berke and Eichenbaum 2009; Schmitzer-Torbert and Redish 2008). In contrast, hippocampal cells show even better spatial representations when the task gets complicated, even when the aspect that makes it complicated is nonspatial (Fenton and others 2010; Wikenheiser and Redish 2011).
What are the properties that we expect the hippocampal map to have to be useful for deliberative decision-making? First, a map must be available as soon as possible. Deliberative decision-making is more flexible than habit and is generally used first when learning new tasks (Killcross and Coutureau 2003; Redish and others 2008). Thus, we would expect the hippocampal map to appear quickly, even if it must settle down to stability over time. Second, one will need multiple maps for planning in different environments and with different reward distributions. Third, the map should go beyond a simple record of previous experiences; it needs to enable prediction of routes or outcomes that have rarely or not yet been experienced.
In fact, the hippocampal place fields have the appropriate representational firing patterns and the correct dynamics to be the map that is searched during deliberation. The place fields appear from the first entry into an environment (Hill 1978; Redish 1999), although they may take time to stabilize, and the stability depends on the need to attend to the task at hand and the presence of dopamine (Kentros and others 2004). In each environment, there is a random mapping from place cell to place field such that each cell has a random chance of having a field in an environment and a random location (or locations) of preferred firing in each environment. If the distribution of goal locations within an environment is changed drastically, one sees a dramatic remapping of the place fields. Finally, the distribution of place fields within an environment is approximately uniform: although there is some evidence that place fields are smaller around goals, producing a concentration of place fields around goals (Hollup and others 2001), place fields do not accumulate around locations that require more complex action-selection information (van der Meer and others 2010).
So, what would deliberation and imagination look like on such a map? During deliberation, animals should pause at choice points, and one should see sequential, serial representations of positions sweeping ahead of the animal. These representations should preferentially occur at choice points and preferentially during deliberative rather than habitual events. This is exactly what is seen. As noted above, when rats come to difficult choice points, they pause and look back and forth, showing a behavioral phenomenon called VTE (Muenzinger and Gentry 1931; Tolman 1932). During these VTE events, the hippocampal place cells with place fields ahead of the animal fire in sequence, first down one path, then down the other (Johnson and Redish 2007). These sequences start at the location of the rat and proceed to the next available goal (see Figure 4). These sequences are significantly ahead of the animal rather than behind it. They are serial, not parallel, and preferentially occur during VTE events at choice points (Johnson and Redish 2007). These are the neural correlates one would expect from a deliberative search process.
In line with these neural dynamics, hippocampal lesions impair the ability of humans to remember the past (episodic memory, Cohen and Eichenbaum 1993), to imagine the future (episodic future thinking, Schacter and Addis 2011), and to plan beyond the present (Hassabis and Maguire 2011). Hippocampal lesions impair the ability of rats to navigate complex spatial environments (O’Keefe and Nadel 1978; Redish 1999) and to place new objects within the environmental context (the schema of the world; Tse and others 2007), and they attenuate VTE events (Hu and Amsel 1995). Thus, the hippocampus implements a searchable map composed of relationships between spatial locations, objects, and contexts. The dynamics of hippocampal representations match the expectations one would see if the hippocampus was involved in planning.
Dorsal Striatum
The functional roles of the striatal subregions reflect the topographical organization of its inputs and outputs (Swanson 2000). A distinction is generally made between the dorsolateral striatum, interconnected with sensory and motor cortex, the dorsomedial striatum, interconnected with associative cortical areas, and the ventral striatum, interconnected with hippocampal and frontal cortical areas; this subdivision should be understood as gradual rather than as clear-cut and abrupt. In addition, this connectivity-based subdivision should also be understood as having additional effects along the anterior-posterior axis (Swanson 2000; Yin and Knowlton 2004).
