Abstract
Objective
This study describes associations of ozone and fine particulate matter with Parkinson’s disease observed among farmers in North Carolina and Iowa.
Methods
We used logistic regression to determine the associations of these pollutants with self-reported, doctor-diagnosed Parkinson’s disease. Daily predicted pollutant concentrations were used to derive surrogates of long-term exposure and link them to study participants’ geocoded addresses.
Results
We observed positive associations of Parkinson’s disease with ozone (OR=1.39; 95% CI: 0.98, 1.98) and fine particulate matter (OR=1.34; 95% CI: 0.93, 1.93) in North Carolina but not in Iowa.
Conclusion
The plausibility of an effect of ambient concentrations of these pollutants on Parkinson’s disease risk is supported by experimental data demonstrating damage to dopaminergic neurons at relevant concentrations. Additional studies are needed to address uncertainties related to confounding and to examine temporal aspects of the associations we observed.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting over one million people in the United States. Because PD risk is strongly associated with older age, its prevalence is expected to increase as the population ages [1]. PD involves loss of the dopaminergic neurons of the substantia nigra as well as injury to dopaminergic neurons in other brain regions and to neuronal populations using other neurotransmitters [2]. PD affects both motor and non-motor function, the latter including abnormalities of sleep, cognition, mood, autonomic function and olfaction [3]. Oxidative stress is a likely mechanism underlying neurodegenerative disease, and the substantia nigra may be particularly sensitive to oxidative stress for several reasons, including the fact that dopamine metabolism is itself an oxidative process [4].
Air pollution is known to have significant effects on respiratory and cardiovascular health. More recently, evidence has linked air pollution to neurologic dysfunction [5]. For example, human studies have found associations of air pollution with cognitive dysfunction [6–13], and neuropathological findings in brains of individuals living in urban areas with high air pollution were similar to those of individuals with PD or Alzheimer’s disease [14]. The only epidemiologic study of air pollution and PD reported a null association between nitrogen dioxide (NO2), a marker of traffic pollution, and physician diagnosed PD; however, manganese in total suspended particulates was associated with PD in one of the two Canadian cities studied [15].
Findings from human studies are supported by toxicological investigations in rodents exposed to ozone and PM2.5 concentrations that are near ambient levels [37, 38]. Studies of ozone and particulate matter are particularly interesting because both pollutants have been linked to brain disease and both contribute to oxidative stress [5, 16]. Studies involving exposure to concentrated ambient particulate matter have demonstrated microglial activation and other signs of inflammation, increased levels of alpha-synuclein in midbrain, and loss of dopaminergic neurons in the substantia nigra [17–21]. Studies of rodents examining the effects of long-term exposure to relatively low levels of ozone have demonstrated progressive damage in various brain regions in conjunction with altered behaviour and changes in microglial activation, changes in cell morphology in the substantia nigra and striatum, and loss of nigral dopaminergic neurons [22–26]. These changes are similar to those found in brains of PD patients.
Our objective was to investigate the associations of PD with exposure to ambient concentrations of ozone and fine particulate matter (PM2.5 – particulate matter with an aerodynamic diameter ≤ 2.5 microns). We selected these pollutants for study because they both cause oxidative damage in the brains of experimental animals at or near ambient exposure concentrations. Our study population was composed of farmers and their spouses enrolled in the Agricultural Health Study (AHS). To date, few studies of air pollution health effects have been designed to include non-urban participants [27–30]. One such study reported an association between PM2.5 exposure and cardiovascular mortality among men enrolled in the AHS cohort [27]. This finding indicates a need to examine the health effects of air pollution in rural populations. The AHS provides an excellent population to perform such an evaluation.
Materials and Methods
Study population (Figure 1)
Figure 1.
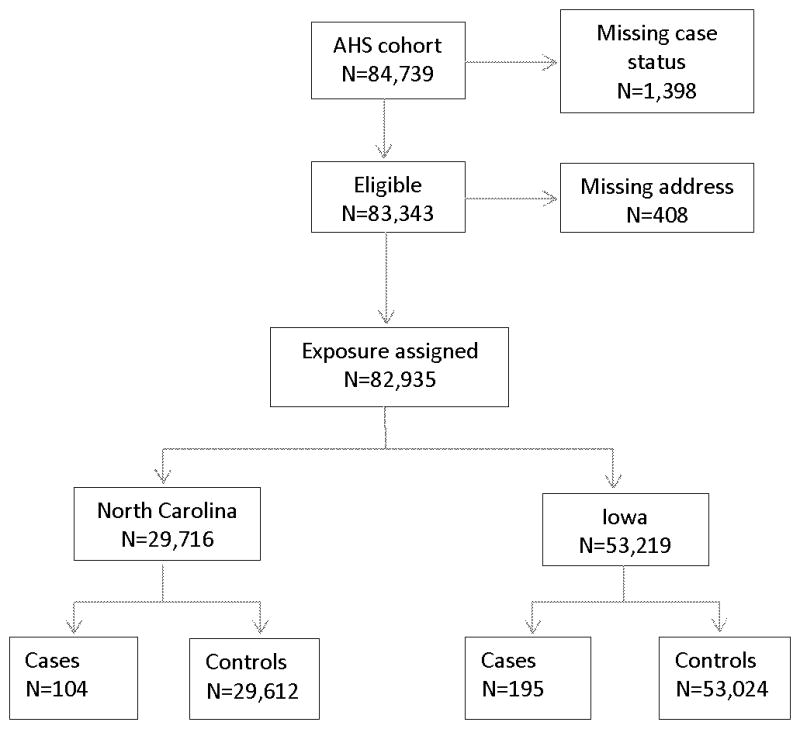
AHS cohort members included in the final logistic regression analyses of the association of Parkinson’s disease (PD) with Ozone and PM2.5.
The AHS cohort was established between 1993 and 1997 in IA and NC [31]. Applicants for certification to use restricted-use pesticides were enrolled in the study. At enrollment, 52,394 private applicators (84% of those eligible) completed a self-administered questionnaire. Enrolled private pesticide applicators who were married (83%), were requested to ask their spouses to participate and 32,345 (74%) of the spouses agreed to participate in the study and completed self-administered or telephone questionnaires. Thus, the cohort included 84,739 private applicators and spouses. Questionnaires completed by applicators and spouses obtained information on pesticide use, demographic factors, lifestyle and medical history, including information on PD.
