Abstract
Natural Abs, which arise without known immune exposure, have been described that specifically recognize cells dying from apoptosis, but their role in innate immunity remains poorly understood. Herein, we show that the immune response to neoantigenic determinants on apoptotic thymocytes is dominated by Abs to oxidation-associated Ags, phosphorylcholine (PC), a head group that becomes exposed during programmed cell death, and malondialdehyde (MDA), a reactive aldehyde degradation product of polyunsaturated lipids produced following exposure to reactive oxidation species. While natural Abs to apoptotic cells in naive adult mice were dominated by PC and MDA specificities, the amounts of these Abs were substantially boosted by treatment of mice with apoptotic cells. Moreover, the relative amounts of PC and MDA Abs was affected by VH gene inheritance. Ab interactions with apoptotic cells also mediated the recruitment of C1q, which enhanced apoptotic cell phagocytosis by immature dendritic cells. Significantly, IgM Abs to both PC and MDA were primary factors in determining the efficiency of serum-dependent apoptotic cell phagocytosis. Hence, we demonstrate a mechanism by which certain natural Abs that recognize neoantigens on apoptotic cells, in naive mice and those induced by immune exposure to apoptotic cells, can enhance the functional capabilities of immature dendritic cells for phagocytic engulfment of apoptotic cells.
The selective and efficient elimination of cell corpses is indispensable for maintaining tissue homeostasis as well as for the resolution of inflammation and the prevention of autoimmune disease. However, apoptosis is an obligatory outcome of development, proliferation, and cell differentiation that continues throughout life, and every day >1011 cells in our bodies die by apoptosis. Apoptotic cells (ACs)3 are therefore ubiquitous and abundant; nonetheless, in health they do not pose an immediate threat to the host because of innate immune processes that safely dispose of dying cells. In many settings the professional phagocytic cells, macrophages, have the primary responsibility for the clearance of cell corpses. However, if efficiency is limited there is greater opportunity for cellular progression to secondary necrosis and release of proinflammatory factors and autoantigens that may select pathogenic B and T cell clones.
Dendritic cells (DCs) at early stages of differentiation (i.e., immature DCs) also phagocytose ACs by a process that enables the constant steady-state sampling and presentation of self-Ags (reviewed in Ref. 1). In vivo experiments have previously demonstrated that these interactions with ACs can induce immunologic tolerance (2, 3). However, DCs also serve as sentinel immune cells, and when induced to fully mature they lose phagocytic capacity, up-regulate costimula-tory molecules and chemokine receptors, migrate to draining lymph nodes, and become potent APCs. Fully activated DCs can also be high-level producers of a range of cytokines and chemokines. As AC ingestion is reported to block the induced maturation of DCs (4), the differentiation status and phagocytic capacity of DCs may be central determinators of the homeostatic set point for the maintenance of immunologic tolerance as well as for the integration of pro- and antiinflammatory responses (1).
The in vivo clearance of dying cells is a specialized and complicated multistep process, which has been conserved since early in evolution, and it involves numerous phagocyte receptors, signaling transducers, and membrane-associated ligands and soluble bridging molecules that provide “eat me” signals for phagocytic cells. Among these signals are recognition molecules, such as complement factors, collectins, ficolins, C-reactive protein, surfactant protein A, surfactant protein D, adiponectin, β2-glycoprotein-1, and galectin, that can be selectively deposited on ACs (reviewed in Refs. 5, 6). These opsonizing factors appear to have redundant functions, and their relative dominance may vary depending on anatomic site and physiologic or pathologic context.
The Ab products of the B cell compartment of the adaptive immune system can also specifically recognize cells dying from apoptotic death and distinguish them from healthy cells (7–10). AC immunization of mice with healthy immune systems has been reported to induce Abs to ACs due to the recognition of a range of self-Ags, which include nuclear Ags, cardiolipin, and ssDNA (11). Other studies have reported that AC immunization induces the production of Abs, which are reactive with the phosphorylcholine (PC) headgroup of phosphatidylcholine, that is exposed following oxidative damage to the polyunsaturated fatty acid side chain in position 2 of the glycerol backbone, such as the reactive aldehyde 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine (POVPC) that is generated during apoptosis (7, 10, 12, 13). AC death can also lead to caspase-3 activation of the calcium-independent phospholipase A2 that can remove the fatty acid at the sn-2 position of phosphatidylcholine to generate lyso-phosphatidylcholine, which is also recognized by PC-specific Abs (9). Importantly, these anti-PC Abs do not bind nonoxidized phosphatidylcholine, which explains why they do not interact with healthy cells. Other oxidation-associated neodeterminants have also been implicated, such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA), which are degradation products of the interactions of unsaturated lipids with reactive oxidation species (14, 15). In fact, MDA production is used as a biomarker for the measurement of oxidative stress (16).
To investigate the potential contributions of Abs to innate immune functions, we have characterized the AC-specific responses in immunocompetent naive mice and after i.v. challenge. As classical complement pathway factors are known to be recruited to Ab-containing immune complexes, while some are also directly deposited on ACs, we therefore assessed whether these anti-AC responses can affect the recruitment of such soluble opsonic eat me signals. Additionally, we studied the influence of these factors on phagocytosis by immature DCs, which may play pivotal roles in the maintanence of peripheral tolerance. Our findings provide a new perspective on the role of natural Abs for AC recognition and opsonization, as well as how these interactions can affect the functional capabilities of DCs.
Materials and Methods
Mice
Age- and gender-matched adult BALB/c or C57BL/6 and congenic B cell-deficient muMT mice (provided by The Jackson Laboratory), congenic S107.1−/− mice (17) (kind gift of J. J. Kenny and R. Fischer, National Institutes of Health, National Institute on Aging), and congenic C3−/− (kind gift of M. Carroll, Center for Blood Research, Boston, MA) mice were bred under specific pathogen-free conditions as supervised by the University of California at San Diego Animal Care Program. All animal protocols were approved by the University of California at San Diego Institutional Animal Care and Use Committee.
Apoptotic cell treatment
Based on pilot studies with outcomes assessed after weekly treatments, groups received i.v. 2.5 × 107 freshly apoptotic (etoposide-treated) thymocytes (see below) in PBS by tail vein injection, with bleeds obtained on day 16. Control studies revealed equivalent results with thymocytes induced to apoptosis by gamma irradiation, or by incubation with etoposide at 10 μM (Sigma-Aldrich), dexamethasone at 1 μM (Sigma-Aldrich), or 50 ng/ml PMA (Sigma-Aldrich) in RPMI 1640/10% FCS for 15 h (not shown). Apoptosis was confirmed based on annexin V staining, TUNEL staining, nuclear condensation, and cytoplasmic bleb formation (not shown).
Antibodies
T15 IgM (from the EO6 hybridoma) (18) and the IgM isotype control from the hybridoma, NC17-D8 (19) (gift of L. Arnold, University of North Carolina, Chapel Hill NC), which both express J chain transcripts (data not shown), were produced under serum-free conditions in hollow-fiber (10,000 MWCO) bio-reactors in hybridoma serum-free media (Invitrogen) to a cell density of ~5– 10 × 108/ml and then maintained for 30–45 days by the National Cell Culture Center (Minneapolis, MN). Supernatants were purified with a 300-kDa tangential flow filtration device, followed by a 10-kDa tangential flow filtration for further concentration, and then dialyzed against PBS (pH 7.2), with documented low endotoxin (<0.5 EU/mg). Aliquots were stored at −80°C. By native PAGE analysis and Western blot, IgM populations were predominantly pentamers with <10% hexamers and without monomeric IgM or other low molecular mass species (data not shown).
Ig immunoassays
Standard sandwich ELISAs were performed with precoats of goat anti-IgM or anti-IgG (Jackson ImmunoResearch Laboratories), PC-BSA (Biosearch Technologies), pneumococcal cell wall polysaccharide (C-PS; Statens Serum Institut, Copenhagen Denmark), MDA-BSA (Academy Biomedical), oxidized low-density lipoprotein (Ox-LDL; gift of J. Witztum, University of California at San Dieto), or BSA (Sigma-Aldrich) for control Ags, with detection with anti-IgM or anti-IgG, as described (20).
Ag microarray studies were performed as recently described, with the same complete Ag ligand panel (21). As described (21), background correction used a Matlab script, and the mean values for replicate spots were determined with JMP 7.0 software (www.jmp.com). Cluster analysis diagrams from comparisons of replicate arrays, after processing with monoclonal IgM or sera, were generated with Cluster 3.0 (Stanford University) and Java TreeView (rana.lbl.gov/EisenSoftware.htm). Background levels were confirmed based on replicate slides developed without sera or IgM (not shown). In these studies, a level of 600 digital fluorescence intensity units was set as a threshold for significant reactivity, which was the mean background ±3 SD. These data have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (accession no. GSE14958; www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc).
ELISPOT analyses
ELISPOT analyses were performed as previously described (20), with pre-coating of membranes at 10 μg/ml overnight at 4°C. Mononuclear cell suspensions from spleen or bone marrow were then added to triplicate wells for each condition at 400,000, 100,000, 25,000, and 6,250 cells/well. Final values were calculated per 106 mononuclear cells.