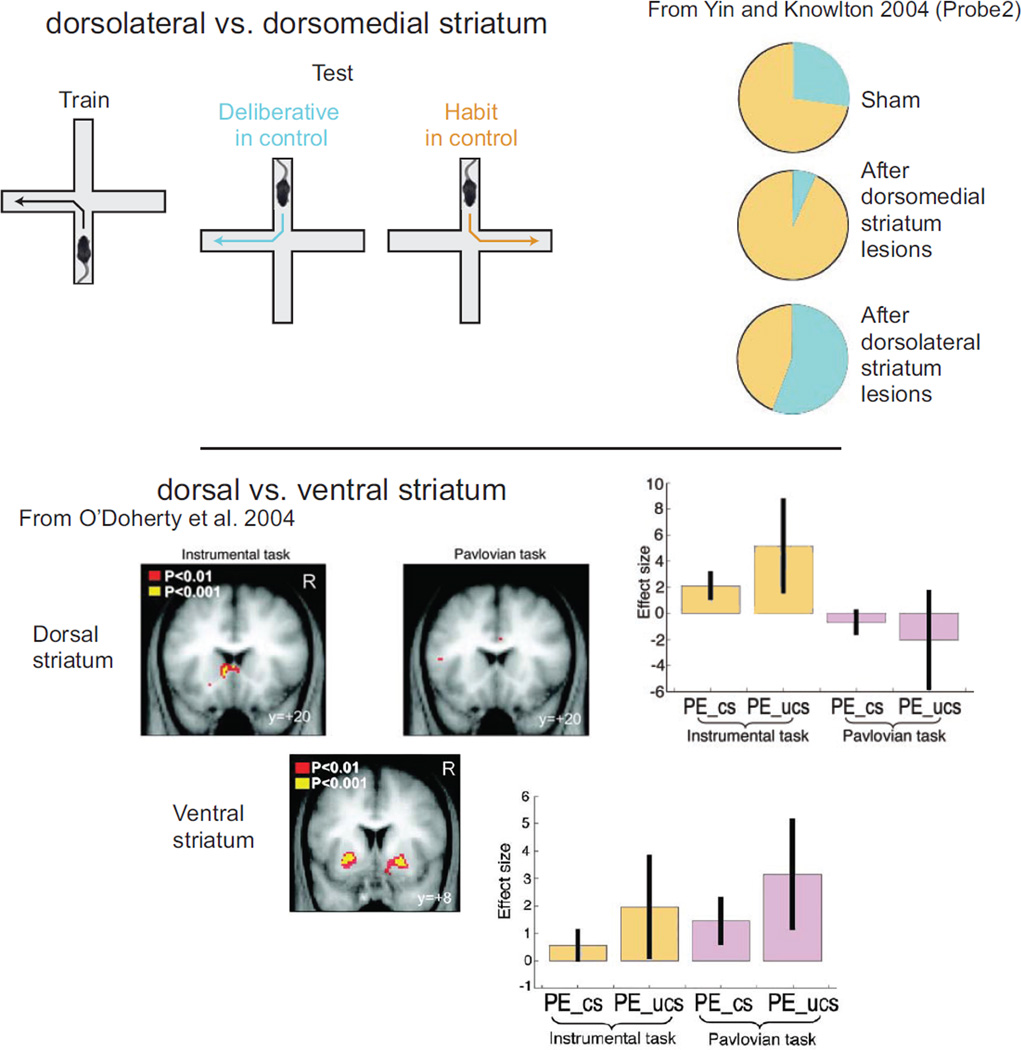
Lesion studies indicate a striking dissociation between dorsolateral and dorsomedial striatum, with dorsolateral striatum being important for the performance of habitual actions and with dorsomedial striatum being important for the performance of deliberative (goal-directed, going to a place rather than making a response) actions (Yin and Knowlton 2004). Strikingly, Atallah and others (2007) found that dorsolateral striatum was required for the performance, but not acquisition, of an instrumental S-A (habit) task.
Recording studies have tended to concentrate on the anterior dorsolateral striatum because lesion studies have found that the anterior dorsolateral striatum produced contrasting effects to hippocampal lesions on tasks that put place-directed (deliberative) strategies in conflict with response-directed (habit) strategies (see Figure 7). Studies in the anterior dorsolateral striatum find that cells learn to encode situation-action pairs such that the situation-correlations depend on the information necessary to find reward (Berke and Eichenbaum 2009; Schmitzer-Torbert and Redish 2008). Cells in the anterior dorsolateral striatum develop task-related firing with experience (Barnes and others 2005; van der Meer and others 2010). This task-related firing tends to occur at task components where habitual decisions needed to be initiated (Barnes and others 2005). There has not been much equivalent recording done in posterior dorsomedial striatum, although one recent study did find that in anterior dorsomedial striatum, cells developed task-related firing more quickly than anterior dorsolateral striatum and that these cells showed firing related to decisions (Thorn and others 2010).
Figure 7. Striatal components.
(A) Lesion studies differentiating anterior dorsolateral striatum from posterior medial striatum find that anterior dorsolateral striatum is critical for response and habit strategies, while posterior medial dorsal striatum is critical for place and deliberative strategies. Right-side panel modified from Yin and Knowlton (2004) with permission of author and publisher. (B) fMRI studies find that ventral striatum is active in Pavlovian and instrumental (deliberative, habit) tasks, while dorsal striatum is only active during instrumental (in this case, habit) tasks. Figures in panel B from O’Doherty and others (2004), reprinted with permission of author and publisher.
An influential model of the learning and performance of habitual actions in the dorsolateral striatum is that it provides situation-action associations in a model-free TDRL algorithm (see Box 2). This conceptualization suggests that the dorsal striatum associates situation information coming from cortical structures with actions as trained by the dopaminergic training signals.
