Abstract
Background
South Asians are at high risk for chronic kidney disease. However, unlike those in the United States and United Kingdom, laboratories in South Asian countries do not routinely report estimated glomerular filtration rate (eGFR) when serum creatinine is measured. The objectives of the study were to: (1) evaluate the performance of existing GFR estimating equations in South Asians, and (2) modify the existing equations or develop a new equation for use in this population.
Study Design
Cross-sectional population-based study.
Setting & Participants
581 participants 40 years or older were enrolled from 10 randomly selected communities and renal clinics in Karachi.
Predictors
eGFR, age, sex, serum creatinine level.
Outcomes
Bias (the median difference between measured GFR [mGFR] and eGFR), precision (the IQR of the difference), accuracy (P30; percentage of participants with eGFR within 30% of mGFR), and the root mean squared error reported as cross-validated estimates along with bootstrapped 95% CIs based on 1,000 replications.
Results
The CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation performed better than the MDRD (Modification of Diet in Renal Disease) Study equation in terms of greater accuracy at P30 (76.1% [95% CI, 72.7%–79.5%] vs 68.0% [95% CI, 64.3%–71.7%]; P <0.001) and improved precision (IQR, 22.6 [95% CI, 19.9–25.3] vs 28.6 [95% CI, 25.8–31.5] mL/min/1.73 m2; P < 0.001). However, both equations overestimated mGFR. Applying modification factors for slope and intercept to the CKD-EPI equation to create a CKD-EPI Pakistan equation (such that eGFRCKD-EPI(PK) = 0.686 × eGFRCKD-EPI1.059) in order to eliminate bias improved accuracy (P30, 81.6% [95% CI, 78.4%–84.8%]; P < 0.001) comparably to new estimating equations developed using creatinine level and additional variables.
Limitations
Lack of external validation data set and few participants with low GFR.
Conclusions
The CKD-EPI creatinine equation is more accurate and precise than the MDRD Study equation in estimating GFR in a South Asian population in Karachi. The CKD-EPI Pakistan equation further improves the performance of the CKD-EPI equation in South Asians and could be used for eGFR reporting.
INDEX WORDS: Glomerular filtration rate (GFR), estimating equations, South Asians, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Pakistan, renal function
Chronic kidney disease (CKD) is now recognized as a global public health challenge.1 The adverse outcomes of CKD, including kidney failure, accelerated cardiovascular disease, and premature mortality, have a profound impact on the national economies of low- and middle-income countries.2 The problem is magnified further in the South Asian populations of India, Pakistan, Bangladesh, and Sri Lanka, where risk factors for CKD, including hypertension and diabetes, are widely prevalent and increasing.3–5
Clinical assessment of kidney function is a routine part of medical practice for overall health evaluation, drug dosing, and administration of intravenous contrast for diagnostic testing and therapeutic procedures.6 Glomerular filtration rate (GFR) is widely regarded as the best indicator of kidney function in both health and disease. GFR can be assessed best by measuring urinary clearance of an ideal exogenous marker like inulin. However, measuring GFR is costly, time consuming, and difficult to do. Thus, GFR estimating equations based on serum creatinine level, age, sex, and body size have been developed and are recommended for routine use in clinical practice.
In the United States and United Kingdom, estimated GFR (eGFR) is reported by >75% of clinical laboratories when serum creatinine is measured, using either the isotope-dilution mass spectrometry (IDMS)-traceable 4-variable MDRD (Modification of Diet in Renal Disease) Study equation or the more recently developed CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.7–12 A recent systematic review showed that this CKD-EPI equation was more accurate in estimating measured GFR (mGFR) than the MDRD Study equation, and a recent meta-analysis showed that GFR estimates based on the CKD-EPI equation more accurately predict the risk of progression to kidney failure and mortality than those based on the MDRD Study equation.13,14 Clinical laboratories in South Asian countries do not routinely report eGFR, in part because both muscle mass and meat intake are lower in South Asian populations compared with North American and European populations, which might lead to inaccuracy of eGFR, and neither of these equations has been validated in South Asian general populations.15 Therefore, we performed a cross-sectional population-based study of adults 40 years and older in Karachi, Pakistan, to: (1) evaluate the accuracy of existing creatinine-based GFR estimating equations in this South Asian population, and (2) improve the accuracy of GFR estimation by modifying existing GFR estimating equations or developing a new equation for use in the South Asian population.
METHODS
Study Design and Participants
Participants were drawn from 2 sources. The first was a population-based sample from 10 randomly selected low- to middle-income communities in Karachi. Because the general population was expected to have few people with decreased kidney function, we enriched our sample with 40 patients with a serum creatinine level ≥2.0 mg/dL from the renal clinic (Fig S1, available as online supplementary material), so that 20% of our study sample would have a GFR <60 mL/min/1.73 m2 (ie, CKD stage 3 or worse). The details regarding recruitment and stratification have been published previously.16 The Aga Khan University Ethics Review Committee approved the study.
Screening Visit and Visit to Research Laboratory
All participants attended the research clinic in the morning after an overnight fast with a single 24-hour urine collection. Details of assessment have been published.16
Laboratory Measurements
As previously described, blood samples were collected for measurement of serum creatinine (Jaffé kinetic rate reaction method with alkaline picrate solution with SynchronCX7 Delta analyzer [Beckman Coulter Inc]), hemoglobin, fasting blood glucose, serum albumin, and serum urea nitrogen. Urine was collected for 24 hours for measurement of urine creatinine, albumin, and urea nitrogen.16 All assessments were performed to a standard protocol that conformed to international standards for definitions, and measurements and external quality control were performed by the Bio-Rad Laboratories Inc, Irvine, CA.
