SUMMARY
The pathogenic bacterium Chlamydia replicates in a eukaryotic host cell via a developmental cycle marked by temporal waves of gene expression. We have previously shown that late genes transcribed by the major chlamydial RNA polymerase, σ66 RNA polymerase, are regulated by a transcriptional repressor EUO. We now report that EUO also represses promoters for a second subset of late genes that are transcribed by an alternative polymerase called σ28 RNA polymerase. EUO bound in the vicinity of six σ28-dependent promoters and inhibited transcription of each promoter. We used a mutational analysis to demonstrate that the EUO binding site functions as an operator that is necessary and sufficient for EUO-mediated repression of σ28-dependent transcription. We also verified specific binding of EUO to σ66-dependent and σ28-dependent promoters with a DNA immunoprecipitation assay. These findings support a model in which EUO represses expression of both σ66-dependent and σ28-dependent late genes. We thus propose that EUO is the master regulator of late gene expression in the chlamydial developmental cycle.
Keywords: sigma 28, transcription factor, promoter, temporal regulation, DNA immunoprecipitation
INTRODUCTION
The human pathogen Chlamydia replicates within an infected host cell via an unusual developmental cycle in which there is conversion between two specialized forms of the bacterium (Abdelrahman & Belland, 2005). The elementary body (EB), which is the infectious form, initiates the intracellular infection by binding and entering the host cell. Early in the infection, within the first few hours of entry, the EB converts into a second morphologic form called a reticulate body (RB). The RB is metabolically active and replicates through multiple rounds of binary fission during midcycle of the infection. At about 18–24 hours post infection (hpi), the intracellular infection enters its late stage when individual RBs convert into an EB, prior to exit from the host cell to infect new cells.
Chlamydial genes are expressed in three main temporal classes that correspond to these three stages of the developmental cycle (Belland et al., 2003, Shaw et al., 2000, Nicholson et al., 2003). Early genes are transcribed within three hours of EB entry, and are believed to be important for establishing the intracellular infection. Midcycle genes are involved in RB growth and replication and make up the large majority of chlamydial genes. Late genes are a small group of specialized genes that are first transcribed or upregulated towards the end of the developmental cycle. Early, midcycle and late genes can all be transcribed by the major chlamydial RNA polymerase, σ66 RNA polymerase (Tan, 2012).
Many late genes are involved in RB-to-EB conversion and EB function. For example, the late operon omcAB encodes two cysteine-rich outer membrane proteins that are highly abundant in EBs (Mukhopadhyay et al., 2006). hctA and hctB are late genes that encode the histone-like proteins HctA (Hc1) and HctB (Hc2), which bind and compact DNA, mediating the DNA condensation that is characteristic of an EB (Barry et al., 1992) (Brickman et al., 1993). Other late genes include tsp, which encodes a chlamydial protease, tlyC_1, which encodes a putative hemolysin, and several genes for the type III secretion apparatus, such as scc2, cdsJ, and cdsU (Yu et al., 2006b, Case et al., 2010). The expression of late genes must be delayed until RBs have replicated in order to prevent premature RB-to-EB conversion.
Late genes transcribed by σ66 RNA polymerase are regulated by a transcription factor called EUO (Rosario & Tan, 2012). EUO provides a mechanism to differentially regulate σ66-dependent late genes from early and midcycle genes that are transcribed by the same form of RNA polymerase. EUO, which stands for early upstream operon, was first identified because its transcript is expressed at very early times in the developmental cycle (Wichlan & Hatch, 1993). It is a DNA binding protein that recognizes a 15 bp A/T-rich consensus sequence (Zhang et al., 1998, Zhang et al., 2000). We have shown that EUO selectively binds to this operator sequence in the vicinity of σ66-dependent late promoters and inhibits their transcription (Rosario & Tan, 2012). These findings support a model in which the early expression of EUO represses transcription of σ66-dependent late genes until they are derepressed at late times by an undefined mechanism.
Not all late genes are controlled by σ66 RNA polymerase, however, for a subset is regulated by an alternative chlamydial RNA polymerase called σ28 RNA polymerase (Yu & Tan, 2003). σ28 RNA polymerase transcribes promoters for three C. trachomatis late genes, hctB, tsp, and tlyC_1 (Yu et al., 2006b). However, σ28 is transcribed from a midcycle gene rpsD (Douglas & Hatch, 2000, Shen et al., 2004), raising the question of whether the activity of this alternative RNA polymerase is temporally regulated.
From these published observations, we hypothesized that the transcription of σ28-dependent late genes is regulated to control their temporal expression. We were particularly interested in EUO as a well-characterized chlamydial transcription factor that controls late gene expression. We found that EUO bound and repressed promoters for all known σ28-dependent genes, including the three late genes and three additional σ28-dependent genes that are expressed prior to late times. Our results demonstrate that EUO regulates both σ66-dependent and σ28-dependent late promoters, supporting a role for this transcriptional repressor as a master regulator of late gene expression in the chlamydial developmental cycle.
RESULTS
EUO binds in the vicinity of chlamydial σ28-dependent promoters
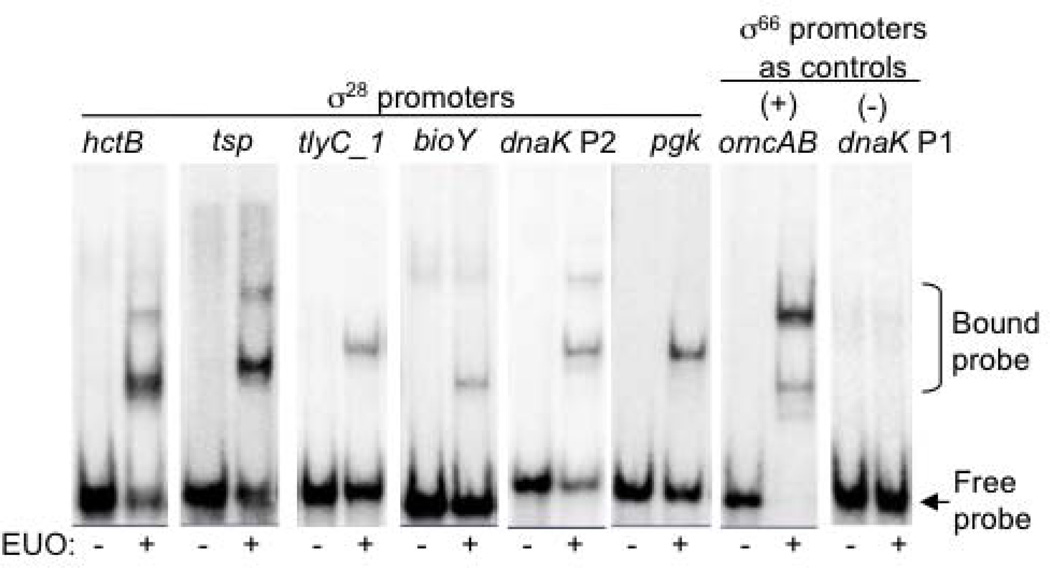
To investigate if EUO has a broader role in regulating late chlamydial gene expression, we examined if this transcriptional repressor of σ66-dependent late genes also binds C. trachomatis promoters transcribed by σ28 RNA polymerase. In an electrophoretic mobility shift assay (EMSA), recombinant C. trachomatis EUO (rEUO) produced a gel shift of two bands with the σ28-dependent hctB promoter (−55 to +5, relative to the transcription start site, +1) (Fig. 1) (Yu & Tan, 2003). This EMSA pattern was similar to the shift produced with the omcAB promoter, which is a σ66-dependent EUO target (Rosario & Tan, 2012). The binding was sequence-specific because there was no binding to the dnaK P1 promoter, which is an early σ66-dependent chlamydial gene that is not regulated by EUO (Fig. 1). EUO also bound to DNA fragments for the tsp, tlyC_1, bioY, dnaK P2 and pgk promoters, which are five other C. trachomatis σ28-regulated promoters that have been experimentally verified (Fig. 1 and Table 1) (Yu et al., 2006b).
