Abstract
The association between primary hyperparathyroidism (PHPT) and acute or chronic pancreatitis is controversial. For this reason, we conducted a review of the literature over the past 30 years to explore the relationship between these 2 disorders. Ten retrospective studies each with >50 patients diagnosed with PHPT were identified. With the notable exception of 2 studies, the rate of pancreatitis among patients with PHPT was higher than that reported in general among hospitalized patients without PHPT. A higher serum calcium level may contribute to pancreatitis in these cases, along with additional genetic or environmental insults. Hypercalcemia may predispose the pancreatic acinar cell to abnormal, sustained calcium levels, lead to premature pancreatic protease activation, and pancreatitis. Although there was only short-term follow-up, most reports cited that definitive treatment of PHPT by parathyroidectomy led to the resolution of pancreatitis attacks. The published cohorts of patients with PHPT and pancreatitis are subject to bias, because serum calcium screening was not universally performed among all control nonpancreatitis patients to evaluate for PHPT. However, the pooled clinical and experimental data suggest an association between PHPT and pancreatitis and implicate hypercalcemia. For clinicians, it is important to recognize pancreatitis in patients with PHPT and, conversely, to consider PHPT by checking serum calcium levels in patients, who present with an unexplained pancreatitis.
Keywords: pancreatitis, primary hyperparathyroidism, parathyroid hormone, hypercalcemia, parathyroidectomy
The most common cause of an elevated serum calcium level is primary hyperparathyroidism (PHPT),1,2 which has an annual incidence of about 30 cases per 100,000 in the United States and Europe. Most instances of hypercalcemia are asymptomatic. However, it can present as disturbances to multiple organ systems including the cardiovascular, renal, neuropsychiatric, and gastrointestinal system. The hypercalcemia seen with PHPT has been associated with both acute and chronic pancreatitis since the mid-20th century. The first report dates back to 1940 by Smith and Cook.3 In 1962, Mixter reviewed 62 cases from the literature of pancreatitis in patients with PHPT.4 Despite numerous other reports since, there continues to be debate about the existence of a true association between these 2 diseases. To address this issue, we performed a Pubmed search with the key words “hyperparathyroidism” and “pancreatitis.” Ten retrospective reviews on the association were identified from the last 30 years. Only patients with PHPT were included. Case reports or small case series, derived from <50 patients with PHPT were excluded. The current review seeks to synthesize the available information on the reported cases of PHPT and pancreatitis and examines: (1) whether there is indeed an association between the 2; (2) whether hypercalcemia is a likely cause; and (3) whether parathyroidectomy reduces pancreatitis recurrence.
PANCREATITIS
Pancreatitis is pathologically defined as the histologic presence of inflammation within the parenchyma of the pancreas.5 Acute pancreatitis is a self-limited process characterized by the presence of interstitial edema, infiltration by acute inflammatory cells and varying degrees of necrosis, apoptosis and hemorrhage. The clinical manifestations of acute pancreatitis include epigastric pain, vomiting, and ileus. About 10% to 15% of affected patients develop multiorgan dysfunction and/or pancreatic necrosis. One quarter of these severe cases succumb to death. In adult patients, obstruction of the common bile duct by a gallstone and alcohol abuse account for up to 80% of cases of pancreatitis.6 In children, the etiologies are more diverse and include pancreaticobiliary malformations, medications, multisystemic disease, trauma, infections, metabolic disease, and familial causes.7 Metabolic causes of acute pancreatitis include diabetic ketoacidosis, hypertriglyceridemia, and hypercalcemia, with or without hyperparathyroidism. Whereas acute pancreatitis is characteristically a reversible process, chronic pancreatitis is a progressive, inflammatory condition in which the