Abstract
Endocannabinoids can affect multiple cellular targets, such as cannabinoid (CB) receptors, transient receptor potential cation channel, subfamily V, member 1 (TRPV1) and peroxisome proliferator-activated receptor γ (PPARγ). The stimuli to induce adipocyte differentiation in hBM-MSCs increase the gene transcription of the CB1 receptor, TRPV1 and PPARγ. In this study, the effects of three endocannabinoids, N-arachidonoyl ethanolamine (AEA), N-arachidonoyl dopamine (NADA) and 2-arachidonoyl glycerol (2-AG), on adipogenesis in hBM-MSCs were evaluated. The adipocyte differentiation was promoted by AEA whereas inhibited by NADA. No change was observed by the treatment of non-cytotoxic concentrations of 2-AG. The difference between AEA and NADA in the regulation of adipogenesis is associated with their effects on PPARγ transactivation. AEA can directly activate PPARγ. The effect of AEA on PPARγ in hBM-MSCs may prevail over that on the CB1 receptor mediated signal transduction, giving rise to the AEA-induced promotion of adipogenesis. In contrast, NADA had no effect on the PPARγ activity in the PPARγ transactivation assay. The inhibitory effect of NADA on adipogenesis in hBM-MSCs was reversed not by capsazepine, a TRPV1 antagonist, but by rimonabant, a CB1 antagonist/inverse agonist. Rimonabant by itself promoted adipogenesis in hBM-MSCs, which may be interpreted as the result of the inverse agonism of the CB1 receptor. This result suggests that the constantly active CB1 receptor may contribute to suppress the adipocyte differentiation of hBM-MSCs. Therefore, the selective CB1 agonists that are unable to affect cellular PPARγ activity inhibit adipogenesis in hBM-MSCs.
Keywords: Endocannabinoids, Cannbinoid type 1 (CB1) receptor, Adipogenesis, Human mesenchymal stem cells, Rimonabant
INTRODUCTION
The discovery of a psychoactive compound in marijuana, Δ9-tetrahydrocannabinol (THC), has contributed to the identification of the endocannabinoid system in mammalian physiology (Di Marzo and Matias, 2005; Klein, 2005; Cluny et al., 2011). The endocannabinoid system regulates various physiological functions such as pain sensation, appetite and inflammation (Song et al., 2011; Silvestri and Di Marzo, 2013). Pharmacological studies on THC and/or its synthetic analogs have identified two cannabinoid (CB) receptors that are members of the G-protein coupled receptor (GPCR) family. Physiological functions of the first cannabinoid receptor, CB1, have been mainly elucidated in central nervous system (CNS) (Pertwee et al., 2010). Due to the neurological functions of CB1, the CB1 receptor antagonists or inverse agonists have been developed as an appetite suppressing anti-obesity drugs (Wiley et al., 2012). The CB1 receptor also exists in peripheral tissues such as heart, liver, small intestine and immune cells (Di Marzo and Matias, 2005; Klein, 2005; Cluny et al., 2011; Liu et al., 2012; Tam et al., 2014). The CB2 receptor is abundantly found in spleen and is also detected in brain, muscle, heart and testis to a lower level (Pertwee et al., 2010). Some selective CB2 receptor agonists have no psychoactive effects (Pacher and Hasko, 2008). Interestingly, a recent report suggested that some physiological stresses induced the up-regulation of the CB2 receptor in brain (Viscomi et al., 2009).
Many endogenous ligands for CB receptors, also called as endocannabinoids, have been identified, such as N-arachidonoyl ethanolamine (anandamide, AEA), 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl dopamine (NADA) (Huang et al., 2002; Di Marzo and Matias, 2005). Endocannabinoids play roles as neurotransmitters and/or neuromodulators in the CNS and also function as autocrine or paracrine mediators in peripheral tissues (Bermúdez-Siva et al.., 2006; Pacher and Hasko, 2008; Pertwee et al., 2010). In addition, endocannabinoids, AEA, 2-AG and NADA can activate the transient receptor potential cation channel subfamily V member 1 (TRPV1) which is important in nociception (Pacher and Hasko, 2008).