Figure 1. EUO binds to C. trachomatis σ28-dependent promoters.
EMSA reactions were performed in the absence or presence of 320 nM rEUO. Each promoter region was contained on a 60 bp DNA probe,with the exception of tsp which was a 90 bp DNA probe (Table 1). Bands corresponding to the bound and free probes are indicated to the right.
Table 1.
C. trachomatis σ28-dependent promoter regions tested in EMSA reactions for EUO binding
| Promoter | Region tested (relative to transcription start site, +1) |
|---|---|
| hctB | −55 to +5 |
| tsp | −55 to +5 |
| −85 to +5 | |
| tly_1 | −55 to +5 |
| −30 to +30 | |
| bioY | −55 to +5 |
| dnaK P2 | −55 to +5 |
| pgk | −55 to +5 |
In these in vitro binding studies, EUO bound the six σ28-dependent promoters in different locations relative to their respective −35 and −10 promoter elements (Table 1). For example, EUO bound within −55 to +5 of the hctB promoter, which encompasses the −35 and −10 elements. However, EUO appears to bind upstream of the −35 element of the tsp promoter because it bound a DNA fragment containing sequences from −90 to +5 (Fig. 1) but not −55 to +5 (data not shown). In contrast, EUO appears to bind downstream of the −10 element of the tlyC_1 promoter because it bound a DNA fragment from −30 to +30 (Fig. 1) but not −55 to +5 (data not shown).
EUO represses transcription of chlamydial σ28-dependent promoters
We next measured the functional effect of EUO binding on these six σ28-dependent promoters with an in vitro transcription assay. These studies were performed with σ28 RNA polymerase reconstituted from recombinant C. trachomatis σ28 and E. coli core enzyme (Yu & Tan, 2003). This heterologous σ28 RNA polymerase has a similar promoter recognition as σ28 RNA polymerase reconstituted from recombinant C. trachomatis σ28 and partially purified C. trachomatis RNA polymerase but has the advantage of lacking other co-purified chlamydial proteins (Yu & Tan, 2003).
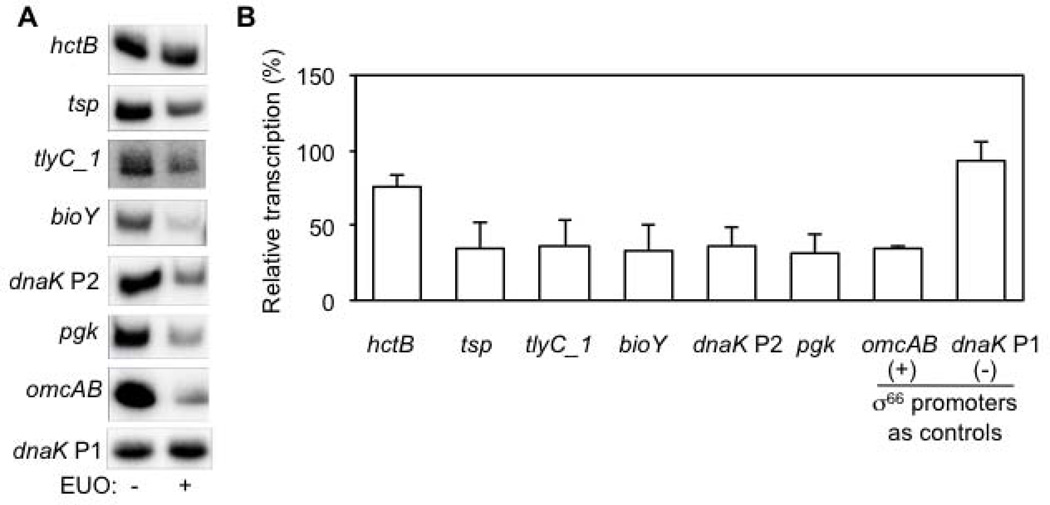
For five of the six σ28 promoters, rEUO decreased transcription to 31–35% relative to transcription in the absence of EUO (Fig. 2). This level of inhibition was similar to repression of the omcAB promoter that was measured in parallel experiments with σ66 RNA polymerase (Fig. 2). In a negative control experiment, EUO did not inhibit the dnaK P1 promoter, which is not regulated by EUO. In contrast to the five other σ28 promoters, EUO only decreased transcription of the hctB promoter to 75% of baseline levels, which was a statistically significant (p < 0.005), but small effect.
Figure 2. EUO represses transcription of σ28-dependent promoters.
A. Representative in vitro transcription assay for each promoter, which was present on a supercoiled transcription plasmid at a concentration of 13 nM. Transcription reactions were performed with σ28 RNA polymerase in the absence or presence of 2.5 µM rEUO. σ66-dependent control promoters were transcribed with E. coli holoenzyme. These gels do not reflect the relative strength of the promoters because different amounts of transcription reaction were used for each promoter in order to have a similar baseline transcription in the absence of EUO. However for a given promoter, the same amount of transcription reaction in the absence or presence of EUO was analyzed. B. Graph of the effect of EUO on transcriptional activity. For each promoter, transcription in the presence of EUO was normalized to baseline transcription in the absence of EUO. Values are from the average of at least three independent experiments with standard deviation indicated by the error bar.