pancreatic parenchyma is permanently destroyed and replaced by fibrous tissue.8 This often results in chronic pain, acute pain from recurrent attacks of acute pancreatitis, malabsorption from pancreatic insufficiency, and a brittle form of diabetes from islet cell destruction. The etiologies of chronic pancreatitis are similar to the acute form and include alcohol abuse, chronic biliary obstruction, medications, certain toxins, genetic causes, a tropical form, hypertriglyceridemia, and hypercalcemia, also with or without hyperparathyroidism. Although their pathologic and clinical manifestations differ, the 2 entities of pancreatitis, acute and chronic, may form a broad continuum of 1 disease process.9
PHPT
PHPT represents a nonphysiological overproduction of parathyroid hormone (PTH). It is most commonly caused by a single adenoma of the parathyroid gland, but less common causes include parathyroid hyperplasia, carcinoma, and multiple endocrine neoplasia (MEN) types 1 and 2A.1 In children, the prevalence of PHPT is not known, but it seems to be rare. Common etiologies include sporadic isolated parathyroid adenoma and parathyroid hyperplasia related to MEN and familial non-MEN hyperparathyroidism. 10 PTH increases renal reabsorption of calcium directly by increasing calcium absorption in the early distal tubule and indirectly by increasing the synthesis of 1-α-hydroxylase in the proximal renal tubule, which promotes the synthesis of calcitriol, the active form of vitamin D. This results in enhanced calcium absorption in the intestine by inducing the transcription of calcium transport proteins such as calbindin. PTH also directly stimulates bone turnover by inducing monocytic stem cells to become osteoclasts, and thus mobilizes calcium from bone. Under normal conditions, these interrelated homeostatic actions of PTH tightly regulate serum calcium levels. However, unregulated production of PTH, such as during PHPT, can lead to persistent elevations in serum calcium.
ASSOCIATION BETWEEN PHPT AND PANCREATITIS
The relationship between PHPT and pancreatitis has been debated for decades. There are at least 10 retrospective studies or case series on pancreatitis associated with hyperparathyroidism since 1980, originating from the United States, India, France, Australia, Spain, and Germany (Table 1). A challenge with making exact comparisons between studies is that the diagnosis of PHPT has evolved over the years. Newer generation PTH assays are more sensitive than the ones from 2 to 3 decades ago. In addition, very few of the studies validated PHPT by measuring urinary calcium. Nonetheless, 8 of the 10 studies suggest an association between PHPT and pancreatitis,14–16,18 whereas 2 refute any relationship.11,13,17,19
TABLE 1.
Rates of Pancreatitis Among Patients With PHPT
| References | Country | All PHPT Patients | PHPT Patients With Pancreatitis, n (%) | Type of Pancreatitis | Patients Diagnosed With PHPT During Pancreatitis Workup, n (%) | PHPT-Pancreatitis Patients With Coexisting Pancreatitis Etiologies, n (%)* | Resolution of Pancreatitis After Parathyroidectomy, n/x (%) |
|---|---|---|---|---|---|---|---|
| Felderbauer et al11 | Germany | 1259 | 57 (4.5) | 16 AP, 15 CP | — | Coexisting etiologies excluded | — |
| Khoo et al12 | USA | 684 | 10 (1.5) | All AP | — | 4 (40) | 0/1 (0) |
| Bhadada et al13 | India | 59 | 9 (15.3) | All CP | — | Coexisting etiologies excluded | 6/6 (100) |
| Jacob et al14 | India | 101 | 13 (12.9) | 6 AP, 6 RP, 1 CP | 12 (92.3) | 5 (38.5) | — |
| Agarwal et al15 | India | 87 | 6 (6.9) | 5 RP, 1 CP | 5 (83.3) | — | 4/5 (80) |
| Carnaille et al16 | France | 1224 | 40 (3.3) | 18 AP, 8 RP, 14 CP | 27 (67.5) | Excluded patients with gallstones | 17/40 (42.5) |
| Shepherd17 | Australia | 137 | 7 (5.1) | All AP | 5 (71.4) | 2 (28.6) | — |
| Koppelberg et al18 | Germany | 234 | 13 (5.6) | 9 AP, 4 CP | 10 (76.9) | 5 (38.5) | 7/10 (70) |
| Sitges-Serra et al19 | Spain | 86 | 7 (8.1) | 3 AP, 1 RP, 3 CP | — | — | — |
| Bess et al20 | USA | 1153 | 17 (1.5) | 10 AP, 7 CP | — | 11 (64.7) | 5/17 (29.4) |
| Total | 5024 | 179 (3.6) | 79 AP, 20 RP, 54 CP | 59 (74.6) | 27 (45) | 39/79 (49.3) |
Primarily gallstones or sludge, alcohol ingestion, and triglyceride elevations.