Recently, CB1 antagonists like rimonabant have been developed as centrally acting anti-obesity drugs (Wiley et al., 2012). The CB receptors exist in various peripheral tissues, in this regard, it is important to understand the extraneural effects of the CB receptor antagonists on peripheral tissues. Recently, the direct effect of THC on adipogenesis of murine preadipocyte cell line 3T3-L1 was reported (Teixeira et al., 2010). THC promotes the gene transcription of both peroxisome proliferator-activated receptor γ (PPARγ) and adiponectin during adipogenesis in 3T3-L1 cells, suggesting the physiological role of endocannabinoids in adipose tissue. In addition, a synthetic CB1 receptor antagonist, rimonabant, was reported to increase adiponectin mRNA expression in the adipose tissue of obese fa/fa rats (Gary-Bobo et al., 2006). The increase in the adiponectin level can be interpreted as either the increase in the number of adipocytes in adipose tissues or the improvement of insulin sensitivity (Lindsay et al., 2002). Because the CB agonist THC promotes adipogenesis, the pharmacological outcomes by the rimonabant-induced up-regulation of adiponectin during adipogenesis are still controversial. Currently, the effects of endocannabinoids on mammalian adipogenesis or on adipose tissue metabolism has not been fully understood. In this study, we examined the effects of three endocannabinoids, AEA, 2-AG and NADA on adipogenesis in human bone marrow mesenchymal stem cells (hBM-MSCs).
MATERIALS AND METHODS
Cell culture and differentiation of hBM-MSCs
hBM-MSCs were purchased from Lonza, Inc. (Walkersville, MD, USA). hBM-MSCs were cultured according to the manufacturer’s instructions with minor modifications. hBM-MSCs were maintained in Dulbecco’s modified eagle’s medium (DMEM) with low glucose (1 g/L glucose) containing 10% fetal bovine serum (FBS) (Lonza), supplemented with antibiotics and GlutamaxTM (Invitrogen, Carlsbad, CA, USA). To induce adipogenesis, hBM-MSCs were cultured to 100% confluence. Three days after the confluence, the medium was exchanged with DMEM with high glucose (4.5 g/L glucose) supplemented with 10% FBS, 10 μg/ml insulin, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (IDX medium). After inducing adipogenesis in hBM-MSCs, IDX media were exchanged at every 72 hours. For the treatment of endocannabinoids, AEA, 2-AG and NADA were freshly added to the IDX medium during the medium exchange. AEA, 2-AG, NADA, capsazepine, rimonabant, glibenclamide, aspirin and troglitazone were purchased from Sigma-Aldrich (Sigma Aldrich, St. Louis, MO, USA).
Cell viability tests
Viability of hBM-MSCs was evaluated using the WST-1 assay according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). 4-3-[4-lodophenyl]-2-4(4-nitrophenyl)-2H-5-tetrazolio-1,3-benzene disulfonate (WST-1; 10 μM pure solution) was treated in hBM-MSCs in culture and further incubated for 2 hours. The absorbance of the samples at 450 nm (A450) was determined.
Oil Red O staining
Adipogenesis-induced hBM-MSCs were washed in PBS, fixed for 30 minutes in 10 % formalin solution in PBS. Fixed cells were washed in 60% isopropyl alcohol and stained at room temperature with Oil Red O (ORO) in 60% isopropyl alcohol. After 5 minute-ORO staining, cells were quickly washed in distilled water. To determine the adipogenic level, adsorbed ORO dissolved with 100% isopropyl alcohol and absorbance was measured at 500 nm.
Total RNA isolation and quantitative real-time reverse transcription polymerase chain reaction (Q-RT-PCR)
Q-RT-PCR was performed using Assays-on-DemandTM Gene Expression kits (Applied Biosystems, Foster City, CA, USA). cDNA samples were analyzed for CB1 receptor (CNR1, Hs01038522_s1), CB2 receptor (CNR2, Hs00275635_m1), TRPV1 (Hs0021 8912_m1), adiponectin (ADIPOQ, Hs00605917_m1), fatty acid binding protein 4 (FABP4, Hs00609791_m1) and peroxisome proliferator activated receptor gamma (PPARγ, Hs00233423_m1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 4333764F) was analyzed as control. The cDNA from the reverse transcription reaction was amplified by PCR to measure the FAM fluorescence of each PCR cycle using ABI7500 Real Time PCR System (Applied Biosystems).