Residential addresses at the time of enrollment were available for all IA participants and 98.6% of NC participants (408 individuals were excluded because they were missing an address). Address records were geocoded using automated batch matching with Geographic Data Technology’s MatchMaker SDK software and street database (Professional Version 4.3 October 2002) to obtain latitudes and longitudes. Approximately 75% of addresses in IA were matched to the street address or nearest intersection whereas approximately 65% of addresses in NC were matched with this level of accuracy. The remaining addresses were matched at the ZIP code centroid level.
Case status was based on self-reports of PD collected at enrollment and in two follow-up interviews in 1999–2003 and 2005–2010. Approximately 73% of the original cohort members (n=62,347) participated in one or more follow-up interviews. At each contact, participants were asked whether they had ever been diagnosed with PD by a doctor or other health professional. As shown in Figure 1, we excluded 1,398 participants with missing or contradictory information on case status (e.g. participants responding yes to doctor diagnosed PD at enrollment but not at follow-up) leaving a total of 83,343 eligible participants. There were 301 cases of PD eligible for this study, 104 in NC and 195 in IA. Cases were compared to participants who did not report PD, 29,612 in NC and 53,024 in IA.
Exposure assessment
We assigned annual averages of daily predicted pollutant concentrations for 12 by 12 kilometer grids covering NC and IA to the individuals in this study using their geocoded addresses. These predicted concentrations were determined using a hierarchical bayesian model that combines monitoring data from the U.S. Environmental Protection Agency’s (EPA) Air Quality System (AQS) with numerical output from EPA’s Community Multiscale Air Quality Model (CMAQ) [32]. This approach weights air monitoring data more heavily than CMAQ model output where monitoring data exists. The model predicts pollutant concentrations using CMAQ input parameters including emissions and meteorological condition data and is useful in providing predicted concentrations for areas in these states where monitoring data are not available. Monitor coverage is more extensive in NC than in IA. Approximately two to three times more counties in NC (30–35%) than in IA (11–15%) have at least one monitor to measure ozone and/or PM2.5. In both states, only a small proportion of study participants resided in grid cells that also contained a monitor (approximately 4–6% in NC and 1% in IA ). Predicted concentrations were available for the years 2002–2006 with 2006 being the most recent year that quality assured emissions data were available for input into CMAQ.
We used daily pollutant concentration predictions to compute several exposure metrics, which were designed to capture the spatial exposure gradients of ozone and PM2.5.These metrics included the annual average concentration (2005) and the 4-year average concentration (2002–2005). Such exposure metrics are typically considered surrogates of long-term exposure in residentially stable populations. They were derived from the 8-hour daily maximum concentrations for ozone and the 24-hour average concentrations for PM2.5. For ozone, we also computed annual and 4-year seasonal average concentrations (April – October) because average ozone concentrations are higher in the warm months compared to year-round averages.
Data analysis
We used logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations of air pollutant concentrations with PD. We modelled this relationship for participants from NC and IA separately because monitoring coverage is less extensive and the spatial variability in pollutant concentration as well as the absolute concentrations are both lower in IA. Ozone and PM2.5 concentrations were modelled as linear terms and ORs are presented per interquartile range (IQR) increase in pollutant concentration.
Information on demographic and lifestyle factors was obtained from questionnaires completed by participants at the time of enrollment. We included factors in the logistic regression models that we hypothesized could potentially confound the association of PD with air pollution, specifically age, sex, smoking status, and pesticide use, and present adjusted estimates for the associations of covariates with PD (Table 1). The associations of PD with cumulative pesticide exposure and exposure to fifteen specific pesticides known to cause mitochondrial dysfunction or oxidative stress, modes of action associated with PD or clinical features of parkinsonism, have been evaluated among AHS participants [33,34]. Because associations of incident PD with cumulative lifetime days of pesticide use [33], and with rotenone (a mitochondrial toxicant) and paraquat (an oxidative stressor) [34], were observed in some analyses of AHS data, we considered the potential for these variables to confound the association between PD and pollutant concentration. Cumulative lifetime days of pesticide use reported at enrollment was specified as a 4-level categorical variable as in Kamel et al. (2007) [33], and variables to indicate ever using paraquat or rotenone at enrollment were also defined. We considered several alternative approaches for modeling age to allow for the non-linear association of age with PD that was previously observed in these data [33]. Because our results did not differ depending on how we specified the term for age, we adjusted our final models for age using the set of indicator variables in Table 1 (referent age category 51–60 years). Smoking was specified as a binary variable to indicate ever smoking. Spouses and applicators were considered in the same models. Because most applicators were male and most spouses were female we opted to adjust models for sex rather than applicator status. We evaluated multicollinearity between variables included in the final models by computing variance inflation factors.
Table 1.
Demographic and lifestyle characteristics of Parkinson’s disease (PD) cases and controls, Agricultural Health Study 1993–2010.