AP indicates acute pancreatitis; CP, chronic pancreatitis; PHPT, primary hyperparathyroidism; RP, recurrent pancreatitis; x, number of patients who underwent surgery with removal of an adenoma.
Among the positive studies, 2 from India reported the highest rate of pancreatitis among patients with PHPT.13,14 Bhadada et al13 found that 15% of PHPT cases (9 of the 59) had chronic pancreatitis. Serum calcium levels were significantly higher in pancreatitis patients with PHPT than in patients with pancreatitis from alcoholic or idiopathic etiologies (Fig. 1A). Jacob et al14 reported that 13% of PHPT cases (13 of the 101) were roughly split between single and recurrent attacks of acute pancreatitis. Only 1 patient had chronic pancreatitis. The prevalence of PHPT-associated pancreatitis was only about 1% of all pancreatitis cases. However, using overall hospital admission rates, there was about a 30-fold higher likelihood of developing pancreatitis among PHPT patients than among the general inpatient population. The authors attributed the relatively high prevalence of pancreatitis among PHPT patients in their report to a predisposition for tropical calcific pancreatitis among residents of the Indian subcontinent.23 The other positive studies reported lower rates of pancreatitis that ranged from 3.3% to 8.1%, but they estimated that these rates were still higher than a general patient population they normally see. Another notable feature is that some of the studies, in which serum calcium levels could be plotted11,12,14,16 (Fig. 1B), demonstrated a trend toward higher serum calcium among patients with PHPT than among PHPT patients without pancreatitis. The results suggest that a threshold level of serum calcium predisposes to pancreatitis among PHPT patients.
FIGURE 1.
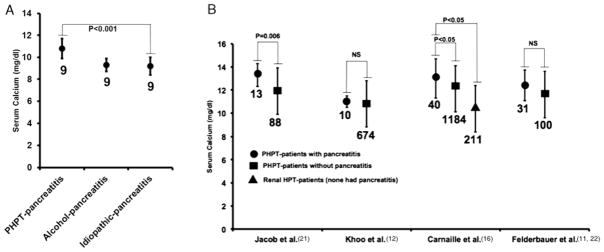
Summary of serum calcium levels. A, Serum calcium levels in PHPT and non-PHPT-pancreatitis patients modified from Bhadada et al13 (n = 9 patients per group). B, Serum calcium levels in PHPT-pancreatitis and PHPT-non-pancreatitis patients as reported by Jacob et al,21 Khoo et al,12 Carnaille et al,16 and Felderbauer et al.11,22 PHPT indicates primary hyperparathyroidism.
The strongest case against an association between PHPT and pancreatitis comes from 2 reports out of the Mayo Clinic in Rochester, MN. Firstly, Bess et al20 argue that it is generally difficult to draw correlations between the 2 disorders among hospitalized patients because of measurement bias. Most cases of detectable PHPT are asymptomatic until late stage. In older reports from western countries, or recent reports from resource-limited areas, serum calcium measurement was not part of a routine admission electrolyte profile. However, serum calcium was routinely performed in patients presenting with pancreatitis. Thus, patients with pancreatitis may have been preferentially screened for PHPT over nonpancreatitis patients. Indeed, 75% of patients (59 of the 79) were diagnosed with PHPT during a pancreatitis workup (Table 1).
