Abstract
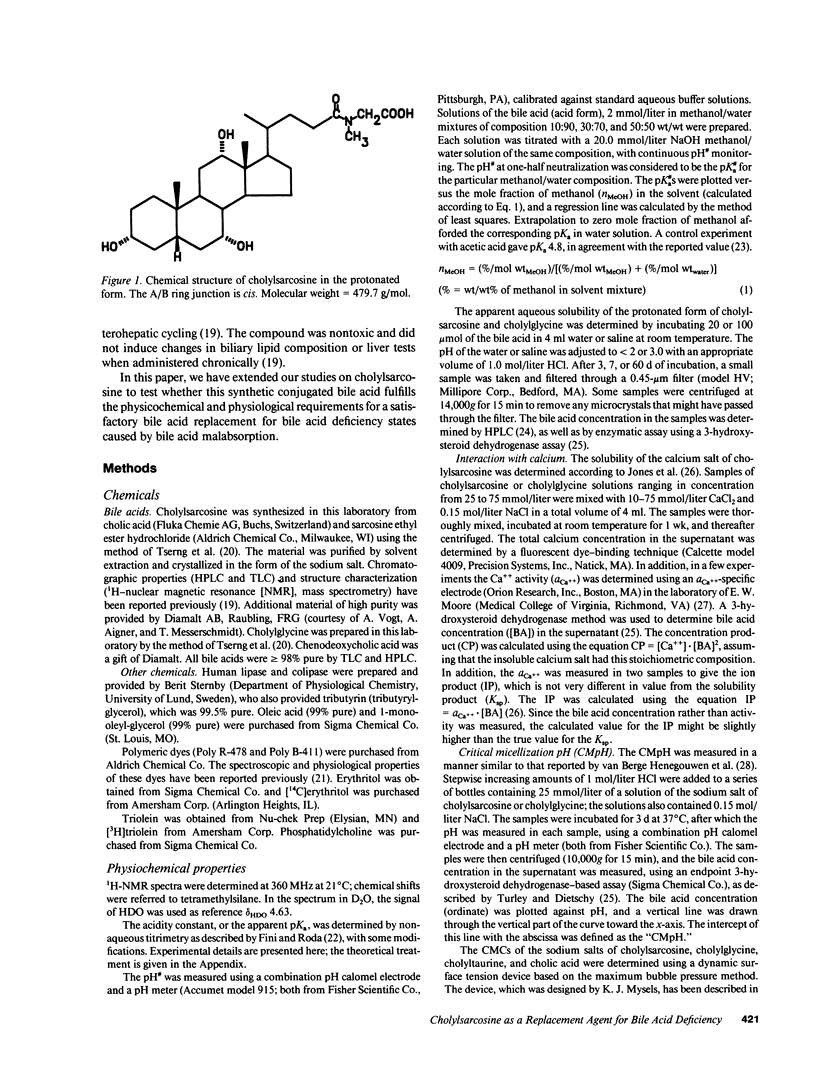
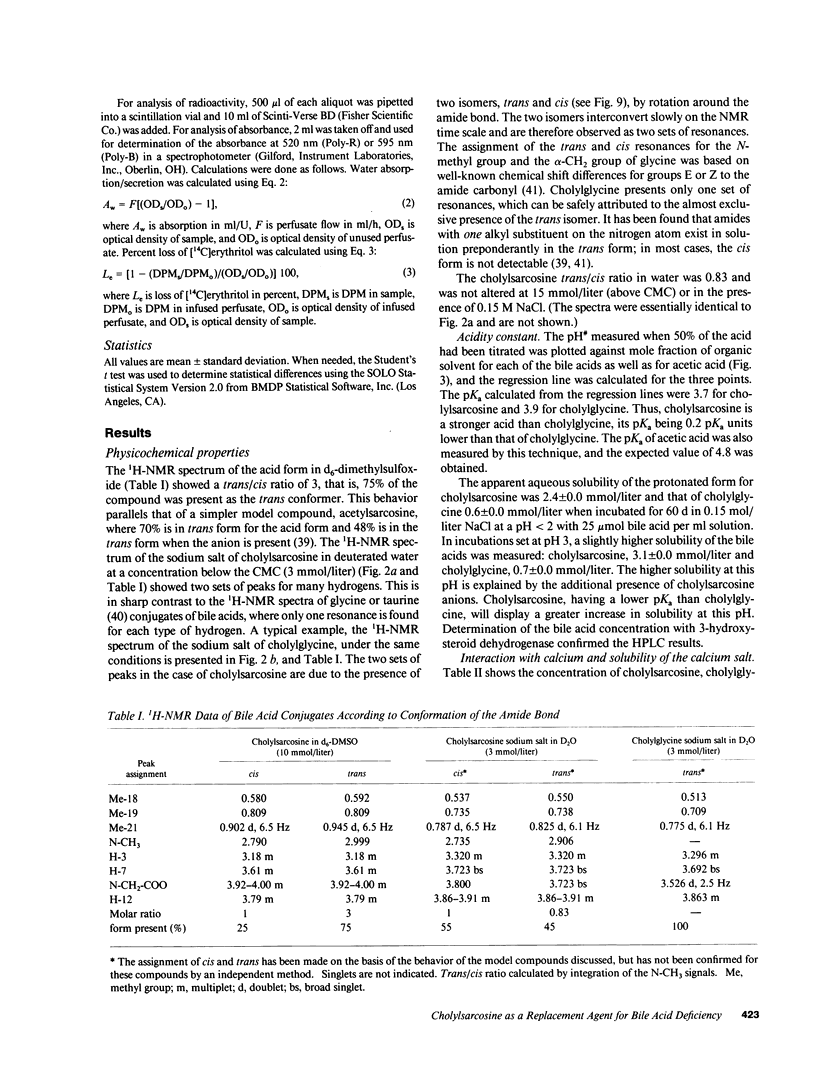
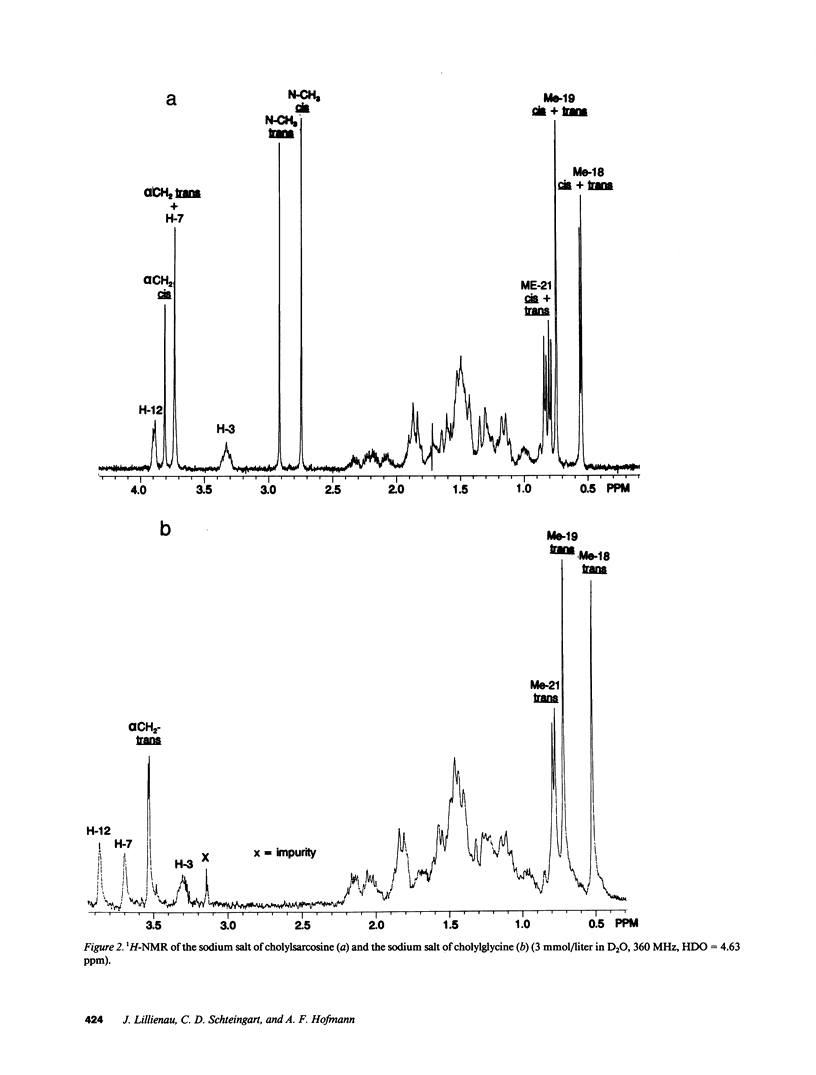
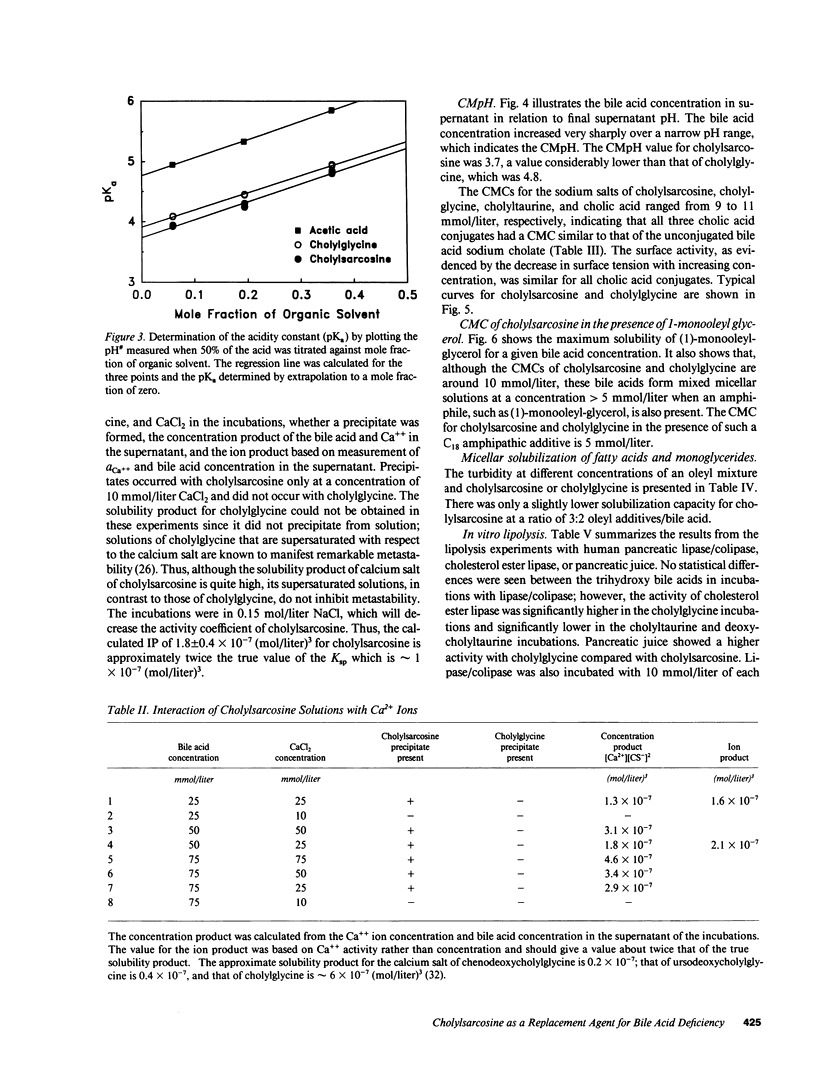
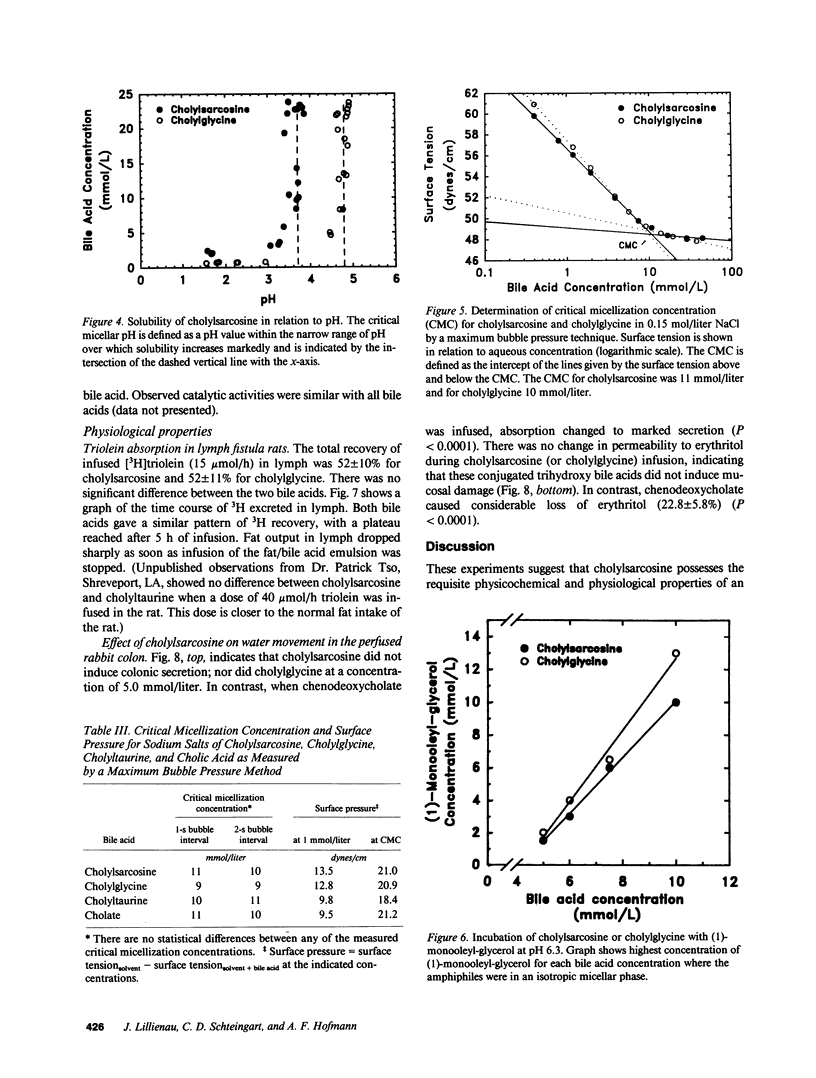
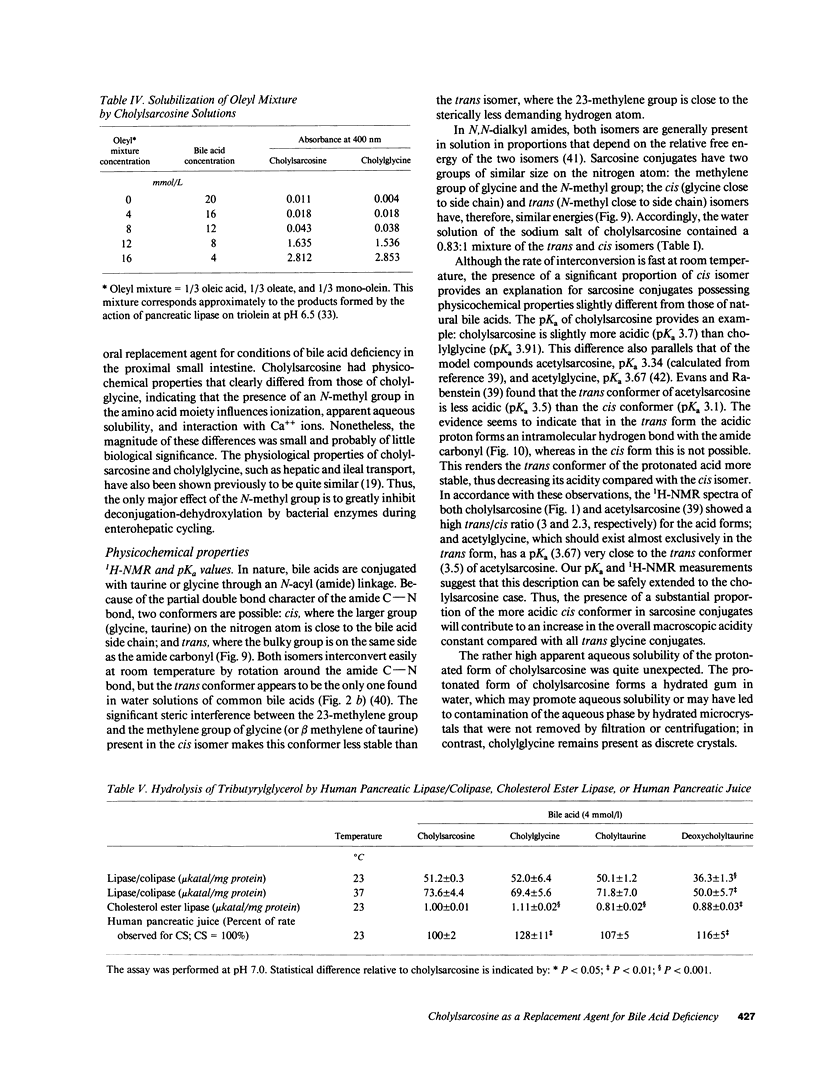
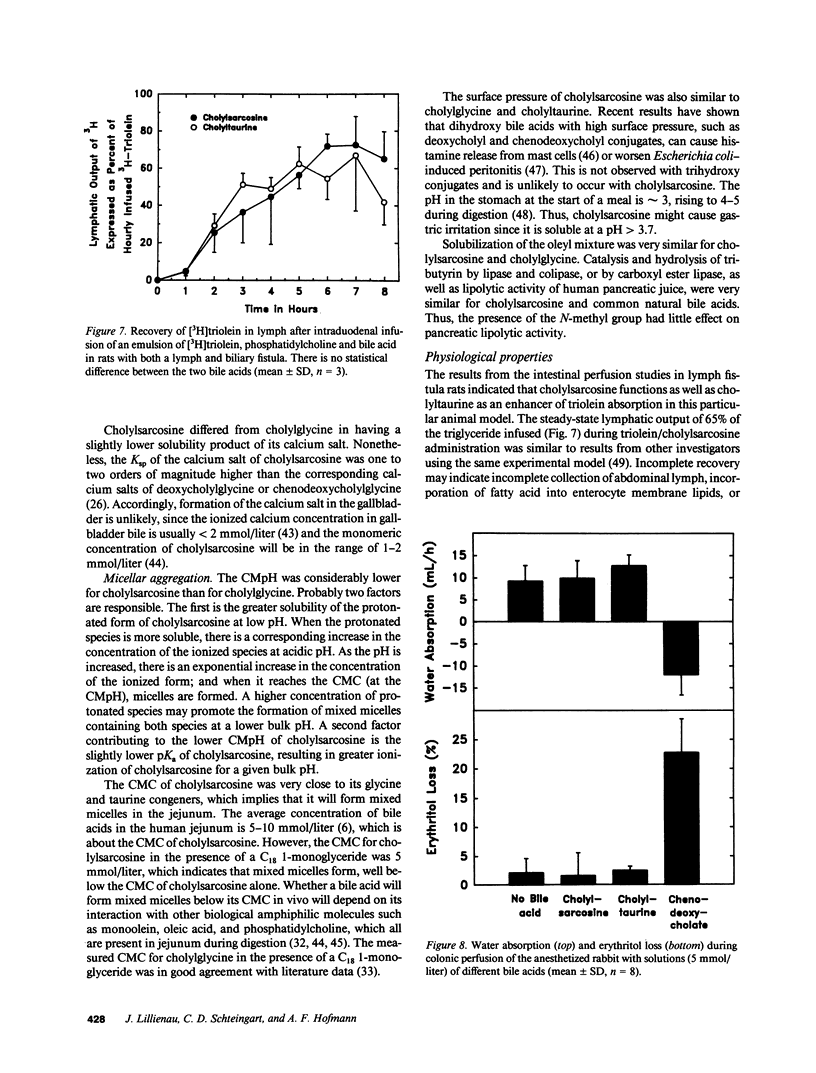
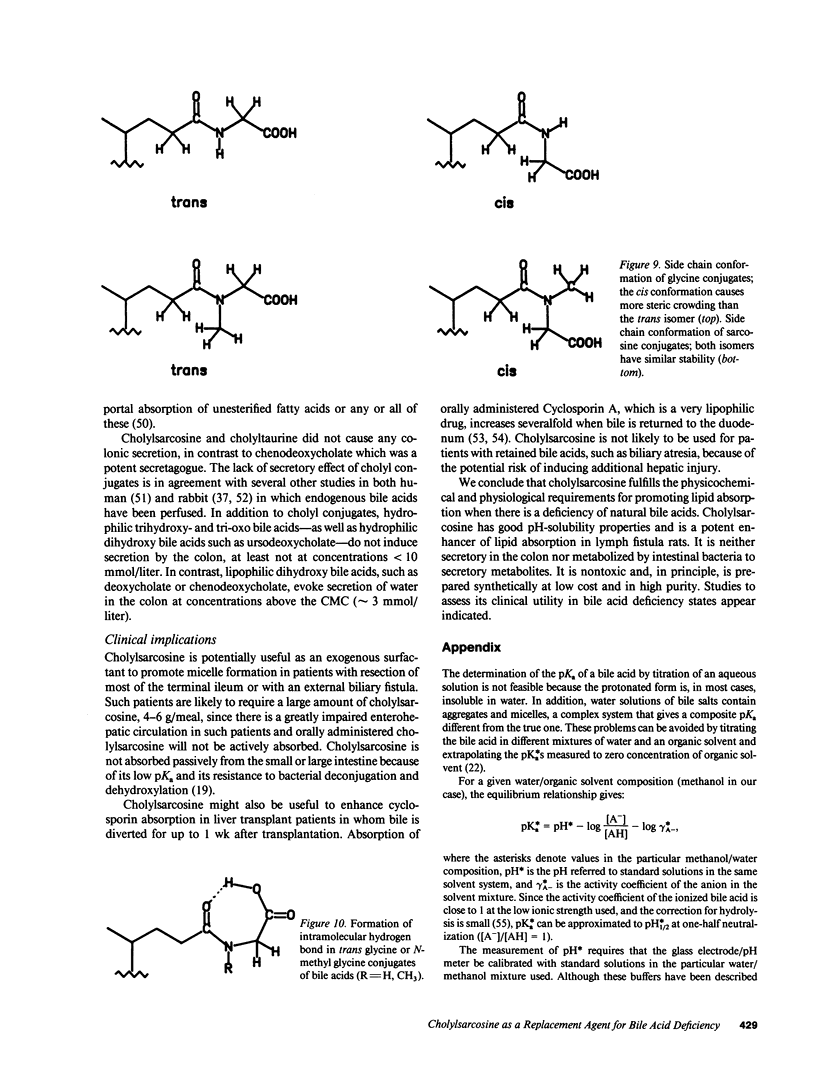
The properties of cholylsarcosine (the synthetic N-acyl conjugate of cholic acid with sarcosine [N-methylglycine]) were examined to determine its suitability as a bile acid replacement agent for conditions of bile acid deficiency in the small intestine, which causes fat malabsorption. Previous studies in rodents had shown that the compound was well transported by the liver and ileum and underwent neither deconjugation nor dehydroxylation during enterohepatic cycling. By 1H-nuclear magnetic resonance, cholylsarcosine was found to exist in dilute aqueous solution as an almost equimolar mixture of two geometric isomers--cis and trans (around the amide bond)--in contrast to cholylglycine, which was present entirely in the trans form. The critical micellization concentration was 11 mmol/liter, similar to that of cholylglycine (10 mmol/liter). By nonaqueous titrimetry, the pKa' of cholylsarcosine was 3.7, only slightly lower than that of cholylglycine (3.9). Cholylsarcosine was poorly soluble below pH 3.7, but highly soluble