Recording studies in anterior dorsolateral striatum have identified striatal activity related to the internal variables needed for the action-selection component of TDRL in both rat (Barnes and others 2005; Berke and Eichenbaum 2009; Schmitzer-Torbert and Redish 2008; van der Meer and others 2010) and monkey (Hikosaka and others 1989; Samejima and others 2005; Lau and Glimcher 2007). A particularly striking effect, observed in different tasks, is the emergence of elevated dorsolateral striatal activity at the beginning and end of action sequences (Barnes and others 2005; Thorn and others 2010) and the separation of action-related and reward-related activity in anterior dorsolateral striatum (Schmitzer-Torbert and Redish 2004, 2008). These results suggest network reorganization with repeated experience consistent with the development of habitual behavior. Comparisons of dorsolateral and dorsomedial striatal activity have yielded mixed results (Kimchi and Laubach 2009; Stalnaker and others 2010; Thorn and others 2010), but generally, these studies have compared anterior dorsolateral striatum with anterior dorsomedial striatum. It is not clear that these studies have directly tested the differences in information processing in different dorsal striatal regions under deliberative and habit-based decision-making.
Ventral Striatum
The ventral aspect of the striatum (encompassing the core and shell of the nucleus accumbens, the ventral caudate/putamen, and the olfactory tubercule) is a heterogenous area anatomically defined through its interconnections with a number of “limbic” areas (Swanson 2000). Historically, ventral striatum has long been seen as the gateway from limbic structures to action-selection (Mogenson and others 1980). Critically, ventral striatum is a major input to dopaminergic neurons in the ventral tegmental area (VTA), which in turn furnishes ventral striatum itself, dorsal striatal areas, prefrontal cortex, and the hippocampus with dopamine signals. The close association with dopamine and convergence of limbic inputs renders vStr a central node in brain networks processing reward- and motivation-related information.
The close anatomical and functional association with dopamine (in terms of providing input to the VTA but also because of its dense return projection; Haber 2009) means that major views of ventral striatum function are intertwined with dopamine function. One such idea is that the ventral striatum computes the value of situations (which includes rewards actually received as well as discounted future rewards expected; see Box 2) to supply one term of the prediction error equation to the VTA. The VTA prediction error in turn serves to update ventral striatal representations of values of given situations as well as dorsal striatal representations of the values of taking actions. This casts the role of ventral striatum as supporting gradual learning from feedback, as is thought to occur in the “habit” system; experimental support for this notion comes, for instance, from inactivation studies that find large effects on acquisition but small effects (if any) on performance (Atallah and others 2007). However, recent demonstrations that the dopamine input to the ventral striatum is not homogenous (Aragona and others 2009) pose a challenge for a straightforward mapping onto TDRL’s conception of the error signal as a single value.
Ventral striatum is also importantly involved in the more immediate modulation of behavior—it mediates aspects of Pavlovian conditioned responding, including autoshaping (Cardinal and others 2002) and sensitivity to devaluation of the US (Singh and others 2010). It is also required for conditioned reinforcement (willingness to work to receive a CS; Cardinal and others 2002). Value representations in ventral striatum are also important for the deliberative and habit systems. Two neuronal firing correlates have been reliably found in ventral striatum: reward-related firing that occurs shortly after an animal receives reward (Lavoie and Mizumori 1994; Taha and Fields 2005; van der Meer and Redish 2009) and “ramp” neurons that increase firing as an animal approaches a reward (Lavoie and Mizumori 1994; van der Meer and Redish 2011).
For representations of potential future states—as required by deliberation and found in the hippocampus— to be useful in deliberative decision-making, some kind of evaluation of the value of these imagined states is required. One possibility for such evaluation is that future states (represented in the hippocampus) function as a cue or state input for the online computation of ventral striatal values. Consistent with this possibility, recording studies in ventral striatum show close association with hippocampal inputs, including reactivation of reward neurons in sync with replay of hippocampal activity during sleep and rest (Lansink and others 2009) and reactivation of reward-related firing on movement initiation and during deliberative decision-making (van der Meer and Redish 2009). Intriguingly, there is evidence that dopamine inputs to ventral striatum are particularly important for the performance of “flexible” approach behavior likely to require such an online evaluation process but not for the performance of a similar, but stereotyped, version of the task (Nicola 2010).
