Abstract
Background
Preclinical studies in rodents and pigs indicate that the self-assembling microtissues known as cardiospheres (CSp) may be more effective than dispersed CSp-derived cells (CDCs). However, the more desirable intracoronary (IC) route has been assumed to be unsafe for CSp delivery: CSp are large (30-150 μm), raising concerns about likely micro-embolization. We questioned these negative assumptions by evaluating the safety and efficacy of optimized IC delivery of CSp in a porcine model of convalescent MI.
Methods and Results
First, we standardized the size of CSp by modifying culture conditions. Then, dosage was determined by infusing escalating doses of CSp in the LAD of naïve pigs, looking for acute adverse effects. Finally in a randomized efficacy study, 14 mini-pigs received allogeneic CSp (1.3×106) or vehicle one month following MI. Animals underwent MRI before infusion and 1 month later to assess left ventricular (LV) ejection fraction (EF), scar mass and viable mass. In the dosing study, we did not observe any evidence of micro-embolization after CSp infusion. In the post-MI study, CSp preserved LV function, reduced scar mass and increased viable mass whereas placebo did not. Moreover, CSp decreased collagen content, and increased vessel densities and myocardial perfusion. Importantly, IC CSp decreased LV end diastolic pressure and increased cardiac output.
Conclusions
Intracoronary delivery of CSp is safe. Intracoronary CSp are also remarkably effective in decreasing scar, halting adverse remodeling, increasing myocardial perfusion and improving hemodynamic status post-MI in pigs. Thus, CSp may be viable therapeutic candidates for IC infusion in selected myocardial disorders.
Keywords: stem cell, cardiospheres, myocardial infarction, adverse remodeling
Ischemic heart disease is the leading cause of death in the world, accounting for at least 7 million deaths per year1,2. New treatments to improve outcome following myocardial infarction (MI) are desirable. Heart-derived cell products have particular promise in this regard3. The first described human cardiac stem cell products were self-assembling multicellular three-dimensional microtissues which Messina et al dubbed “cardiospheres” (CSp)4. These microtissues had diameters of ∼30->200 μm; when injected intramyocardially (IM), CSp improved function and structure in a mouse model of acute MI. Given that capillaries have diameters of only ∼8 μm, intracoronary (IC) delivery of CSp was assumed to be implausible given the likelihood of coronary microembolization5, 6. In contemplating translation to the clinic, we preferred to use the IC route whose safety had been well-validated in prior clinical trials of cell therapy. Thus, we developed cardiosphere-derived cells (CDCs) as a dispersed single-cell product grown from replated CSp. CDCs delivered IC turned out to work quite well, both in animal models and in humans7-11, but we were left wondering whether CSp might have worked even better. When compared head-to-head in small- or large-animal MI models using IM delivery, CSp were at least as effective as CDCs, and often more so. Therefore, in the present study, we questioned our negative assumptions regarding the viability of IC CSp infusion. We reasoned that perhaps, if CSp sizes were standardized and dose/delivery were carefully optimized, we might be able to infuse CSp safely down a coronary artery. We initially optimized CSp size using different culture conditions, and performed a dose-ranging study in minipigs to assess the feasibility and safety of IC CSp infusion. Finally, we performed a blinded, prospective placebo-controlled study in a porcine model of convalescent MI to assess the safety and efficacy of IC CSp infusion.
Methods
For detailed methods please see the supplement material. Animal studies were performed in an American Association for Accreditation of Laboratory Animal Care accredited facility with approval from the Cedars-Sinai Institutional Animal Care and Use Committee.
Growth of CSp and optimization of culture conditions
CDCs were grown from a freshly-explanted heart obtained from a male Sinclair mini-pig, and secondary CSp were made from CDCs at the 5th passage using CSp media as described4, 12. Culture time and cell seeding density were optimized to obtain a minimal percentage of CSp >150 μm (which may cause severe microvascular obstruction) and a high percentage of CSp >50 μm (to avoid the infusion of single cells). For this purpose, we compared 5 different seeding densities and 4 different harvest time points (Figure 1A). Size, number and distribution of the CSp obtained were determined using a particle counter. The CSp generated (from 12M plated CDCs) were counted before and after ex vivo passage through a micro-catheter (Finecross®, Terumo, Tokyo, Japan) to quantify retention of CSp in the catheter.
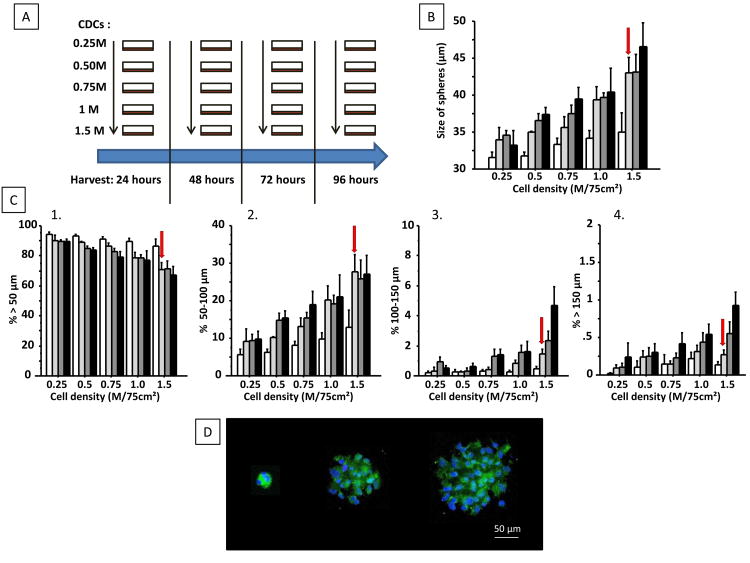
Figure 1.