| Cases (n=301) | Controls (n=83042) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. | % | No. | % | OR1 | 95% CI | |
| Characteristic | ||||||
| Age (years) at Enrollment | ||||||
| 12–50 | 36 | 12 | 51017 | 61 | 0.1 | 0.1,0.2 |
| 51–60 | 96 | 32 | 18115 | 22 | 1.0 | Referent |
| 61–70 | 125 | 42 | 10901 | 13 | 2.1 | 1.6,2.8 |
| 71–92 | 44 | 15 | 3009 | 4 | 2.7 | 1.9,4.0 |
| Sex | ||||||
| Male | 218 | 72 | 50049 | 60 | 1.0 | Referent |
| Female | 83 | 28 | 32993 | 40 | 0.5 | 0.4,0.7 |
| State | ||||||
| Iowa | 195 | 65 | 53024 | 64 | 1.0 | Referent |
| North Carolina | 106 | 35 | 30018 | 36 | 0.7 | 0.6,0.9 |
| Type of Participant | ||||||
| Applicator | 210 | 73 | 51149 | 62 | 1.0 | Referent |
| Spouse | 82 | 27 | 31893 | 38 | 0.6 | 0.4,0.8 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 272 | 90 | 75986 | 92 | 1.0 | Referent |
| Other | 24 | 8 | 5546 | 7 | 1.0 | 0.6,1.6 |
| Missing | 5 | 2 | 1510 | 2 | ||
| Education | ||||||
| <=High School | 192 | 64 | 41409 | 50 | 1.0 | Referent |
| > High School | 92 | 31 | 35578 | 43 | 1.0 | 0.8,1.3 |
| Missing | 17 | 6 | 6055 | 7 | ||
| Smoking Status at Enrollment | ||||||
| Never Smoker | 175 | 58 | 48309 | 58 | 1.0 | Referent |
| Ever Smoker | 122 | 41 | 33795 | 41 | 0.7 | 0.6,0.9 |
| Missing | 4 | 1 | 938 | 1 | ||
| Pack-years | ||||||
| non-smoker | 180 | 60 | 48431 | 58 | 1.0 | Referent |
| >0–10 | 45 | 15 | 14694 | 18 | 0.8 | 0.5,1.1 |
| 10–30 | 42 | 14 | 10689 | 13 | 0.8 | 0.5,1.1 |
| >30 | 19 | 6 | 4876 | 6 | 0.5 | 0.3,0.9 |
| missing | 15 | 5 | 4352 | 5 | ||
| Cumulative days of pesticide use reported at enrollment | ||||||
| 0–64 | 118 | 39 | 36594 | 44 | 1.0 | Referent |
| 65–200 | 43 | 14 | 12404 | 15 | 0.8 | 0.5, 1.1 |
| 201–396 | 61 | 20 | 13986 | 17 | 1.0 | 0.7, 1.4 |
| 397–7000 | 64 | 21 | 14516 | 18 | 0.9 | 0.7, 1.3 |
| missing | 15 | 5 | 5542 | 7 | ||
All Models included age, sex, smoking status, smoking pack-years and state
We present results by state for all participants as well findings from sensitivity analyses of various subsets of participants. The objective of these sensitivity analyses was to evaluate the possible impact of omitting these factors from consideration in the analysis. The rationale for each of our sensitivity analyses is described below.
First, we evaluated our assumption of residential stability by computing the proportion of participants who reported changing their residence between enrollment and follow-up. Our estimate of the proportion of movers was conservative in that those whose rural route was updated to a street address were counted as movers even though their physical address did not in fact change. We also conducted a sensitivity analysis of the association between PD and air pollution among those with geocoded addresses that were matched with the highest level of accuracy (exact street address or closest intersection).
Because applicators and spouses are likely to have different air pollution exposures due to their different time and activity patterns, we conducted analyses to determine whether the association of PM2.5 and ozone with PD were different depending on status as an applicator or a spouse. Because predicted pollutant concentrations were not available prior to 2002, we assumed that the correlation between annual and multiyear average over time was high, as has been consistently shown in other datasets [35–36]. We tested this assumption by conducting a sensitivity analysis excluding cases diagnosed prior to 2002. To evaluate whether differential loss to follow-up influenced our results we also conducted a sensitivity analysis of the association between self-reported PD and air pollution among those participating in the second follow-up interview conducted between 2005 and 2010.
Since the distributions of ozone and PM2.5 typically show a strong geographic pattern, we anticipated potentially strong correlations between type of farming, pesticides used, and pollutant concentrations. To evaluate these correlations we developed predictive models using logistic regression to determine the association of predicted PM2.5 and ozone concentrations with pesticide use variables. We also conducted analyses restricting the data to those with relatively low reported cumulative days of pesticide use (at the 75th percentile of the distribution or lower), or to those who did not report that they had ever used paraquat or rotenone to address concern about possible confounding of the air pollution – PD relationship by pesticides.
The institutional review boards (IRBs) of the National Institutes of Health and its contractors approved the AHS. At enrollment, the study was explained to potential participants, who indicated consent by returning questionnaires. Additional approval specific to the analyses described here, which involve the use of geocoded addresses, was obtained through the IRB of the University of North Carolina on behalf of the U.S. Environmental Protection Agency (EPA).
Results
The associations of PD with demographic and lifestyle characteristics are summarized in Table 1. The risk of PD increased with increasing age and was lower in women, in residents of NC compared to IA, and in ever compared to never smokers. Other covariates, including education, were not significantly associated with PD nor did inclusion of these variables in logistic regression models change the observed association between PD and ozone or PM2.5.