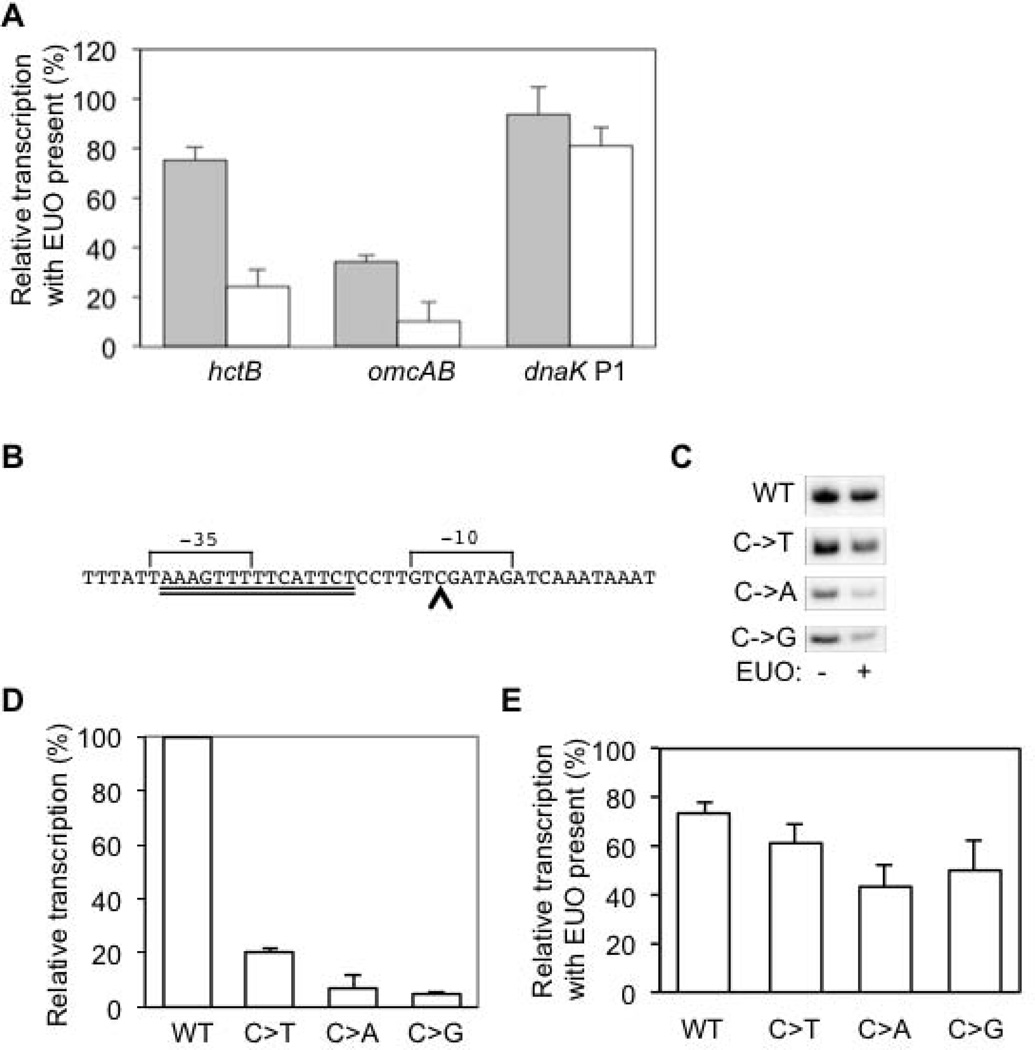
We performed additional studies on the hctB promoter to determine if it is regulated by EUO. This promoter is highly transcribed by chlamydial σ28 RNA polymerase in vitro and its sequence closely resembles the bacterial consensus σ28 promoter sequence (Yu & Tan, 2003). In a first approach, we tested the effect of EUO at a higher EUO:DNA ratio, which we achieved by using less transcription template. At a plasmid DNA concentration of 3 nM, instead of 13 nM, EUO decreased transcription of the hctB promoter to 25% of baseline, providing evidence that it is an EUO target promoter and is repressed similarly to the other σ28-dependent promoters (Fig. 3A).
Figure 3. Regulation of the hctB promoter by EUO.
A. The hctB promoter is repressed at a higher EUO:DNA ratio. Graph showing the effect of 2.5 µM rEUO on transcription of the hctB promoter at a plasmid DNA concentration of 13 nM (gray bars) or 3 nM (white bars). The hctB promoter was transcribed by σ28 RNA polymerase, while σ66-dependent control promoters (omcAB and dnaK P1) were transcribed with E. coli holoenzyme. For each promoter, transcription in the presence of EUO was normalized to baseline transcription in the absence of EUO. Values are from the average of at least three independent experiments with standard deviations indicated by error bars. B. DNA sequence of the C. trachomatis hctB promoter, with the putative 15 bp core EUO-binding site indicated with a double underline, and the C residue at position −12 marked with a carat. The −35 and −10 promoter elements are labeled. C. Representative gels showing in vitro transcription of the wild type (WT) hctB promoter and mutant promoters containing a single nucleotide substitution of the C at position −12. Transcriptions were performed with σ28 RNA polymerase in the absence or presence of 2.5 µM rEUO. Only 1/4 of the transcription reactions with the WT promoter were loaded on the gel because this promoter was transcribed at much higher levels than the mutant promoters. D. Graph showing quantification of these transcription results. Transcript levels from the WT promoter were defined as 100%, and transcript levels from each mutant hctB promoter were normalized to the WT promoter. E. Graph showing effect of EUO on transcription of WT and mutant hctB promoters. For each promoter, transcription in the presence of EUO was normalized to baseline transcription in the absence of EUO. Values are from the average of at least three independent experiments with standard deviation indicated by the error bar.
In a complementary approach, we tested the effect of EUO on three mutant hctB promoters that have reduced promoter activity because of a point substitution at position −12 (Fig. 3B, 3C) (Yu et al., 2006a). EUO caused inhibition, though modest, of these weaker promoters, reducing transcription to 61%, 43%, and 50% of baseline for a C-to-T, C-to-A and C-to-G substitution, respectively (Fig. 3E). Together these results provide evidence that hctB is regulated by EUO. The differences among the six promoters, in the extent of repression by EUO, are consistent with previous observations of promoter-specific effects on the efficiency of repressor action (Lanzer & Bujard, 1988).