Schematic representation of the in vivo study (A); 5 different seeding densities and 4 different times for harvest were used to optimize cardiosphere (CSp) size. Increasing cell density increases the average size of CSp (B), decreases the proportion of small CSp (<50 μm) (C1) and increases the proportion of 50-100 μm CSp (C2). In contrast, increasing culture duration increases the number of CSp in the 100-150 μm range (C3) and CSp>150 μm (C4). The conditions chosen for the in vivo studies are those giving a high numbers of CSp > 50 μm with few CSp > 150 μm (labeled with red arrow in panel B and C1-4). Images of a small, mid-size and large CSp are shown in (D); nuclei are stained with DAPI (blue) and cell membranes are stained with calcein acetomethoxy (green)”.
For in vivo studies, secondary CSp from 12.5M CDCs were frozen and thawed the day of infusion. Viability of the thawed CSp was assessed using homo-ethidium bromide (which stains dead nuclei red).
Study design
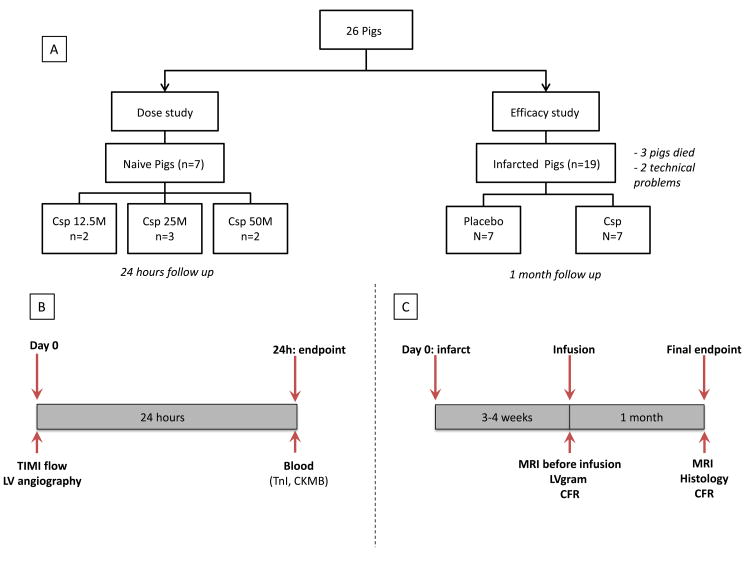
Two separate experimental protocols were performed as depicted in Figure 2. Briefly, a study was first performed to determine the maximal feasible dose and, in a second step, a blinded placebo-controlled study was performed to assess efficacy of infused CSp. A total of 26 Yucatan mini-pigs were used: 7 completed the dose study and 14 completed the efficacy study. Three pigs died, 2 following MI creation and 1 following placebo infusion. Two pigs were excluded because of technical issues during MI creation (one balloon deflation leading to inadequate MI, and one left anterior descending artery [LAD] dissection).
Figure 2. Design of the whole study (A) and of the feasibility (B) and efficacy (C) studies.
MI creation and CSp infusion
For the feasibility study, increasing doses of CSp were administered in naïve Yucatan minipigs. The doses were defined by the number of single cells used to manufacture the CSp (single-cell equivalent [SCE]). Pigs were infused with 12.5×106, 25×106 and 50×106 SCE, corresponding to ∼3.25×105, 6.5 ×105 and 1.3×106 multicellular particles respectively (Supplemental Figure 1). All IC infusions were performed using continuous flow (no flow interruption during infusion, no balloon inflation), with a microcatheter (Finecross®, Terumo, Tokyo, Japan) placed in the mid-LAD. Safety was assessed by adverse events during infusion (e.g., arrhythmias, dissection, hypotension), TIMI flow after infusion, left ventricular ejection fraction (LVEF) measured by LVgram before and after infusion (to detect potential myocardial stunning related to micro-embolization), and troponin I increase 24 hours after infusion.
For the efficacy study, MIs were created in female adult Yucatan mini-pigs by inflating an angioplasty balloon in the mid-LAD just after the 1st diagonal branch for 2.5 hours. Three weeks later, animals were randomized to receive CSp (50×106 SCE, 1.3×106 particles) or vehicle using continuous flow infusion. Safety was assessed as in the dose study. LV end-diastolic pressure was recorded using a pigtail catheter placed into the LV cavity. Left ventriculography was then performed to assess LV function. Minipigs were euthanized one month after infusion. All procedures and analysis were performed blinded to treatment allocation.
MRI
MRI was performed on a 3.0 Tesla MRI scanner (Siemens Magnetom Verio, Erlangen, Germany) at baseline (3 weeks following MI, before infusion) and one month post-infusion.
Coronary flow reserve (CFR) measurement
Coronary flow reserve was quantified with a Doppler wire as described in the supplemental material.
Histology
Histological analyses were performed on 8 μm sections from myocardial samples (fixed in 10% formalin and embedded in paraffin) obtained from infarct and border zone (3-6 slides per heart) and remote zone (2 slides per heart).