IgG depletion studies
To deplete IgG from serum samples, 200-μl aliquots of sera from AC-treated adult BALB/c mice were equilibrated with a protein G-Sepharose column (Millipore) for 1 h at room temperature, and the flow-through sample was then isolated for further analysis. As a control, a serum aliquot from the same mouse was instead passaged over BSA-Sepharose column by the same technique. Samples were normalized for IgM content by ELISA and then used in side-by-side assays, along with an untreated serum aliquot.
Complement deposition studies
For in vitro complement deposition, apoptotic thymocytes were incubated at 37°C with IgM at 20 μg/ml in TBS alone or TBS with 20% Ig-deficient plasma for complement/opsonins. After 40 min, cells were washed and studied for apoptosis (7-aminoactinomycin D and annexin V) and with allophycocyanin-labeled goat anti-IgM and FITC-labeled rat anti-mouse C1q (Cedarlane Laboratories) in the presence of Fc block (22).
Phagocytosis studies
With regard to apoptotic clearance assays, and by adapting previously described methods, for assays of DC phagocytosis of ACs (4, 23–25), bone marrow cells from femurs and tibiae were collected by flushing with RPMI 1640 media, and disaggregate cells were passed through a 70-μm nylon cell strainer (BD Falcon) to remove cell clumps. Cells were washed with RPMI 1640 media and centrifuged at 1200 rpm for 10 min and then re-suspended and cultured at 1 × 106/ml with complete RPMI 1640 with 10% heat-inactivated FBS, 10 mM HEPES, 100 U/ml penicillin plus 100 μg/ml streptomycin, 2 mM glutamine, and 50 μM 2-ME, containing GM-CSF 10 ng/ml (eBioscience) and cultured at 37°C with 5% CO2 for 72 h. We then added 25 ml of fresh complete supplemented RPMI 1640 with 10 ng/ml GM-CSF for another 48 h. Nonadherent cells were then collected and DCs selected in the presence of Fc block with magnetic anti-CD11c beads using LS magnetic columns (Miltenyi Biotec) to >98% CD11c+ purity, as well as undetectable B220 staining. These immature conventional DCs were then washed thrice and used as described below.
To assays for phagocytic activity, isolated thymocytes were labeled with CFSE stock solution, using a modified manufacturer's protocol (Molecular Probes) at a final concentration of 107 cells/ml and a final CFSE concentration of 5 μM at room temperature in the dark for 20 min. Cells were then quenched by the addition of 5 vol of ice-cold complete RPMI 1640 media, and then cells were washed three times. In most experiments, apoptosis of thymocytes was induced by incubation at 2 × 106/ml with complete RPMI 1640 media with etoposide (Sigma-Aldrich) at 10 μM in 5% CO2 incubator at 37°C overnight. Before use, apoptotic thymocytes were washed four times in complete media. Etoposide was preferred due to generally greater homogeneity by flow cytometry with >92% annexin V+ (i.e., apoptotic) with low levels (<25%) of 7-aminoactinomycin D+ cells.
Equal numbers of DCs and ACs (each at 5 × 105) were then cultured in serum-free media (StemSpan SF expansion; StemCell Technologies) for 60 min at 37°C with 5% CO2, or as indicated. In pilot time-course studies, conditions were determined for optimal discrimination of DC subpopulations (not shown), as previously described (24). To quantitate uptake of ACs by DCs during data analysis, we first excluded cell fragments and blebs with a wide mononuclear cell scatter gate (i.e., forward light scatter vs side light scatter) and then gated on all DCs, based on CD11c-allophycocyanin or MHC II-allophycocyanin staining (BD Biosciences).
Immunofluorescence studies
For immunofluorescence microscopic studies, cytospins were prepared after culture with CFSE-labeled thymocytes and stained with allophycocyanin rat anti-mouse CD11c (BD Biosciences). In these DC/AC preps, >700 DCs were counted per condition by deconvoluted microscopy by a Delta-Vision deconvolution instrument with a Nikon TE-200 microscope (×20) with images collected within the linear range of the CoolSNAP camera. To quantitate these results, microscopic studies determined the percentage of DCs that had ingested one or more AC or apoptotic fragments that were >50% internalized, and not just associated with the DC membrane (26), by the formula: % DCs = (no. of DCs with engulfed ACs)/(total number of DCs). Certain assays used purified human C1q (Quidel), which is reported to be functionally equivalent to murine C1q (25).
Statistical analysis
Values are reported as means ± SD unless otherwise stated. Significance was assigned for p < 0.05 by two-tailed t test, with Welsh correction, or ANOVA, as appropriate (InStat; GraphPad Software).
Results
PC and MDA reactivity dominate Ab responses to ACs
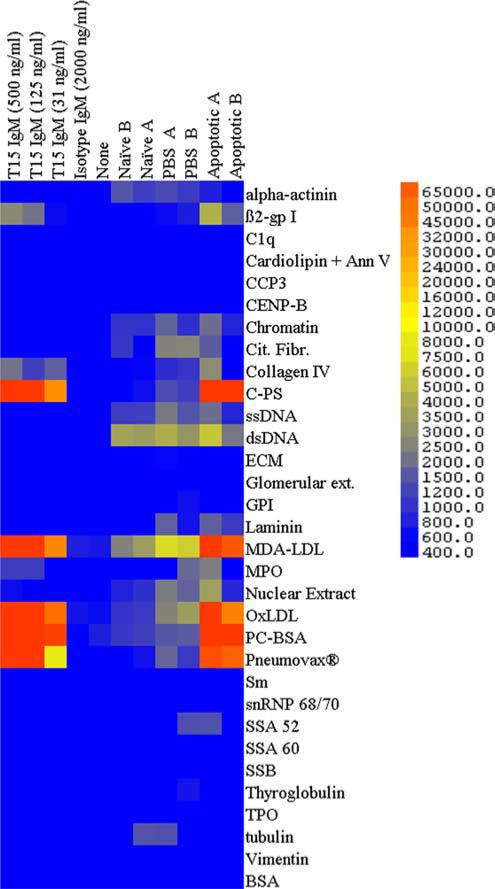
To investigate the binding specificity of natural Abs that recognize ACs, surveys were performed with an 800-feature proteomic Ag array that encompassed >100 different Ags and control ligands (21). These included a broad range of determinants associated with autoimmune pathogenesis, as well as known targets of natural Abs and antimicrobial responses (Fig. 1).
FIGURE 1.
AC treatment induces Abs that predominantly recognize PC and MDA Ags in multiplex Ag arrays. Binding of IgM is shown with replicate autoantigen microarrays, with mAbs, T15 IgM, or isotype control (at concentrations in ng/ml as shown) or adult B6 sera at 1/200, unless otherwise indicated. Sera were obtained from individual naive age-matched adult B6 mice or at day 16 from mice that received three infusions of saline control treatment (PBS A and PBS B), or apoptotic thymocytes on days 0, 7, and 14 (Apoptotic A and Apoptotic B). Results are representative of two independent experiments.
As a control, we first tested the reactivity of purified monoclonal T15 IgM, a natural Ab product of a prototypic clonal set, which is defined by specific canonical Ab gene usage (27) that resides within the B-1 cell subset (28). As expected, the T15 natural Ab displayed strong reactivity with a range of PC-containing Ags that included the PC-BSA conjugate, the purified pneumococcal C-PS, in which PC is an immunodominant epitope (29), and the pneumococcal polysaccha-ride vaccine, Pneumovax. Reiterating past reports, T15 IgM also recognizes copper oxidized LDL and MDA-modified LDL, in which both MDA and PC determinants are displayed (18). Hence, these studies confirm the binding specificity of T15 IgM for exogenous and endogenous Ags that express PC determinants, while the VH11/Vκ9-expressing B-1 cell-derived isotype control IgM, NC-17D8 (19), was nonreactive with all tested Ags.
From autoantigen microarray surveys of sera of adult C57BL/6 (B6) mice, we found that i.v. infusions of apoptotic thymocytes induced increased levels of circulating IgM that also predominantly recognized PC-BSA, C-PS, Pneumovax, Ox-LDL, and MDA-LDL, and with limited reactivity with DNA-containing Ags (e.g., ssDNA, dsDNA, chromatin, and nucleolar extract) and a few other Ags (Fig. 1). In contrast, sera from naive and control-treated (i.e., saline, PBS) mice demonstrated a pattern of only weak reactivity with MDA-LDL, other PC-containing Ags, and DNA-containing Ags (Fig. 1). Hence, AC infusions do not induce nonselective autoantibody responses. Instead, ACs represent potent immunogens for the induction of IgM Abs that prominently recognize the oxidation-associated immunodominant phospholipid neoantigens PC and MDA.