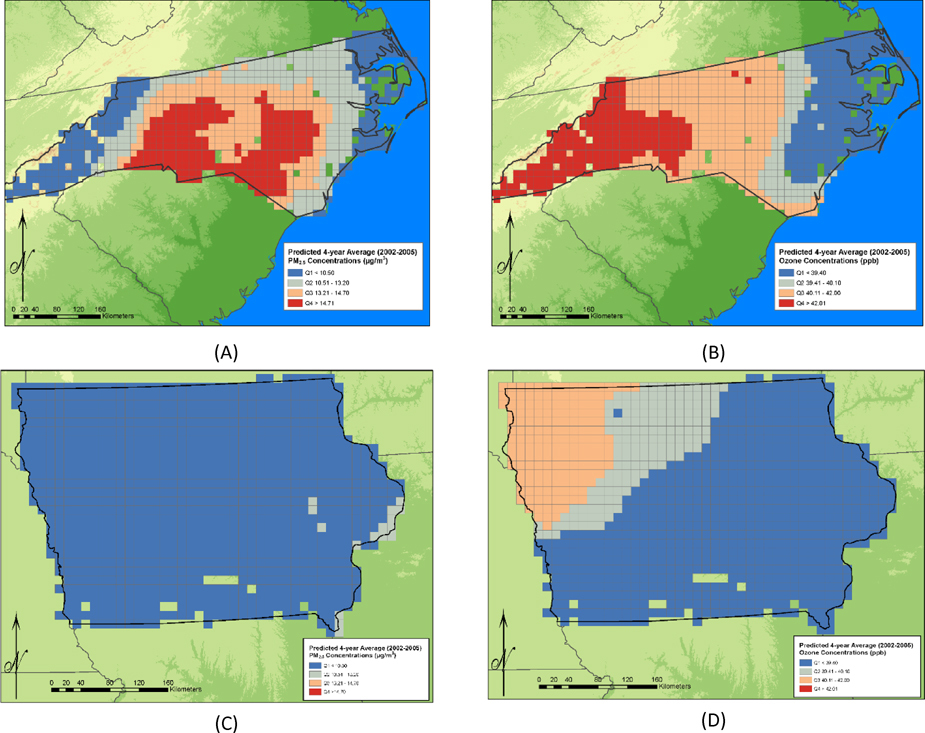
The concentrations of 4-year average predicted ozone and PM2.5 concentration in IA and NC are shown in Figures 2a–2d. The distributions for each of the exposure metrics examined are further described in Table 2. The absolute concentrations and the concentration gradients of both ozone and PM2.5 were greater in NC than in IA. For example, the maximum of the 4-year average predicted ozone concentration assigned to an IA participant was 48 ppb compared to 54 ppb in NC. The IA maximum is below the median warm season average concentration for the U.S. of 49 ppb while the NC maximum is approximately comparable to the 70th percentile concentration for the U.S [37]. The maximum of the 4 year average predicted PM2.5 concentration assigned to an Iowa participant was 11.5 μg/m3 compared to 17.7 μg/m3 in NC. The NC maximum is approximately comparable to the 75th percentile of nationwide concentration distribution for 2005 [38].
Figure 2.
(concentrations are shown only for grid cells in which AHS participants reside). (A) Predicted 4-year average PM2.5 concentrations in North Carolina during the period 2002-2005 (B) Predicted 4-year average ozone concentrations in North Carolina during the period 2002-2005. (C) Predicted 4-year average PM2.5 concentrations in Iowa during the period 2002-2005. (D) Predicted 4-year average ozone concentrations in Iowa during the period 2002-2005.
Table 2.
Distribution of predicted ozone and PM2.5 concentrations assigned to Agricultural Health Study participants in North Carolina and Iowa.
| Iowa | North Carolina | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure metric | Mean (SD) | Median | 95th | Max | IQR | Mean (SD) | Median | 95th | Max | IQR |
| 4-y Avg Ozone (2002–2005) | 39.0 (1.1) | 38.8 | 41.2 | 41.5 | 1.65 | 40.6(1.6) | 40.7 | 43.1 | 46.5 | 2.63 |
| 4-y Seasonal Avg Ozone (April-October, 2002–2005) | 45.6(1.0) | 45.5 | 47.4 | 48.0 | 0.80 | 46.7(2.0) | 47.2 | 49.5 | 53.6 | 3.45 |
| 4-y Avg PM2.5 (2002–2005) | 8.9 (0.5) | 8.7 | 9.9 | 11.5 | 0.71 | 12.6(2.4) | 13.2 | 15.8 | 17.7 | 4.17 |
Ozone concentrations are reported in ppb and PM2.5 concentrations are reported in μg/m3.
In both NC and IA, annual average predicted concentrations assigned to study participants were highly correlated with 4-year average predicted concentrations for both ozone and PM2.5 (r values ≥ 0.97, p-values < 0.01). Because of these high correlations, associations of PD with annual average and 4-year average concentrations were not meaningfully different, and only the results for associations with 4 year average concentrations are presented. Predicted PM2.5 concentrations were not highly correlated with the predicted ozone concentrations assigned to study participants. The correlations between ozone and PM2.5 concentrations in NC ranged from a weak inverse correlation (r=−0.15, p<0.01) to a small positive correlation (r=0.06, p<0.01), depending on the exposure metric. In IA we observed weak positive correlations (r=0.23, p < 0.01) to moderate negative correlations (r=−0.43, p<0.01) between ozone and PM2.5 concentrations.
The associations of air pollution exposures metrics with PD, including findings from sensitivity analyses, are presented in Table 3. Results from models that are adjusted for age, sex and smoking status (Model A) as well as models adjusted for these covariates plus cumulative days of pesticide use (Model B) are presented for NC and IA separately. Despite the link between pesticide use and ozone and PM2.5 levels, the associations between PD and predicted pollutant concentrations were not changed in either state after adjustment for cumulative days of pesticide use. Similarly, sensitivity analyses restricting the population to those with low cumulative days of pesticide use, or never reporting use of rotenone or paraquat did not explain the associations of air pollution with PD.
Table 3.