above pH 4. In vitro, cholylsarcosine behaved as cholylglycine with respect to promoting lipolysis by lipase/colipase. There was little difference between cholylsarcosine and cholylglycine in their solubilization of an equimolar mixture of oleic acid, oleate, and monoolein (designed to simulate digestive products of triglyceride) or in their solubilization of monooleyl-glycerol alone. When a [3H]triolein emulsion with either cholylsarcosine or cholyltaurine was infused intraduodenally in biliary fistula rats, recovery of 3H in lymph was 52 +/- 10% (mean +/- SD) for cholylsarcosine and 52 +/- 11% for cholyltaurine. When perfused into the colon of the anesthetized rabbit, cholylsarcosine (5 mmol/liter) did not influence water absorption or permeability to erythritol, in contrast to chenodeoxycholate, which induced vigorous water secretion and caused erythritol loss. We conclude that cholylsarcosine possesses the physicochemical and physiological properties required for a suitable bile acid replacement in deficiency states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. V., Phillips S. F. Inhibition of colonic water and electrolyte absorption by fatty acids in man. Gastroenterology. 1973 Nov;65(5):744–749. [PubMed] [Google Scholar]
- Andersson R., Tranberg K. G., Lillienau J., Schalén C., Srinivas U., Larsson L., Sonesson A., Bengmark S. Influence of individual bile acids in Escherichia coli peritonitis. Scand J Gastroenterol. 1990 Nov;25(11):1129–1136. doi: 10.3109/00365529008998545. [DOI] [PubMed] [Google Scholar]
- Austad W. I., Lack L., Tyor M. P. Importance of bile acids and of an intact distal small intestine for fat absorption. Gastroenterology. 1967 Apr;52(4):638–646. [PubMed] [Google Scholar]
- BOYD H., HELFRICK F. Celiac disease treated with polyoxyethylene sorbitan monooleate. J Pediatr. 1951 Apr;38(4):493–497. doi: 10.1016/s0022-3476(51)80035-0. [DOI] [PubMed] [Google Scholar]
- Bergstedt S. E., Hayashi H., Kritchevsky D., Tso P. A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. Am J Physiol. 1990 Sep;259(3 Pt 1):G386–G393. doi: 10.1152/ajpgi.1990.259.3.G386. [DOI] [PubMed] [Google Scholar]
- Bright-Asare P., Binder H. J. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973 Jan;64(1):81–88. [PubMed] [Google Scholar]
- Chadwick V. S., Gaginella T. S., Carlson G. L., Debongnie J. C., Phillips S. F., Hofmann A. F. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979 Nov;94(5):661–674. [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology. 1977 Aug;73(2):247–251. [PubMed] [Google Scholar]
- DUBOIS J. J., HOLT P. R., KURON G. W., HASHIM S. A., VANITALLIE T. B. EFFECT OF TWEEN 80 ON CHOLESTYRAMINE-INDUCED MALABSORPTION. Proc Soc Exp Biol Med. 1964 Oct;117:226–229. doi: 10.3181/00379727-117-29542. [DOI] [PubMed] [Google Scholar]