Enzyme linked immunosorbent assay (ELISA)
Adiponectin levels in hBM-MSC culture supernatants were measured by ELISA. Adiponectin ELISA was performed according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA).
PPARγ transactivation assay
A luciferase reporter gene assay was performed as previously reported (Shin et al., 2009). Briefly, CV-1 cells were transfected with a DNA mixture containing PPARγ responsive elements (PPRE)-luciferase reporter plasmid, pcDNA3-hPPARγ, and internal control plasmid pRL-SV-40, using the TransFastTM transfection reagent (Promega, Madison, WI, USA). After 24 hours of transfection, cells were treated with troglitazone, rimonabant or endocannabinoids for additional 24 hours. The activity of luciferase in each cell lysate was measured using the Dual-Luciferase® Reporter Assay System (Promega), according to the manufacturer’s instructions.
Statistical analyses
All statistical analyses were performed with MINITAB® software (Minitab Inc. State College, PA, USA). One ANOVA was used and Bonferroni’s post-tests were used for further analysis. The threshold of significance was set at p<0.05.
RESULTS
The CNR1 and TRPV1 were up-regulated by the induction of adipogenesis in hBM-MSCs
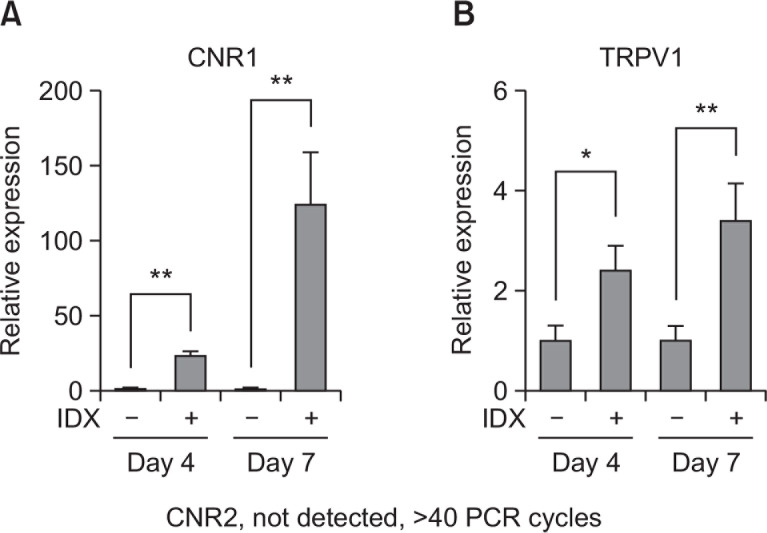
To evaluate the effects of endocannabinoids on adipogenesis in hBM-MSCs in culture, we first determined whether the receptors for endocannabinoids were expressed in differentiated adipocytes. The mRNA levels of the CB1 receptor (CNR1) were upregulated during the adipogenesis of hBM-MSCs in progress (Fig. 1A). However, the signal of the CB2 receptor (CNR2) expression was undetected (Fig. 1A). In addition, TRPV1 gene transcription was also upregulated as the number of differentiated adipocytes in hBM-MSC culture was increased (Fig. 1B). Therefore, hBM-MSCs can express both CB1 receptor and TRPV1 by the induction of adipogenesis.
Fig. 1.
Transcriptional expression profile of CNR1, CNR2 and TRPV1 during adipogenesis in hBM-MSCs. Adipocyte differentiation was induced in hBM-MSCs by exchanging culture media supplemented with 1 μg/ml insulin, 0.1 μM dexamethasone and 0.5 mM isobutylmethylxanthine (IDX). At the seventh day after the induction of adipogenesis, total RNA was extracted and Q-RT-PCR analysis was performed for (A) CNR1 and (B) TRPV1. Values represent the mean expression ± SE of the mRNA of the various genes relative to human GAPDH expression (n=3), *p≤0.05, **p≤0.01.