To address this potential confounder, Khoo et al12 analyzed a population-based database from the Rochester Epidemiology Project, which captures a wide sample of residents from Olmsted County, MN. They found that the calculated incidence of acute pancreatitis among PHPT patients was actually lower than a randomly selected group of control subjects with PHPT; it was 114 per 100,000 person-years versus 140 per 100,000 person-years among the controls. The overall rate of pancreatitis with PHPT (1.5%) was as low as that reported by Bess et al.20 Even though there seems to be a 10-year overlap of the same patient population between the 2 studies, if we count each of them independently, a cumulative rate of pancreatitis among these and the other large studies, weighted by patient number, comes approximately to 3.6%. The number is still higher than the 2.3% incidence of pancreatitis reported among the control non-PHPT patients by Khoo and colleagues. Ultimately, prospectively enrolled, well-controlled, multicenter studies examining the incidence of pancreatitis among PHPT patients will help resolve the debate about whether there is a true association. It would also be informative to know whether there is a higher prevalence of PHPT in patients with pancreatitis versus a randomly selected nonpancreatitis population, and whether the duration or intensity of hypercalcemia impacts the chances of developing pancreatitis.
The second major argument made by the Mayo reports is that 40% to 65% of PHPT-associated pancreatitis cases had at least 1 concomitant etiology for pancreatitis, such as gallstones, alcohol abuse, or triglyceride elevations. This could suggest that PHPT is a coincidental association. Alternatively, it could signify that multiple pancreatitis-causing exposures are often necessary to develop the disease. The latter posit would explain why only a minority of patients with PHPT develop pancreatitis; that is, they need to succumb to ≥1 hits along with PHPT to manifest the disease. Along the lines of a multihit hypothesis for pancreatitis, Felderbauer et al11,22 pooled whether known pancreatitis-associated gene mutations were more frequent among PHPT patients with pancreatitis versus nonpancreatitis PHPT patients. They demonstrated that the former group harbored a greater frequency of serine protease inhibitor kazal type 1, cystic fibrosis transmembrane conductance regulator, and possible chymotrypsin C mutations, although the latter finding fell short of statistical significance. Thus, it seems that patients with PHPT may require multiple genetic and environmental influences to develop pancreatitis.
PHPT HYPERCALCEMIA AND PANCREATITIS?
Assuming there is an association, is there a causal relationship between PHPT and pancreatitis? There is no experimental evidence to suggest that pancreatitis triggers PHPT. However, the converse may be operational; that is, PHPT seems to predispose to pancreatitis. As discussed, the primary defect in PHPT is an inappropriate secretion of PTH and resultant hypercalcemia. Thus, PTH levels are a consideration. However, patients with elevations in PTH but without hypercalcemia, for example, in the setting of renal hyperparathyroidism are not at risk for pancreatitis.16
The serum calcium level, on the other hand, is significantly higher among PHPT patients with pancreatitis compared with nonaffected PHPT patients (Fig. 1B). This finding is consistent with clinical observations that iatrogenic calcium infusions induce pancreatic injury. Examples include intravenous calcium administration during cardiac surgery24 or with parenteral nutrition.25 It is well known that the most common cause of hypercalcemia in the hospitalized patient is a malignancy.26 There are several reports of pancreatitis associated with calcium-secreting tumors.27–29 Thus, hypercalcemia may mediate the development of pancreatitis in these situations.
HOW MIGHT HYPERCALCEMIA PREDISPOSE TO PANCREATITIS?