Calibration of Serum Creatinine Assays
Serum creatinine assays were calibrated to the Roche enzymatic method (Roche-Hitachi P-Module instrument with Roche Creatininase Plus assay; Hoffman-La Roche Ltd), traceable to National Institute of Standards and Technology (NIST) creatinine standard reference material 967 at the Cleveland Clinic.17 The values from Pakistan were higher than the Roche enzymatic method at the Cleveland Clinic by a mean of 0.21 (standard error, 0.01) mg/dL and hence a calibration factor [(−0.1256) + 0.9557×] based on a significant intercept and slope was derived (Fig S2).
GFR Measurement
GFR was measured (mGFR) using urinary clearance of inulin as the reference standard.16 A loading dose of 30 mg/kg of Inutest 25% (Fresenius Kabi, Austria) was given, followed by a continuous infusion of 40 mg/kg of inulin diluted in a 10% mannitol solution. Two clearance periods of 30 minutes each were analyzed after an equilibration period of 45 minutes. If urine flow rate differed by 30 mL between the 2 periods, a third 30-minute clearance was performed. Inulin in plasma and urine samples was assayed using an enzymatic method at the Renal Laboratories, Saint-Etienne Hospital, University of Jean Monnet, Saint-Etienne, France. GFR was measured as the mean of at least 2 urinary clearance periods of inulin and expressed per 1.73 m2 of body surface area (BSA) by multiplying measured values by 1.73/BSA (BSA =height0.725[cm] × body weight0.425[kg] × 0.007184). mGFR adjusted for BSA served as the gold standard for comparison.
Statistical Analysis
Analyses were performed using Stata Statistical Software, release 12.1 (StataCorp LP). Baseline characteristics of the study population were presented as overall and compared across the 3 categories of mGFR (ie, ≥90, 60–89, and <60 mL/min/1.73 m2). Mean values and proportions were compared using one-way analysis of variance and χ2 tests, respectively, whereas the median eGFRs for the MDRD Study and CKD-EPI equations were compared using the Kruskal-Wallis test. Performance of the MDRD Study and CKD-EPI equations was assessed for the entire data set of 581 participants. Using the performance metrics, the correction factors for modification of the CKD-EPI equation were derived, new estimating equations were developed, and their performance was assessed.
Modification of the CKD-EPI Equation
Correction factors for the CKD-EPI equation were derived from the linear regression models of ln(mGFR) versus ln(eGFR) calculated by the CKD-EPI equation. The statistically significant (P <0.05) intercept and slopes were back transformed to exponential form and used as correction factors to modify the equation to the CKD-EPI Pakistan equation, referred to here as CKD-EPIPK (eGFRs calculated with this equation denoted as eGFRCKD-EPI(PK)).
Development of New Estimating Equations
We developed new GFR estimating equations based on this Pakistani population to assess whether their performance was substantially better than the existing or modified equations. Because a 2-slope spline for serum creatinine cannot be assessed in the same model as linear or other functions of linear terms of the same variable, 2 regression models were built. Each equation was developed using stepwise linear regression to relate mGFR to serum creatinine level and clinical characteristics available in the data set with forward selection using an entry and removal criteria of P = 0.05 and P = 0.20, respectively. We transformed mGFR and serum creatinine level to the natural logarithmic scale to reflect their multiplicative (inverse) relationship and stabilize variance across the range of GFRs. For the first model, spline terms of the natural logarithm of serum creatinine were used. For the second model, we used a linear term for the natural logarithm of serum creatinine along with quadratic and cubic terms as the main predictors. Details of these models are presented in Item S1. Other covariates for both models were age, sex, body mass index (BMI), waist circumference, total body fat, hemoglobin level, serum albumin level, serum urea nitrogen level, fasting blood glucose level, blood pressure, hypertension, and diabetes status. All continuous variables were used in their natural (untransformed) form.
Metrics for Equation Performance
The metrics for comparison of estimation equations with mGFR were bias, precision, root mean squared error (RMSE), and accuracy with 95% confidence intervals (CIs). Bias was expressed as the median of the differences between mGFR and eGFR (ie, mGFR − eGFR), with negative values indicating overestimation of GFR. Precision was expressed as the inter-quartile range (IQR) of the differences of mGFR and eGFR. RMSE was defined as the square root of the average squared difference of mGFR and eGFR. Accuracy (P20 and P30) was defined as the percentage of individuals with eGFRs within 20% and 30% of mGFR, respectively. The CIs for all these estimates were computed with normal approximation by the bootstrap method based on 1,000 replications with predefined seed. P values for assessing differences in RMSE, and IQR, of various GFR estimating equations were computed by paired t test on 1,000 bootstrapped estimates, whereas P values for P20 and P30 were computed by McNemar test on the observed data. The differences between estimates of various estimating equations were considered statistically significant when 95% CIs were nonoverlapping or P values were <0.05, and substantially different when both criteria were met.
We also compared eGFR and mGFR for each participant graphically by plotting the bias (mGFR − eGFR) against eGFR to assess the performance of each equation. The smoothed regression lines were plotted using 95% of the data and the 95% CIs were based on the quantile regressions of the 10th and 90th percentiles of the bias.
Cross-validation of Modified and New Equations
We bootstrapped 500 samples with replacement from the original data (N = 581) to internally validate the modified CKD-EPI equation and new estimating equations developed in this data set.18,19 Based on the method described by Harrell et al,20 estimates and 95% CIs were validated by adding or subtracting the optimism indexes calculated as the differences of estimates drawn from the equations developed on bootstrapped samples and after applying those on the original data set. Details of cross-validation are given in Item S1 and tables a-d of Item S2. The comparison of performance was done in the overall study population, as well as in participants with eGFRCKD-EPI(PK) ≥90 and <90 mL/min/1.73 m2.