VH S107.1 is needed for efficient recognition of PC, but not MDA, epitopes on ACs
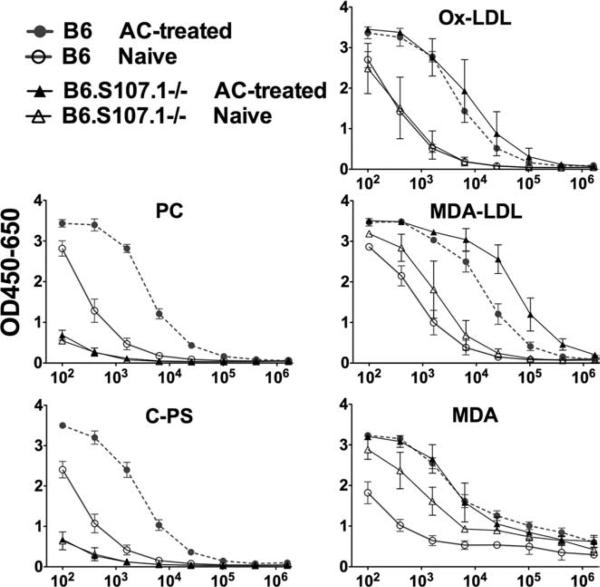
To better understand the basis for immune recognition of phospholipid-associated neodeterminants on ACs, we compared the Ab responses of B6 mice with congenic mice that have a targeted homologous recombination that deletes the inherited germline VHS107.1 gene segment (B6.S107.1−/−). The VHS107.1 gene is known to be required for the generation of T15-related anti-PC Abs (17). Confirming the above-described microarray findings, ELISA studies with a PC-BSA conjugate showed that naive B6 mice had substantial levels of IgM anti-PC Abs, while AC immunization induced a mean 40-fold increase (Fig. 2). There was a comparable induction of the Ab response to PC-containing pneumococcal C-PS. In contrast, naive B6.S107.1−/− mice, which cannot produce T15-related Abs, displayed a profound deficiency in PC reactivity, demonstrated by greatly diminished responses to both PC-BSA and C-PS, which were not substantially boosted by AC treatment (Fig. 2). These findings document that the VHS107.1 gene is required for robust B cell responses to PC determinants on ACs, in the same way as it is required for immune recognition of PC determinants on pneumococci (17).
FIGURE 2.
Serum IgM reactivity of B6 and congenic S107.1-deficient adult mice with PC and MDA Ags. For these studies, wells were coated with PC-BSA, C-PS, Ox-LDL, MDA-LDL, or MDA-BSA and developed with enzyme-conjugated anti-IgM Ab. Serum values for individual mice were determined with depicted values means ± SD (n = 3). Sera were obtained on day 16 from naive 12-wk-old mice, or after bleeds on day 16 following treatment with 25 × 106 ACs on days 0, 7, and 14. Levels of IgM anti-MDA Abs were higher in naive B6.S107.1−/− mice than in B6 mice at 1/400 dilution (p < 0.005, two-tailed t test).
Importantly, in contrast to the differential PC reactivity, both B6 and B6.S107.1−/− mice displayed substantial IgM reactivity with the MDA-BSA conjugate, and levels in naive mice of both strains were significantly increased by AC treatment (Fig. 2). In fact, mean levels of IgM anti-MDA Abs were significantly higher in naive B6.S107.1−/− (i.e., T15-deficient) mice than in naive wild-type B6 mice (Fig. 2) ( p < 0.005). While both strains of mice displayed comparable naive and post-AC IgM responses to the more antigenically complex Ox-LDL, the T15-deficient B6.S107.1−/− mice instead showed higher naive ( p = 0.03) and postimmune ( p < 0.003) responses than did B6 mice to MDALDL, a form of LDL modified to enhance MDA antigenic expression.
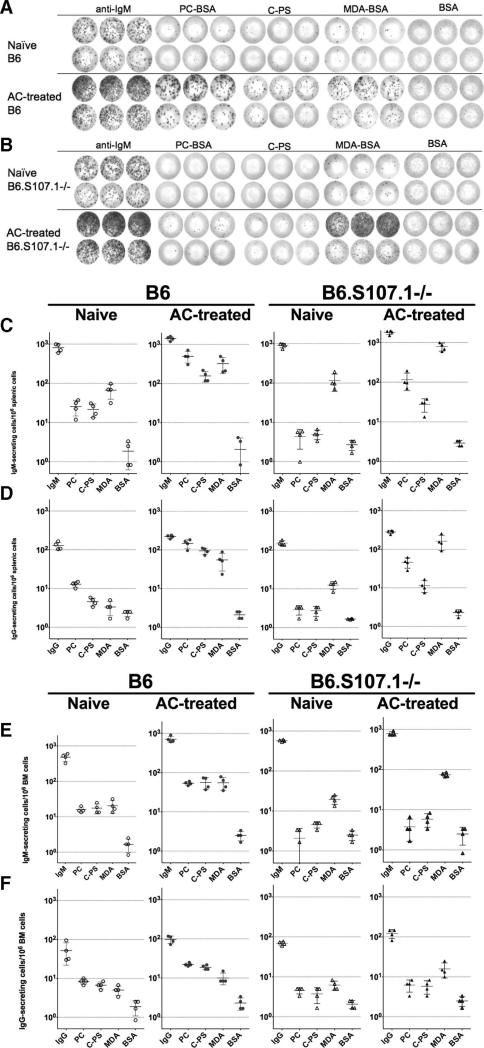
To understand the cellular origins and anatomic distribution of Ab-producing B cells induced by AC immunization, we performed ELISPOT analyses on mononuclear cells from spleen and bone marrow, major sites of natural Ab production (20). As illustrated in Fig. 3A, a significant proportion of splenic IgM-secreting cells in naive B6 mice bound to PC Ags (i.e., PC-BSA 3.1 ± 1.7%; C-PS 2.6 ± 0.5%; mean ± SD) (Fig. 3C). In naive B6 mice, the levels of splenic IgM-anti-MDA-secreting cells were significantly higher (8.29 ± 3.18%) ( p = 0.02) than anti-PC responses, while reactivity levels with both types of apoptosis-associated Ags were well above the observed levels of interaction with the control Ag, unmodified BSA (0.3 ± 0.2%) (Fig. 3C).
FIGURE 3.
Preferential induction of PC- and MDA-reactive Ig-secreting cells by apoptotic cell treatment. A, Representative ELISPOT results compare splenic IgM-secreting cells from a naive (top) and AC-treated (bottom) adult C57BL/6 (B6) mice, and (B) from congenic homozygotic S107.1-deficient (B6.S107.1−/−) mice. These studies depict results from triplicate wells coated with anti-IgM (total IgM-secreting cells), PC-BSA (PC), pneumococcal C-PS, MDA-BSA (MDA), and BSA (albumin) as the negative control. Each dot represents a distinct Ig-secreting cell after addition of 100,000 (top rows) or 25,000 (bottom rows) splenocytes. Data are depicted for estimated rates of IgM-secreting (C and E) and IgG-secreting cells (D and F) for mononuclear cells harvested from the spleens (C and D) and bone marrows (E and F), with values per million cells presented. Values for individual mice are depicted, with mean values of four mice per group shown as a horizontal bar. Results are representative of two independent experiments.
Compared with naive mice (809 ± 192/106 splenocytes), AC treatment resulted in only modest but significant increases in the levels of total IgM-secreting cells (1418 ± 175/106 splenocytes, p = 0.003), while there was a great induction in the relative representation of anti-PC (PC-BSA 35.9 ± 12.3%, p < 0.002; C-PS 14.5 ±5.9%, p = 0.007) and anti-MDA IgM-secreting cells (23.5 ± 12.2%, p < 0.05) (Fig. 3, A and C).
While B6.S107.1−/− mice displayed comparable overall levels of splenic IgM-secreting cells, the frequencies of anti-PC IgM-secreting cells (0.5 ± 0.3% and 0.57 ± 0.2%) in naive mice were only modestly above the level of reactivity with the control Ag, BSA (0.3 ± 0.13%) (Fig. 3, B and D), while levels of MDA-reactive IgM-secreting cells (12.6 ± 5.6%) in naive B6.S107.1−/− mice were comparable to those in naive B6 mice. After AC treatment, anti-PC levels in B6.S107.1−/− mice (PCBSA 7.3 ± 4.9%; C-PS 1.6 ± 0.7%) remained significantly lower than in B6 mice ( p = 0.014 and 0.005, respectively). Importantly, even though anti-PC responses were greatly diminished in B6.S107.1−/− mice, AC treatment did significantly increase levels of MDA-reactive IgM-secreting cells in these T15-deficient mice (44.5 ± 13.7%) strains ( p = 0.005) (Fig. 3). Furthermore, although the overall frequency of total IgM- and Ag-specific IgM-secreting cells were several fold lower in the bone marrow than in the spleen (Fig. 3E), the same general patterns were demonstrated (Fig. 3, C and E). Therefore, these ELISPOT data reiterate patterns seen in the above-described ELISA findings; as compared with B6 mice, the B6.S107.1−/− mice were confirmed to have a profound defect in the capacity to recognize and respond to PC determinants on ACs, while anti-MDA responses were generally unimpaired or even increased.