The parenchymal cell of the pancreas, the acinar cell, is the primary initiating site for pancreatitis.30 It has been speculated that the calcium-sensing receptor (CaSR) may play a pathologic role within the acinar cell during hypercalcemia. 31 The CaSR is the dominant calcium sensor on the surface of chief cells of the parathyroid gland, which regulates PTH secretion in response to changing serum calcium concentrations. Patients with a heterozygous loss of function mutation in CaSR develop a condition known as familial benign hypocalciuric hypercalcemia. Yet despite the hypercalcemia, it is unclear why they are generally thought to be protected against pancreatitis.32 An emerging hypothesis is that CaSR expressed in the acinar cell33,34 is necessary to sensitize the acinar cell to injury from extracellular calcium.35 Thus, loss of function mutations might lead to hypercalcemia due to a defect in the parathyroid gland, however, but the same defect in acinar cell CaSR would protect the patient from the injurious effects of high calcium on the pancreas. If the notion is correct, then, conversely, gain of function mutations in the CaSR might predispose patients to pancreatitis, even under conditions of normocalcemia. Indeed, a few studies36–39 identified CaSR mutations in some patients with pancreatitis compared with control subjects. Two of the mutations may confer a gain of function. Felderbauer and colleagues reported an R896H CaSR mutant in a chronic pancreatitis patient in combination with a serine protease inhibitor kazal type 1 mutation. Stepanchick et al40 recently showed that the consequence of this mutation is increased targeting to the plasma membrane of an otherwise functional CaSR, thus suggesting a gain of function phenotype. Muddana and colleagues found that the R990G CaSR mutation was associated with chronic pancreatitis, particularly alcohol related. This mutant seems to have increased sensitivity to calcium or calcimimetics.41 The functional significance of CaSR mutations in the context of pancreatitis has yet to be elucidated and may offer a clue to the unsettled question of how hypercalcemia might predispose to pancreatitis. CaSR mutations, however, were not increased in patients with PHPT and pancreatitis compared with PHPT without pancreatitis.42
It is also possible that independent of the CaSR, high extracellular calcium could lead to elevations in the cytosolic calcium signals.43 A greater rise in cytosolic calcium can activate calcium proteins, such as calcineurin, and lead to pathologic intra-acinar activation of pancreatic proteases, particularly trypsin,44,45 or can cause NF-κB activation, 46 leading to pancreatic inflammation and altered stress responses. Indeed, in experimental models induction of acute hypercalcemia causes intrapancreatic trypsin activation. 47 In addition, aberrant acinar calcium signals are observed in several forms of experimental pancreatitis.48–51
There is also some thought, based on in vitro enzymatic studies, that high cellular calcium might lead to elevated levels of calcium within intra-acinar vesicles, and thereby inhibit the autodegradation of trypsin either directly or indirectly through another enzyme such as chymotrypsin C.31 However, it is unclear whether the calcium dependency of pancreatic enzymes in isolated cell-free systems operates in cellular environments or whether the relatively small increases in extracellular calcium during hypercalcemia can directly affect those vesicular compartments. Thus, hypercalcemia seems to induce pancreatic injury and likely sensitizes patients with PHPT to pancreatitis, but the mechanism of calcium-induced injury is not clearly defined.
IDENTIFYING PHPT IN PATIENTS WITH PANCREATITIS
Beyond the distinguishing biochemical features of PHPT such as high serum calcium and a low phosphate in the setting of inappropriately high or normal PTH, there are few clinical clues to help identify this condition among patients with pancreatitis. Patients who present with long-standing PHPT may have calcium oxalate or calcium phosphate renal stones, renal dysfunction or colic, or bone defects such as pathologic fractures, osteoporosis, or an uncommon entity termed osteitis fibrosa cystica.52 Patients rarely have palpable neck nodules or profound psychiatric disturbances. The latter is difficult to discern in patients with chronic pancreatitis, who often suffer from depression and chronic pain syndromes.53 Nonetheless, the presence of these signs and symptoms may suggest a diagnosis of PHPT in patients who present with pancreatitis.
TREATING PATIENTS WITH PHPT AND PANCREATITIS
The presence of PHPT does not alter the acute management of pancreatitis episodes, which should focus on diligent supportive care.6 After the acute attack has resolved, patients should undergo elective parathyroidectomy to definitively treat the PHPT. The introduction of minimally invasive techniques54 and recent data from a 10-year prospective study55 demonstrating that parathyroid surgery improves bone mineral density, have helped shape guidelines that recommend parathyroidectomy in virtually all patients with PHPT, who have no contraindications to surgery.