Comparison of Modified and Newly Developed Estimating Equations by Subgroups
In order to assess generalizability among various subgroups, we evaluated the modified CKD-EPI (CKD-EPIPK) and the new estimating equations in subgroups defined by clinical characteristics, including the presence or absence of hypertension or/ and diabetes, as well as factors related to muscle mass and diet, including BMI (<18.5, 18.5–24.9, 25.0–29.9, and ≥30.0 kg/m2) and tertiles of urine creatinine and urine urea nitrogen excretion.14,15
RESULTS
Participant Characteristics
Characteristics of the study population are described in Table 1. Of 581 enrolled participants, 50% were men. Median mGFR, eGFRMDRD, and creatinine-based eGFRCKD-EPI values were 91.0 (IQR, 36.7), 100.5 (IQR, 40.4), and 104.4 (IQR, 25.4) mL/min/1.73 m2, respectively. Participants with mGFR <60 mL/min/ 1.73 m2 were older than those with higher mGFRs (P <0.001) and had greater waist circumference (P = 0.02), lower lean body mass (P = 0.007), lower hemoglobin level (P <0.001), and higher systolic blood pressure (P = 0.001) compared with those with higher mGFRs.
Table 1.
Characteristics of Study Participants
| Characteristics | Overall (N = 581) | mGFR (mL/min/1.73 m2)
|
Pa | ||
|---|---|---|---|---|---|
| ≥90 (n = 300) | 60–89 (n = 188) | <60 (n = 93) | |||
| Age (y) | 50.6 ± 10.0 | 47.2 ± 7.2 | 51.8 ± 10.3 | 59.0 ± 11.7 | <0.001 |
| Male sex | 292 (50.3) | 144 (48.0) | 100 (53.2) | 48 (51.6) | 0.5 |
| Clinical status | <0.001 | ||||
| Hypertension onlyb | 197 (33.9) | 99 (33.0) | 69 (36.7) | 29 (31.2) | |
| Diabetes onlyc | 163 (28.1) | 99 (33.0) | 42 (22.3) | 22 (23.7) | |
| Both | 33 (5.7) | 9 (3.0) | 3 (1.6) | 21 (22.6) | |
| Neither | 188 (32.4) | 93 (31.0) | 74 (39.4) | 21 (22.6) | |
| Weight (kg) | 66.1 ± 13.2 | 66.9 ± 12.4 | 65.0 ± 13.6 | 66.0 ± 14.9 | 0.3 |
| Height (cm) | 159.7 ± 9.1 | 159.8 ± 8.7 | 159.9 ± 9.8 | 158.7 ± 8.8 | 0.6 |
| Body surface area (m2) | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 0.4 |
| Body mass index (kg/m2) | 26.0 ± 5.1 | 26.2 ± 4.9 | 25.4 ± 4.8 | 26.2 ± 6.0 | 0.2 |
| Waist circumference (cm) | 93.3 ± 11.7 | 93.0 ± 10.7 | 92.4 ± 11.8 | 96.3 ± 14.1 | 0.02 |
| Total body fat (%) | 34.8 ± 9.1 | 34.2 ± 8.9 | 34.8 ± 9.0 | 36.6 ± 10.2 | 0.08 |
| Lean body mass (%) | 65.0 ± 9.4 | 65.8 ± 8.9 | 65.2 ± 9.0 | 62.3 ± 11.5 | 0.007 |
| Systolic BP (mm Hg) | 130 ± 21 | 127 ± 19 | 131 ± 23 | 136 ± 22 | 0.001 |
| Diastolic BP (mm Hg) | 81 ± 12 | 81 ± 11 | 80 ± 12 | 79 ± 13 | 0.3 |
| Hemoglobin (g/dL) | 12.5 ± 1.8 | 12.7 ± 1.8 | 12.6 ± 1.8 | 11.6 ± 1.8 | <0.001 |
| Serum albumin (g/dL) | 3.7 ± 0.3 | 3.7 ± 0.3 | 3.7 ± 0.3 | 3.5 ± 0.4 | <0.001 |
| Fasting plasma glucose (mg/dL) | 127 ± 65.2 | 136.8 ± 71.8 | 115.2 ± 50.1 | 119.1 ± 65.6 | 0.001 |
| Urine creatinine (g/d) | 1.0 ± 2.8 | 1.2 ± 3.9 | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.4 |
| Urine creatinine (mg/kg/d)d | 13.9 ± 5.0 | 14.4 ± 5.1 | 14.1 ± 5 | 11.9 ± 4.1 | <0.001 |
| Urine urea nitrogen (g/d)d | 5.1 ± 3.1 | 5.3 ± 2.5 | 4.8 ± 2.3 | 4.9 ± 5.6 | 0.3 |
| 24-h urine volume (L/d) | 1.4 ± 0.6 | 1.5 ± 0.6 | 1.3 ± 0.5 | 1.5 ± 0.6 | 0.01 |
| Serum creatinine (mg/dL)e | 0.9 ± 0.7 | 0.7 ± 0.2 | 0.8 ± 0.2 | 2.0 ± 1.4 | <0.001 |
| Serum urea nitrogen (mg/dL) | 13.3 ± 10.1 | 10.3 ± 3.7 | 11.5 ± 3.9 | 26.6 ± 18.6 | <0.001 |
| mGFR (mL/min/1.73 m2) | 91.0 [36.7] | 109.5 [24.5] | 80.8 [12.5] | 34.6 [32.0] | — |
| eGFRMDRD (mL/min/1.73 m2) | 100.5 [40.4] | 114.3 [25.3] | 93.4 [31.7] | 43.8 [47.5] | <0.001 |
| eGFRCKD-EPI (mL/min/1.73 m2) | 104.4 [25.4] | 110.2 [14.8] | 97.9 [23.8] | 45.0 [54.0] | <0.001 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factors for units: serum creatinine in mg/dL to μmol/L, ×88.4; serum urea nitrogen in mg/dL to mmol/L, ×0.357; fasting plasma glucose in mg/dL to mmol/L, ×0.05551; urine creatinine in g/d to mmol/d, ×8.84; urine urea nitrogen in g/d to mmol/d, ×35.7.