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for the association of Prevalent Parkinson’s disease with Interquartile Range (IQR) 1 increase in pollutant concentration, among residents of North Carolina, Iowa and North Carolina and Iowa Combined, 1993–2010
| North Carolina | Iowa | |||||||
|---|---|---|---|---|---|---|---|---|
| Model A 2 | Model B 3 | Model A 2 | Model B 3 | |||||
| Main Results | ||||||||
| Air pollution exposure metric | OR | 95% CI | OR | 95% CI | ||||
|
| ||||||||
| 4-y Avg Ozone (2002–2005) | 1.09 | 0.99, 1.18 | 1.11 | 0.80, 1.54 | 0.88 | 0.72, 1.09 | 0.88 | 0.71, 1.09 |
| 4-y Warm Season4 Avg Ozone | 1.34 | 0.99, 1.18 | 1.39 | 0.98, 1.98 | 0.93 | 0.83, 1.03 | 0.94 | 0.84, 1.05 |
| 4-y Avg PM2.5 (2002–2005) | 1.32 | 0.93, 1.88 | 1.34 | 0.93, 1.93 | 0.90 | 0.75, 1.09 | 0.91 | 0.75, 1.11 |
|
| ||||||||
| Sensitivity Analyses | ||||||||
| Sub-group examined | ||||||||
|
| ||||||||
| Non-movers | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.42 | 0.91, 2.21 | 1.52 | 0.96, 2.42 | 0.86 | 0.75, 0.99 | 0.86 | 0.75, 0.99 |
| 4-y Avg PM2.5 (2002–2005) | 1.20 | 0.75, 1.91 | 1.19 | 0.74, 1.91 | 1.12 | 0.89, 1.41 | 1.15 | 0.91, 1.45 |
|
| ||||||||
| Address matched with the highest level of accuracy (street address or closest intersection) | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.30 | 0.82, 2.05 | 1.40 | 0.87, 2.25 | 0.92 | 0.82, 1.03 | 0.92 | 0.82, 1.04 |
| 4-y Avg PM2.5 (2002–2005) | 1.33 | 0.86, 2.06 | 1.34 | 0.85, 2.09 | 0.89 | 0.72, 1.11 | 0.92 | 0.74, 1.14 |
|
| ||||||||
| Applicators | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.52 | 1.02, 2.26 | 1.49 | 1.00, 2.23 | 0.97 | 0.85, 1.10 | 0.97 | 0.85, 1.10 |
| 4-y Avg PM2.5 (2002–2005) | 1.21 | 0.75, 1.96 | 1.24 | 0.83, 3.56 | 0.90 | 0.72, 1.13 | 0.90 | 0.72, 1.13 |
|
| ||||||||
| Spouses | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 0.93 | 0.49, 1.79 | 1.09 | 0.52, 2.31 | 0.85 | 0.71, 1.02 | 0.87 | 0.71, 1.06 |
| 4-y Avg PM2.5 (2002–2005) | 1.71 | 0.83, 3.56 | 1.84 | 0.79, 4.28 | 0.90 | 0.63, 1.29 | 0.94 | 0.64, 1.38 |
|
| ||||||||
| Diagnosed in 2002 or later | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.46 | 0.91, 2.33 | 1.54 | 0.94, 2.53 | 0.93 | 0.82, 1.06 | 0.95 | 0.83, 1.09 |
| 4-y Avg PM2.5 (2002–2005) | 1.39 | 0.92, 2.09 | 1.41 | 0.92, 2.15 | 0.93 | 0.74, 1.16 | 0.95 | 0.76, 1.19 |
|
| ||||||||
| Participated in the second follow-up interview | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.28 | 0.85, 1.93 | 1.32 | 0.83, 2.04 | 0.98 | 0.85, 1.14 | 1.01 | 0.87, 1.17 |
| 4-y Avg PM2.5 (2002–2005) | 1.12 | 0.74, 1.69 | 1.14 | 0.74, 1.74 | 0.91 | 0.72, 1.16 | 0.93 | 0.72, 1.11 |
|
| ||||||||
| Never reporting use of rotenone/paraquat | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.32 | 0.87, 1.98 | 1.38 | 0.89, 2.13 | 0.91 | 0.81, 1.02 | 0.93 | 0.83, 1.05 |
| 4-y Avg PM2.5 (2002–2005) | 1.35 | 0.89, 2.04 | 1.38 | 0.90, 2.13 | 0.85 | 0.69, 1.05 | 0.86 | 0.69, 1.06 |
|
| ||||||||
| Low cumulative pesticide use | ||||||||
|
| ||||||||
| 4-y Warm Season4 Avg Ozone | 1.42 | 0.96, 2.08 | 1.52 | 1.02, 2.28 | 0.89 | 0.79, 1.01 | 0.99 | 0.88, 1.13 |
| 4-y Avg PM2.5 (2002–2005) | 1.36 | 0.92, 2.01 | 1.37 | 0.92, 2.01 | 0.82 | 0.66, 1.03 | 0.83 | 0.66, 1.04 |
NC: IQR 2.63 ppm, 4-y avg Ozone; 3.45 ppb 4-year warm season avg Ozone; 4.17 μg/m3 4-y avg PM2.5; Iowa: IQR=1.65 ppb, 4-y avg ozone; IQR=0.8 ppb, 4-y warm season avg ozone; IQR=0.7 μg/m3, 4-y avg PM2.5 μg/m3;
adjusted for age, sex and smoking status
adjusted for age, sex, smoking status, cumulative days of pesticide use reported at enrollment
April – October
After adjustment for age, sex, and smoking, and pesticide use we observed positive associations between ozone and PD among NC participants, (e.g., Odds Ratio [OR] =1.39, 95% Confidence Interval [CI]: 0.98, 1.98 per IQR increase in four-year warm season average ozone concentration) (Table 3). We also observed non-significant positive associations of PM2.5 with elevated PD risk in NC (e.g. OR=1.34, 95% CI: 0.93, 1.93 per IQR increase in 4-year average PM2.5 concentration). The associations of PD with ozone were stronger after restricting the population to non-movers, while the associations of PD with PM2.5 were attenuated (Table 3). Associations were similar after restricting the analysis to those with geocoded addresses matched with the highest level of accuracy. Analyses of the NC participants that were stratified by applicator status (applicator vs. spouse) showed that the association of ozone with PD was stronger among the applicators (OR=1.49 [95%CI 1.02, 2.26]) and substantially attenuated among the spouses (OR=1.09 [95%CI 0.52, 2.31]. The associations of PD with PM2.5 remained positive but lost precision for both applicators (OR=1.24 [95% CI 0.82, 1.85]) and spouses (OR=1.84 [95% CI: 0.79, 4.28]). The associations of both PM2.5 and ozone with PD in NC were generally robust in the other sensitivity analyses.
In IA, null associations or relatively weak (compared to NC) inverse associations of PD with both ozone and PM2.5 concentrations were observed (Table 3). After restricting the population to non-movers, inverse associations of PD with ozone were strengthened while the association with PM2.5 became positive. After restricting the analysis to those with geocoded addresses matched with the highest level of accuracy, the inverse associations observed in IA were closer to the null value. The associations of both PM2.5 and ozone with PD were generally robust or closer to the null in the other sensitivity analyses.
We evaluated the associations of air pollution with pesticide use in predictive models because both show considerable geographic variation. Ozone and PM2.5 were strong independent predictors of pesticide use. After adjusting for age, sex, and smoking status, cumulative lifetime days of pesticide use, ever using paraquat, and ever using rotenone were all positively and significantly associated with 4-year average PM2.5 concentrations in NC (data not shown). In contrast, we observed significant inverse associations of 4-year warm season average ozone concentrations with cumulative lifetime days of pesticide use and ever using paraquat but not with ever using rotenone in NC (data not shown). In IA, we observed a similar pattern of associations.