Although the overall frequencies of induced IgG-secreting cells were considerably lower than for IgM-secreting cells (Fig. 3, D and F), we found similar patterns in the spleen and bone marrow, with significantly lower levels of PC-specific IgG-secreting cells in the T15-deficient B6.S107.1−/− mice than wild-type mice after AC immunization ( p < 0.001). Levels were also assessed in naive mice, but were too low for meaningful comparisons. However, there was a trend in the B6.S107.1−/− mice toward higher AC-induced MDA-specific IgG-secreting cells compared with B6 mice ( p < 0.16) (Fig. 3). Notably, these induced IgG Ab responses were overwhelmingly of the IgG3 subclass (data not shown), which suggested that akin to T15 responses to pneumococcal immunization (30, 31), i.v. AC infusions without adjuvant also induces T cell-independent type II responses. Taken together, these findings indicate that immune development in a setting of paucity of VHS107.1-expressing B cell precursors leads to anti-AC immune responses with relative expansion of MDA-reactive B cells.
T15 IgM Ab enhances the recruitment of C1q to ACs
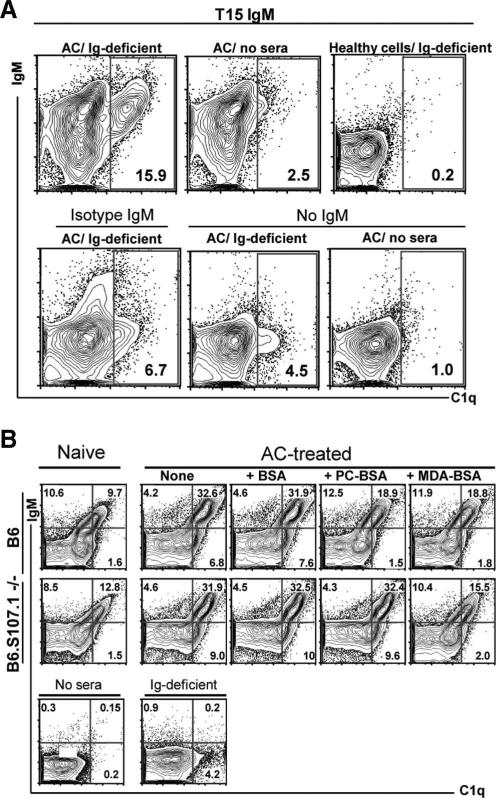
To better understand the potential functional capabilities of anti-AC Abs, we examined the capacity of these responses to recruit complement. Earlier reports have shown that direct interactions of ACs with C1q, the recognition molecule of the classical complement pathway, can play a prominent role in the phagocytic clearance of ACs (32–35). We found that incubation with sera from muMT mice (36), which provides a source of soluble opsonizing factors devoid of Ig (i.e., Ig-deficient sera), resulted in the deposition of C1q onto ACs but not healthy cells (Fig. 4A), which confirmed that C1q can interact with apoptosis-associated motifs, such as exposed phosphatidylserine residues (37, 38). Importantly, the addition of purified T15 IgM Ab, which recognizes cells dying of apoptosis in a PC-inhibitable fashion (8), induced much greater increases in C1q deposition on ACs (Fig. 4A). In contrast, T15 AB neither recognized freshly isolated healthy thymocytes nor enhanced C1q deposition on these cells (Fig. 4A). Additionally, the IgM isotype control, which displayed only limited binding interactions with ACs at late stage of apoptosis (not shown), induced only minor differences in C1q deposition (Fig. 4A). These studies document the capacity of the T15 clonotypic anti-PC Ab to greatly enhance C1q deposition on ACs, with levels of C1q on ACs directly correlating with relative levels of bound T15 Ab (Fig. 4A).
FIGURE 4.
Deposition of C1q onto apoptotic thymocytes is primarily mediated by anti-AC IgM Abs. A, Representative flow cytometric studies demonstrate low but detectable levels of direct deposition of C1q from Ig-deficient sera onto thymocytes undergoing etoposide-induced apoptosis (i.e., ACs), but not to healthy freshly isolated thymocytes (i.e., healthy cells). Apoptosis was confirmed by annexin V staining (not shown). In the presence of T15 IgM, but not isotype control, there is a distinct AC subset with high levels of bound IgM that has a proportionate high level of deposition of C1q. Without Ig-deficient sera there is little or no reactivity with the anti-C1q detection reagent, and the background for the detection signal is demonstrated with an isotype control for the C1q detection in the presence of T15 IgM and sera (not shown). B, Top, Incubation of naive (left) or AC-treated B6 sera (right) results in deposition on AC of IgM and proportionate deposition of C1q primarily in the IgM-associated ACs. While levels are higher with AC-treated B6 sera, IgM and also C1q binding are reduced by preincubation with PC-BSA (PC) or MDA-BSA (MDA) but not by BSA alone. Bottom, Incubation of naive (left) or AC-treated B6.S107.1−/− sera (right) also results in deposition on AC of IgM and C1q, with higher levels of both associated with AC-treated sera. Here, binding of IgM and C1q is reduced by preincubation with MDA-BSA but not by PC-BSA or BSA alone. Results are representative of three independent studies.
Natural Abs to PC and MDA recruit C1q to ACs
We next evaluated the properties of natural Abs in the preimmune repertoire of naive mice, and found that naive sera from both B6 and B6.S107.1−/− mice have IgM natural Abs that bind a subset of apoptotic thymocytes (Fig. 4B). Moreover, compared with the level of C1q that bound AC in the presence of Ig-deficient sera, incubation with sera from naive B6 and B6.S107.1−/− mice resulted in much higher levels of C1q deposition, which was primarily associated with the subset of ACs with bound IgM (Fig. 4B). Furthermore, compared with naive mice, sera from AC-treated mice produced even greater increases in IgM binding of ACs, and these levels were also directly proportional to concordant increases in C1q binding to ACs (Fig. 4B). In general, despite the above-described differences in the expressed apoptosis-associated binding specificities, the overall levels of IgM anti-AC Ab and C1q binding were similar in naive B6 and B6.S107.1−/− mice, and there were also comparable increases in the proportion of ACs with associated IgM and C1q after incubation with sera from AC-treated mice (Fig. 4B).
To assess the binding specificity of these anti-AC Abs, we performed Ag inhibition studies. As shown in a representative study (Fig. 4B), we found that incubation of AC-treated B6 sera with PC-BSA resulted in inhibition of the levels of IgM (~15%) and C1q deposition (~48%) on ACs, compared with the AC-treated sera alone or after control incubation with BSA. Similarly, incubation with MDA-BSA also inhibited (~16%) IgM and (~47%) C1q deposition onto ACs (Fig. 4B). These studies therefore confirm that in AC-treated wild-type B6 mice, Ab recognition of ACs is dominated by anti-PC and MDA Abs, which are responsible for marked increases in the levels of associated C1q when compared with incubation with Ig-deficient sera. Similar levels of inhibition by PC-BSA and MDA-BSA were also found with naive B6 sera (not shown). Notably, incubation of AC-treated B6.S107.1−/− sera with MDA-BSA also greatly inhibited the deposition of IgM (30%) and C1q (58%) on ACs. In contrast, incubation of this T15-deficient B6.S107.1−/− serum with PC-BSA did not affect binding of IgM Ab to AC or C1q deposition (<1%) (Fig. 4B), with similar findings found with naive sera (not shown).
Taken together, these findings demonstrate that these anti-AC responses are Ag specific and do not represent nonspecific polyclonal responses. We also found that even though B6.S107.1−/− mice are deficient in anti-PC Abs, their expression of MDA-inhibitable Abs is unimpaired, which is consistent with the above-described direct-binding specificity studies. Moreover, the recognition of particular neoepitopes on ACs was shown to be determined by VH gene inheritance, as the absence of a single VHS107.1 germline gene resulted in a shift of in vivo immune recognition away from PC-related and toward MDA-related determinants. However, it appeared that there are compensatory changes, as the anti-AC responses in S107.1-deficient mice have the same effector capabilities for augmenting the deposition of C1q onto the membranes of cells undergoing apoptotic death.
Natural Abs enhance AC phagocytosis by immature DCs
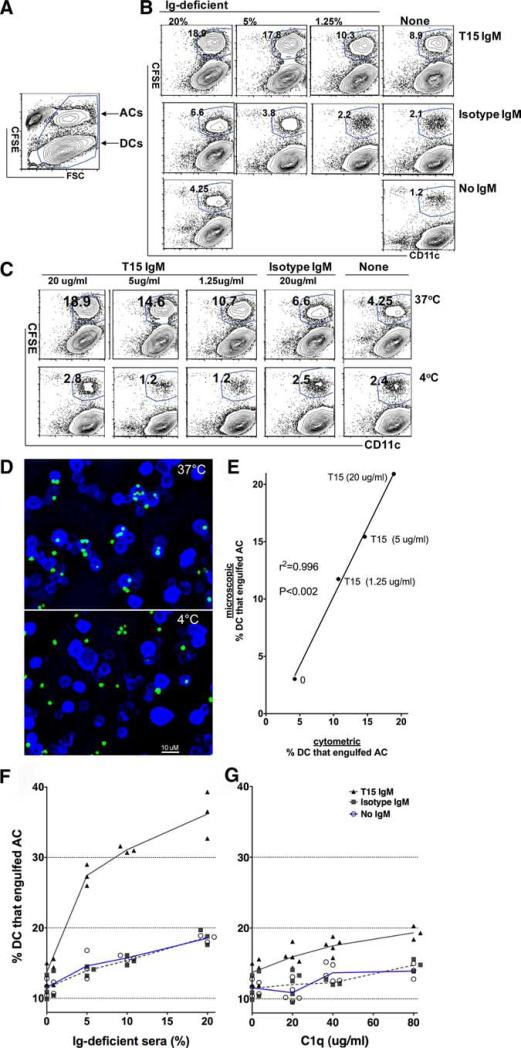
To evaluate whether these Ab responses can affect the innate immune function of AC clearance, we adapted an earlier described assay for quantifying the phagocytic capacity of bone marrow-derived conventional CD11c+ immature DCs (4, 24, 25). In a representative flow cytometry study, after incubation of immature DCs (>98% CD11b+CD11c+) with CFSE-labeled ACs (Fig. 5A and data not shown), DCs are here discriminated based on their larger size (i.e., forward light scatter), or based on CD11c staining (Fig. 5B and data not shown). Importantly, this approach enables identification of a distinct immature DC subpopulation that has ingested CFSE-tagged ACs due to their associated shift in fluorescence.