The course of pancreatitis in patients with PHPT who undergo parathyroidectomy is not clear because there are no long-term studies. Most reports had a follow-up of only about 2 years. Notwithstanding the short-time frame, they cited a 42% to 100% resolution in pancreatitis recurrence. Bhadada et al13 mentions that even chronic pancreatitis pain improved, although there were no quantitative measures provided. In contrast, none of the chronic pancreatitis patients in the study by Carnaille et al16 had improvement of disease after parathyroidectomy. The 2 studies from the Mayo Clinic showed either recurrence of pancreatitis in the 1 patient who underwent resection12 or attributed some of the improvement to a reduction in heavy alcohol use.20 Overall, in the short term there might be an improvement in pancreatitis patients who undergo parathyroidectomy. However, longitudinal, prospective data are necessary to adequately ascertain a benefit.
CONCLUSIONS
A cumulative review of the available large case series or cohorts with PHPT and pancreatitis over the last 30 years suggests that patients with PHPT develop a higher rate of pancreatitis than hospitalized patients without PHPT. However, data are confounded in many instances by measurement bias and thus lack appropriate control subjects who have universally undergone serum calcium testing. Several lines of experimental evidence do suggest, though, that hypercalcemia can lead to intra-pancreatic trypsin activation and pancreatic injury and can sensitize the pancreas to pancreatitis. Pancreatitis in this setting is likely the result of additional genetic and environmental influences. It is important to check serum calcium in cases of pancreatitis and to consider pancreatitis in PHPT patients who present with abdominal symptoms.
Acknowledgments
Supported by National Institutes of Health Grants DK093491, DK083327, HD001401 (Yale Child Health Research Center), DK34989 (Yale Liver Center), and a Children’s Digestive Health and Nutrition Young Investigator Award (to S.Z.H.).
The authors thank Dr Mark Lowe for providing helpful comments and Dr Lauren Wilson for providing German language translation assistance during the preparation of this manuscript.
Footnotes
The authors declare that they have nothing to disclose.
References
- 1.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 2.Al-Azem H, Khan A. Primary hyperparathyroidism. CMAJ. 2011;183:E685–E689. doi: 10.1503/cmaj.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith FB, Cook RT. Acute fatal hyperparathyroidism. Lancet. 1940;2:650. [Google Scholar]
- 4.Mixter CG, Keynes WM, Cope O. Further experience with pancreatitis as a diagnostic clue to hyperparathyroidism. N Engl J Med. 1962;266:265–272. [Google Scholar]
- 5.Whitcomb DC. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 6.Frossard J-L, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 7.Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52:262–270. doi: 10.1097/MPG.0b013e3182061d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet. 2011;377:1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 9.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 10.Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115:974–980. doi: 10.1542/peds.2004-0804. [DOI] [PubMed] [Google Scholar]
- 11.Felderbauer P, Karakas E, Fendrich V, et al. Multifactorial genesis of pancreatitis in primary hyperparathyroidism: evidence for “protective” (PRSS2) and “destructive” (CTRC) genetic factors. Exp Clin Endocrinol Diabetes. 2011;119:26–29. doi: 10.1055/s-0030-1255106. [DOI] [PubMed] [Google Scholar]
- 12.Khoo TK, Vege SS, Abu-Lebdeh HS, et al. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab. 2009;94:2115–2118. doi: 10.1210/jc.2008-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhadada SK, Udawat HP, Bhansali A, et al. Chronic pancreatitis in primary hyperparathyroidism: comparison with alcoholic and idiopathic chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:959–964. doi: 10.1111/j.1440-1746.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacob JJ, John M, Thomas N, et al. Does hyperparathyroidism cause pancreatitis? A south indian experience and a review of published work. Anz j surg. 2006;76:740–744. doi: 10.1111/j.1445-2197.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, George RK, Gupta SK, et al. Pancreatitis in patients with primary hyperparathyroidism. Indian J Gastroenterol. 2003;22:224–225. [PubMed] [Google Scholar]