Statistical analysis
Continuous variables are presented as mean ± standard deviation in the text and mean ± standard error in the figures. Categorical variables are expressed as absolute number and percentage. Independent groups (CSp and placebo) were compared using Mann-Whitney U test. Wilcoxon test was used to compare paired groups (changes from baseline). All P values are two sided, and a P value <0.05 was considered statistically significant.
Results
CSp culture optimization
Figure 1B shows a histogram of CSp size distribution at various times and seeding densities. Increased seeding density is associated with a higher proportion of spheres >50 μm (Figure 1C) whereas longer culture time mostly increased the percentage of very big particles (>150 μm; Figure 1D). Therefore a high seeding density (1.5×106 CDCs in 75 cm2 flasks, i.e. 20×103 CDCs/cm2) and short culture duration (48 hours) were deemed optimal for CSp manufacturing. The number and size of secondary CSp manufactured from 12.5M CDCs using these conditions are shown in Supplemental Figure 1.
Using optimally-manufactured CSp, we observed 5±9% total retention of CSp in the catheter and ∼10% retention for CSp>50 μm (Supplemental Figure 1). Therefore, for the in vivo study we decided to increase the doses by 10% to offset in-catheter retention. Fortuitously, the extent of loss dramatically increased with particle size, reaching ∼70% for CSp>150 μm, providing an additional safeguard against microvascular occlusion.
Safety of CSp infusion
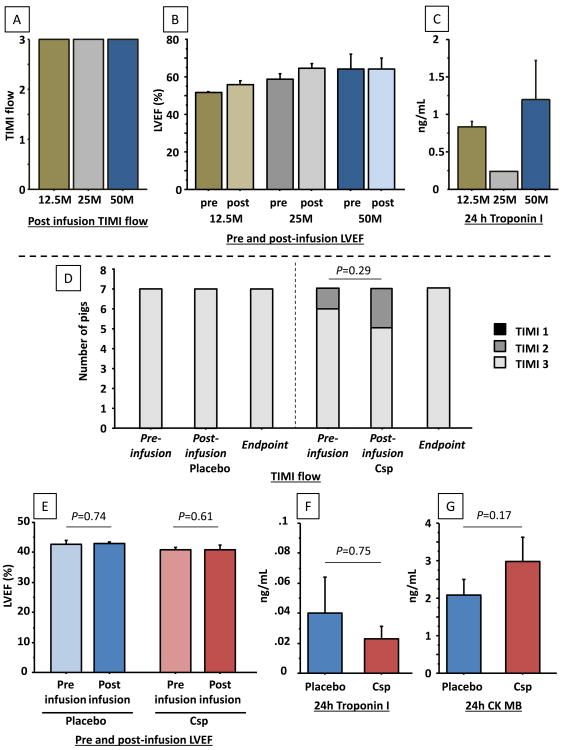
A dose escalation study was performed in 7 naïve animals to assess feasibility and safety. Pigs were infused with 12.5×106 SCE (n=2), 25×106 SCE (n=3) and 50×106 SCE (n=2). No arrhythmia occurred during CSp infusion and no impairments of TIMI flow or LVEF following infusion were observed (Figure 3 A, B). Troponin increase 24 hours after infusion was low without any difference between the 3 doses (Figure 3C). To further assess safety, the heart of a pig infused with 50M SCE and euthanized 24 hours after infusion was stained with TTC. No myocardial necrosis was evident, either in the infused region or in remote areas (Supplemental Figure 2). Therefore the highest dose (50M SCE) was used for further studies.
Figure 3.
Intracoronary infusion of CSp is safe in both naïve pigs and in pigs with convalescent myocardial infarction. TIMI flow (A), LVEF (B) and troponin I (C) following infusion of increasing doses of CSp (dose labeled in single-cell equivalents, SCE) in naïve pigs (feasibility study). In infarcted pigs (efficacy study), no differences were observed in TIMI flow (D), Troponin I (E), CK MB (F) or LVEF (G) following infusion of CSp or placebo (N=7 per groups).
Safety was then confirmed in infarcted animals. Following infusion of CSp (dose of 50M SCE) we observed no impairment of LVEF when compared to vehicle infusion; also, troponin I and CK at 24 hours were similar in CSp and placebo groups (Figure 3 D-G). No impairment in TIMI flow was observed after vehicle infusion, whereas it decreased from TIMI 3 to TIMI 2 in 1 of 7 pigs infused with CSp. This decrease in TIMI flow was most likely related to coronary spasm, for three reasons: 1) coronary flow normalized immediately after nitroglycerin infusion, along with visible dilatation of the epicardial vessel; 2) there was no decrease in LV function; 3) troponin did not increase at 24 hours. These considerations exclude micro-embolization as the cause of the decrease in TIMI flow grade. No other adverse events occurred during CSp infusion, whereas one pig died of severe hypotension followed by cardiac arrest immediately after placebo infusion. Analysis of the MRI performed in that pig before infusion revealed a major myocardial scar involving both ventricles with severe bi-ventricular dysfunction. Therefore, only 14 pigs were analyzed at the 1 month post-infusion endpoint.
Preclinical study: Benefits associated with CSp infusion
Having established a safe dose, we performed a preclinical study in which animals were blindly allocated to CSp (50 ×106 SCE, 1.3 CSp) (n=7) or vehicle infusion (n=8). The average diameter of the CSp infused was 45±23 μm; 30.2±6.8%, 3.4±1.8% and 0.24±0.17% of the CSp were >50, 100 and 150 μm respectively. Viability of the infused CSp was 92.1±3.9%. MRI characteristics of the animals before treatment are shown in Supplemental Table 1.