FIGURE 5.
T15 IgM binds apoptotic cells and by recruitment of serum factors enhances phagocytosis by conventional DCs. A, Flow cytometric analysis of a representative study of purified bone marrow-derived conventional immature DCs after incubation with CFSE-labeled apoptotic thymocytes. Healthy DCs are shown to be larger (i.e., forward light scatter (FSC)), while the smaller apoptotic thymocytes have been labeled with CFSE, which allows identification of the subset of DCs that have ingested ACs. B, Using this gating, representative results compare outcome after incubation of DCs and ACs for 45 min at 37°C, without or with T15 IgM or isotype control at 20 μg/ml. C, Representative results from cultures with 20% Ig-deficient sera and addition of IgM (T15, isotype control, or none) after 45 min at either 37°C or 4°C. Compared with the limited level at 4°C, phagocytosis is greatly up-regulated at 37°C, with greatest activity with the highest level of T15 IgM and Ig-deficient sera. D, Representative deconvoluted microscopy images (×20) of cytospins of replicate cultures with T15 IgM at 20 μg/ml and Ig-deficient sera after incubation at either 37°C (top) or 4°C (bottom) for 45 min. A 10-μm bar is indicated for size comparisons. E, Flow cytometric determination of values for the percentage of immature DCs that have engulfed ACs are plotted against values obtained from microscopic evaluation of >700 DCs per condition, from replicate cultures after 45 min. Data were compliled from percentage of DCs that have engulfed ACs after 1 h at 37°C, with titrated T15 IgM concentrations. F, Addition of Ig-deficient sera results in dose-dependent increases in phagocytosis, with lower levels in the absence of Ig or in the presence of the IgM isotype control after incubation for 1 h at 37°C. Higher levels are associated with the T15 IgM at 20 μg/ml. G, The addition of purified C1q results in dose-dependent increases in phagocytosis, with much lower levels in the absence of Ig, or presence of the IgM isotype control, compared with higher levels with the T15 IgM at 20 μg/ml after incubation for 1 h at 37°C. Results are representative of three independent studies.
In control studies, under serum-free conditions (i.e., devoid of Ig and opsonins) at 37°C the same low level of phagocytosis was found for labeled healthy thymocytes (11.1 ± 1.7%, n = 3) as was documented for apoptotic thymocytes (11.6 ± 0.9%, n = 7) (Fig. 5F). Importantly, while the addition of 20% Ig-deficient sera significantly increased the level of phagocytosis of ACs at 1 h at 37°C (18.5 ± 0.5%, p < 0.0001, t test with Welsh correction, n = 3), compared with serum-free conditions, these conditions were not associated with enhanced phagocytosis of CFSE-labeled healthy thymocytes (not shown). These findings confirm the importance of serum opsonins for enhancing the phagocytosis of cells undergoing apoptotic death.
In studies that assessed the role of anti-AC Abs to DC phagocytosis, we found that in the absence of serum factors the addition of T15 IgM resulted in only modest increases of DC phagocytosis (mean 13.7 ± 1.4%, n = 7). Significantly, in the presence of Igdeficient sera the addition of T15 IgM resulted in significant serum-dependent increases in the levels of AC engulfment (mean 36.1 ± 3.3%, n = 3) ( p < 0.01) (Fig. 5F), as 20% Ig-deficient sera and T15 IgM were associated with a >2.5-fold increase in phagocytosis compared with the absence of IgM, or with the IgM isotype control (Fig. 5F). Importantly, engulfment was temperature-dependent, as following incubation at 4°C with the T15 anti-PC Ab and 20% Ig-deficient sera, we found only very few (<3%) DCs had ingested CFSE-tagged ACs as detected by flow cytometry (Fig. 5B).
To better examine the actual interactions between DCs and ACs that were detected in the flow cytometric studies, cytospins of these same samples were visualized by deconvoluted microscopy (Figs. 5C–E). After incubation at 37°C, we found that many DCs had ingested one or more labeled ACs or cell fragments, while after incubation at 4°C labeled ACs were rarely found fully within the DCs (Fig. 5D). Further documenting the accuracy of this assay for quantifying DCs that engulf ACs, the results from flow cytometric and digital confocal studies had remarkably high positive correlation (r2 = 0.996, p < 0.002; Fig. 5E). Hence, this flow cytometry-based assay was therefore shown to be highly quantitative for assessing the relative efficiency for AC engulfment, and not just membrane association, with conventional immature DCs.
C1q enhances natural Ab-mediated AC phagocytosis
We also looked for potential interactions of serum anti-AC Abs with C1q, the recognition factor of the classical complement pathway that has well-known roles in immune complex functions. Compared with serum-free media, the addition of C1q to serum-free media provided a dose-dependent increase in DC phagocytosis, which reiterated overall patterns seen with the addition of Ig-deficient sera. Moreover, the level of phagocytosis after addition of C1q was similar whether incubated in the presence of the IgM isotype control or without Ig (Fig. 5G). Strikingly, the addition of T15 IgM further enhanced the capacity of DCs for AC phagocytosis, which was dependent on the relative concentration of C1q. However, while C1q alone enhanced T15 IgM-dependent increases in phagocytosis, there were still much greater increases with Ig-deficient sera. These findings suggest that C1q alone can convey natural Ab effector functions required for enhanced DC phagocytosis, although the full level of phagocytosis associated with Ig-deficient sera appears to involve additional factors.
Based on reports that myeloid cells can themselves produce complement and likely other opsonic factors (39, 40), we repeated studies of the same design with bone marrow-derived DCs made from homozygotic C3-deficient congenic mice, but we found neither differences from wild-type DCs in the baseline level of phagocytosis without Abs or serum factors (i.e., only serum-free media) (C3−/−, 10.9 ± 0.1%, n = 3 vs B6 11.6 ± 0.9%, n = 7, NS) nor the levels attained with 10% Ig-deficient sera (15.1 ± 0.7% n = 3 vs 15.7±0.35%, n = 3, NS) or with the addition of T15 IgM at 20 μg/ml along with serum opsonins (29.4 ± 2.9% vs 31.1 ± 0.45%, n = 3, NS). These findings document that anti-AC Abs enhance phagocytosis by a process that can be mediated by C1q alone, and they also suggest that sera likely contain additional soluble factors, other than C3, that can further enhance natural Ab-mediated DC phagocytosis of ACs.
Oxidation-associated determinants inhibit natural Ab-dependent phagocytosis
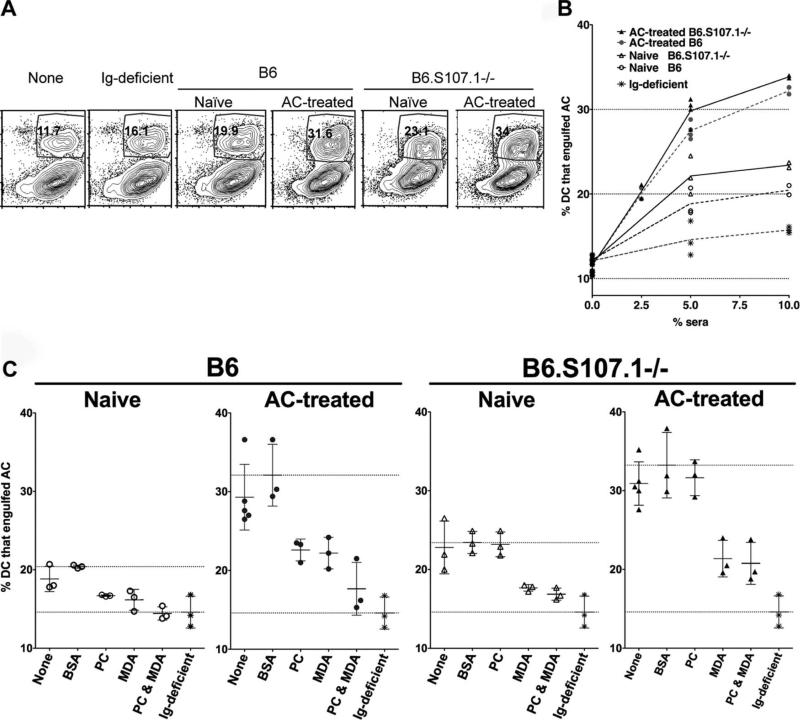
To better assess the potential physiologic relevance of these findings, we next evaluated the effect on DC phagocytosis of sera that vary in content of anti-AC Abs. As described above, compared with the baseline activity levels associated with serum-free media, phagocytosis was increased by addition of Igdeficient sera (Figs. 5E and 6, A and B), while addition of Ig-containing sera from either naive B6 or B6.S107.1−/− mice resulted in even greater dose-dependent increases in DC phagocytosis of ACs ( p < 0.01) (Fig. 6, A and B). In comparison, addition of sera from AC-treated mice, which have much greater concentrations of anti-AC, was associated with even higher levels of DC phagocytosis (Fig. 6B). Hence, sera from B6 and B6.S107.1−/− mice conveyed a level of activity in an AC phagocytosis assay that was directly proportional to the associated IgM anti-AC binding activity (Figs. 4B and 6B).