- Duane W. C. The intermicellar bile salt concentration in equilibrium with the mixed-micelles of human bile. Biochim Biophys Acta. 1975 Aug 25;398(2):275–286. doi: 10.1016/0005-2760(75)90143-5. [DOI] [PubMed] [Google Scholar]
- Dupas J. L., Moreau M., Hofmann A. F. Polymeric dyes: useful nonabsorbable reference markers for intestinal perfusion studies in animals. J Pharm Sci. 1985 Mar;74(3):328–330. doi: 10.1002/jps.2600740322. [DOI] [PubMed] [Google Scholar]
- Erlanson C., Borgström B. Purification and further characterization of co-lipase from porcine pancreas. Biochim Biophys Acta. 1972 Jul 21;271(2):400–412. doi: 10.1016/0005-2795(72)90215-2. [DOI] [PubMed] [Google Scholar]
- Evans C. A., Rabenstein D. L. Nuclear magnetic resonance studies of the acid-base chemistry of amino acids and peptides. II. Dependence of the acidity of the C-terminal carboxyl group on the conformation of the C-terminal peptide bond. J Am Chem Soc. 1974 Nov 13;96(23):7312–7317. doi: 10.1021/ja00830a023. [DOI] [PubMed] [Google Scholar]
- Fini A., Roda A. Chemical properties of bile acids. IV. Acidity constants of glycine-conjugated bile acids. J Lipid Res. 1987 Jul;28(7):755–759. [PubMed] [Google Scholar]
- Fordtran J. S., Bunch F., Davis G. R. Ox bile treatment of severe steatorrhea in an ileectomy-ileostomy patient. Gastroenterology. 1982 Mar;82(3):564–568. [PubMed] [Google Scholar]