NADA inhibited adipogenesis in hBM-MSCs whereas AEA promoted
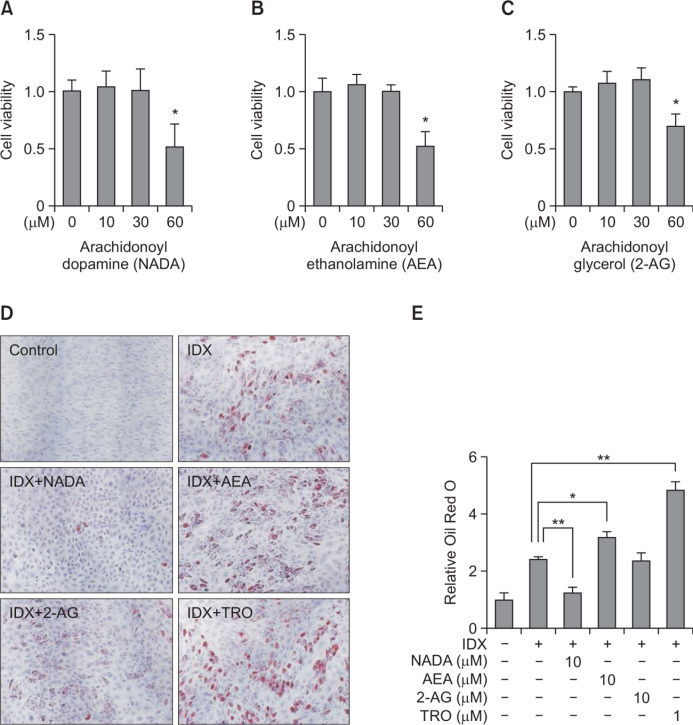
The cell viability was tested for various concentrations of three major endocannabinoids, NADA, AEA and 2-AG, in hBM-MSC culture to determine the non-cytotoxic concentration. All three endocannabinoids exhibited cytotoxicity in hBM-MSC culture at the 60 μM concentration (Fig. 2A–C). At the noncytotoxic concentration (10 μM), the effects of NADA, AEA or 2-AG were evaluated during adipogenesis in hBM-MSCs (Fig. 2). In ORO staining analysis, we found that NADA significantly inhibited adipogenesis in hBM-MSCs compared to that in the IDX control (Fig. 2D, 2E). In contrast, AEA promoted adipogenesis in hBM-MSCs, although its pharmacological activity was far lower than that of troglitazone (Fig. 2D, 2E). 2-AG had no effect on adipogenesis in hBM-MSCs (Fig. 2). In further analysis, 30 μM of 2-AG unaffected the adipocyte differentiation of hBM-MSCs (data not shown).
Fig. 2.
Effects of endocannabinoids on adipogenesis in hBM-MSCs. hBM-MSCs were cultured in 24 well plates. When confluent, the cell viability effects of endocannabinoids were evaluated in hBM-MSCs. NADA (A), AEA (B) and 2-AG (C), were treated for 72 hours in hBM-MSC culture. The cell viability was determined with a detecting reagent, 10 μM of WST-1. Adipogenesis was induced in hBM-MSCs under the presence of the IDX adipocyte differentiation inducing medium. After treating NADA, AEA and 2-AG, in every two or three day, media were exchanged. At the 7th days in culture, lipid droplets in differentiated adipocytes were stained with Oil Red O (ORO) (D). After dissolving the ORO in isopropyl alcohol, the level of staining was quantified at 500 nm using a spectrometer. Data were normalized by setting the control as 1 (E). Results are the mean ± standard deviation (SD) of three measurements using independent hBM-MSCs from three different donors (n=3). *p≤0.05 and **p≤0.01.
NADA decreased the expression of adipocyte differentiation markers in a concentration-dependent manner
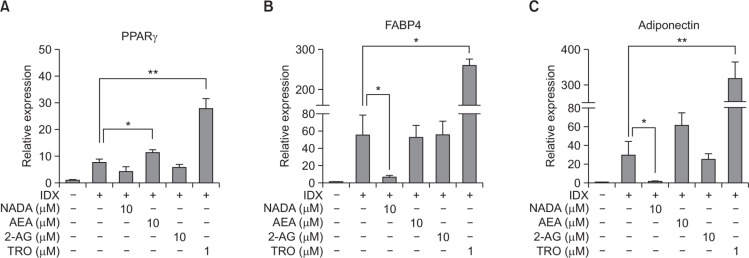
The mRNA levels of major adipocyte differentiation markers, PPARγ, FABP4 and adiponectin, were measured in hBM-MSCs treated with NADA, AEA or 2-AG (Fig. 3). AEA upregulated the expression of PPARγ and FABP4, which was correlated to the ORO staining data (Fig. 2D). AEA showed the tendency to increase mRNA level of adiponectin, however, the statistical significance was not observed (Fig. 3). NADA significantly decreased the gene transcription of both FABP4 and adiponectin during adipogenesis in hBM-MSCs (Fig. 3). Although the p value for the difference in the PPARγ expression was greater than 0.05, the mRNA level in the NADA treated cells was 32% lower than that in the control (Fig. 3A). As the ORO staining result, no effect on the adipocyte marker expression was observed by the 2-AG treatment (Fig. 3). The protein level of adiponectin expression was also measured hBM-MSC culture supernatants by ELISA (Fig. 4). As consistent with the mRNA level, AEA upregulated adiponectin production during adipogenesis in hBM-MSCs whereas NADA decreased (Fig. 4A).