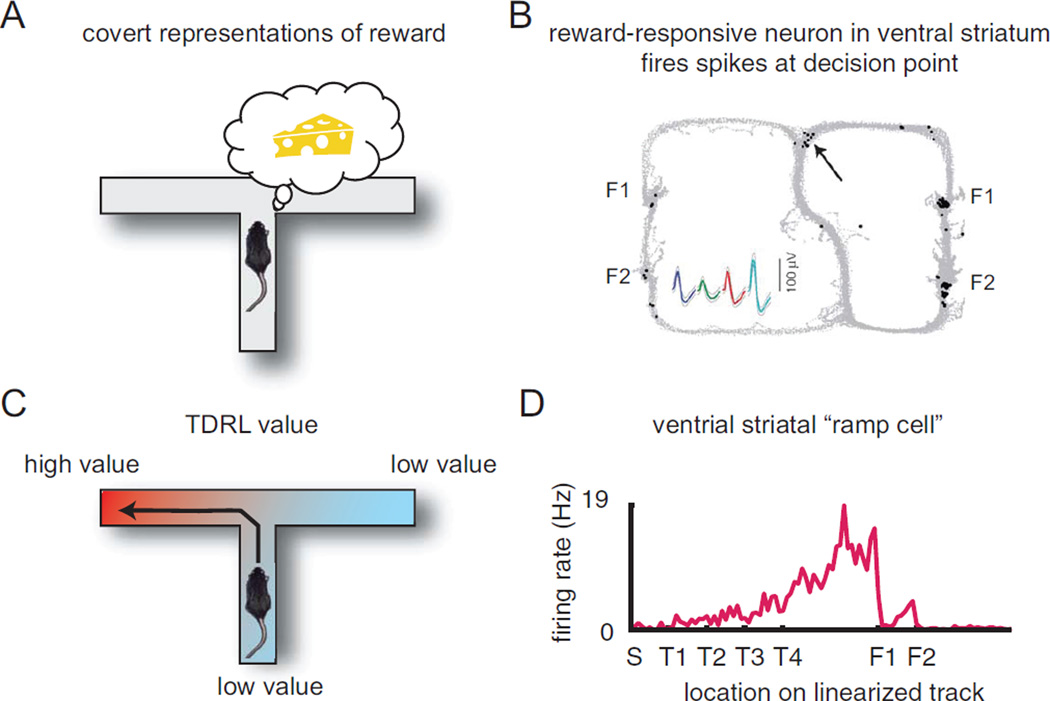
To train the habit-based situation-action association, one would also need a value signal, such as that provided by the TDRL value-learning system. Ventral striatal ramp cells show the right firing patterns to provide this signal (see Figure 6). Although ventral striatum is often necessary for learning, it is not necessary for performance of habit-based instrumental decision tasks (Atallah and others 2007).
Figure 6. Ventral striatal contributions to decision-making.
(A) Value plays a role in deliberative decision-making in that it is a necessary step during deliberative events. (B) Ventral striatal reward-related cells show extra activity during deliberative events. Gray dots show the same MT task seen in earlier figures. Black dots show locations of the animal when this single cell fired its spikes. Note that most spikes are fired at the feeder locations (two locations each on the right and left return rails). But a few extra spikes occur at the choice point where deliberation occurs (arrow). The cell was recorded from a tetrode (four channels per electrode), so there are four waveforms for the single cell, one from each channel. (C) Value plays a role in habit decision-making in that it is necessary to develop a continuous function that encodes the value of each situation. (D) Ventral striatal “ramp” cells show increasing activity to reward sites. (Animals are running the same task as in panel B. F1 = food site 1, approximately one-third the way down the return rail; F2 = food site 2, approximately two-thirds the way down the return rail.) Data in panel B from van der Meer and Redish (2009). Data in panel D from van der Meer and Redish (2011).
In sum, value plays a central role in Pavlovian, habit, and deliberative systems alike, and as a central node in reward processing, it appears that ventral striatum plays a role in all three systems. To what extent this role reflects unitary processing (the same computational role) or different processing for each system and how this relates to known heterogeneities, such as core/shell, are important, unanswered, current research topics.
Discussion
Decision-Making and Neuroeconomics
Neuroeconomics attempts to study decision-making starting from the point of view of microeconomics, relating neuroscientific results to economic variables. The neuroeconomic view of decisions is that each available outcome is evaluated to a scalar “value” or “utility,” and these scalars are compared, with a preference for choosing higher-value outcomes.
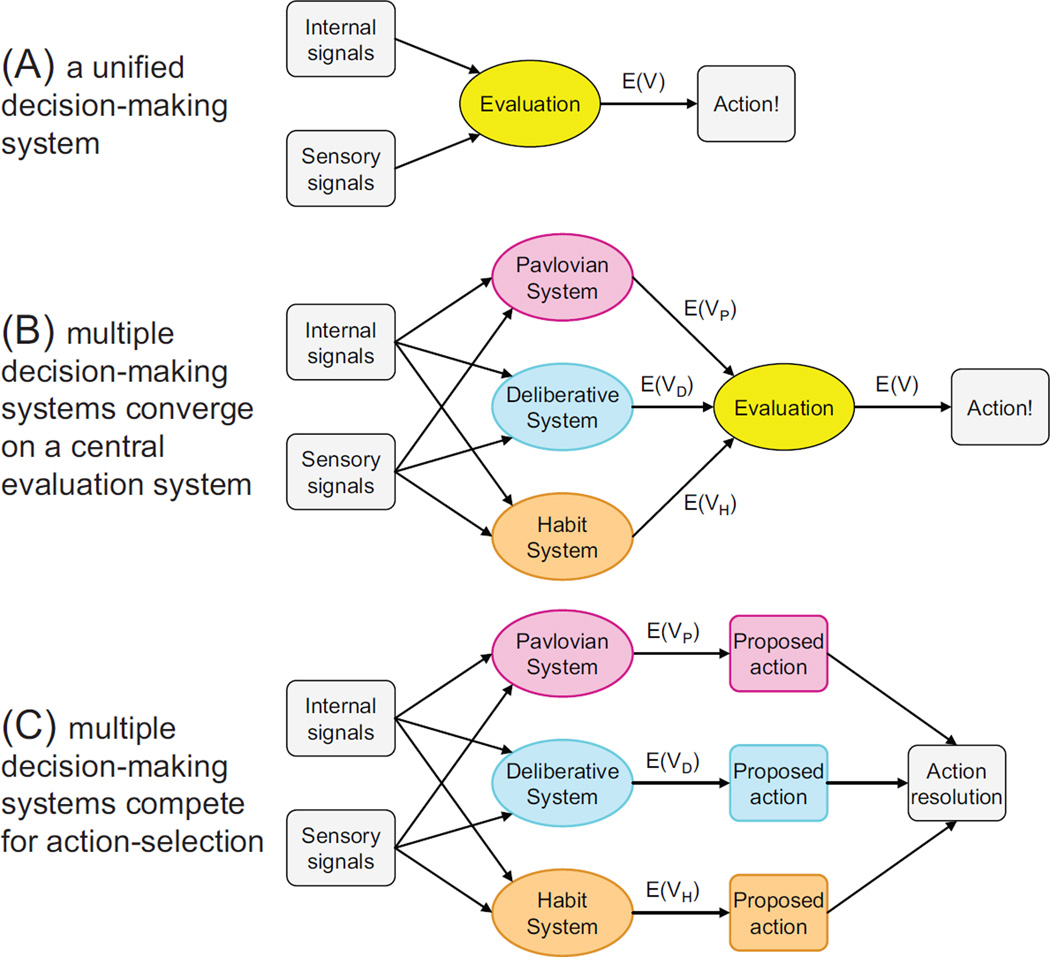
The multiple systems theory postulates that each system has its own decision-making algorithms, which compete and interact to produce the actual decision. This seems to be at odds with the neuroeconomic view that there is a unitary evaluation of each outcome. One can imagine at least two different ways of reconciling these views. Perhaps neuroeconomic valuation is a descriptive approximation for the overall behavior that emerges from multiple systems interacting. Or, perhaps neuroeconomic valuation is used within some of the multiple decision-making systems but can be violated when other systems take over (Figure 8). We suggest that the latter is the case.
Figure 8. Multiple decision-making systems and neuroeconomics.