- HOFMANN A. F. THE FUNCTION OF BILE SALTS IN FAT ABSORPTION. THE SOLVENT PROPERTIES OF DILUTE MICELLAR SOLUTIONS OF CONJUGATED BILE SALTS. Biochem J. 1963 Oct;89:57–68. doi: 10.1042/bj0890057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison W. G., Rosenberg I. H. Bile-salt deficiency in the steatorrhea following resection of the ileum and proximal colon. N Engl J Med. 1967 Aug 17;277(7):337–342. doi: 10.1056/NEJM196708172770704. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F., Poley J. R. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972 May;62(5):918–934. [PubMed] [Google Scholar]
- Huijbregts A. W., Cox T. M., Hermsen J., van Berge Henegouwen G. P., van Schaik A., Chadwick V. S. Micellar solubilization of intestinal lipids after ursodeoxycholic acid therapy in short bowel patients and healthy controls. Neth J Med. 1981;24(3):108–113. [PubMed] [Google Scholar]
- King R. F., Howdle P. D., Kelleher J., Losowsky M. S. Synthetic detergents in bile-salt-deficient steatorrhoea. Clin Sci (Lond) 1979 Mar;56(3):273–281. doi: 10.1042/cs0560273. [DOI] [PubMed] [Google Scholar]
- LaRusso N. F., Thistle J. L. Ursodeoxycholic acid ingestion after ileal resection. Effect on biliary bile acid and lipid composition. Dig Dis Sci. 1981 Aug;26(8):705–709. doi: 10.1007/BF01316859. [DOI] [PubMed] [Google Scholar]