- 16.Carnaille B, Oudar C, Pattou F, et al. Pancreatitis and primary hyperparathyroidism: forty cases. Aust N Z J Surg. 1998;68:117–119. doi: 10.1111/j.1445-2197.1998.tb04719.x. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd JJ. Hyperparathyroidism presenting as pancreatitis or complicated by postoperative pancreatitis. Aust N Z J Surg. 1996;66:85–87. doi: 10.1111/j.1445-2197.1996.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 18.Koppelberg T, Bartsch D, Printz H, et al. Pancreatitis in primary hyperparathyroidism (phpt) is a complication of advanced phpt. Dtsch Med Wochenschr. 1994;119:719–724. doi: 10.1055/s-2008-1058752. [DOI] [PubMed] [Google Scholar]
- 19.Sitges-Serra A, Alonso M, de Lecea C, et al. Pancreatitis and hyperparathyroidism. Br J Surg. 1988;75:158–160. doi: 10.1002/bjs.1800750224. [DOI] [PubMed] [Google Scholar]
- 20.Bess MA, Edis AJ, van Heerden JA. Hyperparathyroidism and pancreatitis. Chance or a causal association? JAMA. 1980;243:246–247. [PubMed] [Google Scholar]
- 21.Jacob JJ, Chacko A, Selvan B, et al. Primary hyperparathyroidism and chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:164. doi: 10.1111/j.1440-1746.2007.05233.x. [DOI] [PubMed] [Google Scholar]
- 22.Felderbauer P, Karakas E, Fendrich V, et al. Pancreatitis risk in primary hyperparathyroidism: relation to mutations in the spink1 trypsin inhibitor (n34s) and the cystic fibrosis gene. Am J Gastroenterol. 2008;103:368–374. doi: 10.1111/j.1572-0241.2007.01695.x. [DOI] [PubMed] [Google Scholar]
- 23.Tandon RK. Tropical pancreatitis. J Gastroenterol. 2007;42:141–147. doi: 10.1007/s00535-006-1930-y. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-del Castillo C, Harringer W, Warshaw AL, et al. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991;325:382–387. doi: 10.1056/NEJM199108083250602. [DOI] [PubMed] [Google Scholar]
- 25.Izsak EM, Shike M, Roulet M, et al. Pancreatitis in association with hypercalcemia in patients receiving total parenteral nutrition. Gastroenterology. 1980;79:555–558. [PubMed] [Google Scholar]
- 26.Jacobs TP, Bilezikian JP. Clinical review: rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90:6316–6322. doi: 10.1210/jc.2005-0675. [DOI] [PubMed] [Google Scholar]
- 27.Marroni A. The role of the divers alert network europe in underwater emergencies. Minerva Anestesiol. 1991;57:1609–1611. [PubMed] [Google Scholar]
- 28.Mantadakis E, Anagnostatou N, Smyrnaki P, et al. Life-threatening hypercalcemia complicated by pancreatitis in a child with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2005;27:288–292. doi: 10.1097/01.mph.0000165131.94544.a6. [DOI] [PubMed] [Google Scholar]
- 29.Nabi G, Dogra PN, Chowdhary A. Renal cell carcinoma presenting as acute pancreatitis. Urol Int. 2002;68:202–203. doi: 10.1159/000048452. [DOI] [PubMed] [Google Scholar]
- 30.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 31.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–424. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 32.Stuckey BG, Gutteridge DH, Kent GN, et al. Familial hypocalciuric hypercalcaemia and pancreatitis: no causal link proven. Aust N Z J Med. 1990;20:718–719. 725. doi: 10.1111/j.1445-5994.1990.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 33.Bruce JI, Yang X, Ferguson CJ, et al. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 34.Racz GZ, Kittel A, Riccardi D, et al. Extracellular calcium sensing receptor in human pancreatic cells. Gut. 2002;51:705–711. doi: 10.1136/gut.51.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaRusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011;27:467–474. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murugaian EE, Premkumar RM, Radhakrishnan L, et al. Novel mutations in the calcium sensing receptor gene in tropical chronic pancreatitis in india. Scand J Gastroenterol. 2008;43:117–121. doi: 10.1080/00365520701580413. [DOI] [PubMed] [Google Scholar]