Three potential reconciliations between the multiple decision-making system theory and neuroeconomics. (A) Microeconomic valuation is a description of the overall behavior but is not applicable to neuroscience. (B) Each of the multiple decision-making systems proposes a valuation of a potential option, which is then compared and evaluated in a single evaluation system. (C) Each action-selection system proposes an action, which is then selected through some nonmicroeconomic mechanism. The mechanism can be some function of the internal confidence of each system, measured, for example, by the internal self-consistency of each system’s action proposal (Johnson and others 2008) or through explicit arbitration by another support system (such as prefrontal cortex; Rich and Shapiro 2009). The potential inclusion of reflexes as a fourth action-selection system, which clearly does not use microeconomic valuation in its action-selection algorithm, suggests panel C as the most likely hypothesis.
Many of the experiments identifying neural correlates of value use habitual tasks and carefully eliminate Pavlovian influences. However, Pavlovian influences can undermine neuroeconomic valuation. For example, real options (such as a physical candy bar) are harder to reject than linguistically labeled options (Boysen and Berntson 1995; Bushong and others 2010)—that is, it is easier to say “I will keep my diet and not eat that candy bar” when the candy bar is not in front of you. Similarly, pigeons cannot learn to withhold pecking in order to get reward (Breland and Breland 1961), and cockerel chicks cannot learn to run away from food to get food (Hershberger 1986). The unified neuroeconomic account would indicate that once the animals have learned the task contingencies, they should make the action that leads to the larger reward. Thus, it would follow from a neuroeconomic standpoint that the animals are simply unable to learn the task contingency, a possibility made less likely by the fact that, in the Boysen and Berntson (1995) experiment, the same chimpanzees could learn to point to an Arabic numeral to receive the larger pile of candy. The multiple systems theory provides the more satisfying account: that animals do learn the task, but when a food reward is within pointing distance, a Pavlovian UR (reaching/pointing) is released, which wins out over a more rational choice in the competition between systems.
Of course, the organism remains a unitary being— eventually, there must be a decision made at the level of the muscles. An interesting (and yet unanswered) question is whether the integration of the multiple decision-making systems happens at the level of the brain, before action-commands are sent down to the spinal cord, or whether the final integration only happens at the level of the motor commands themselves. (Most likely, some integration happens at each stage.)
Computational Psychiatry
The ability to identify specific mechanisms of decision-making provides a potential mechanistic language to address how decisions can go wrong (Huys and others 2011; Maia and Frank 2011; Redish and others 2008). Psychiatry has historically been based on categorizations of observable symptoms, which may or may not have direct relevance to underlying mechanistic causes (McHugh and Slavney 1998). The multiple decision-making systems theory provides a level of structure to connect information processing mechanisms in the brain with observable behavior. Now that we can talk about specific mechanisms, it becomes possible in this mechanistic language to describe various things that can go wrong.