LV function preservation and scar reduction
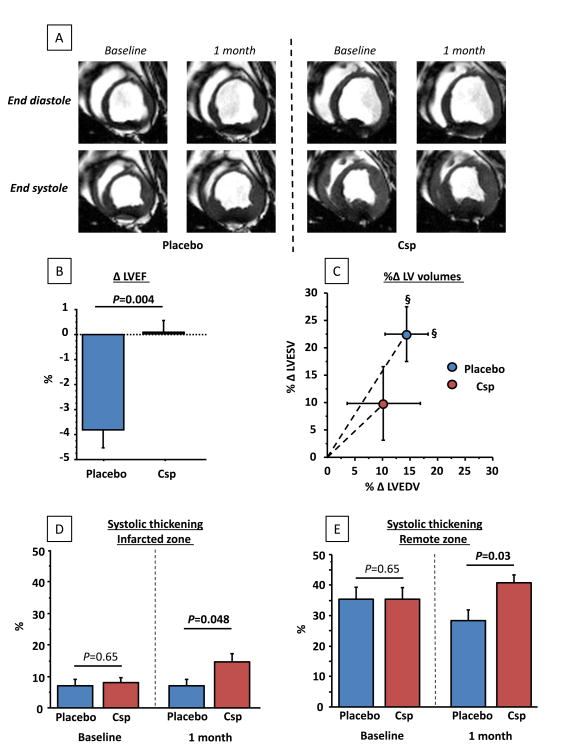
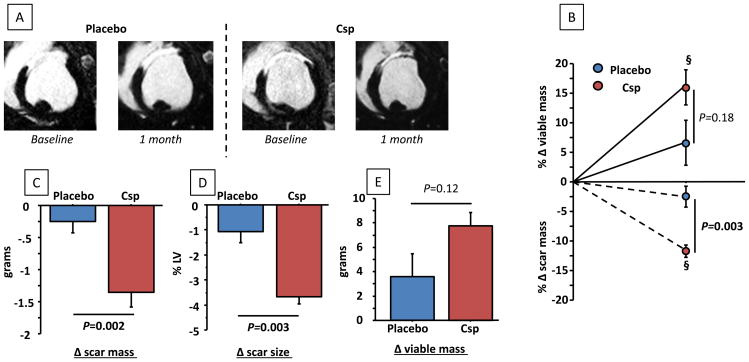
Figure 4A shows representative non-enhanced MR images at end-diastole and end-systole in placebo (left) and CSp groups (right). Pooled data (Figure 4B) reveal that, at baseline, LVEF was similar in the two groups. At 1 month follow up, however, LVEF was preserved in CSp-treated animals (P=0.35 within group) whereas it significantly decreased in placebo (P=0.02 within group, P=0.004 between groups). In parallel, both indexed LV end systolic and end-diastolic volumes significantly increased in the placebo group but not in the CSp group (Figure 4C), indicative of attenuated adverse remodeling with CSp treatment. In accordance with preservation of LV volumes, CSp-treated pigs showed better 1 month regional function (assessed by end systolic thickening) compared to placebo both in infarcted and remote zones (Figure 4D,E).
Figure 4.
Cardiopheres preserve LV function and stop adverse remodeling compared to control. (A) Matched representative end-diastolic and end-systolic MR images of placebo and CSp-treated minipigs before infusion and at 1 month follow-up. Changes in LVEF (B), relative changes in LV end-diastolic and end-systolic volumes (indexed/BSA) (C) and changes in regional function from the infarcted (D) and remote (E) zones in CSp- and placebo-treated pigs. (N=7 per groups)
Analysis of late Gd-enhanced images (Figure 5A) revealed that scar mass decreased significantly in CSp-treated animals (P=0.02 within group) but not in placebo (P=0.18 within group, P=0.002 between group) leading to a five-fold higher relative decrease in the CSp group (-11.6±2.7% vs. -2.3±4.6%, P=0.003) despite similar scar mass at baseline (Figure 5B, C). Consistently, the decrease in scar size (scar mass divided by total LV mass; Figure 5D) was significantly greater in CSp group than in control. In addition to the reduction of scar mass, we observed a strong trend toward a higher increase in viable mass in CSp-treated animals (Figure 5B, D). We have previously noted that viable mass recovery tends to lag behind the reduction of scar, such that changes in viable mass that are not quite significant at one month (as is the case here) tend to increase with longer follow-up 11, 13, 14.
Figure 5.