FIGURE 6.
MDA- and PC-inhibitable Abs to apoptotic cells recruit C1q and enhance phagocytosis by DCs. A, Representative studies of IgM and C1q binding to ACs after incubation with 10% sera from naive or AC-treated wild-type adult mice or mice deficient in the VHS107.1 gene (B6.S107.1−/−). The percentage of DCs that ingested ACs is indicated, after incubation for 1 h at 37°C. B, Results from replicate immature DC cultures, after incubation for 1 h at 37°C, showed consistent direct correlations of the proportion of added sera with the resulting level of enhanced AC apoptosis in replicate cultures. Such differences were consistently documented in comparisons of replicate cultures containing different proportions of sera. C, To determine the Ag specificity of serum Abs that affect DC phagocytosis, sera were first preincubated with PC-BSA, MDA-BSA, or BSA at 50 μg/ml for 1 h at 37°C before addition to cultures for 1 h at 37°C. Bars represent mean values and SDs for triplicate cultures. Top horizontal line is the mean level after serum incubation with BSA, while the bottom horizontal line is the mean level obtained with Ig-deficient sera (i.e., Ig-independent activity level). Results are representative of three independent studies.
As AC treatment increased Ab responses to PC and MDA determinants (Figs. 1–3 and 4B), we evaluated the contribution of such Abs to the efficiency of DC phagocytosis. In control experiments, there was no reduction in DC phagocytosis levels of ACs when 10% Ig-deficient sera were incubated with BSA or MDA-BSA or PC-BSA conjugates (not shown). In contrast, incubation of either PC-BSA or MDA-BSA conjugates with naive B6 sera, which contains natural Abs, significantly inhibited DC phagocytosis, causing 64% ( p < 0.05) and 73% ( p < 0.001) reduction, respectively (Fig. 6C). Moreover, when MDA-BSA was added together with PC-BSA to naive B6 sera, there was greater inhibition than with either alone (i.e., ~100% inhibition of the Ig contribution) ( p < 0.001), as DC phagocytosis was reduced to the level associated with the same dilution of Igdeficient sera. Sera from AC-treated B6 mice, which conveyed higher levels of phagocytosis, were also significantly inhibited by either PC-BSA and MDA-BSA, representing 54% ( p < 0.05) and 57% inhibition ( p < 0.05), respectively. Once again, when PC-BSA and MDA-BSA were added together to AC-treated B6 sera, there was a greater inhibition (80.6%, p < 0.01) than with either conjugate alone, which suggested that the sera contains non-cross-reacting Abs to MDA and PC determinants that separately enhance phagocytosis (Fig. 6C).
Studies of sera from B6.S107.1−/− mice reiterated the same strain-specific patterns demonstrated in Ab-binding studies, as there was no inhibition of DC phagocytosis by BSA alone or by the PC-BSA conjugate (Figs. 2, 3, and 6C), as these mice have defective anti-PC responses. In contrast, DC phagocytosis was significantly inhibited by addition of MDA-BSA (65%, p < 0.01), while there was no significant difference in the level of inhibition after the addition of PC-BSA and MDA-BSA together (74%) compared with MDA-BSA alone. Again, as levels of anti-AC Abs were increased after AC treatment (Fig. 4B), we confirmed our prediction that sera from AC-treated B6.S107.1−/− mice also conveyed higher levels of DC phagocytosis than sera from naive mice ( p < 0.02) (Fig. 6). While PC-BSA or BSA did not affect the level of phagocytosis associated with sera from AC-treated B6.S107.1−/− sera, MDA- BSA did significantly inhibit DC phagocytosis of ACs (mean, 60.1%; p < 0.01), but there was no further decrease by preincubation of MDA-BSA together with PC-BSA (mean, 66.9%). Taken together, these studies confirm the contributions of anti-AC Abs and the dominance of PC- and MDA-related AC determinants to the enhancement of the phagocytic function of immature DCs. Strikingly, in the absence of the single VHS107.1 gene, PC-inhibitable determinants were no longer immunodominant and major contributors to Ab-mediated DC phagocytosis, while the relative contributions of MDA-inhibitable determinants on ACs were unaffected or even increased.
Even though AC immunization induced an IgM response that was much greater than the IgG response, we sought to evaluate whether IgG Abs contributed to the Ig-dependent enhancement of AC phagocytosis. For these studies, serum aliquots obtained after AC treatment were passaged through a protein G-Sepharose column, which reduced IgG content by >96% without affecting IgM activity (data not shown). After normalizing samples based on total IgM concentration, we performed AC phagocytosis studies and found no significant differences in the proportion of immature DCs that had engulfed apoptotic thymocytes after 1 h in the presence of IgG-depleted sera (protein G passaged (7.21 ± 0.9%) vs untreated (6.89 ± 0.1%), NS, or vs control column passaged (7.89 ± 0.6%), n = 3, NS). However, because different cellular ratios were used, these specific values were not comparable to the above-described studies. Importantly, these findings confirm that under these conditions the IgM response to apoptosis-associated determinants is the key determinant for the efficiency of serum-dependent DC phagocytosis, and the minor AC-induced IgG response, of the IgG3 subclass, is not a major contributor.
Discussion
The capacity to dispose of dead and dying cells is essential for the host, and while a range of contributory molecules and pathways has been characterized, the potential roles of host Ab responses in these key processes have generally been overlooked. Herein, we now demonstrate that naive mice have circulating natural Abs to ACs that can enhance the capacity of immature DCs to phagocytose ACs, and this fundamental process can be further enhanced by Ab responses induced by i.v. challenge with ACs. We also provide the first evidence that genetic inheritance, in this case VH germline gene usage, determines the dominance of the Ags recognized on ACs. These findings were confirmed by Ag-specific inhibition of Ab binding and the associated recruitment of C1q to ACs, as well as by documenting Ag-specific inhibition of Ab-mediated enhancement of DC phagocytosis of ACs. Hence, in contrast to an earlier report that T15 IgM instead inhibited phagocytic clearance by elicited macrophages (7), our results document that the T15 IgM, as well as anti-AC natural Abs, can significantly enhance DC phagocytosis of ACs by a process that is highly dependent on the availability of serum factors. Taken together, our studies document the potent functional contributions of Ab responses for determining the AC phagocytic capacity of immature DCs.
Earlier surveys demonstrated that AC immunizations of healthy mice can induce Abs to a range of nuclear and membrane-associated self-Ags (117, 9, 10). As the relative contribution of distinct binding specificities to the in vivo Ab recognition of ACs has been uncertain, we used a highly multiplex Ag array in our investigations, and to our knowledge performed the most extensive surveys performed to date. Our studies also provided the first quantification of the frequency of Ag-reactive B cells in antiapoptotic thymocyte responses. Hence, based on a range of direct-binding and Ag-inhibition studies, we showed that these responses in C57BL/6 mice are highly dominated by immune recognition of AC membrane oxidation-associated PC and MDA neodeterminants.
Remarkably, in AC-treated mice >35% of the splenic IgM-secreting cells recognized PC determinants and ~20% recognized MDA determinants (Fig. 3, A and C). Furthermore, based on the capacity of PC and MDA ligands for additive inhibition, Ab responses to these determinants do not appear to be highly cross-reactive. Hence, despite the complexity of molecules that are displayed on these cells, PC and MDA determinants appear to dominate Ab responses to apoptotic thymocytes in B6 mice, as together these represent more than half of the functional anti-AC B cell responses to these dying cells (Fig. 3C). This antigenic dominance does not appear to be specific for death induced by etopo-side, as our control studies showed that MDA and PC determinants are also prominent in the recognition of thymocytes dying from exposure to gamma-irradiation or dexamethasone (data not shown), similar to findings previously reported with porcine aortic endothelial cells dying from dexamethasone exposure (8, 18) (data not shown). The above-described findings therefore refute the notion that natural Ab responses are always inherently polyreactive, or that ACs are inherently “sticky”, and that Ab binding to AC is nonspecific. Nonetheless, although PC and MDA are the major neoantigens on dying thymocytes, other self or altered self-Ags might be predominantly expressed during the death of other cell types (reviewed in Ref. 41).