- Maislos M., Shany S. Bile salt deficiency and the absorption of vitamin D metabolites. In vivo study in the rat. Isr J Med Sci. 1987 Nov;23(11):1114–1117. [PubMed] [Google Scholar]
- McLeod G. M., Wiggins H. S. Bile-salts in small intestinal contents after ileal resection and in other malabsorption syndromes. Lancet. 1968 Apr 27;1(7548):873–876. doi: 10.1016/s0140-6736(68)90235-3. [DOI] [PubMed] [Google Scholar]
- Mehta M. U., Venkataramanan R., Burckart G. J., Ptachcinski R. J., Delamos B., Stachak S., Van Thiel D. H., Iwatsuki S., Starzl T. E. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988 May;25(5):579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekjian H. S., Phillips S. F., Hofmann A. F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971 Aug;50(8):1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. W. The role of calcium in the pathogenesis of gallstones: Ca++ electrode studies of model bile salt solutions and other biologic systems. With an hypothesis on structural requirements for Ca++ binding to proteins and bile acids. Hepatology. 1984 Sep-Oct;4(5 Suppl):228S–243S. [PubMed] [Google Scholar]
- Naoumov N. V., Tredger J. M., Steward C. M., O'Grady J. G., Grevel J., Niven A., Whiting B., Williams R. Cyclosporin A pharmacokinetics in liver transplant recipients in relation to biliary T-tube clamping and liver dysfunction. Gut. 1989 Mar;30(3):391–396. doi: 10.1136/gut.30.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poley J. R., Hofmann A. F. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology. 1976 Jul;71(1):38–44. [PubMed] [Google Scholar]
- Quist R. G., Ton-Nu H. T., Lillienau J., Hofmann A. F., Barrett K. E. Activation of mast cells by bile acids. Gastroenterology. 1991 Aug;101(2):446–456. doi: 10.1016/0016-5085(91)90024-f. [DOI] [PubMed] [Google Scholar]
- Roda A., Hofmann A. F., Mysels K. J. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem. 1983 May 25;258(10):6362–6370. [PubMed] [Google Scholar]
- Rossi S. S., Converse J. L., Hofmann A. F. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J Lipid Res. 1987 May;28(5):589–595. [PubMed] [Google Scholar]
- Schmassmann A., Angellotti M. A., Ton-Nu H. T., Schteingart C. D., Marcus S. N., Rossi S. S., Hofmann A. F. Transport, metabolism, and effect of chronic feeding of cholylsarcosine, a conjugated bile acid resistant to deconjugation and dehydroxylation. Gastroenterology. 1990 Jan;98(1):163–174. doi: 10.1016/0016-5085(90)91306-q. [DOI] [PubMed] [Google Scholar]