Of course, this only works as long as the description still maps on to the way things are functioning. For example, the pathology could be massive brain trauma or neurodegeneration to such an extent that “Pavlovian decision-making” is no longer a meaningful description of the biological system. But we suggest that many psychiatric disorders, including autism, borderline personality disorder, depression, and addiction are meaningfully described as parameter variations within multiple decision-making systems (Huys 2007; Kishida and others 2010; Redish and others 2008).
In classic psychiatry, disease states are clustered by their distance in a symptom space, which arose historically by phenomenological description (McHugh and Slavney 1998). Additionally, the same binary diagnosis can be given for very different symptom combinations, because diagnoses are made when the number of symptoms present crosses a numerical threshold. For example, there are nine criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders for borderline personality disorder, and there is a positive diagnosis when five or more of these criteria are met. Thus, two people could have only one criterion in common and receive the same diagnosis of borderline personality disorder (and likely be offered the same pharmacological treatment, when the underlying anatomical and neuromodulatory pathologies may be completely different). The superficiality of the symptom space is analogous to diagnosing “chest pain.” A deeper understanding of mechanism reveals that either acid reflux or heart disease can cause chest pain. Likewise, computational psychiatry argues that psychiatric disorders ought to be classified based on their distance in “causal” or “functional” space and treated on the basis of an understanding of the links between the anatomy and physiology of the brain and the dimensions of this mechanistic space.
In each decision-making system in the brain, there are parameters that, when set inappropriately, produce maladaptive decisions—in other words, vulnerabilities (Redish and others 2008). Drug addiction, for example, has been partially modeled as a disorder of the habit (cached-action) system (Redish 2004). We saw that the phasic firing of dopaminergic neurons encodes the reward prediction error signal of TDRL (Schultz and others 1997). Since many drugs of abuse share the common mechanism of boosting phasic dopamine firing to mediate their reinforcing effects, it is logical that these drugs are pharmacologically manipulating the computations in the learning process to produce an uncompensable prediction error such that the reward expectation following drug-seeking actions is perpetually revised upward. However, there are features of addiction that extend beyond habit. Addicts will sometimes engage in complex planning (deliberation) to obtain drugs (Heyman 2009). There are differences in how important the dopamine signal is to different users’ taking of different drugs (Badiani and others 2011). This suggests that addiction is also accessing vulnerabilities in the deliberative and other systems (Redish and others 2008).
Similarly, depression has also been suggested to have roots in deliberative decision-making processes (Huys 2007). In deliberative decision-making, the agent attempts to make inferences about the future consequences of its actions. A key feature of depression is the sense of “helplessness”: a belief that the agent has little control over the future reinforcers it will receive. Thus, if we make the assumption of normative inference, we can predict the types of prior beliefs (perhaps genetically modulated) or the kinds of experiences that would lead an agent into periods of depression (Huys 2007).
Although we have not discussed the “support structures” of motivation and situation-categorization here, both systems can have their own failure modes, which can drive decision-making errors (Flagel and others 2011; Redish and others 2007). For example, both the cached-action and deliberative systems require some form of dimensionality reduction of the input space to learn situation-action mappings (cached-action) or to search over (deliberative). Pathologies in this cognitive state classification system can also be described computationally. It has been proposed that in problem gambling, agents classify wins as consequences of their actions but attribute losses to ancillary factors (Langer and Roth 1975).
With an understanding of psychiatric conditions at this mechanistic level, we can start to make more reasoned predictions about what kinds of treatment will be most effective for each individual. The multiple decision-making theory takes us one step closer to that mechanistic level.
Supplementary Material
Figure 1. Pavlovian action-selection.
(A) Anatomy of the Pavlovian action-selection system in rat (left) and human (right). (B) We can write Pavlovian action-selection as an association between stimulus (S) and outcome (O) that releases an action (a) associated with that outcome. (C) Seen from the point of view of temporal-difference reinforcement learning (TDRL), situations (indicated by circles in the top panel and corresponding colored locations in the bottom panel) are associated with inherent valuations. Animals approach stimuli with inherent value. (D) This becomes a problem in sign tracking where animals approach and interact with cueing stimuli rather than using those cueing stimuli to predict the location of goal tracking. Histological slices from www.thehumanbrain.info and brainmaps.org, used with permission. Abbreviations: PFC, prefrontal cortex; OFC, orbitofrontal cortex; MC, motor cortex; dmStr, dorsomedial striatum; dlStr, dorsolateral striatum; vStr, ventral striatum; HC, hippocampus; AMG, amygdala; PAG, periaquaductal gray; VTA, ventral tegmental area; SNc, substantia nigra pars compacta.
Figure 5. Dorsolateral striatal contributions to decision-making.