We also investigated the relationship between the immune recognition of ACs and B cells that express T15 clonally related Abs. T15 clonotypic B-1 cells spontaneously arise and become highly represented during the first week of life, even in mice raised under germ-free conditions (42). Subsequently, T15 idiotypically related Abs dominate immune responses to experimental and microbial PC Ags, and they provide optimal protection from systemic pneumococcal infection (43). In addition to the paired canonical VHS107.1 and Vκ22 L chain rearrangements used in the classical T15 B-1 cell clone, there are also anti-PC Abs that are reported to express VHS107.1 H chain rearrangements paired with a range of other light chains (44–48). In contrast, structurally diverse anti-PC Abs without restricted Ab gene usage are instead produced during T cell-dependent responses to PC-protein conjugates (reviewed in Ref. 49). Importantly, no matter what their Ab gene usage or presumed mature B cell subset of origin, in a previous report we found that all tested anti-PC Abs could recognize ACs and discriminate them from healthy cells (8).
To assess the importance of T15-related Abs for in vivo responses to AC neodeterminants, we used the VHS107.1-deficient murine strain developed by Kenny and colleagues (17). The present studies showed that sera from VHS107.1-deficient mice, both naive and after AC treatment, contained Abs that recognize ACs, with the capacity to enhance AC phagocytosis by immature DCs to levels comparable with wild-type mice. Nonetheless, in both the pre- and postexposure repertoires, mice without this single inherited VH gene had a marked paucity of anti-PC Ig-secreting cells and dramatically reduced levels of PC-reactive Abs. Hence, our studies clearly demonstrated that the capacity for robust responses to PC determinants on ACs requires the S107.1 VH gene.
Our studies extend earlier reports that the S107.1 gene plays an essential role in the recognition of PC determinants on microbial pathogens (17), and we now demonstrate its unique and irreplaceable role in the recognition of PC determinants on ACs. In fact, the germline configuration of S107.1 is extremely well suited for binding of PC determinants, as many replacement mutations have been shown to result in impaired PC binding, while none has been found that improve it (50, 51). Consequently, we postulate that the germ-line S107.1 gene sequence has been selected during immune evolution for its relationship with this neo-self AC-binding specificity. Significantly, in the absence of the VHS107.1 gene, we found a concurrent and compensatory expansion of MDA-reactive Ig-secreting B cells, which highlights the importance of this parallel set of anti-AC Ab-secreting cells and the Abs to ACs. Our findings therefore document that even when the availability of inherited gene elements limits the range of neoepitopes that can be recognized, and there is a proportionate reduction in the diversity of preimmune B cell precursors, there remains a biologic pressure to maintain comparable overall levels of anti-AC B cells and IgM Abs. We interpret these findings as evidence of an immunologic drive based on homeostatic functions that requires the maintenance of high levels of these “autoreactive” B cells to ACs.
Our studies also illustrate that not all B-1 cell natural Abs, even among those that bind phospholipid-related Ags, can recognize apoptotic thymocytes and facilitate their clearance. As an isotype control IgM, we used a representative of another prototypic B-1 cell set (52, 53), which expresses the characteristic VH11 rearrangement and recognizes bromelain-treated red cells (19). While such Abs have been postulated to mediate in vivo removal of senescent red cells, we found that this IgM neither recognized phosphorylcholine Ags nor significantly bound apoptotic thymocytes or affected their phagocytosis. Nonetheless, it remains possible that such Abs, while they display little or no activity in our assays with apoptotic thymocytes, might be active in other systems, perhaps involving senescent red cells.
Complement factors have previously been implicated in the pathways by which polyclonal IgM responses may affect the phagocytic functions of macrophages. Notably, the capacity to induce C3 deposition on ACs was found to directly correlate with the efficiency of AC phagocytosis by macrophages (26, 54). In fact, several reports have pointed to a central role of the receptors CD11b/CD18 (i.e., complement receptor 3, CR3) and CD11c (i.e., CR4) for C3 breakdown products, such as iC3b and C3b, in AC immune recognition and clearance, especially in DCs. However, these studies have also been criticized for the predominant use of artificial experimental Ags and blocking Abs (35, 55–58).
There is also substantial evidence that C1q, independent of being an upstream initiator of C3 activation, plays important direct roles in phagocyte recognition and engulfment of ACs (32–34, 59). C1q deficiency results in significant defects in the clearance of ACs by stimulated and by resting peritoneal macrophages (35) and, importantly, mice deficient in C1q have increased numbers of glomerular apoptotic bodies independent of C3 activation (35, 60, 61). However, these studies did not take into consideration the potential contribution of natural Abs in the serum. To reconsider this key issue of whether purified C1q can affect Ab-mediated phagocytic clearance by immature DCs, we used a reductionistic and highly quantitative in vitro phagocytosis assay with serum-free media. Notably, C1q can be recruited to many, but not all, Ab-containing immune complexes, but even IgM Abs differ widely in their capacity to recruit complement (62). Our studies confirmed that purified C1q, under serum-free and Ig-free conditions, is directly deposited onto the cell membranes of apoptotic but not healthy thymocytes. However, we also demonstrated that T15 IgM fostered much higher levels of C1q deposition and phagocytosis of ACs, compared with the absence of Ig, or in the presence of the isotype IgM control that did not bind ACs. Moreover, T15 IgM displayed a dose-dependent deposition onto ACs of purified C1q, or of C1q from serum. At physiologically relevant levels, C1q also imparted dose-dependent enhancement of T15 IgM-mediated DC phagocytosis (Fig. 5G). Notably, our in vitro assay also demonstrated that C3 is not essential for the recognition and engulfment of apoptotic thymocytes by conventional DCs, which is consistent with a recent report (63).
Our studies therefore demonstrate that AC phagocytosis is greatly enhanced by naturally arising Abs, as well as AC-induced Abs, by a process that can be dependent on C1q recruitment. However, C1q at physiologic concentrations, akin to levels found in sera, did not attain the levels of apoptotic phagocytosis associated with the addition of sera (64). It is possible that greater activity could be attained with other C1q preparations or with the use of an anti-AC IgM with greater C1q-binding activity. However, as there are many other known op-sonic factors, we postulate that there are other eat me factors, in addition to C1q, that contribute to natural Ab-mediated enhancement of DC phagocytosis. While we were surprised to discover detectable levels of AC phagocytosis even in the absence of serum proteins, it is possible that these ACs might have become opsonized during the period in culture in the presence of serum, before their use in phagocytosis assays. Indeed, this background level of phagocytosis could also be due to interactions with the opsonins that may be locally produced by DCs themselves, as phagocytes are reported to produce a range of opsonic and bridging molecules, such as C1q, C3, and even MBL, among others (39, 40, 65–67).
There is increasing evidence that Abs to ACs are also commonly found in humans and play roles in health and disease. In fact, in our recently published serologic survey of adult systemic lupus erythematosus patients, we found evidence that human anti-MDA responses were more prominent than anti-PC responses (21). Our present findings suggest that the prominence of anti-PC responses in mice might be caused by the dominant role of S107.1-encoded Ig products, while the absence of a functionally equivalent human VH gene may explain why there is a greater dominance of anti-MDA responses in the human anti-AC repertoire. Moreover, our recent systemic lupus erythematosus studies also included three relevant cases in which higher IgM anti-MDA and/or anti-PC responses correlated with protection of individuals, who were otherwise genetically predisposed, from the development of clinically active systemic lupus erythematosus (21).
These findings have led us to consider how anti-AC responses may be involved in autoimmune pathogenesis. It is therefore intriguing that inflammatory conditions are reported to be associated with reduced levels of such IgM Abs (68, 69). Furthermore, the experimental regimen used herein, in which infusion of autologous apoptotic mononuclear cells induced an IgM anti-AC Ab response that enhances apoptotic clearance, is essentially equivalent to that used in extracorporeal photopheresis, a Food and Drug Administration-approved treatment with documented efficacy in diseases that include graft-vs-host disease and allograft rejection (70). Hence, our findings suggest an alternative mechanistic basis for benefits from extracorporeal photopheresis, and they provide a rationale for further investigations into how Abs to apoptosis-associated neodeterminants can affect the pathogenesis of inflammatory and autoimmune diseases. Additionally, Abs to PC and MDA bind to modified LDL and atherosclerotic plaques (8). Hence, these natural Ab responses may also affect the pathogenesis of atherosclerosis (18, 71), a chronic inflammatory disease that is the most common cause of morbidity and mortality in Western society. Further investigations are therefore required to assess the contributions of natural anti-AC Abs, and perhaps DCs, to other fundamental innate immune functions that may affect homeostasis and the pathogenesis of inflammatory and autoimmune diseases.
Acknowledgments
This work was performed in the Laboratory of B-cell Immunobiology at the University of California at San Diego. We appreciate the assistance and support of J. Feramisco, K. Pestonjamasp, and the Digital Imaging Shared Resource of the National Institutes of Health– National Cancer Institute-supported University of California at San Diego Moores Cancer Center, as well as helpful discussions with Drs. Andrea Tenner, V. Michael Holers, Carolyn Mold, Terry DuClos, C. Gronwall, J. Vas, M. P. Corr, and D. Kono.