Mapping the location of the EUO operator for a σ28-dependent promoter
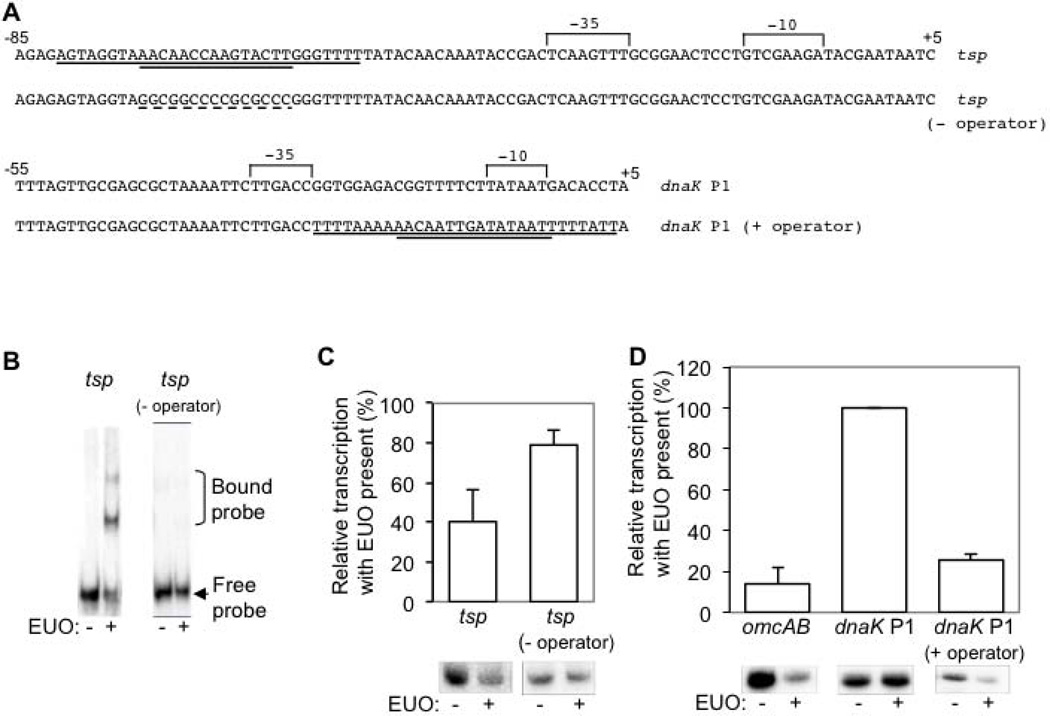
We used a mutational approach to verify that an EUO binding site is necessary for repression. We chose to map the operator for tsp because its location appeared, from our EMSA studies, to be farthest from the σ28 promoter. We identified a candidate site from −73 to −59 (Fig. 4A) based on its resemblance to the EUO consensus binding sequence (Zhang et al., 2000). In our mutational studies, we were guided by previous observations that showed that nine, but not three, nucleotide substitutions were necessary to greatly reduce EUO binding to the C. trachomatis omcAB promoter (Rosario & Tan, 2012). Therefore, we introduced nucleotide substitutions in 10 of the 15 bp of the predicted tsp operator, replacing adenine or thymine residues with guanine or cytosine, respectively (Fig. 4A). EUO was unable to bind a tsp promoter template containing this mutant operator (Fig. 4B) nor inhibit its transcription by σ28 RNA polymerase (Fig. 4C). These results provide evidence for an operator upstream of the −35 element of the tsp promoter that is necessary for repression by EUO.
Figure 4. The EUO operator is necessary and sufficient for repression.
A. DNA sequence of wild type and mutant tsp and dnaK P1 promoters used in the mutational analyses of the EUO operator. The putative 15 bp core EUO-binding site (double underline) with flanking sequence (single underline) is indicated, and the mutated sequence is indicated by a dashed underline. The −35 and −10 promoter elements are labeled, and nucleotide positions relative to the transcription start site (+1) are indicated. The tsp (− operator) mutant promoter has nucleotide substitutions in 10/15 bp of the core EUO-binding site. The dnaK P1 (+ operator) mutant promoter contains the EUO binding site from the omcAB promoter on a 30 bp insert. This insert preserved both the sequence of the −10 promoter element and its spacing relative to the −35 promoter element. B. EMSA experiments performed with 90 bp DNA fragments containing either the wild type tsp promoter, with its operator located from −76 to −59, or a mutant promoter [labeled as tsp promoter (− operator)] with substitutions in 10 of 15 nucleotides of the operator. Experiments were performed in the absence or presence of 320 nM EUO. Bands corresponding to the bound and free probes are indicated to the right. C. Graph and representative gels showing effect of EUO on transcription of the wild type or mutant tsp promoter templates by σ28 RNA polymerase. D. Graph and representative gels showing effect of EUO on transcription by σ66 RNA polymerase for the positive control omcAB promoter, the negative control wild type dnaK P1 promoter, which lacks an EUO operator, and a mutant dnaK P1 promoter containing the EUO operator of the omcAB promoter located in the center of a 30 nucleotide sequence (dnaK P1 promoter + operator). For each promoter, transcription by RNA polymerase in the presence of 2.5 µM EUO was normalized to baseline transcription in the absence of EUO. Values are reported as the average of at least three independent experiments with standard deviation indicated by the error bar.
The EUO operator is sufficient for EUO-mediated repression
We next examined if a promoter could be converted into an EUO-regulated promoter by addition of an EUO operator. We placed the omcAB EUO operator into the EUO-independent dnaK P1 promoter and tested for binding by EMSA (Fig. 4A). The core 15 bp of the omcAB EUO operator was not sufficient for EUO binding (data not shown), which is consistent with published observations (Zhang et al., 2000). Instead EUO binding required the 15 bp core EUO operator to be present in the middle of a 30 bp omcAB fragment (data not shown), suggesting that additional sequences flanking the core operator are also important for binding (Fig. 4A). EUO reduced transcription of this operator-containing dnaK P1 promoter to 26% of transcription in the absence of EUO, but did not inhibit transcription of the wild type dnaK P1 promoter (Fig. 4D). These results demonstrate that a 30 bp nucleotide sequence containing the EUO operator is sufficient for EUO-mediated repression.