(A) The dorsolateral striatum associates situations with actions. On a spatial task, different situations recall different actions. (B) The dorsolateral striatal neural ensemble only encodes spatial information on spatial tasks. On the Multiple-T (MT) task, rats run through the central sequence and turn left or right for reward. The correct action is completely determined by the position of the animal on the maze. On the Take-5 (T5) task, rats must run five sides around a rectangle to get food. This means that the food location changes on each trial, which dissociates the position of the animal from the appropriate action. Because decoding quality (R2 correlation between decoded position and actual position) depends on the number of cells in the ensemble, the correct measure of the decoding quality is the slope as a function of the number of cells in the ensemble. Dorsal striatum reliably encodes spatial position on MT but not T5. In both cases, the striatal ensemble reliably encodes reward delivery information. Panel B from Schmitzer-Torbert and Redish (2008), reprinted with permission from the publisher.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH research grants MH080318 and DA024080 (ADR, ZKN) and by the Canada Research Chairs Program through the National Science and Engineering Research Council (NSERC, Tier II), VENI award 863.10.013 from the Netherlands Organisation for Scientific Research (NWO) and the University of Waterloo (MvdM).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Technically, a reflex is also a decision, as it entails the taking of an action in response to stimuli. The fact that a reflex is a decision can be seen in that a reflex only takes the action under certain conditions and it interacts with the other decision-making systems (e.g., it can be overridden by top-down processes). In the language of decision-making systems developed here, a reflex is a specific action taken in response to a triggering condition. Both the triggering condition and the action taken are learned over evolutionary timescales. The anatomy, mechanism, and specific stimulus/response pairs associated with reflexes are well understood and available in most primary textbooks and are not repeated here.
There is some evidence that this experience can be achieved without repeating the actual experience through a consolidation process in which the experience is replayed internally (Morris and others 1982; Redish and Touretzky 1998; Sutherland and McNaughton 2000). Computationally, learning through imagined repetition of a specific experience is similar to increasing the learning rate; however, if there is noise in the replayed memories, this can aid in generalization processes in the situation-recognition and association components (Samsonovich and Ascoli 2005).
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30(10):1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah EH, Lopeq-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nature Neuroscience. 2007;10(1):126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12(11):685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Bellman R. On a routing problem. Quart J Appl Math. 1958;16(1):87–90. [Google Scholar]
- Berke JD, Eichenbaum H. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol. 2009;101(3):1575–1587. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: a contemporary synthesis. Sunderland (MA): Sinauer Associates; 2007. [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) J Exp Psychol Anim Behav Process. 1995;21(1):82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Breland K, Breland M. The misbehavior of organisms. Am Psychol. 1961;16(11):682–684. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bushong B, King LM, Camerer CF, Rangel A. Pavlovian processes in consumer choice: the physical presence of a good increases willingness-to-pay. Am Econ Rev. 2010;100(4):1556–1571. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge (MA): MIT Press; 1993. [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dayan P, Niv Y. Reinforcement learning: the good, the bad and the ugly. Curr Opin Neurobiol. 2008;18(2):185–196. doi: 10.1016/j.conb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Dayan P, Niv Y, Seymour B, Daw ND. The misbehavior of value and the discipline of the will. Neural Netw. 2006;19:1153–1160. doi: 10.1016/j.neunet.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck-Santini PP, Zinyuk LE, Kubik S, et al. Attention-like modulation of hippocampus place cell discharge. J Neurosci. 2010;30(13):4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011;21(3):381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Gupta AS. PhD thesis. Pittsburgh (PA): Carnegie Mellon University; 2011. Behavioral correlates of hippocampal neural sequences. [Google Scholar]
- Haber SN. Anatomy and connectivity of the reward circuit. In: Dreher JC, Tremblay L, editors. Handbook of reward and decision making. New York: Academic Press; 2009. p. 327. [Google Scholar]
- Hassabis D, Maguire EA. The contruction system in the brain. In: Bar M, editor. Predictions in the brain: using our past to generate a future. Oxford: Oxford University Press; 2011. pp. 70–82. [Google Scholar]
- Hershberger WA. An approach through the looking glass. Learn Behav. 1986;14(4):443–451. [Google Scholar]
- Heyman G. Addiction: a disorder of choice. Cambridge (MA): Harvard University Press; 2009. [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. J Neurophysiol. 1989;61(4):780–832. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62:282–297. doi: 10.1016/0014-4886(78)90058-4. [DOI] [PubMed] [Google Scholar]
- Hill C. The rationality of preference construction (and the irrationality of rational choice) Min J Law Sci Technol. 2008;9(2):689–742. [Google Scholar]
- Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci. 2001;21(5):1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Amsel A. A simple test of the vicarious trial-and-error hypothesis of hippocampal function. Proc Natl Acad Sci U S A. 1995;92:5506–5509. doi: 10.1073/pnas.92.12.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJM. PhD thesis. Gatsby Computational Neuroscience Unit, University College London; 2007. Reinforcers and control: towards a computational ætiology of depression. [Google Scholar]