Discussion
We found positive associations between PD and predicted ambient ozone and PM2.5 concentrations in NC. In IA, associations were generally weakly inverse or null. Concentrations of ozone and PM2.5 in IA were low relative to the national average concentrations and generally lower and less variable than those observed in NC. The low variation in exposure may have limited the ability to detect associations with air pollution in IA.
Because air quality monitors are usually located in densely populated areas where exposures are typically higher, air pollution studies have typically focused on urban or semi-urban populations. However, an association between PM2.5 exposure and cardiovascular mortality, which is well documented in the literature, was reported among members of the AHS [26], demonstrating the value of studies of rural populations. In our study, we used concentration predictions for 12 by 12 kilometer grid cells covering the entire surfaces of NC and IA so that we could examine associations of air pollution with PD among the rural population enrolled in the AHS. PD status was reported at enrollment in 1993 or during follow-up through 2010, but daily predicted air pollutant concentrations were available only for the period from 2002 to 2005. Therefore, we used the annual average and 4-year average (2002–2005) pollutant concentrations as surrogates for long term exposure in our analyses. This was justified because high correlations among year to year pollutant levels are consistently reported in the literature [35–36], and using annual or multi-year average concentrations as surrogates for long-term air pollutant exposure is common practice in epidemiologic studies. However, because ambient pollutant concentration data were not available to test the correlations among annual average concentrations during the AHS follow-up period from 1993 to 2010, we conducted a sensitivity analysis to evaluate whether our associations would persist if we limited our data to include only cases diagnosed from 2002 onward. We found that the associations of ozone and PM2.5 with PD in NC persisted after restricting the data in this manner, suggesting that annual and 4-year average concentrations are useful surrogates for long-term exposure in this as in other studies.
In studies of air pollution, geographic movement of individuals can theoretically introduce a considerable amount of exposure misclassification. Farmers in general, and AHS study participants in particular, are a residentially stable population. Approximately 62% of participants were still at their enrollment address through the end of follow-up. This is a conservative estimate of the proportion of participants that had moved because changes in mailing address that occur with no change in physical address were counted as “movers”. In NC, analyses restricted to non-movers, the associations of ozone concentration with PD were stronger and more precise, although the association of PM2.5 with PD was somewhat attenuated. Thus, using data from 2002–2005 as a surrogate for longer-term exposure most likely results in minimal misclassification due either to air pollution levels changing or to participants moving during the follow-up period in NC. In IA, inverse associations were strengthened in non-movers.
There is human and animal evidence suggesting mechanisms through which air pollution could contribute to the development of PD. A study of children and young adults who died suddenly in Mexico City found that those residing in highly polluted urban areas, compared to those residing in less polluted areas, showed increased neuroinflammation indicated by the presence of activated microglia and the accumulation of proteins including alpha-synuclein and amyloid β42 (Aβ42) [14]. Uptake of particles to the brain was detected in this study [14] and in another study of dogs exposed to urban air pollution in Mexico City [16]. Respiratory inflammation, capillary pathology in the olfactory bulb and frontal cortex, and evidence that this damage caused breaching of the blood brain barrier was also found in the dogs [16]. Further, a gradient in the accumulation of metals with the highest concentrations in the olfactory mucosa, lower concentrations in the olfactory bulb and the lowest concentrations in the frontal cortex was demonstrated in these dogs [39]. These findings are notable given olfactory involvement in early PD pathophysiology [3].
Several toxicology studies found that ozone and PM exposure to the brain is associated with progressive, cumulative damage in the brains of rodents [22–26], with two studies demonstrating loss of dopaminergic neurons in the substantia nigra [22, 26]. In addition, a recent inhalation study demonstrated the loss of dopaminergic neurons in the substantia nigra of mice with chronic exposure to fine concentrated ambient particles [21], and another study found generalized and mid-brain specific neuroinflammation with an increase in alpha synuclein and Aβ42 in rats exposed via inhalation to diesel exhaust [19, 20]. One epidemiologic study of air pollution and PD reported that PD was not associated with traffic generated NO2, although the manganese component of PM was associated with PD risk in one of the cities studied [15]. There is also evidence from studies of animals and children that the endotoxin component of air pollution can lead to chronic brain inflammation, which is mechanistically linked to the development of PD [40, 41].
PD can be definitively confirmed only after death through a pathological exam. Clinical diagnoses of PD range in accuracy depending on the training of the clinician performing those diagnoses. Schrag et al. (2008) reported that 83% of cases diagnosed as PD by general practitioners could be confirmed by experts trained to distinguish various parkinson-like movement disorders [42]. Although doctor-diagnosed PD was self-reported in our study, the resulting misclassification is not likely to be large. Tanner et al. found that 84% of AHS self-reports were confirmed following evaluation of in-person exams and medical records by movement disorder specialists [34]. Similar findings are reported in other cohorts. For example, upward of 85% of self-reported doctor-diagnosed PD cases were confirmed by the treating neurologist (and further validated through a medical record review) among participants of the Health Professionals Follow-up and Nurses’ Health Studies [43] and among participants in the American Association of Retired Persons Diet and Health Study [44] Additionally, we observed the associations of self-reported doctor-diagnosed PD with age, smoking and sex that are well established in the literature [1], further suggesting that misclassification of case status is likely to be minimal.
Another issue regarding our PD case group should also be considered. We included prevalent PD in some analyses to maximize the number of cases available for analysis. Consequently, the composition of the case group reflects survival as well as incidence. However, the observed associations persisted when we restricted the analysis to cases diagnosed after 2002, alleviating this concern.