Abbreviations: BP, blood pressure; eGFR, glomerular filtration rate; eGFRCKD-EPI, eGFR calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine equation; eGFRMDRD, eGFR calculated by Modification of Diet in Renal Disease Study equation; mGFR, measured glomerular filtration rate.
P for differences across mGFR categories.
Hypertension defined as systolic BP ≥ 140 or diastolic BP ≥ 90 mm Hg or receiving antihypertensive medications.
Diabetes defined as fasting blood glucose ≥126 mg/dL or receiving antidiabetic medications.
Three missing values (n = 578).
Local serum creatinine assays were calibrated to isotope-dilution mass spectrometry at the Cleveland Clinic Research Laboratory for using Roche enzymatic method: y = [−0.1256 + (0.9557×)]; where y = standardized serum creatinine and x = local serum creatinine.
CKD-EPIPK and New Estimating Equations
Table 2 lists coefficients for the modified and newly developed equations. The regression of ln(mGFR) on ln(eGFR) computed using the CKD-EPI equation had intercept of −0.376 and slope of 1.059, both significantly different from 0 and 1, respectively (P <0.001). Therefore, the “Pakistani correction factor” for the CKD-EPI equation can be rendered as eGFRCKD-EPI(PK) = 0.686 × eGFRCKD-EPI1.059.
Table 2.
Regression Coefficients for CKD-EPIPK and New GFR Estimating Equations Developed in a Pakistani Population
| Variables | CKD-EPIPK | New Estimating Equation 1 | New Estimating Equation 2 |
|---|---|---|---|
| Constant | 0.686 (0.560 to 0.841) | 114.6 (87.0 to 150.9) | 97.0 (73.0 to 128.8) |
| CKD-EPI equation | 1.059 (1.014 to 1.104) | — | — |
| Age (y) | — | 0.992 (0.990 to 0.994) | 0.992 (0.99 to 0.994) |
| Sex | — | 0.902 (0.852 to 0.955) | 0.741 (0.694 to 0.791) |
| Body mass index (kg/m2) | — | 1.015 (1.007 to 1.022) | 1.015 (1.007 to 1.023) |
| Waist circumference (cm) | — | 0.996 (0.993 to 1.000) | 0.996 (0.993 to 1.000) |
| Hemoglobin (g/dL) | — | 1.016 (1.002 to 1.031) | 1.016 (1.001 to 1.031) |
| Fasting blood glucose (mg/dL) | — | 1.001 (1.000 to 1.001) | 1.001 (1.000 to 1.001) |
| Serum urea nitrogen (mg/dL) | — | 0.994 (0.990 to 0.998) | 0.993 (0.988 to 0.997) |
| Serum creatinine (mg/dL)a | — | −0.947 (−1.063 to −0.831) | |
| If ≤0.83 (for male)a | — | −0.286 (−0.442 to −0.130) | — |
| If >0.83 (for male)a | — | −1.064 (−1.174 to −0.953) | — |
| If ≤0.64 (for female)a | — | −0.286 (−0.442 to −0.130) | — |
| If >0.64 (for female)a | — | −1.064 (−1.174 to −0.953) | — |
| (ln[SCr])2,b | — | — | 0.762 (0.701 to 0.827) |
| (ln[SCr])3,c | — | — | 1.154 (1.068 to 1.246) |
Note: Values in parentheses are 95% confidence intervals.
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIPK, CKD-EPI equation with Pakistani correction factors; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
Regression coefficients in natural logarithm form whereas for all other variables, coefficients are transformed to exponential form.
Square of ln(SCr).
Cube of ln(SCr).
In addition, 2 new GFR estimating equations were developed based on this Pakistani population: equation 1 with a single sex-specific knot for serum creatinine level (for men, 0.83 mg/dL; and for women, 0.64 mg/dL), and equation 2 with the linear term of the natural logarithm of serum creatinine along with quadratic and cubic terms. Other statistically significant (P < 0.05) variables in both models were age, sex, BMI, waist circumference, hemoglobin level, fasting blood glucose level, and serum urea nitrogen level (Table 2).
Formulas for the existing MDRD Study and CKD-EPI equations, CKD-EPIPK, and new estimating equations are reported in Box 1.
Box 1. Existing, Modified, and New GFR Estimating Equations.
| Existing Equations | |
| MDRD Study equation: | |
|
| |
| CKD-EPI equation: | |
|
| |
| Modified Equation | |
| CKD-EPIPK equation: | |
|
| |
| New Equations | |
| Equation 1: | |
|
| |
| Equation 2: | |
|
|
Note: Modified and new estimating equations developed in a Pakistani population; see Methods.
Abbreviations: BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration CKD-EPIPK, CKD-EPI equation with Pakistani correction factors; FBG, fasting blood glucose; Hb, hemoglobin; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine; SUN, serum urea nitrogen; WC, waist circumference.
Comparison of Performance
Table 3 and Figs 1 and 2 compare the performance among equations.
Table 3.