Cardiospheres but not placebo decreased scar size and tended to increase viable mass at 1 month. (A) Representative MR images showing late gadolinium enhancement for placebo and CSp-treated pigs. Changes in scar mass (B), scar size (scar mass/total LV mass) (C) and viable mass (D). Relative changes in scar mass and viable mass (E). § P<0.05 vs. baseline (paired analysis). (N=7 per groups)
Myocardial perfusion increase
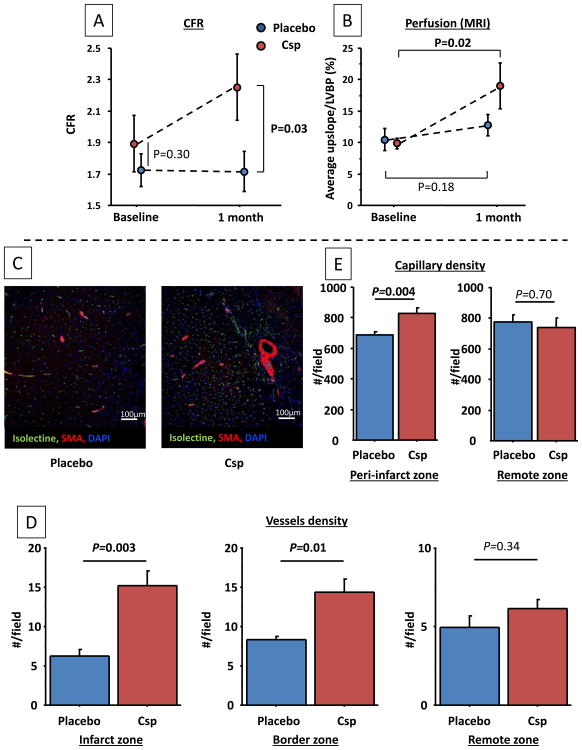
Myocardial perfusion was assessed using 2 different methods: 1) CFR of the infarct-associated artery before CSp infusion and 1 month later using a Doppler wire, and 2) First-pass MRI to quantify perfusion in the infarcted area. CFR values at endpoint were higher in CSp-treated pigs than in control despite similar baseline values (Figure 6A). Consistent with these findings, MRI first-pass sequences revealed a significant increase in myocardial perfusion at endpoint in the CSp group but not in placebo controls (Figure 6B; P=0.02 in CSp, vs. P=0.18 in control, P=0.13 for intergroup comparison).
Figure 6.
Cardiospheres increase myocardial perfusion of the infarcted zone assessed by CFR (A) and MRI (1st pass sequences) (B). Representative images of arterioles and capillaries in the peri-infarcted area in CSp- and placebo-treated pigs (C). Arteriolar density is increased in the infarcted (D) and border zones but not in the remote zone. Capillary density (E) is increased in the peri-infarct zone but not in the remote zone. (N=7 per groups)
Histology: fibrosis, vascular density and architecture
Functional measurements reviewed above indicate an increase in myocardial perfusion with CSp treatment. Consistent with these findings, histological analysis revealed twofold higher arteriolar density (i.e., isolectin and α-smooth muscle actin-positive vascular structures) in the infarcted and border zones of CSp-treated pigs compared to controls, but no differences in the remote zone (Figure 6C,D). Capillary density was also higher in the peri-infarct zone, but not in the remote zone (Figure 6E).
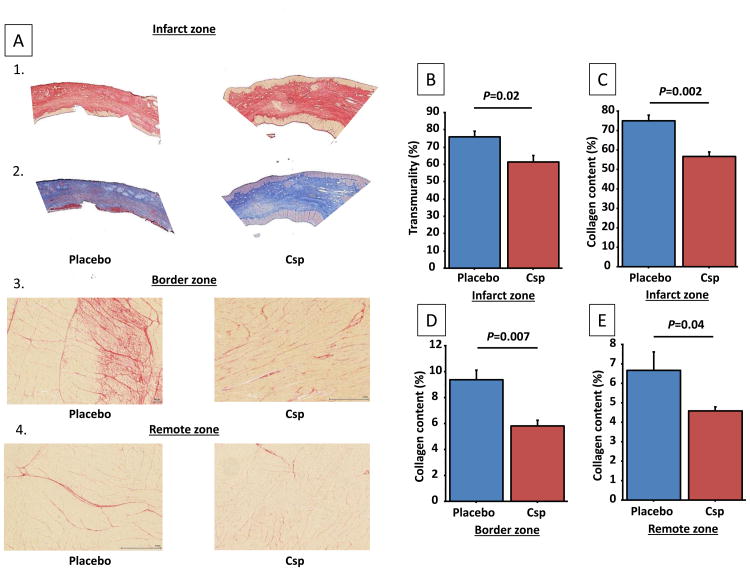
MRI also revealed a decrease in myocardial scar after CSp infusion. To confirm this histologically, we first evaluated the transmurality of the scar using Masson trichrome staining. Then, collagen content in the infarcted, border and remote areas was quantified using Picrosirius red (which offers superior contrast between fibrotic and non-fibrotic areas in the remote and border zones relative to Masson trichrome, as evident from the representative images in Figure 7A1-4). Scar transmurality was less in CSp-treated pigs vs. placebo (Figure 7B). In parallel, collagen content was reduced in the CSp-treated pigs in the infarct zone and in border zone, but also in the remote zone (Figure 7C-E).
Figure 7.
Cardiosphere treatment decreases fibrosis in the heart. (A) Collagen-stained sections of infarct zone by Picrosirius red (A1) and Masson trichrome staining (A2), and of border (A3) and remote (A4) zones by Picrosirius red. Transmurality of the scar (B) and collagen content of the infarcted (C), border (D) and remote (E) zones. (N=7 per groups)
These differences in fibrosis and vascularity were not accompanied by cardiomyocyte hypertrophy, as demonstrated by the similar cross-sectional areas of cardiomyocytes from CSp and placebo pigs (Supplemental Figure 3).