Fig. 3.
Effects of endocannabinoids on adipocyte differentiation marker expression during adipogenesis in hBM-MSCs. When hBM-MSCs were in confluent state, adipogenesis was induced by exchanging media with the IDX adipogenic cocktail. At the 7th days in culture, total RNA samples were extracted and Q-RT-PCR was performed for PPARγ (A), FABP4 (B) and adiponectin (C). GAPDH was used as an internal control for Q-RT-PCR standardization. Values represent the mean expression ± standard deviation (SD) (n=3). *p≤0.05 and **p≤0.01.
Fig. 4.
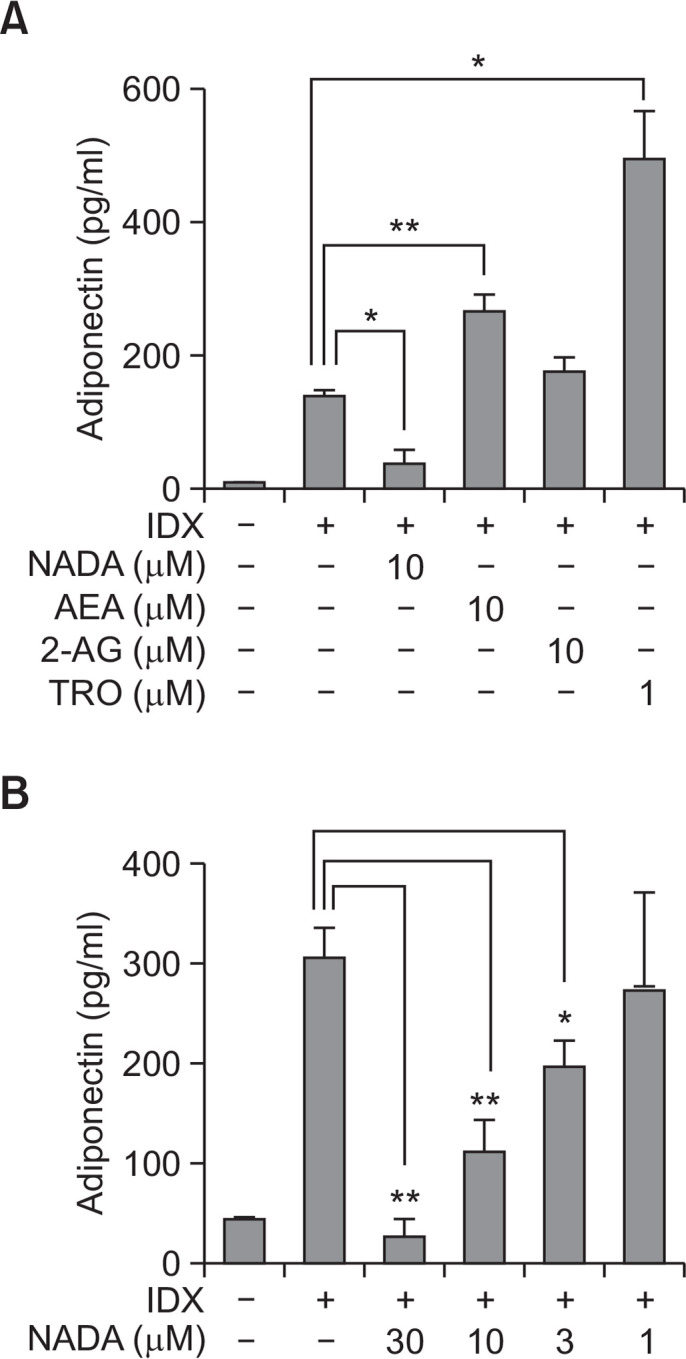
Effects of endocannabinoids on adiponectin production during adipogenesis in hBM-MSCs. When hBM-MSCs were in confluent state, adipogenesis was induced by exchanging media with the IDX adipogenic cocktail. ELISA was performed to measure the concentration of adiponectin (A) accumulated in cell culture supernatants for 48 hours after the last medium exchange. The concentration-dependent effect of arachidonyl dopamine (NADA) on the inhibition of adipogenesis was evaluated (B). Values represent the mean expression ± standard deviation (SD) (n=3). *p≤0.05 and **p≤0.01.