Performance of GFR Estimating Equations
| GFR Estimating Equation | RMSEa | Biasb | Precisionc | Accuracyd
|
|
|---|---|---|---|---|---|
| P20 | P30 | ||||
| Overall Study Population (N = 581) | |||||
| MDRD Study equation | 0.295 (0.269 to 0.321) | −8.5 (−10.1 to −6.8) | 28.6 (25.8 to 31.5) | 54.7 (50.9 to 58.6) | 68.0 (64.3 to 71.7) |
| CKD-EPI equation | 0.265 (0.240 to 0.290) | −6.8 (−8.2 to −5.4) | 22.6 (19.9 to 25.3) | 57.8 (53.8 to 61.8) | 76.1 (72.7 to 79.5) |
| CKD-EPIPK equation | 0.265 (0.240 to 0.290) | NA | 22.8 (20.3 to 25.2) | 65.9 (62.1 to 69.7) | 81.6 (78.4 to 84.8) |
| New estimating equation 1 | 0.259 (0.234 to 0.284) | NA | 22.8 (20.6 to 25.0) | 66.8 (62.9 to 70.7) | 82.7 (79.8 to 85.7) |
| New estimating equation 2 | 0.263 (0.238 to 0.288) | NA | 23.1 (20.4 to 25.7) | 68.7 (65.0 to 72.4) | 82.5 (79.4 to 85.5) |
| Participants With eGFRCKD-EPI(PK) ≥ 90 mL/min/1.73 m2(n= 300) | |||||
| MDRD Study equation | 0.247 (0.215 to 0.280) | −14.6 (−17.9 to −11.3) | 35.2 (30.6 to 39.8) | 51.0 (45.8 to 56.2) | 64.8 (59.9 to 69.6) |
| CKD-EPI equation | 0.246 (0.215 to 0.278) | −9.9 (−12.9 to −7.0) | 26.6 (22.4 to 30.9) | 59.0 (53.8 to 64.2) | 77.9 (73.7 to 82.2) |
| CKD-EPIPK equation | 0.226 (0.194 to 0.258) | NA | 32.6 (28.7 to 36.5) | 68.1 (63.0 to 73.2) | 86.6 (82.5 to 90.6) |
| New estimating equation 1 | 0.218 (0.187 to 0.250) | NA | 29.3 (25.6 to 33.0) | 71.9 (66.9 to 76.9) | 90.3 (86.8 to 93.9) |
| New estimating equation 2 | 0.221 (0.189 to 0.253) | NA | 30.5 (26.5 to 34.5) | 71.4 (66.5 to 76.3) | 89.7 (86.1 to 93.4) |
| Participants With eGFRCKD-EPI(PK) < 90 mL/min/1.73 m2 (n = 281) | |||||
| MDRD Study equation | 0.304 (0.264 to 0.344) | −1.1 (−4.0 to 1.8) | 18.4 (15.3 to 21.5) | 60.3 (54.0 to 66.7) | 72.8 (67.2 to 78.4) |
| CKD-EPI equation | 0.291 (0.251 to 0.331) | −4.8 (−6.9 to −2.8) | 16.8 (13.9 to 19.8) | 56.0 (49.7 to 62.4) | 73.3 (67.8 to 78.8) |
| CKD-EPIPK equation | 0.316 (0.275 to 0.358) | NA | 11.8 (9.1 to 14.5) | 63.3 (57.2 to 69.3) | 74.2 (69.1 to 79.4) |
| New estimating equation 1 | 0.307 (0.267 to 0.346) | NA | 12.5 (9.5 to 15.6) | 57.7 (51.7 to 63.6) | 72.7 (67.6 to 77.8) |
| New estimating equation 2 | 0.302 (0.262 to 0.343) | NA | 10.9 (7.8 to 14.1) | 58.3 (52.2 to 64.4) | 73.8 (68.7 to 79.0) |
Note: Values in parentheses are 95% confidence intervals.
Abbreviations and definitions: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIPK, CKD-EPI equation with Pakistani correction factors; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; mGFR, measured GFR; NA, not applicable, bias is expected to be zero; RMSE, root mean squared error.
Square root of the average squared difference of mGFR and eGFR.
Median of mGFR − eGFR, expressed in mL/min/1.73 m2.
IQR of difference of mGFR and eGFR, expressed in mL/min/1.73 m2.
Accuracy (P20 and P30) refers to percentages of participants with eGFRs within 20% and 30% of mGFR, respectively; the 95% CIs are based on bootstrapping by 1,000 replications.
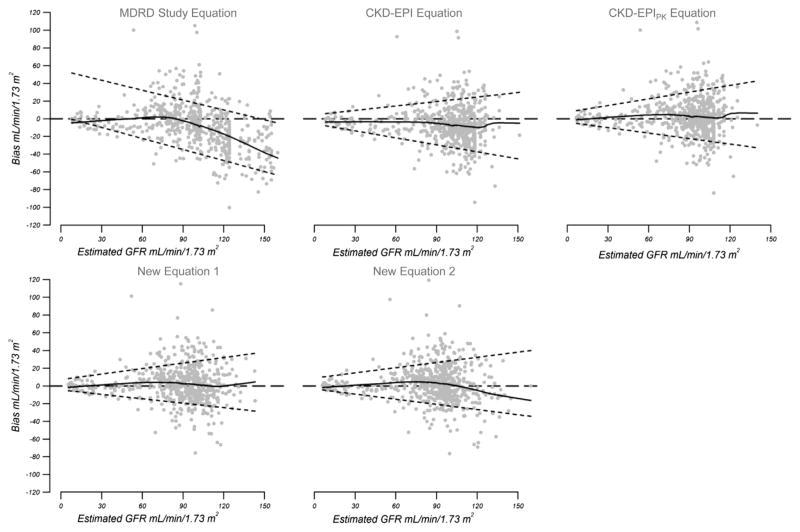
Figure 1.