Footnotes
This work was supported by the Alliance for Lupus Research, and by Grants AI40305 and AI46637 from the National Institutes of Health–National Institute of Allergy and Infectious Diseases, an Innovative Research Grant from the Arthritis Foundation and funding by the American College of Rheumatology Research and Education Foundation's Within Our Reach: Finding a Cure for Arthritis campaign (to G.J.S.).
Abbreviations used in this paper: AC, apoptotic cell; C-PS, cell wall polysaccharide; DC, dendritic cell; LDL, low-density lipoprotein; MDA, malondialdehyde; Ox-LDL, oxidized LDL; PC, phosphorylcholine.
Disclosures
GJS has received consulting fees from Neostasis Inc. UCSD has submitted a patent.
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi M, Kobayashi Y. Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells. Cell. Immunol. 2003;226:105–115. doi: 10.1016/j.cellimm.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr. Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 6.Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr. Biol. 2008;18:R76–R79. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw PX, Goodyear CS, Chang M-K, Witztum J, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J. Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA2 activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J. Exp. Med. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shvedova AA, Tyurina YY, Tyurin VA, Kikuchi Y, Kagan VE, Quinn PJ. Quantitative analysis of phospholipid peroxidation and antioxidant protection in live human epidermal keratinocytes. Biosci. Rep. 2001;21:33–43. doi: 10.1023/a:1010430000701. [DOI] [PubMed] [Google Scholar]
- 13.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids: importance of Schiff base formation and aldol concentration. J. Biol. Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 14.Pryor WA, Stanley JP. Letter: a suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids: nonenzymatic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- 16.Moore K, Roberts LJ., 2nd. Measurement of lipid peroxidation. Free Radical Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- 17.Mi QS, Zhou L, Schulze DH, Fischer RT, Lustig A, Rezanka LJ, Donovan DM, Longo DL, Kenny JJ. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc. Natl. Acad. Sci. USA. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J. Exp. Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman GJ, Cary SP, Dwyer DC, Luo L, Wagenknecht R, Curtiss VE. A B cell superantigen-induced persistent “Hole” in the B-1 repertoire. J. Exp. Med. 2000;192:87–98. doi: 10.1084/jem.192.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman GJ, Srikrishnan R, Germar K, Goodyear CS, Andrews KA, Ginzler EM, Tsao BP. Genetic imprinting of autoantibody repertoires in SLE patients. Clin. Exp. Immunol. 2008;153:102–116. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur. J. Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 23.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–285. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J. Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 27.Lee W, Cosenza H, Kohler H. Clonal restriction of the immune response to phosphorylcholine. Nature. 1974;247:55–57. doi: 10.1038/247055a0. [DOI] [PubMed] [Google Scholar]
- 28.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int. Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 29.Cohn M, Notani G, Rice SA. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969;6:111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- 30.Fung J, Kohler H. Immune response to phosphorylcholine, VII: Functional evidence for three separate B cell subpopulations responding to TI and TD PC-antigens. J. Immunol. 1980;125:640–646. [PubMed] [Google Scholar]
- 31.Slack J, Der-Balian GP, Nahm M, Davie JM. Subclass restriction of murine antibodies, II: The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J. Exp. Med. 1980;151:853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 33.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 37.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J. Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 38.Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–1290. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 39.Bensa JC, Reboul A, Colomb MG. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem. J. 1983;216:385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reboul A, Prandini MH, Bensa JC, Colomb MG. Characterization of C1q, C1s and C-1 Inh synthesized by stimulated human monocytes in vitro. FEBS Lett. 1985;190:65–68. doi: 10.1016/0014-5793(85)80428-2. [DOI] [PubMed] [Google Scholar]
- 41.Lutz HU. Homeostatic roles of naturally occurring antibodies: an overview. J. Autoimmun. 29:287–294. doi: 10.1016/j.jaut.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Sigal NH, Gearhart PJ, Klinman NR. The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J. Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- 43.McDaniel LS, Benjamin WHJ, Forman C, Briles DE. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J. Immunol. 1984;133:3308–3312. [PubMed] [Google Scholar]
- 44.Gearhart PJ, Sigal NH, Klinman NR. The monoclonal anti-phosphorylcholine antibody response in several murine strains: genetic implications of a diverse repertoire. J. Exp. Med. 1977;145:876–891. doi: 10.1084/jem.145.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crews S, Griffin J, Huang H, Calame K, Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981;25:59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 46.Guo WX, Burger AM, Fischer RT, Sieckmann DG, Longo DL, Kenny JJ. Sequence changes at the V-D junction of the VH1 heavy chain of anti-phosphocholine antibodies alter binding to and protection against Streptococcus pneumoniae. Int. Immunol. 1997;9:665–677. doi: 10.1093/intimm/9.5.665. [DOI] [PubMed] [Google Scholar]
- 47.Kenny JJ, Moratz CM, Guelde G, O'Connell CD, George J, Dell C, Penner SJ, Weber JS, Berry J, Claflin CL, Longo DL. Antigen binding and idiotype analysis of antibodies obtained after electroporation of heavy and light chain genes encoding phosphocholine-specific antibodies: a model for T15-idiotype dominance. J. Exp. Med. 1992;176:1637–1643. doi: 10.1084/jem.176.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, Roberts VA, Stevens S, Brown M, Stenzel-Poore MP, Rittenberg MB. Enhancement and destruction of antibody function by somatic mutation: unequal occurrence is controlled by V gene combinatorial associations. EMBO J. 1995;14:2784–2794. doi: 10.1002/j.1460-2075.1995.tb07278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenzel-Poore MP, Bruderer U, Rittenberg MB. The adaptive potential of the memory response: clonal recruitment and epitope recognition. Immunol. Rev. 1988;105:113–136. doi: 10.1111/j.1600-065x.1988.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 50.Brown M, Rittenburg MB, Chen C, Roberts VA. Tolerance of single, but not multiple, amino acid replacements in antibody VH CDR 2: a means of minimizing B cell wastage from somatic hypermutation? J. Immunol. 1996;156:3285–3291. [PubMed] [Google Scholar]
- 51.Wiens GD, Heldwein KA, Stenzel-Poore MP, Rittenberg MB. Somatic mutation in VH complementarity-determining region 2 and framework region 2: differential effects on antigen binding and Ig secretion. J. Immunol. 1997;159:1293–1302. [PubMed] [Google Scholar]
- 52.Carmack CE, Shinton SA, Hayakawa K, Hardy RR. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J. Exp. Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline: relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 55.Behrens EM, Sriram U, Shivers DK, Gallucci M, Ma Z, Finkel TH, Gallucci S. Complement receptor 3 ligation of dendritic cells suppresses their stimulatory capacity. J. Immunol. 2007;178:6268–6279. doi: 10.4049/jimmunol.178.10.6268. [DOI] [PubMed] [Google Scholar]
- 56.Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N. The apoptotic-cell receptor CR3, but not αvβ5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–955. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr., Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 58.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, Kutikov I, Gill O, Mevorach D. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J. Exp. Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gershov D, Kim S, Brot N, Elkon KB. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell DA, Taylor PR, Cook HT, Moss J, Bygrave AE, Walport MJ, Botto M. Cutting edge: C1q protects against the development of glomerulonephritis independently of C3 activation. J. Immunol. 1999;162:5676–5679. [PubMed] [Google Scholar]
- 61.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos RM, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 62.Hughey CT, Brewer JW, Colosia AD, Rosse WF, Corley RB. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J. Immunol. 1998;161:4091–4097. [PubMed] [Google Scholar]
- 63.Behrens EM, Ning Y, Muvarak N, Zoltick PW, Flake AW, Gallucci S. Apoptotic cell-mediated immunoregulation of dendritic cells does not require iC3b opsonization. J. Immunol. 2008;181:3018–3026. doi: 10.4049/jimmunol.181.5.3018. [DOI] [PubMed] [Google Scholar]
- 64.Cortes-Hernandez J, Fossati-Jimack L, Carugati A, Potter PK, Walport MJ, Cook HT, Botto M. Murine glomerular mesangial cell uptake of apoptotic cells is inefficient and involves serum-mediated but complement-independent mechanisms. Clin. Exp. Immunol. 2002;130:459–466. doi: 10.1046/j.1365-2249.2002.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 66.Ezekowitz RA. Local opsonization for apoptosis? Nat. Immunol. 2002;3:510–512. doi: 10.1038/ni0602-510. [DOI] [PubMed] [Google Scholar]
- 67.Ezekowitz RA, Sim RB, MacPherson GG, Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro: role of type 3 complement receptors and macrophage-derived complement. J. Clin. Invest. 1985;76:2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padilla ND, Ciurana C, van Oers J, Ogilvie AC, Hack CE. Levels of natural IgM antibodies against phosphorylcholine in healthy individuals and in patients undergoing isolated limb perfusion. J. Immunol. Methods. 2004;293:1–11. doi: 10.1016/j.jim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 70.Bladon J, Taylor PC. Extracorporeal photopheresis: a focus on apoptosis and cytokines. J. Dermatol. Sci. 2006;43:85–94. doi: 10.1016/j.jdermsci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Binder C, Hörkkö S, Dewan A, Chang M-K, Kieu E, Goodyear C, Shaw P, Palinski W, Witztum J, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between oxidized LDL and Streptococcus pneumoniae. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]