Hemodynamic improvement
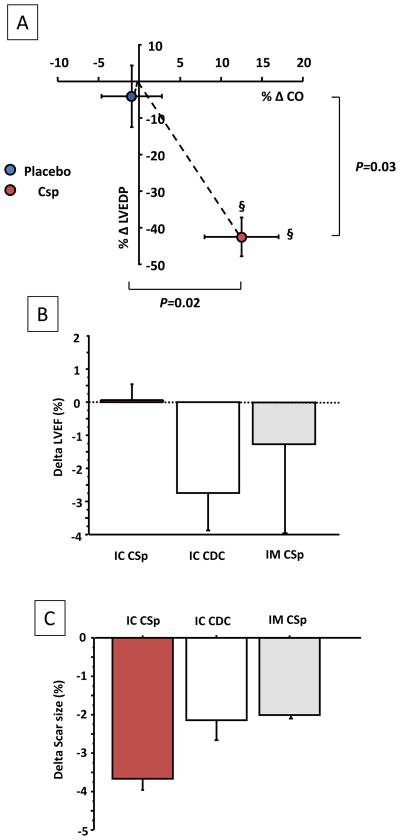
To investigate whether the observed functional and architectural improvements were associated with hemodynamic improvement, we measured LV end diastolic pressure (LVEDP) and cardiac output (CO, from stroke volume and heart rate). At baseline, LVEDP and CO were similar in CSp- and placebo-treated pigs. After treatment, LVEDP significantly decreased in CSp but not in placebo, while CO increased only in the CSp group (Figure 8A; Table). We did not observe any differences in systolic or diastolic blood pressure or heart rate that might have confounded the LVEDP and CO measurements (Table).
Figure 8.
(A) Cardiospheres increase cardiac output and decrease LV end diastolic pressure compared to placebo. Relative changes are shown. § P<0.05 vs. baseline (paired analysis) (N=7 per groups). (B) and (C) Effects on LVEF preservation (B) and scar size decrease (C) of IC CSp compared to IC CDC and to intra-myocardial injection (NOGA) of a comparable dose of Csp (45M SCE). The results for IC CDC and intra-myocardial CSp are derived from previous studies (no statistical comparisons were therefore performed) 6, 11
Table.
Baseline and endpoint values of Left Ventricle End-Diastolic Pressure (LVEDP), Cardiac Output (CO), Systolic blood pressure (SBP), Diastolic Blood Pressure (DBP) and Heart Rate (HR) in CSp- and Placebo-treated pigs (N=7 per group).
| Baseline | Endpoint | Delta | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Placebo | CSp | P | Placebo | CSp | P | Placebo | CSp | P | |
| LVEDP (mmHg) | 16.4±2.5 | 18.2±1.8 | 0.31 | 15.0±3.3 | 11.8±4.5 | 0.06 | -1.4±3.7 | -6.3±3.8 | 0.02 |
| CO (L/Min) | 3.5±0.6 | 3.6±0.3 | 0.65 | 3.5±0.4 | 4.1±0.5 | 0.05 | -0.1±0.3 | 0.5±0.5 | 0.01 |
| SBP (mmHg) | 71±11 | 71±8 | 0.61 | 86±4 | 93±14 | 0.25 | 18±16 | 21±8 | 0.65 |
| DBP (mmHg) | 51±5 | 53±7 | 0.89 | 63±5 | 67±16 | 0.36 | 13±3 | 14±16 | 0.58 |
| HR (bpm) | 108±11 | 111±27 | 0.84 | 104±16 | 98±7 | 0.40 | -6±18 | -10±11 | 0.65 |
Immunological safety: cellular infiltration and allo-antibodies
Histological analysis revealed more lymphocyte infiltration in the infarcted and border zone of the treated animals compared to placebo (5/7 animals vs. 0/7 animals, P=0.002). Importantly, no area of cardiomyocyte damage related to immune response was observed, indicating that no animal had a grade of immune reaction >1 (Supplemental Figure 4). Slightly higher levels of allo-antibodies were observed in CSp-treated animals as compared to placebo (Supplemental Figure 4), but the significance of this observation is uncertain.
Discussion
Adverse remodeling is a major contributor to MI-related mortality and morbidity15, 16. Despite the improvement of care and faster reperfusion, mortality remains >10% at 1 year 2. Here, we demonstrated the ability of 3-dimensional multicellular cardiospheres to halt the ongoing process of adverse remodeling in a clinically-relevant porcine model of convalescent MI. Furthermore, we established the safety of the desirable IC route for the administration of this cell product.
Safety of intracoronary infusion
Intracoronary infusion is an easy, convenient and widely-available technique for delivery of biological products. Prior to this study, IC infusion of CSp had been avoided, because of the prevailing assumption that these multicellular microtissues may cause microvascular obstruction resulting in myocardial damage. Indeed, some authors have raised the concern that IC infusion of particles >10 μm may be unsafe because of microvascular obstruction 17. However, the safety of IC infusion of large particles (CellBeads of 160-400μm) has previously been reported, although the number of particles infused was low (up to 60000), and those studies aimed to cause a certain degree of micro-vascular occlusion 18-20. Here, we have been able to deliver IC >1×106 CSp with an average diameter of 45 μm without any adverse events. The first study evaluating IC infusion of dispersed CDCs used minipigs with convalescent MI and traditional stop-flow delivery; IC infusion of ≥ 25×106 CDCs, which have a diameter ∼20 μm, led to elevation of serum troponin I at 24 hrs, establishing the maximal safe dose as 12.5×106 (or ∼300,000 CDCs/kg)8. The combination of careful CSp formulation and the use of continuous-flow infusion21 allowed us to deliver up to an equivalent of 50×106 cells IC in the same model. This ability to deliver a higher number of cells may augment efficacy, although cell therapy does not necessarily follow conventional dose-response relationships22-24.