EUO selectively binds both σ28-dependent and σ66-dependent late promoters
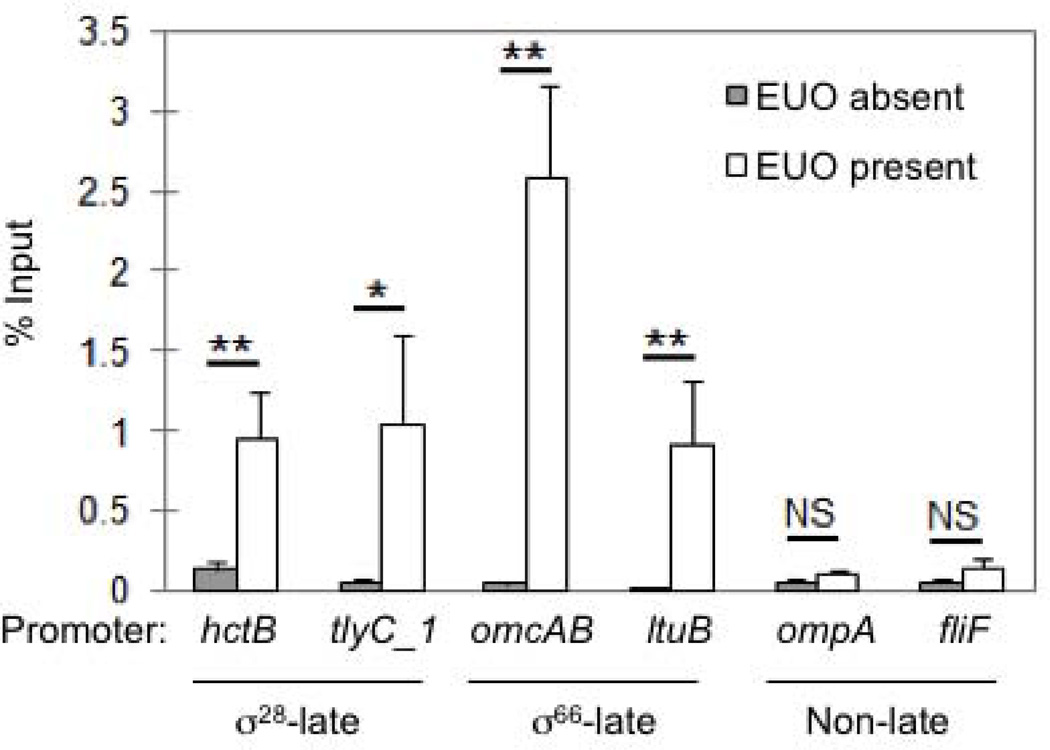
We used DNA immunoprecipitation to provide further evidence that EUO selectively regulates late genes. C. trachomatis genomic DNA was incubated in the presence or absence of rEUO, and EUO-bound DNA fragments were isolated by immunoprecipitation and quantified by qPCR. In control experiments without EUO, we recovered < 0.15% of input DNA for all of the six promoters tested, providing a measure of background DNA immunoprecipitation. EUO binding to specific promoters was detected by DNA recovery above this background threshold and by enrichment when comparing recovery in the presence and absence of EUO.
EUO bound to two σ28-dependent late promoters with a 7.3-fold enrichment for hctB (0.95% of input in the presence of EUO versus 0.13% in the absence of EUO), and a 21-fold enrichment for tlyC1 (1.04% versus 0.05% input) (Fig. 5). EUO also bound to two σ66-dependent late promoters, with a 65-fold enrichment for omcAB (2.6% versus 0.04% input) and a 46-fold enrichment for ltuB (0.91% versus 0.02% input) (Fig. 5). EUO addition produced a modest enrichment for two non-late promoters, with a 2-fold enrichment for ompA (0.1% versus 0.05% input), and a 2.8-fold enrichment for fliF (0.14% versus 0.05% input) (Fig. 5). However, this DNA recovery, even with the addition of EUO, was below the background threshold, consistent with a lack of specific binding to these non-late promoters. These immunoprecipitation studies demonstrate that EUO specifically binds both σ28-dependent and σ66-dependent late genes.
Figure 5. EUO selectively binds to both σ28-dependent and σ66-dependent late promoters.
DNA immunoprecipitation was performed in the absence (grey bars) or presence of 20 nM rEUO (white bars). Immunoprecipitated DNA was amplified with promoter-specific primers and calculated as a percentage of input DNA as described in the Experimental Procedures. Values are reported as the average of three independent experiments with standard deviation indicated by the error bars. Statistical analysis: * indicates p < 0.01, ** indicates p < 0.005, and NS indicates no statistically significant difference.
DISCUSSION
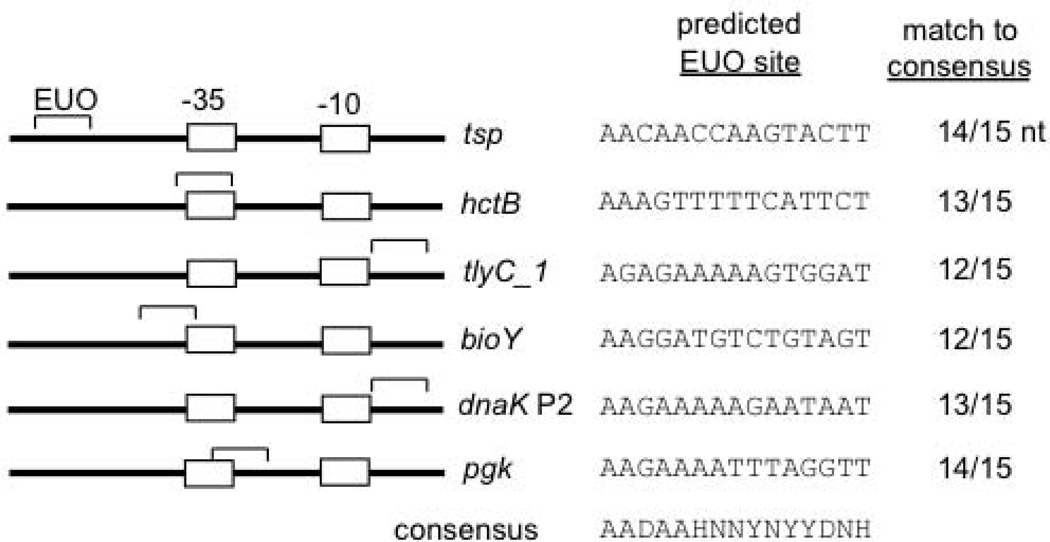
This study provides evidence that the chlamydial transcriptional repressor EUO regulates promoters transcribed by σ28 RNA polymerase. EUO bound and repressed the six known chlamydial σ28-dependent promoters (Yu et al., 2006b), and we identified a putative EUO binding site in the vicinity of each promoter (Fig. 6). In addition, we demonstrated that the EUO binding site functions as an operator that is necessary and sufficient for EUO-mediated repression. Binding and transcriptional inhibition of σ28 promoters by EUO, as well as the location of the operator near the promoter, were similar to EUO-mediated repression of σ66 late promoters (Rosario & Tan, 2012).