- 37.Felderbauer P, Klein W, Bulut K, et al. Mutations in the calcium-sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006;41:343–348. doi: 10.1080/00365520510024214. [DOI] [PubMed] [Google Scholar]
- 38.Kapoor A, Satishchandra P, Ratnapriya R, et al. An idiopathic epilepsy syndrome linked to 3q13.3-q21 and missense mutations in the extracellular calcium sensing receptor gene. Ann Neurol. 2008;64:158–167. doi: 10.1002/ana.21428. [DOI] [PubMed] [Google Scholar]
- 39.Muddana V, Lamb J, Greer JB, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor kazal 1type and alcohol. World J Gastroenterol. 2008;14:4486–4491. doi: 10.3748/wjg.14.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stepanchick A, McKenna J, McGovern O, et al. Calcium sensing receptor mutations implicated in pancreatitis and idiopathic epilepsy syndrome disrupt an arginine-rich retention motif. Cell Physiol Biochem. 2010;26:363–374. doi: 10.1159/000320560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terranegra A, Ferraretto A, Dogliotti E, et al. Calcimimetic r-568 effects on activity of r990g polymorphism of calcium-sensing receptor. J Mol Endocrinol. 2010;45:245–256. doi: 10.1677/JME-10-0034. [DOI] [PubMed] [Google Scholar]
- 42.Felderbauer P, Karakas E, Fendrich V, et al. Pancreatitis in primary hyperparathyroidism-related hypercalcaemia is not associated with mutations in the CASR gene. Exp Clin Endocrinol Diabetes. 2007;115:527–529. doi: 10.1055/s-2007-981455. [DOI] [PubMed] [Google Scholar]
- 43.Sutton R, Criddle D, Raraty MG, et al. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497–505. doi: 10.1159/000075581. [DOI] [PubMed] [Google Scholar]
- 44.Husain SZ, Grant WM, Gorelick FS, et al. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1594–G1599. doi: 10.1152/ajpgi.00500.2006. [DOI] [PubMed] [Google Scholar]
- 45.Shah AU, Sarwar A, Orabi AI, et al. Protease activation during in vivo pancreatitis is dependent upon calcineurin activation. Am J Physiol Gastrointest Liver Physiol. 2009;5:6967–6973. doi: 10.1152/ajpgi.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hietaranta AJ, Saluja AK, Bhagat L, et al. Relationship between nf-[kappa]b and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388–395. doi: 10.1006/bbrc.2000.4120. [DOI] [PubMed] [Google Scholar]
- 47.Mithofer K, Fernandez-del Castillo C, Frick TW, et al. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995;109:239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 48.Sutton R, Petersen OH, Pandol SJ. Pancreatitis and calcium signalling: report of an international workshop. Pancreas. 2008;36:e1–14. doi: 10.1097/MPA.0b013e3181675010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mooren F, Hlouschek V, Finkes T, et al. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem. 2003;278:9361–9369. doi: 10.1074/jbc.M207454200. [DOI] [PubMed] [Google Scholar]
- 50.Perides G, Laukkarinen JM, Vassileva G, et al. Biliary acute pancreatitis in mice is mediated by the g protein-coupled cell surface bile acid receptor gpbar1. Gastroenterology. 2009;2:715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward JB, Sutton R, Jenkins SA, et al. Progressive disruption of acinar cell calcium signaling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology. 1996;111:481–491. doi: 10.1053/gast.1996.v111.pm8690215. [DOI] [PubMed] [Google Scholar]
- 52.Heath H., III Clinical spectrum of primary hyperparathyroidism: evolution with changes in medical practice and technology. J Bone Miner Res. 1991;6(suppl 2):S63–S70. doi: 10.1002/jbmr.5650061415. discussion S83–S64. [DOI] [PubMed] [Google Scholar]
- 53.Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol. 2010;24:323–335. doi: 10.1016/j.bpg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Bellantone R, Raffaelli M, Traini E, et al. Minimally-invasive parathyroid surgery. Acta Otorhinolaryngol Ital. 2011;31:207–215. [PMC free article] [PubMed] [Google Scholar]
- 55.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]