Approximately 27% of those initially enrolled in the AHS did not participate in follow-up interviews. If a higher proportion of individuals destined to become PD cases than controls dropped out of the study, and loss to follow-up was also differential with respect to air pollution, this could bias our estimates of relative risk. However, we found that after restricting the data set to those participating in the second follow-up interview the association of 4-year average warm season ozone was robust despite a loss of precision. Further, a previous study found that in the AHS loss to follow-up was not associated with either pesticide exposures or health outcomes [44]. The association of loss to follow-up with air pollution has not been specifically evaluated, however.
Misclassification of case status could be different depending upon air pollution concentration if access to specialty medical care is associated with exposure to air pollution in this cohort. The fact that associations between both ozone and PM2.5 concentration are observed in NC despite the different spatial patterns that these pollutants exhibit (Figures 2a and 2b), argues against this possibility, however.
Cumulative days of pesticide use generally as well as specific pesticides (i.e. rotenone and paraquat) are associated with the occurrence of PD among participants in the AHS [33–34]. In addition, the spatial distributions of ozone and PM2.5 concentration were correlated with pesticide use. Specifically, both pollutants were significant predictors of the pesticide use variables evaluated as potential confounders in this analysis but the direction of the associations was not consistent (e.g. PM2.5 was positively associated with several pesticide use metrics while ozone was inversely associated the same metrics). Despite the potential for confounding by pesticide use to occur, the associations of PM2.5 and ozone with PD observed in both states were robust to adjustment for cumulative days of pesticide use. Further, sensitivity analyses indicated that high cumulative days of pesticide use, using paraquat or using rotenone did not explain the associations of PD with ozone concentration observed in these data. However, we found differences in air pollution associations depending on status as an applicator or spouse. Time spent outside is associated with exposure to ambient pollution and may also be associated with being an applicator or a spouse as well as exposure to other sources of PM exposure (e.g. farm equipment exhaust. Therefore, we cannot exclude the possibility of residual confounding by pesticide exposure or confounding by other occupational risk factors for PD that are different in applicators and spouses.
Major strengths of the AHS are its large size and the detailed information collected on potential confounders. Moreover, use of an internal comparison group minimizes the possibility of confounding by lifestyle or other factors. In addition, our exposure assessment approach allowed high spatial and temporal resolution of predicted air pollutant concentration estimates in rural areas across NC and IA. The cohort of outdoor workers studied is likely to spend a relatively large proportion of time working outside close to their homes compared to the general population. Homes of farmers enrolled in the AHS are typically located on the land that they work, while the average travel time to work among the U.S. population is approximately twenty-five minutes according to the Census Bureau [46]. Therefore, exposure misclassification stemming from the assignment of exposure based on residential address is potentially reduced.
Conclusions
We observed positive yet imprecise associations of ozone and PM2.5 concentrations with PD in NC while generally weak inverse or null associations were observed in IA, where the spatial exposure gradient is far less pronounced. Results from our sensitivity analyses increased our confidence that the associations we observed in NC were not due to exposure misclassification or selection bias. Further, the plausibility of an association of ambient concentrations of these pollutants with PD is supported by experimental data demonstrating that long-term exposure to PM2.5 and ozone can damage dopaminergic neurons and lead to chronic brain inflammation. However, additional studies are needed to address uncertainties in our analysis related to potential confounding by pesticide use and by correlated air toxicants such as endotoxin. In addition, research designed to examine the temporal aspects of observed associations is needed to further our understanding of relevant windows of exposure involved in the development of PD, including those during childhood.
Acknowledgments
Source of Funding
This work was supported in part by the intramural research program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES049030) and National Cancer Institute (Z01-CP010119). Data were obtained from Agricultural Health Study data release versions P1REL0906.00 (for phase 1), P2REL0907.00 (phase 2) and P3RREL0901 (phase 3).
Abbreviations
- AHS
Agricultural Health Study
- NC
North Carolina
- IA
Iowa
- PM2.5
particulate matter with an aerodynamic diameter ≤ 2.5 microns
- PD
Parkinson’s disease
- NO2
Nitrogen Dioxide
- OR
Odds Ratio
- CI
Confidence Interval
- μg/m3
micrograms per cubic meter
- ppb
parts per billion
- Q
quartile
Footnotes
Conflicts of Interest
Dr. Caroline Tanner has served as a consultant to Pfizer Pharmaceuticals, a manufacturer of treatments for Parkinson’s disease. No other potential conflicts of interest were declared.
Disclaimer: The views expressed in this abstract are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Contributor Information
Ellen F. Kirrane, Email: kirrane.ellen@epa.gov.
Christal Bowman, Email: christal.bowman@gmail.com.
J. Allen Davis, Email: davis.allen@epa.gov.
Jane A. Hoppin, Email: jahoppin@ncsu.edu.
Aaron Blair, Email: blaira@mail.nih.gov.
Honglei Chen, Email: chenh2@niehs.nih.gov.
Molini M. Patel, Email: patel.molini@epa.gov.
Dale P. Sandler, Email: sandler@niehs.nih.gov.
Caroline M. Tanner, Email: caroline.tanner@ucsf.edu.
Lisa Vinikoor-Imler, Email: vinikoor-imler.lisa@epa.gov.
Mary H. Ward, Email: wardm@mail.nih.gov.
Thomas J. Luben, Email: luben.tom@epa.gov.
Freya Kamel, Email: kamel@niehs.nih.gov.