Figure 6. Identification of putative EUO-binding sites for σ28-dependent promoters.
Diagram showing the location of each EUO-binding site (marked by a bracket) relative to the −35 and −10 promoter elements (marked by boxes). For comparison, the consensus EUO-binding site is shown below (D = not C, H = not G, N = G, A, T, C, Y = C or T) (Zhang et al., 2000). For each promoter, the DNA sequence of the predicted EUO-binding site and the number of nucleotides that match the 15 nt consensus EUO binding sequence are shown.
The mechanism of EUO-mediated repression is not known. The location of many EUO operators in the close vicinity of their promoter, and often overlapping the −35 and −10 elements (Rosario & Tan, 2012 and Fig. 6), suggests that EUO may repress these promoters throught the classic mechanism of steric hindrance. A noteable exception is the tsp operator, which is located relatively far upstream of its promoter. The 15 bp core tsp operator from −73 to −59 has a limited predicted overlap with the region of σ28 RNA polymerase-promoter binding, which has been mapped in E. coli from −62 to +14 by DNase I footprinting (Kundu et al., 1997, Payankaulam et al., 2010). However, the region of EUO binding may be larger because the EUO DNAse I footprint covers 27–37 bp (Zhang et al., 2000), and we found that additional flanking sequences were important for EUO-mediated binding and repression. Alternatively, it is possible that EUO represses the tsp promoter via mechanisms subsequent to RNA polymerase-promoter binding. For example, EUO may inhibit promoter melting or clearance, which are more likely to be rate-limiting steps in transcription initiation for the highly-transcribed tsp promoter (Rojo, 2001).
These findings support a role for EUO as the master regulator of late gene expression in the chlamydial developmental cycle. Late genes can be divided into two subsets that are transcribed by either σ66 RNA polymerase, which is the major chlamydial RNA polymerase, or σ28 RNA polymerase (Tan, 2012). We previously showed that EUO regulates σ66-dependent late genes because it repressed transcription of six late promoters by σ66 RNA polymerase and bound the promoter regions of four additional σ66-dependent late genes (Rosario & Tan, 2012). By showing that EUO also controls six σ28-dependent promoters, we now provide evidence that EUO regulates both subsets of late chlamydial genes. Our data indicate that the EUO regulon consists of at least 16 operons encoding 17 late genes in C. trachomatis. The total number of EUO target genes is not known, however, and it is difficult to accurately identify EUO operators in the genome because of their degenerate A/T-rich sequence. It is also not yet known whether EUO is solely responsible for late gene expression during the developmental cycle.
EUO provides an elegant mechanism to coordinately regulate the temporal expression of multiple chlamydial late genes. EUO is expressed from very early times in the chlamydial developmental cycle with transcripts and protein detected within 1 hour post infection (Zhang et al., 2000). We have proposed that this early expression of EUO inhibits late promoters until its transcriptional repression is relieved at late times by an as-yet-undefined mechanism (Rosario & Tan, 2012). As a transcriptional repressor, EUO can regulate multiple promoters provided that each target gene has an operator in the vicinity of its promoter for EUO binding. As we have shown in the current study, this mechanism of promoter-specific regulation does not depend on the form of RNA polymerase that transcribes each target late gene. There is precedent for a transcription factor to regulate two forms of RNA polymerase because Klebsiella pneumoniae NAC (nitrogen assimilation control) represses both σ54- and σ70-dependent transcription (Rosario et al., 2010).
We propose that EUO-mediated repression may be calibrated so that individual promoters are repressed to different extents. In this study, and in our previous studies with σ66-dependent promoters (Rosario & Tan, 2012), we observed that the level of binding and transcriptional inhibition by EUO varied by promoter. Thus it is likely that target genes within the EUO regulon will not have an identical temporal profile even though they are all late genes that are regulated by EUO. For example, strongly repressed genes may have an off-on expression pattern, while partially repressed genes may be transcribed at baseline levels and then upregulated when EUO-mediated repression is relieved. These predictions are consistent with the observed expression patterns of late genes, which include genes that are first transcribed at late times, as well as genes that are upregulated to higher expression levels at late times (Belland et al., 2003).
As the master regulator of late gene expression, EUO is likely to play a critical role in the chlamydial developmental cycle. Proteins encoded by late genes are important for RB-to-EB conversion, which is a terminal differentation step. For example, the σ28-dependent late gene hctB encodes the histone-like protein HctB which is proposed to mediate the compaction of chlamydial DNA into the condensed chromatin of an EB (Brickman et al., 1993). σ66-dependent late genes include omcAB, which are the genes for two EB-specific outer membrane proteins (Liu et al., 2010, Clarke et al., 1988), and scc2 and cdsU, which encode components of the Type III secretion system that is utilized for EB entry into a new host cell (Betts-Hampikian & Fields, 2010). It is not known why late genes are transcribed by two forms of RNA polymerase, but the timing of all late genes must be regulated to prevent premature RB-to-EB conversion before RBs have undergone multiple rounds of replication. We propose that EUO coordinates the expression of the two subsets of late genes, and in doing so controls the balance between chlamydial replication and the production of infectious progeny. This important regulatory role could be used as the basis for a novel anti-chlamydial antibiotics strategy in which EUO is targeted to limit chlamydial replication by promoting premature RB-to-EB conversion.
EXPERIMENTAL PROCEDURES
Construction of in vitro transcription plasmids
Promoter sequences were amplified by PCR from C. trachomatis serovar D UW-3/Cx genomic DNA or produced by annealing complementary oligonucleotides. Mutant hctB promoter mutants were generated as previously described (Yu et al., 2006a). The mutant tsp promoter, lacking its operator, was generated by PCR using an oligo containing substitutions of ten nucleotides in the predicted EUO operator (Fig. 4). The mutant dnaK P1 promoter, containing the omcAB operator, was generated by annealing complimentary 60 bp oligonucletoides containing the nucleotide substitutions shown in Fig. 4. DNA fragments was collected through a mini Quick Spin DNA column (Roche). Each promoter sequence was cloned upstream of the promoterless G-less cassette transcription template pMT1125 as previously described (Wilson & Tan, 2002). All constructs were verified by sequencing (Genewiz). Plasmids used in this study are listed in Table 2.
Table 2.