Both AEA and NADA are classified as endocannabinoids, however, they showed opposite effects on adipogenesis in hBM-MSCs. AEA was reported to increase PPARγ transactivation in murine 3T3-L1 preadipocytes (Bouaboula et al., 2005). Therefore, the effect of AEA on adipogenesis was also confirmed in human cells (Fig. 2–4). To confirm the effect of NADA, the concentration-effect analysis was performed for NADA. NADA inhibited adipogenesis in hBM-MSCs in a concentration-dependent manner (Fig. 4).
The NADA-induced inhibition of adipogenesis was antagonized by the CB1 receptor antagonist rimonabant
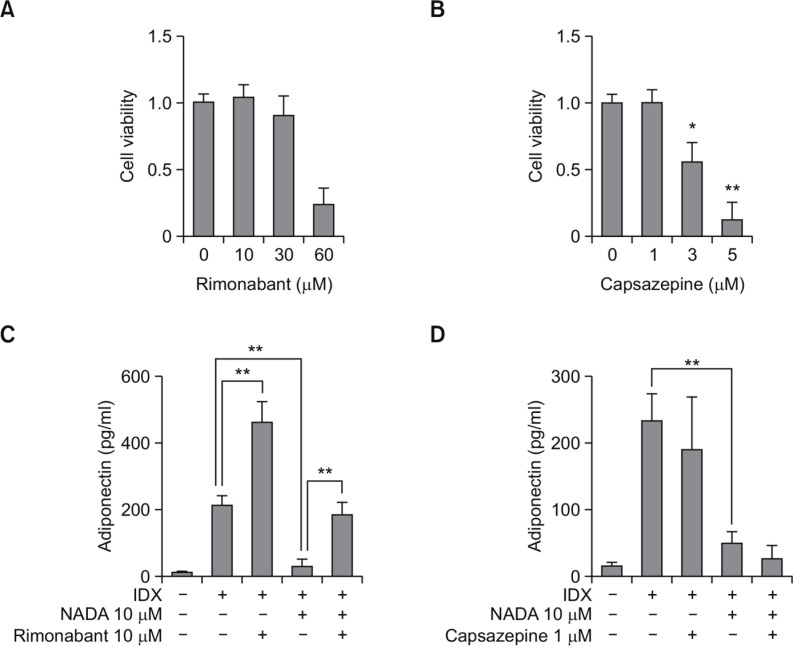
NADA can activate cellular signaling pathways triggered by both CB receptors and TRPV1 (Pacher and Hasko, 2008). To investigate whether NADA inhibited adipogenesis by the receptor-mediated effect, the CB1 antagonist, rimonabant, and the TRPV1 antagonist, capsazepine, were co-treated with NADA during adipogenesis in hBM-MSCs (Fig. 5). The non-cytotoxic concentrations were determined for rimonabant and capsazepine (Fig. 5A, B). At the non-cytotoxic concentration, rimonabant (10 μM) significantly antagonized the NADA-induced effect on hBM-MSCs (Fig. 5C) whereas casazepine had no effect (Fig. 5D). Therefore, the inhibitory effect of NADA on adipogenesis in hBM-MSCs is affected by the CB1 receptor-mediated signal transduction and not by the TRPV1-induced activity.
Fig. 5.