References
- 1.Bronstein J, Carvey P, Chen H, Cory-Slechta D, DiMonte D, Duda J, et al. Environ Health Perspect; Meeting Report: Consensus Statement – Parkinson’s Disease and the Environment: Collaborative on Health and the Environment and Parkinson’s Action Network (CHE PAN) Conference; 26–28 June 2007; pp. 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinger KA. Psychiatry of Parkinson’s Disease. Eur J Neurol. 2012;19(6):1468–1331. [Google Scholar]
- 3.Doty RL. Olfactory dysfunction in Parkinson’s disease. Nat Rev Neurol. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 4.Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. J Bioenerg Biomembr. 2009;41(6):469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- 5.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JC, Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, III, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Int Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ailshire JA, Crimmins EM. Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. Am J Epidemiol. 2014 Jun 24; doi: 10.1093/aje/kwu155. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonne C, Elbaz A, Beevers S, Singh-Manoux A. Traffic-related Air Pollution in Relation to Cognitive Function in Older Adults. Epidemiology. 2014 Jul 16; doi: 10.1097/EDE.0000000000000144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ailshire JA, Clarke P. Fine Particulate Matter Air Pollution and Cognitive Function among U.S. Older Adults. J Gerontol B Psychol Sci Soc Sci. 2014 Jun 6; doi: 10.1093/geronb/gbu064. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatto NM, Henderson VW, Hodis HN, St John JA, Lurmann F, Chen JC, Mack WJ. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology. 2014 Jan;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic Pathology. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104(3):420–32. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Calderón-Garcidueñas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, et al. Air pollution and brain damage. Toxicologic Pathology. 2002;30:373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 17.Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman MT, Araujo JA, Nel A, Sioutas C, Campbell A, Cong PQ, et al. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicology Letters. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levesque S, Surace MJ, McDonald J, Block ML. Air Pollution and the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. Journal of Neuroinflammation. 2011;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ, Block ML. Diesel exhaust activates & primes microglia: Air pollution, neuroinflammation, & regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119:1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veronesi B, Makwana O, Pooler M, Chen LC. Effects of subchronic exposures to concentrated ambient particles: VII. Degeneration of dopaminergic neurons in Apo E−/− mice Inhalation. Toxicology. 2005;17:235–241. doi: 10.1080/08958370590912888. [DOI] [PubMed] [Google Scholar]
- 22.Angoa-Pérez M, Jiang H, Rodríguez AI, Lemini C, Levine RA, Rivas-Arancibia S. Estrogen counteracts ozone-induced oxidative stress and nigral neuronal death. Neuro Report. 2006;17:629–633. doi: 10.1097/00001756-200604240-00014. [DOI] [PubMed] [Google Scholar]
- 23.Guevara-Guzmán R, Arriaga V, Kendrick KM, Bernal C, Vega X, Mercado-Gómez OF, Rivas-Arancibia S. Estradiol prevents ozone-induced increases in brain lipid peroxidation and impaired social recognition memory in female rats. Neuroscience. 2009;159:940–950. doi: 10.1016/j.neuroscience.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Pereyra-Muñoz N, Rugerio-Vargas C, Angoa-Pérez M, Borgonio-Pérez G, Rivas-Arancibia S. Oxidative damage in substantia nigra and striatum of rats chronically exposed to ozone. J Chem Neuroanat. 2006;31:114–123. doi: 10.1016/j.jchemneu.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Gomes MZ, Angoa-Pérez M, Raisman-Vozari R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci. 2010;113:187–197. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- 26.Santiago-López D, Bautista-Martínez JA, Reyes-Hernandez CI, Aguilar-Martínez M, Rivas-Arancibia S. Oxidative stress, progressive damage in the substantia nigra and plasma dopamine oxidation, in rats chronically exposed to ozone. Toxicology Letters. 2010;197:193–200. doi: 10.1016/j.toxlet.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Weichenthal S, Villeneuve PJ, Burnett RT, van Donkelaar A, Martin RV, Jones RR, et al. Long-term exposure to fine particulate matter: association with nonaccidental and cardiovascular mortality in the Agricultural Health Study Cohort. Environ Health Perspect. 2014;122:609–615. doi: 10.1289/ehp.1307277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacks JD, Rappold AG, Davis JA, Jr, Richardson DB, Waller AE, Luben TJ. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in North Carolina. Environ Health Perspect. 2014;122:506–512. doi: 10.1289/ehp.1306940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120:708–714. doi: 10.1289/ehp.1104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correia AW, Pope CA, III, Dockery DW, Yun Wang, Ezzati M, Dominici F. Effect of Air Pollution Control on Life Expectancy in the United States An Analysis of 545 US Counties for the Period from 2000 to 2007. Epidemiology. 2013;24:23–31. doi: 10.1097/EDE.0b013e3182770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMillan NJ, Holland DM, Morara M, Feng JY. Combining numerical model output and particulate data using Bayesian space-time modeling. Environmetrics. 2010;21:48–65. [Google Scholar]
- 33.Kamel F, Tanner CM, Umbach DM, Hoppin JA, Alavanja MCR, Blair A, et al. Pesticide Exposure and Self-reported Parkinson’s Disease in the Agricultural Health Study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 34.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, Paraquat and Parkinson’s Disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. NEJM. 2007;356 (5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 37.U.S. EPA. Air quality criteria for ozone and related photochemical oxidants. 2006 [Google Scholar]
- 38.U.S. EPA. Integrated science assessment for Particulate Matter. 2009 [PubMed] [Google Scholar]
- 39.Calderón-Garcidueñas L, Maronpot RR, Torres-Jardon R, Henríquez-Roldán C, Schoonhoven R, Acuña–Ayala H, et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–38. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 40.Villarreal-Calderon R, Torres-Jardón R, Palacios-Moreno J, Osnaya N, Pérez-Guillé B, Maronpot RR, Reed W, Zhu H, Calderón-Garcidueñas L. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol. 2010;29(6):604–15. doi: 10.1177/1091581810383587. [DOI] [PubMed] [Google Scholar]
- 41.Calderon-Garciduenas L, Franco-Lira M, Mora-Tiscareno A, Medina-Cortina H, Torres-Jardon R, Kavanaugh M. Early Alzheimer’s and Parkinson’s disease pathology in urban children: Friend versus Foe responses--it is time to face the evidence. Biomed Res Int. 2013:161687. doi: 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatry. 2002;73:529–534. doi: 10.1136/jnnp.73.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective Study of Caffeine Consumption and Risk of Parkinson’s Disease in Men and Women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A. Smoking duration, intensity and risk of Parkinson’s disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery MP, Kamel F, Hoppin JA, Beane-Freeman LE, Alavanja MCR, Sandler DP. Effects of self-reported health conditions and pesticide exposures on probability of follow-up in a prospective cohort study. Am J Ind Med. 2010;53(5):486–96. doi: 10.1002/ajim.20789. U.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Census Bureau. Commuting in the United States. American Community Survey Reports, ACS-15. Washington, DC: 2009. [Google Scholar]