C. trachomatis transcription templates used in this study.
| Plasmid | Promoter (nucleotides relative to transcription start site, +1) |
Reference |
|---|---|---|
| pMT1150 | omcAB promoter region from −122 to +5 | (Wilson & Tan, 2002) |
| pMT1212 | hctB promoter region from −164 to +5 | (Yu & Tan, 2003) |
| pMT1228 | hctB promoter region from −39 to +6 | (Yu et al., 2006a) |
| pMT1232 | tlyC_1 promoter region from −230 to +5 | (Yu et al., 2006b) |
| pMT1234 | pgk promoter region from −266 to +5 | Hilda Yu (unpublished) |
| pMT1236 | tsp promoter region from −273 to +5 | (Yu et al., 2006b) |
| pMT1272 | hctB promoter region from −39 to +6; nucleotide substitution at position −12 from C to A | (Yu et al., 2006a) |
| pMT1273 | hctB promoter region from −39 to +6; nucleotide substitution at position −12 from C to T | (Yu et al., 2006a) |
| pMT1274 | hctB promoter region from −39 to +6; nucleotide substitution at position −12 from C to G | (Yu et al., 2006a) |
| pMT1456 | bioY promoter region from −219 to +5 | (Yu et al., 2006b) |
| pMT1457 | dnaK P2 promoter region from −269 to +5 | (Yu et al., 2006b) |
| pMT1647 | tsp promoter region from −85 to +5 | This work |
| pMT1648 | tsp promoter region from −85 to +5 containing a 10 bp nucleotide substitution in the EUO operator site | This work |
| pMT1662 | dnaK P1 promoter region from −55 to +5 | This work |
| pMT1663 | dnaK P1 promoter region from −55 to +5 containing a 30 bp nucleotide substitution with omcAB EUO-binding operator at positions −26 to +4 in dnaK | This work |
Purification of recombinant EUO
Purification of recombinant His-tagged EUO (rEUO) was previously described (Rosario & Tan, 2012). E. coli strain BL21 was transformed with pMT1181, which is an expression plasmid encoding C. trachomatis EUO with a 6×His tag at the C-terminus. Transformed cells were grown in 1 L LB containing 100 µg/ml ampicillin to mid-logarithmic growth and induced with 1 mM IPTG for 2 h at 37°C. Pelleted cells were resuspended in buffer N [10 mM Tris-HCl (pH 8.0), 0.3 M NaCl, 10 mM 2-mercaptoethanol] containing 20 mM imidazole, and sonicated twice with a Branson digital sonifier 250D for 30 s at 22% output. The material was centrifuged and the supernatant was incubated with a 1 ml slurry of Ni-NTA beads (Qiagen) for 1 h at 4°C. The beads were then washed with 500 ml of buffer N containing 20 mM imidazole, and protein was eluted with 6 bed volumes of buffer N containing 250 mM imidazole. The eluted protein was dialyzed overnight against 1 L of storage buffer [10 mM Tris HCl (pH 8.0), 10 mM MgCl2, 0.1 mM EDTA, 100 mM NaCl, 10 mM 2-mercaptoethanol, 30% (v/v) glycerol]. Dialyzed protein was aliquoted and stored at −70°C.
Electrophoretic mobility shift assays (EMSA)
Annealed 60 bp complementary primers were labelled by T4 polynucleotide kinase (New England Biolabs) with approximately 30 µCi [γ-32P]-ATP (10 mCi mmol−1; MP Biomedicals). Free nucleotides were removed with a mini Quick Spin DNA column (Roche). Approximately 0.5 nM labelled DNA was incubated with 320 nM rEUO in binding buffer [40 mM Tris-HCl (pH 8.0), 4 mM MgCl2, 70 mM KCl, 125 µM EDTA, 100 µM dithiothreitol, 7.5% glycerol, 10 ng of salmon sperm DNA] at room temperature for 20 min. Samples were loaded onto a 6% polyacrylamide EMSA gel at 150 V in 0.5× Tris-borate-EDTA (TBE) buffer (Read, 1996). After electrophoresis, the gel was dried on Whatman paper and exposed to a phosphorimager screen, which was scanned with a Bio-Rad Personal FX scanner.
In vitro transcription assays
In vitro transcription of σ28-dependent promoters was performed as previously described (Yu et al., 2006b) with slight modifications. Approximately 13 nM (or 3 nM in Fig. 3) plasmid DNA containing the transcription template was incubated with 2.5 µM rEUO at room temperature for 15 minutes, and then transcription was initiated with σ28 RNA polymerase consisting of 0.4 U E. coli core enzyme (Epicentre) and 1 µl C. trachomatis recombinant His-tagged σ28. σ66-dependent promoters were transcribed with 0.4 U E. coli RNA polymerase holoenzyme (Epicentre). The transcripts were resolved on an 8 M urea-6% polyacrylamide gel. The amount of transcripts loaded onto the gel was adjusted to give similar intensities among the different promoter constructs. After electrophoresis, the gel was fixed, dried and exposed to a phosphorimager screen. The screen was scanned with a Bio-Rad Personal FX scanner, and the amount of each transcript was quantified using Quantity One software (Bio-Rad). For each promoter, the relative transcription was calculated by measuring transcript levels in the presence of EUO and normalizing to levels in the absence of EUO. Values are reported as the mean of the relative transcript levels with standard deviation from at least three individual experiments.
Micrococcal Nuclease digestion
C. trachomatis RBs (serovar L2 434/Bu) were purified on a renografin gradient as previously described (Zhang et al., 1998). Genomic DNA was isolated using a DNeasy Blood and Tissue Kit (Qiagen). 2 µg genomic DNA was digested to 300–1200 bp fragments by incubating with 1.5 units of Micrococcal Nuclease (NEB) at 37°C for 5 minutes.
DNA Immunoprecipitation
For each immunoprecipitation, 30 µl of Protein-G beads (GE Healthcare) were prepared at 4°C by preincubating with 5 µg of anti-His antibody (GE Healthcare) for 30 minutes, blocking with 500 µl of 5% BSA containing 200 µg/ml sheared salmon sperm DNA for 30 minutes, and then washing twice with 250 µl of Wash Buffer (40 mM Tris pH 8, 4 mM MgCl2, 70 mM KCl, and 7.5% glycerol).
Digested C. trachomatis serovar L2 434/Bu genomic DNA (10 pM) was incubated in the presence or absence of rEUO (20 nM) on ice for 30 minutes and then incubated with an aliquot of prepared Protein-G beads for a further 30 minutes. The supernatant was discarded and the beads were washed four times with 250 µl of Wash Buffer. Protein/DNA complexes were eluted from the beads with 100 µl of 0.1 M glycine pH 2.5, followed by addition of 12 µl of 1 M Tris pH 8.5 to neutralize the pH. Samples were heated to 95°C for 10 minutes to denature the protein. The DNA immunoprecipitation was performed as three independent experiments.