Bias (measured glomerular filtration rate [GFR] − estimated GFR) by levels of estimated GFR. Solid curved lines indicate smoothed regression lines created using 95% of the data by smoothing function (LOWESS), short dashed lines are the quantile regressions of 10th and 90th percentiles of bias; and dashed horizontal lines represent a reference. Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKD-EPIPK, CKD-EPI equation with Pakistani correction factors; MDRD, Modification of Diet in Renal Disease.
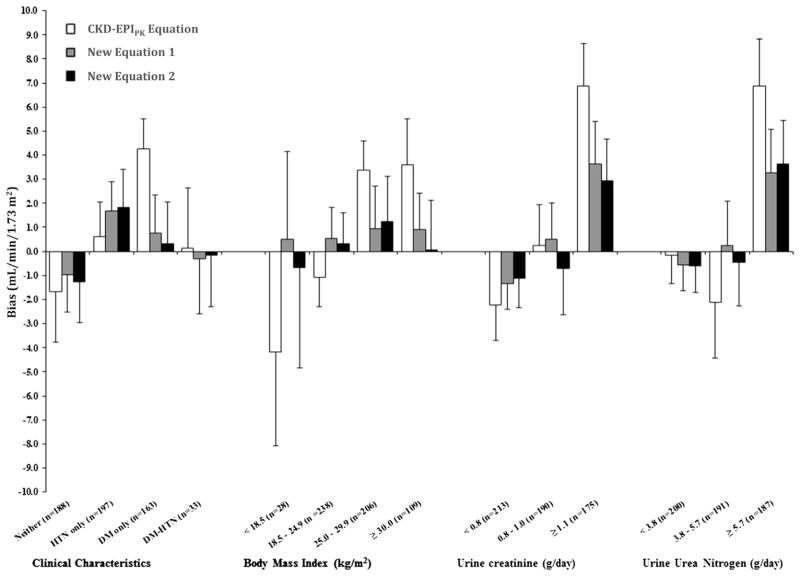
Figure 2.
Comparison of bias (measured glomerular filtration rate [GFR] − estimated GFR) for CKD-EPIPK (CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] equation with Pakistani correction factors) equation and new estimating equations 1 and 2 among subgroups. Bars denote median bias, error bars represent standard error of median bias based on 1,000 bootstrapped samples. Bias values that are negative represent overestimation. Conversion factors for units: urine creatinine in g/d to mmol/d, ×8.84; urine urea nitrogen in g/d to mmol/d, ×35.7. Abbreviations and definitions: Neither, nonhypertensive nondiabetic; HTN only, hypertensive; DM only, diabetic; DM-HTN, diabetic and hypertensive.
CKD-EPI and MDRD Study Equations
The CKD-EPI creatinine equation had significantly greater accuracy (P30, 76.1% [95% CI, 72.7%–79.5%]) and better precision (IQR, 22.6 [95% CI, 19.9–25.3] mL/min/1.73 m2; P < 0.001) compared to the MDRD Study equation (P30, 68.0% [95% CI, 64.3%–71.7%; IQR, 28.6 [95% CI, 25.8–31.5] mL/ min/1.73 m2; Table 3). However, both equations overestimated mGFR; bias (ie, median mGFR− eGFR values) for the CKD-EPI and MDRD Study equations were −6.8 (95% CI, −8.2 to −5.4) and −8.5 (95% CI, −10.1 to −6.8) mL/min/1.73 m2, respectively. Figure 1 shows less variation in bias across the range of eGFRs with the CKD-EPI equation versus MDRD Study equation.
Among participants with eGFRCKD-EPI(PK) ≥ 90 mL/min/1.73 m2, the performance of CKD-EPI was better than that of the MDRD Study equation, whereas in participants with eGFRCKD-EPI(PK) <90 mL/min/ 1.73 m2, there was no difference between them.
CKD-EPIPK Equation
As expected, modification of the CKD-EPI equation with the Pakistan correction factor eliminated bias and there was no apparent variation across the range of eGFRs (Fig 1). Furthermore, accuracy improved; P20 was 65.9% (95% CI, 62.1%–69.7%) versus 57.8% (95% CI, 53.8%–61.8%; P <0.001), and P30 was 81.6% (95% CI, 78.4%–84.8%) versus 76.1% (95% CI, 72.7%–79.5%; P < 0.001), respectively, for the CKD-EPIPK and CKD-EPI equations, respectively. Although the 95% CIs of P30 were overlapping, the difference was statistically significant on the basis of P value (see Table 3 and Table S1 for P values).
Among participants with eGFRCKD-EPI(PK) ≥ 90 mL/min/1.73 m2, performance of the CKD-EPIPK equation was better than the CKD-EPI equation itself (P30, 86.6% [95% CI, 82.5%–90.6%] vs 77.9% [95% CI, 73.7%–82.2%], respectively), whereas in participants with eGFRCKD-EPI(PK) <90 mL/min/1.73 m2, there was no difference between them.
New Estimating Equations
Although both new estimating equations developed in this Pakistani population (equations 1 and 2) had significantly better P20 and P30 accuracy compared to the original CKD-EPI equation (P < 0.001 for each estimate), their performance was comparable to that of the CKD-EPIPK equation in terms of all parameters with overlapping 95% CIs (RMSE, precision [IQR], and accuracy [P20 and P30]) across the range of eGFRs (ie, overall, as well as eGFRCKD-EPI(PK) ≥ 90 and <90 mL/min/1.73 m2; see Table 3 and Table S1 for P values). The new estimating equation 2 appeared to overestimate at higher GFRs compared to equation 1 (Fig 1).