- Huys QJM, Moutoussis M, Williams J. Are computational models of any use to psychiatry. Neural Netw. 2011;24(6):544–551. doi: 10.1016/j.neunet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Johnson A, Crowe DA. Revisiting Tolman, his theories and cognitive maps. Cogn Crit. 2009;1:43–72. [Google Scholar]
- Johnson A, Jackson J, Redish AD. Measuring distributed properties of neural representations beyond the decoding of local variables—implications for cognition. In: Hölscher C, Munk MHJ, editors. Mechanisms of information processing in the brain: encoding of information in neural populations and networks. Cambridge: Cambridge University Press; 2008. pp. 95–119. [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27(45):12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontral cortex of rats. Cereb Cortex. 2003;13(8):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kimchi EY, Laubach M. Dynamic encoding of action selection by the medial striatum. J Neurosci. 2009;29(10):3148–3159. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, King-Casas B, Montague PR. Neuroeconomic approaches to mental disorders. Neuron. 2010;67(4):543–554. doi: 10.1016/j.neuron.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Nelson Z, Redish AD. Modeling decision-making systems in addiction. In: Gutkin B, Ahmed SH, editors. Computational neuroscience of drug addiction. New York: Springer; 2012. [Google Scholar]
- Langer EJ, Roth J. Heads I win, tails it’s chance: the illusion of control as a function of the sequence of outcomes in a purely chance task. J Pers Soc Psychol. 1975;32(6):951–955. [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CMA. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7(8):e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci. 2007;27(52):14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Mizumori SJY. Spatial-, movement- and reward-sensitive discharge by medial ventral striatum neurons in rats. Brain Res. 1994;638:157–168. doi: 10.1016/0006-8993(94)90645-9. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The synaptic self. New York: Penguin; 2002. [Google Scholar]
- Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14(2):154–165. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Lucantonio F, Burke KA, Niv Y, Schoenbaum G. Ventral striatum and orbitofrontal cortex are both required for model-based, but not model-free, reinforcement learning. J Neurosci. 2011;31(7):2700–2705. doi: 10.1523/JNEUROSCI.5499-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh PR, Slavney PR. The perspectives of psychiatry. Baltimore, MD: Johns Hopkins; 1998. [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muenzinger KF, Gentry E. Tone discrimination in white rats. J Comp Psychol. 1931;12(2):195–206. [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30(49):16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10(8):375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Redish AD. Beyond the cognitive map: from place cells to episodic memory. Cambridge (MA): MIT Press; 1999. [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306(5703):1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–487. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A, Kurth-Nelson Z. Reconciling reinforcement learning models with behavioral extinction and renewal: Implications for addiction, relapse, and problem gambling. Psychol Rev. 2007;114(3):784–805. doi: 10.1037/0033-295X.114.3.784. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10(1):73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian Conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokesy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29(22):7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Samsonovich AV, Ascoli GA. A simple neural network model of the hippocampus suggesting its pathfinding role in episodic memory retrieval. Learn Mem. 2005;12(2):193–208. doi: 10.1101/lm.85205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. In: Bar M, editor. Predictions in the brain: using our past to generate a future. Oxford: Oxford University Press; 2011. pp. 58–69. [Google Scholar]
- Schmitzer-Torbert NC, Redish AD. Neuronal activity in the rodent dorsal striatum in sequential navigation: Separation of spatial and reward responses on the multiple-T task. J Neurophysiol. 2004;91(5):2259–2272. doi: 10.1152/jn.00687.2003. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert NC, Redish AD. Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience. 2008;153(2):349–360. doi: 10.1016/j.neuroscience.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Singh T, McDannald MA, Haney RZ, Cerri DH, Schoenbaum G. Nucleus accumbens core and shell are necessary for reinforcer devaluation effects on Pavlovian conditioned responding. Front Integr Neurosci. 2010;4:126. doi: 10.3389/fnint.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker T, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Neural correlates of stimulus-response and response-outcome associations in dorsolateral versus dorsomedial striatum. Front Integr Neurosci. 2010;19:12. doi: 10.3389/fnint.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton BL. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10(2):180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: an introduction. Cambridge (MA): MIT Press; 1998. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25(5):1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Seymour B, Dayan P, Dolan RJ. Human pavlovian-instrumental transfer. J Neurosci. 2008;28(2):360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66(5):781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Purposive behavior in animals and men. New York: Appleton-Century-Crofts; 1932. [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- van der Meer MAA, Johnson A, Schmitzer-Torbert NC, Redish AD. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67(1):25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MAA, Redish AD. Covert expectation-of-reward in rat ventral striatum at decision points. Front Integr Neurosci. 2009;3(1):1–15. doi: 10.3389/neuro.07.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MAA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. J Neurosci. 2011;31(8):2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD. Changes in reward contingency modulate the trial-to-trial variability of hippocampal place cells. J Neurophysiol. 2011;106(2):589–598. doi: 10.1152/jn.00091.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11(4):459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.