- Stark R. E., Storrs R. W., Levine S. E., Yee S., Broido M. S. One- and two-dimensional NMR relaxation studies of dynamics and structure in bile salt-phosphatidylcholine mixed micelles. Biochim Biophys Acta. 1986 Aug 21;860(2):399–410. doi: 10.1016/0005-2736(86)90536-5. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Hachey D. L., Klein P. D. An improved procedure for the synthesis of glycine and taurine conjugates of bile acids. J Lipid Res. 1977 May;18(3):404–407. [PubMed] [Google Scholar]
- Tso P., Lindström M. B., Borgström B. Factors regulating the formation of chylomicrons and very-low-density lipoproteins by the rat small intestine. Biochim Biophys Acta. 1987 Dec 14;922(3):304–313. doi: 10.1016/0005-2760(87)90053-1. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978 Sep;19(7):924–928. [PubMed] [Google Scholar]
- Van Deest B. W., Fordtran J. S., Morawski S. G., Wilson J. D. Bile salt and micellar fat concentration in proximal small bowel contents of ileectomy patients. J Clin Invest. 1968 Jun;47(6):1314–1324. doi: 10.1172/JCI105823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. W., Meldrum S. J., Riddle H. C., Brown R. L., Sladen G. E. pH profile of gut as measured by radiotelemetry capsule. Br Med J. 1972 Apr 8;2(5805):104–106. doi: 10.1136/bmj.2.5805.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berge-Henegouwen G. P., Hofmann A. F., Gaginella T. S. Pharmacology of chenodeoxycholic acid. I. Pharmaceutical properties. Gastroenterology. 1977 Aug;73(2):291–299. [PubMed] [Google Scholar]