Figure 2 compares bias among the CKD-EPIPK equation and the new estimating equations in subgroups by hypertension and diabetes status and for different ranges of BMI, urine creatinine excretion, and urea nitrogen excretion. Although the new estimating equations appeared to have slightly lower bias compared to the CKD-EPIPK equation, all 3 equations over- or underestimated GFR to some extent according to participant characteristics, and this was more pronounced in individuals with low BMI and obesity, diabetes, high urine creatinine excretion, and high urine urea nitrogen excretion.
DISCUSSION
The new KDIGO CKD guideline recommends using the CKD-EPI creatinine equation to report eGFR unless an alternative equation has been shown to be more accurate in the local population.21 This is the first report of the performance of existing GFR estimating equations in the general population from a South Asian country using a directly measured GFR method as a reference. Using urinary inulin clearance as the gold standard, we found that the CKD-EPI creatinine equation is significantly more accurate and more precise compared to the MDRD Study equation in this largely unselected South Asian population in Karachi, Pakistan. However, both equations overestimated mGFR at the high levels observed in this population. The Pakistan correction factors in the CKD-EPIPK equation reduced bias and improved accuracy in estimating GFR compared to the original equation in the overall population, mainly due to improvement in individuals with eGFRCKD-EPI(PK) ≥90 mL/min/ 1.73 m2. Performance of the CKD-EPIPK equation was comparable to that of the new estimating equations developed in this Pakistani population, which did not offer substantial advantage and required using additional variables that are not routinely collected by clinical laboratories. Our findings have potentially significant implications for clinical practice and public health policy in South Asians.
The better performance of the CKD-EPI equation versus the MDRD Study equation in South Asians in Pakistan is consistent with observations in whites22 and Southeast Asian populations.14,23–25 However, the correction factor in our study accounted for both the slope and the intercept in the regression analysis and therefore had 2 terms (0.686 × eGFRCKD-EPI1.059) instead of the single terms proposed for correcting the MDRD Study or CKD-EPI equations in Japanese and Chinese individuals.23,26,27 Nevertheless, the terms potentially are readily programmable in the equations for automated reporting of eGFR. The new estimating equations described here include terms for individual characteristics (eg, BMI and waist circumference) not included in the CKD-EPI or MDRD Study equations, which would be more difficult for clinical laboratories implementing automatic eGFR reporting.
Compared to the original CKD-EPI creatinine equation, the improvements in accuracy for the CKD-EPIPK equation and the new estimating equations were significant; however, some variations in their performance remained across categories of BMI, urine excretion of creatinine, and urea nitrogen, and the variations were more apparent at the extremes (Fig 2). This is consistent with the expectation that difference in muscle mass and diet affect serum creatinine concentration independently of GFR. These findings support the contention that no creatinine-based equation will perform equally well across the full spectrum of clinical characteristics observed in any clinical population.14
The main strength of this study was its community-based (door-to-door) sampling frame, which is representative of the general population. These equations previously have been evaluated predominantly in patients with CKD or kidney donors. Thus, our findings would be generalizable to the general population. Moreover, we recruited additional participants from the clinic so our results also apply to South Asian patients with advanced CKD. Second, this is the first evaluation of estimation equations from a South Asian country. Furthermore, we used optimal approaches to measure GFR (inulin clearance), urinary inulin clearance as the gold-standard GFR reference method, standardized serum creatinine assays (traceable to the IDMS procedure), and the unbiased cross-validated estimates based on 500 bootstrapped samples. Furthermore, the significance of comparison metrics relied on 1,000 bootstrapped replications for 95% CIs and parametric tests for pairwise comparisons. Finally, the performance of the CKD-EPI creatinine equation in our study generally is consistent with the observations in other populations24,25,28,29 (Table S2). Thus, we have confidence in the accuracy of our GFR estimating equation and believe it can be generalized to the general population for estimating GFR in health screening programs and epidemiologic studies in Pakistan and possibly other South Asian countries.
Our study has limitations. The MDRD Study and CKD-EPI equations were developed using iothalamate clearance to measure GFR, whereas we used urinary clearance of inulin in our study; thus, some of the differences in bias may be due to GFR measurement method.30,31 Second, there was no external validation data set. However, cross-validation using bootstrapping is recommended as a method for obtaining unbiased results.18–20 Moreover, this limitation would not apply to our finding that the original CKD-EPI equation outperformed the original MDRD Study equation. Third, the few participants with low GFR limited evaluation of performance in participants with various stages of advanced CKD. However, the main objective of the study was to test the application of the GFR estimating equations in the general population from Karachi, Pakistan, which we were able to achieve. Last, urine samples for inulin clearance were collected by spontaneous voiding and not by catheterization. Thus, incomplete bladder emptying is a potential source of measurement bias. However, use of a mean of 2 inulin clearance periods and a mean of 3 periods in case the difference in volume was > 30 mL would minimize this limitation.
The Pakistan coefficient will need to be tested in other South Asian populations to assess whether this equation can serve as a common correction factor for wider application of the CKD-EPI creatinine equation across South Asia and whether it also enhances prediction of risk of progression to kidney failure, onset of cardiovascular disease, and mortality better than the original CKD-EPI equation in this population.13 Such information relating estimates closer to mGFR with outcomes will enable evaluation of the clinical utility and efficiency of the CKD-EPIPK equation in Pakistan. At the same time, it is also important to emphasize that with overall accuracy of 82%, this CKD-EPIPK equation has substantial room for further improvement in GFR estimation, especially in those with low GFRs. One possibility involves the addition to the equation of other filtration markers, including the combination of creatinine and cystatin C, which has been shown to perform better and is recommended as a confirmatory test in the subgroup of patients of European origin with low GFRs.32
In conclusion, our findings suggest that modification of the CKD-EPI equation with a Pakistan correction factor is the most accurate and practicable creatinine-based GFR estimating equation for the South Asian population, at least in Pakistan. The performance of new estimating equations that were developed in this Pakistani population and that incorporate creatinine and additional variables was comparable to that of the CKD-EPIPK equation. The need for efficient decision making in clinical practice and public health policy requires implementation of eGFR reporting with a single equation across a wide region. Our results suggest that CKD-EPIPK, the CKD-EPI equation modified for Pakistan, would be the most valid and easy to implement in South Asia. We suggest that these findings be shared with clinical laboratories in Pakistan and neighboring countries to facilitate reporting of eGFR when serum creatinine is measured. These efforts ultimately may translate into better management and improved patient outcomes.