Anti-fibrotic and pro-angiogenic properties of CSp
CDCs have been extensively studied pre-clinically, leading to several Phase I/II clinical trials involving first autologous and, more recently, allogeneic products9, 25. Thus, the ability of CDCs to decrease scar mass and to increase viable tissue has been thoroughly validated. In the porcine post-MI model, we confirmed the impressive anti-remodeling properties of CSp that had been observed by IM injection in small-animal models13, 14, 26-28. Indeed, CSp infusion completely halted further LVEF deterioration. Moreover, we observed an improvement in regional function and a decrease in fibrosis both in infarcted and in non-infarcted zones, indicating a positive effect on adverse global remodeling, and not just a decrease in scar content of the infarcted area. These “global” effects on LV remodeling and fibrosis may be related to the higher engraftment and survival of CSp as compared to CDCs and to the secretion of growth factors, endoglin and MMP as described 12, 13, 27, 29, 30. Taken together, these properties may enable a more sustained and diffuse paracrine effect. Paracrine signaling is a major contributor to cardiac regeneration following cell therapy29, 31-35, as benefits far outlast measurable cell engraftment7, 10, 28. It seems likely that the benefit observed in this study might increase over time8,10 and potentially surpass the benefit observed with CDCs11. The benefits seen here are indeed generally superior to those seen 1 month after IC infusion of CDCs or after intramyocardial injection of a comparable dose of CSp (comparison with historical results from Yee et al.6, and unpublished 1-month follow-up results from the study by Malliaras et al.11 are summarized in Figure 8B,C). However, a direct comparison was beyond the scope of the present study.
In addition to this anti-fibrotic effect, we describe a strong angiogenic effect following CSp infusion. Indeed, we observed a twofold greater number of arterioles in the infarcted and border zones of CSp-treated animals compared to placebo. This increased arteriolar number was associated with improved perfusion in the infarcted area as assessed by two different methods (invasive and non-invasive). Given the chronicity of the model, the increase in myocardial perfusion is likely attributable to the growth of new vessels, not to preservation of existing vasculature. This impressive angiogenic effect might be useful to recruit in selected clinical situations, e.g. in patients with intractable angina and/or micro-circulatory deficits.
Hemodynamic improvement
Consistent with the structural changes, we observed hemodynamic improvements following CSp infusion, including increased CO and, importantly, decreased LVEDP. High LVEDP is a strong predictor of adverse events (i.e. acute heart failure or death)36-39; if reproduced in patients, the twofold decrease observed in this study could be highly meaningful clinically.
Immunological safety
The structural and functional improvements associated with allogeneic CSp infusion were associated with minimal immune reaction, confirming the safety of allogeneic heart-derived cell therapy10, 11, 28. While no immunosuppressive treatment was given, we did not observe any reaction >grade 1 ISHLT in the tissue, and the level of allo-antibodies that were detected was not very different than in the negative control.
Limitations
This study has several limitations. First, the MI model, although mimicking human adverse remodeling, is made on a background of young healthy animals. Therefore their ability to recover and generate new cardiac tissues might be superior to what can be observed in patients with cardiovascular disease risk factors and other comorbidities. Second, engraftment was not quantified in this study; engraftment of CSp has been shown to be greater than that of CDCs, but only in intra-myocardial injection models 6, 12, 27. However, it is not clear whether the benefit of CSp is related to the higher engraftment, to properties of the secretome, or both. While engraftment correlates roughly with efficacy, the relationship is vague, particularly over the longer term8, 10, 11. Nevertheless, increased short-term engraftment would be expected to boost the availability of secreted factors, so the two possibilities are undoubtedly intertwined.
Conclusions
We have demonstrated the safety of IC infusion of multicellular 3-dimensional cardiospheres. Moreover, CSp treatment decreases scar, attenuates adverse remodeling, increases myocardial perfusion and improves hemodynamics in a pig model of convalescent MI. Cardiosphere infusion may deserve testing as a therapeutic approach to attenuate adverse remodeling and/or to achieve angiogenesis in humans.
Supplementary Material
Acknowledgments
Sources of Funding: This study was funded by the Cedars-Sinai Board of Governors Heart Stem Cell Center. General support for the laboratory is provided by NIH. RG received a grant from the French Society of Cardiology. JD is supported by NIH T32HL116273.
Footnotes
Disclosures: EM and LM own equity in Capricor Inc. LM is employed by Capricor Inc. The other authors report no conflicts.