Quantitative PCR
DNA immunoprecipitation products were analyzed with quantitative PCR on a BioRad iCycler using the iQ SYBR Green Supermix (BioRad) with promoter-specific primers (Table S1). For each target promoter, the Ct value for 100% of input DNA (10 pM) was determined. Using the same primer pair, the Ct value for the immunoprecipitated DNA was then measured and normalized to the input DNA and reported as a percentage. The fold enrichment for each promoter was calculated from the ratio of DNA immunoprecipitated in the presence of EUO: DNA immunoprecipitated in the absence of EUO. Results are reported as the mean of three independent DNA immunoprecipitation experiments and the calculated standard deviation.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Kirsten Johnson, Jennifer Lee, and Emilie Orillard for critical reading of the manuscript. This work was supported by a grant from the NIH (AI44198). B.R.H. was supported by NRSA postdoctoral fellowship F32-AI108097.
REFERENCES
- Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–959. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Barry C, Hayes S, Hackstadt T. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science. 1992;256:377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- Belland RJ, Zhong G, Crane DD, Hogan D, Sturdevant D, Sharma J, Beatty WL, Caldwell HD. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts-Hampikian HJ, Fields KA. The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Frontiers in Microbiology. 2010;1 doi: 10.3389/fmicb.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman TJ, Barry CE, 3rd, Hackstadt T. Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. Journal of bacteriology. 1993;175:4274–4281. doi: 10.1128/jb.175.14.4274-4281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case ED, Peterson EM, Tan M. Promoters for Chlamydia type III secretion genes show a differential response to DNA supercoiling that correlates with temporal expression pattern. J Bacteriol. 2010;192:2569–2574. doi: 10.1128/JB.00068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IN, Ward ME, Lambden PR. Molecular cloning and sequence analysis of a developmentally regulated cysteine-rich outer membrane protein from Chlamydia trachomatis. Gene. 1988;71:307–314. doi: 10.1016/0378-1119(88)90047-9. [DOI] [PubMed] [Google Scholar]
- Douglas AL, Hatch TP. Expression of the transcripts of the sigma factors and putative sigma factor regulators of Chlamydia trachomatis L2. Gene. 2000;247:209–214. doi: 10.1016/s0378-1119(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Kundu T, Kusano S, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase sigmaF holoenzyme involved in transcription of flagellar and chemotaxis genes. J Bacteriol. 1997;179:4264–4269. doi: 10.1128/jb.179.13.4264-4269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Afrane M, Clemmer DE, Zhong G, Nelson DE. Identification of Chlamydia trachomatis outer membrane complex proteins by differential proteomics. Journal of bacteriology. 2010;192:2852–2860. doi: 10.1128/JB.01628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Good D, Miller RD, Graham JE, Mathews SA, Timms P, Summersgill JT. Identification of Chlamydia pneumoniae proteins in the transition from reticulate to elementary body formation. Molecular & cellular proteomics: MCP. 2006;5:2311–2318. doi: 10.1074/mcp.M600214-MCP200. [DOI] [PubMed] [Google Scholar]
- Nicholson TL, Olinger L, Chong K, Schoolnik G, Stephens RS. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J Bacteriol. 2003;185:3179–3189. doi: 10.1128/JB.185.10.3179-3189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payankaulam S, Li LM, Arnosti DN. Transcriptional repression: conserved and evolved features. Curr Biol. 2010;20:R764–R771. doi: 10.1016/j.cub.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read M. Electrophoretic mobility shift assay (EMSA) In: Docherty K, editor. Gene Transcription: DNA Binding Proteins. Chichester, West Sussex, UK: John Wiley & Sons; 1996. pp. 6–11. [Google Scholar]
- Rojo F. Mechanisms of transcriptional repression. Curr Opin Microbiol. 2001;4:145–151. doi: 10.1016/s1369-5274(00)00180-6. [DOI] [PubMed] [Google Scholar]
- Rosario CJ, Janes BK, Bender RA. Genetic analysis of the nitrogen assimilation control protein from Klebsiella pneumoniae. Journal of bacteriology. 2010;192:4834–4846. doi: 10.1128/JB.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario CJ, Tan M. The early gene product EUO is a transcriptional repressor that selectively regulates promoters of Chlamydia late genes. Molecular microbiology. 2012;84:1097–1107. doi: 10.1111/j.1365-2958.2012.08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw EI, Dooley CA, Fischer ER, Scidmore MA, Fields KA, Hackstadt T. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol Microbiol. 2000;37:913–925. doi: 10.1046/j.1365-2958.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- Shen L, Li M, Zhang YX. Chlamydia trachomatis sigma28 recognizes the fliC promoter of Escherichia coli and responds to heat shock in chlamydiae. Microbiology. 2004;150:205–215. doi: 10.1099/mic.0.26734-0. [DOI] [PubMed] [Google Scholar]
- Tan M. Temporal gene regulation during the chlamydial development cycle. In: Tan M, Bavoil PM, editors. Intracellular Pathogens I: Chlamydiales. Washington DC: ASM Press; 2012. pp. 149–169. [Google Scholar]
- Wichlan DG, Hatch TP. Identification of an early-stage gene of Chlamydia psittaci 6BC. Journal of bacteriology. 1993;175:2936–2942. doi: 10.1128/jb.175.10.2936-2942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AC, Tan M. Functional analysis of the heat shock regulator HrcA of Chlamydia trachomatis. J Bacteriol. 2002;184:6566–6571. doi: 10.1128/JB.184.23.6566-6571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Di Russo EG, Rounds MA, Tan M. Mutational analysis of the promoter recognized by Chlamydia and Escherichia coli sigma(28) RNA polymerase. Journal of bacteriology. 2006a;188:5524–5531. doi: 10.1128/JB.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Kibler D, Tan M. In silico prediction and functional validation of sigma28-regulated genes in Chlamydia and Escherichia coli. Journal of bacteriology. 2006b;188:8206–8212. doi: 10.1128/JB.01082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HHY, Tan M. Sigma 28 RNA polymerase regulates hctB a late developmental gene in Chlamydia. Mol. Micro. 2003;50:577–584. doi: 10.1046/j.1365-2958.2003.03708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Douglas AL, Hatch TP. Characterization of a Chlamydia psittaci DNA binding protein (EUO) synthesized during the early and middle phases of the developmental cycle. Infect Immun. 1998;66:1167–1173. doi: 10.1128/iai.66.3.1167-1173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Howe MM, Hatch TP. Characterization of in vitro DNA binding sites of the EUO protein of Chlamydia psittaci. Infect Immun. 2000;68:1337–1349. doi: 10.1128/iai.68.3.1337-1349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.