Supplementary Material
Acknowledgments
We thank all research staff for their assistance and acknowledge the cooperation of Mr Ibrahim Mustafa at the Aga Khan University Hospital for logistical assistance with the GFR clinic for research participants and Dr Lise Thibaudin for inulin assays at Renal Laboratories, Saint-Etienne Hospital, University of Jean Monnet, Saint-Etienne, France. We also thank Dr Syed Mansoor Ahmed Shah from Aga Khan University Hospital and Drs Aasim Ahmad and Kiran Nasir from the Kidney Center, Karachi, for referring patients to the study.
Support: The study was supported by a research award (1R03TW007588-01A1) from the National Institutes of Health, Fogarty International Center. The design, conduct, analysis, interpretation, and presentation of the data were the responsibility of the authors with no involvement from the funder.
Footnotes
The abstracts based on this work were presented at the Joint Statistical Meetings on August 6, 2013, in Montreal, Canada; World Congress of Nephrology on June 2, 2013 in Hong Kong, and 44th Annual Renal Week on November 11, 2011, in Philadelphia, PA.
Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Kunitoshi Iseki, MD) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Table S1: P values for comparison of metrics among GFR estimating equations.
Table S2: Performance of CKD-EPI equation in Pakistani and other Asian populations.
Figure S1: Study flow chart.
Figure S2: Jaffe vs Roche enzymatic methods for measuring serum creatinine.
Item S1: Model development and cross-validation for new estimating equations for Pakistan.
Item S2: Cross-validation on 500 bootstrapped samples.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2013.07.023) is available at www.ajkd.org
References
- 1.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH. The growing burden of chronic kidney disease in Pakistan. N Engl J Med. 2006;354(10):995–997. doi: 10.1056/NEJMp058319. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract. 2009;111(3):c197–c203. doi: 10.1159/000199460. discussion c203. [DOI] [PubMed] [Google Scholar]
- 5.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53(1):166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Gurm HS, Dixon SR, Smith DE, et al. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58(9):907–914. doi: 10.1016/j.jacc.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Hall RK, Wang V, Jackson GL, et al. Implementation of automated reporting of estimated glomerular filtration rate among Veterans Affairs laboratories: a retrospective study. BMC Med Inform Decis Mak. 2012;12(1):69. doi: 10.1186/1472-6947-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47(9):1017–1019. doi: 10.1515/CCLM.2009.264. [DOI] [PubMed] [Google Scholar]
- 9.Miller G. [Accessed March 14, 2013];Current status of reporting eGFR. http://www.cap.org/apps/docs/committees/chemistry/current_status_reporting_egfr_09.pdf.
- 10.Accetta NA, Gladstone EH, DiSogra C, Wright EC, Briggs M, Narva AS. Prevalence of estimated GFR reporting among US clinical laboratories. Am J Kidney Dis. 2008;52(4):778–787. doi: 10.1053/j.ajkd.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick ES, Verrill H. A national audit of estimated glomerular filtration rate and proteinuria reporting in the UK. Ann Clin Biochem. 2011;48(pt 6):558–561. doi: 10.1258/acb.2011.011083. [DOI] [PubMed] [Google Scholar]
- 12.British Medical Association. [Accessed March 14, 2013];Chronic Kidney Disease FAQs. http://www.nhsemployers.org/SiteCollectionDocuments/Chronic_Kidney_Disease_FAQs_cd_110209.pdf.
- 13.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795. W-270–W-278. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 15.Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- 16.Jafar TH, Islam M, Jessani S, et al. Level and determinants of kidney function in a South Asian population in Pakistan. Am J Kidney Dis. 2011;58(5):764–772. doi: 10.1053/j.ajkd.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 18.Mooney CZ, Duval RD. Bootstrapping: A Nonparametric Approach to Statistical Inference (Quantitative Applications in the Social Sciences) Newbury Park, CA: Sage Publications Inc; 1993. [Google Scholar]
- 19.Davison AC, Hinkley DV. Bootstrap Methods and Their Application (Cambridge Series in Statistical and Probabilistic Mathematics, No 1) New York, NY: Cambridge University Press; 1997. [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD Epidemiology Collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56(1):32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 24.Praditpornsilpa K, Townamchai N, Chaiwatanarat T, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol Dial Transplant. 2011;26(9):2780–2785. doi: 10.1093/ndt/gfq815. [DOI] [PubMed] [Google Scholar]
- 25.Teo BW, Xu H, Wang D, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58(1):56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 26.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung BH, Yu JH, Cho HJ, et al. Comparison of estimating equations for the prediction of glomerular filtration rate in kidney donors before and after kidney donation. PLoS One. 2013;8(4):e60720. doi: 10.1371/journal.pone.0060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rule AD, Teo BW. GFR Estimation in Japan and China: what accounts for the difference? Am J Kidney Dis. 2009;53(6):932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463–472. doi: 10.1053/j.ajkd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.