References
- 1.World Health Organisation. Global atlas on cardiovascular disease prevention and control. 2011 [Google Scholar]
- 2.Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. Bmj. 2012;344:e356. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marban E. Breakthroughs in cell therapy for heart disease: focus on cardiosphere-derived cells. Mayo Clinic proceedings. 2014;89:850–8. doi: 10.1016/j.mayocp.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation research. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 5.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 6.Yee K, Malliaras K, Kanazawa H, Tseliou E, Cheng K, Luthringer DJ, Ho CS, Takayama K, Minamino N, Dawkins JF, Chowdhury S, Duong DT, Seinfeld J, Middleton RC, Dharmakumar R, Li D, Marban L, Makkar RR, Marban E. Allogeneic Cardiospheres Delivered via Percutaneous Transendocardial Injection Increase Viable Myocardium, Decrease Scar Size, and Attenuate Cardiac Dilatation in Porcine Ischemic Cardiomyopathy. PloS one. 2014;9:e113805. doi: 10.1371/journal.pone.0113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marban E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. European heart journal. 2015;36:751–62. doi: 10.1093/eurheartj/ehu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83. doi: 10.1161/CIRCULATIONAHA.108.816058. 7 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. The Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–12. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marban L, Marban E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–75. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marban E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. Journal of the American College of Cardiology. 2011;57:455–65. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Tseliou E, de Couto G, Terrovitis J, Sun B, Weixin L, Marban L, Marban E. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PloS one. 2014;9:e88590. doi: 10.1371/journal.pone.0088590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseliou E, Reich H, de Couto G, Terrovitis J, Sun B, Liu W, Marban E. Cardiospheres reverse adverse remodeling in chronic rat myocardial infarction: roles of soluble endoglin and Tgf-beta signaling. Basic research in cardiology. 2014;109:443. doi: 10.1007/s00395-014-0443-8. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 16.St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moye LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S, Menapace FJ, Parker JO, Lewis S, Sestier F, Gordon DF, McEwan P, Bernstein V, Braunwald E. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–31. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong R, van Hout GP, Houtgraaf JH, Kazemi K, Wallrapp C, Lewis A, Pasterkamp G, Hoefer IE, Duckers HJ. Intracoronary infusion of encapsulated glucagon-like Peptide-1-eluting mesenchymal stem cells preserves left ventricular function in a porcine model of acute myocardial infarction. Circulation Cardiovascular interventions. 2014;7:673–83. doi: 10.1161/CIRCINTERVENTIONS.114.001580. [DOI] [PubMed] [Google Scholar]
- 19.Houtgraaf JH, de Jong R, Monkhorst K, Tempel D, van de Kamp E, den Dekker WK, Kazemi K, Hoefer I, Pasterkamp G, Lewis AL, Stratford PW, Wallrapp C, Zijlstra F, Duckers HJ. Feasibility of intracoronary GLP-1 eluting CellBead infusion in acute myocardial infarction. Cell transplantation. 2013;22:535–43. doi: 10.3727/096368912X638973. [DOI] [PubMed] [Google Scholar]
- 20.Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, Holt CM. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem cells translational medicine. 2012;1:759–69. doi: 10.5966/sctm.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki G, Weil BR, Leiker MM, Ribbeck AE, Young RF, Cimato TR, Canty JM., Jr Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PloS one. 2014;9:e113009. doi: 10.1371/journal.pone.0113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. European heart journal. 2008;29:251–9. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 23.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. European heart journal. 2009;30:2722–32. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseliou E, Dawkins JF, Gallet R, Kreke M, Smith RR, Middleton R, Valle J, Cingolani E, Kar S, Marban L, Marban E. Dose-Escalation Study Using Novel Continuous Flow Intracoronary Delivery of Allogeneic Cardiosphere-Derived Stem Cells: Is There a Threshold for Cell Therapy? AHA scientific Sessions 2014 Abstract 16451. 2014 [Google Scholar]
- 25.Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR) ( NCT01458405) doi: 10.3727/096368916X692933. Available at: http://clinicaltrials.gov/ct2/show/NCT01458405?term=allstar&rank=1. [DOI] [PMC free article] [PubMed]
- 26.Cho HJ, Lee HJ, Youn SW, Koh SJ, Won JY, Chung YJ, Cho HJ, Yoon CH, Lee SW, Lee EJ, Kwon YW, Lee HY, Lee SH, Ho WK, Park YB, Kim HS. Secondary sphere formation enhances the functionality of cardiac progenitor cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1750–66. doi: 10.1038/mt.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marban E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem cells. 2010;28:2088–98. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marban L, Luthringer D, Marban E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. Journal of the American College of Cardiology. 2013;61:1108–19. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 29.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circulation research. 2010;106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marban E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PloS one. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation research. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO molecular medicine. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circulation research. 2011;109:1044–54. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 34.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation research. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, Li T, Lu L, Lu G, Marban E. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem cells. 2014;32:2397–406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang HY, Cauduro SA, Pellikka PA, Bailey KR, Grossardt BR, Yang EH, Rihal C, Seward JB, Miller FA, Abraham TP. Comparison of usefulness of echocardiographic Doppler variables to left ventricular end-diastolic pressure in predicting future heart failure events. The American journal of cardiology. 2006;97:866–71. doi: 10.1016/j.amjcard.2005.09.136. [DOI] [PubMed] [Google Scholar]
- 37.Trenouth RS, Rosch J, Antonovic R, Chaitman BR, Rahimtoola SH. Ventriculography and coronary arteriography in the acutely III patient. Complications, extent of coronary arterial disease, and abnormalities of left ventricular function. Chest. 1976;69:647–54. doi: 10.1378/chest.69.5.647. [DOI] [PubMed] [Google Scholar]
- 38.Sharma GV, Woods PA, Lindsey N, O'Connell C, Connolly L, Joseph J, McIntyre KM. Noninvasive monitoring of left ventricular end-diastolic pressure reduces rehospitalization rates in patients hospitalized for heart failure: a randomized controlled trial. Journal of cardiac failure. 2011;17:718–25. doi: 10.1016/j.cardfail.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Spevack DM, Karl J, Yedlapati N, Goldberg Y, Garcia MJ. Echocardiographic left ventricular end-diastolic pressure volume loop estimate predicts survival in congestive heart failure. Journal of cardiac failure. 2013;19:251–9. doi: 10.1016/j.cardfail.2013.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.