Effects of rimonabant and capsazepine on the NADA-dependent inhibition of adipogenesis in hBM-MSCs. hBM-MSCs were cultured in 24 well plates. When confluent, the cell viability effects were evaluated for rimonabant (A) and capsazepine (B), both of which were treated for 72 hours in hBM-MSC culture. The cell viability was determined by WST-1 assay. For testing the effects of capsazepine (C) and rimonabant (D) on the NADA-induced inhibition on the adipogenesis in hBM-MSCs, cell culture supernatants were harvested for the measurement of adiponectin by ELISA at the 7th days after exchanging media containing with IDX. Values represent the mean expression ± standard deviation (SD) (n=3). *p ≤0.05 and **p≤0.01.
Rimonabant promoted adipogenesis in hBM-MSCs in a concentration dependent manner
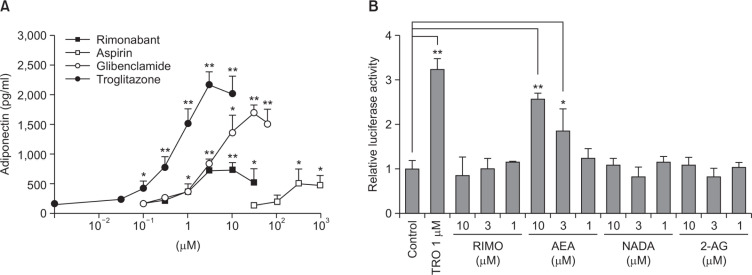
Notably, rimonabant significantly promoted adiponectin production when co-treated with the IDX adipogenic medium (Fig. 5C). The adipogenesis promoting effect of rimonabant was compared with those of other drugs, such as troglitazone, glibenclamide and aspirin by measuring adiponectin concentrations in hBM-MSC culture supernatants (Fig. 6A). The adipogenesis promoting activity of rimonabant showed the concentration dependency and was more potent than that of aspirin (Fig. 6A). However, the maximum activity reached by the treatment of rimonabant was less than those of troglitazone, a PPARγ agonist, and glibenclamide, a sulfonylurea anti-diabetic drug, suggesting that their intrinsic activity of rimonabant is regulated by the different molecular pathway in hBM-MSCs.
Fig. 6.
The effects of rimonabant on adipogenesis in hBM-MSCs and PPARγ transactivation. (A) The concentration-dependent effect of rimonabant on adipogenesis in hBM-MSCs was evaluated in 24 well plates. The effect of rimonabant was compared with those of aspirin, glibenclamide and troglitazone. Cell culture supernatants were harvested for the measurement of adiponectin by ELISA at the 7th days after exchanging media containing with IDX. (B) CV-1 cells were transiently cotransfected with the PPARγ expression vector and the PPRE-TK-Luciferase reporter, and then treated with vehicle, rimonabant (RIMO), AEA, NADA and 2-AG (1, 3, and 10 μM) or troglitazone (TRO, 1 μM). Values represent the mean expression ± standard deviation (SD) (n=3). *p≤0.05 and **p≤0.01.
Because it has been reported that endocannabinoids affect the cellular PPARγ activity (Pertwee, 2005; Fong and Heymsfield, 2009), an in vitro PPARγ transactivation assay was performed to determine whether the effect of rimonabant or other endocannabinoids was mediated by affecting the PPARγ activity (Fig. 6B). AEA significantly increased the PPARγ transactivation signal in a concentratin dependent manner. However, rimonabant, NADA and 2-AG had no effect on the PPARγ transactivation (Fig. 6B).
DISCUSSION
The cellular expression of the CB1 receptor and TRPV1 was upregulated during adipogenesis in hBM-MSCs (Fig. 1). This result suggests that endocannabinoids may affect physiological functions of hBM-MSCs related to the adipocyte biology. We evaluated the effects of three endocannabinoids, AEA, NADA and 2-AG, which can activate both CB receptors and TRPV1, on the adipocyte differentiation of hBM-MSCs. In this study, we found that AEA promoted adipogenesis in hBM-MSCs whereas NADA inhibited. The inhibition of NADA on adipogenesis was antagonized by rimonabant, a CB1 antagonist and not by capsazepine, a TRPV1 antagonist. We also demonstrated that rimonabant promoted adipogenesis in hBM-MSCs.
Both THC and AEA, CB agonists, increase the transactivation of PPARγ and adiponectin production in adipocytes and/or preadipocyte cell lines (Bouaboula et al., 2005; Teixeira et al., 2010). THC and AEA can directly bind to PPARγ as well as CB1 (O’Sullivan and Kendall, 2010). AEA was reported to competitively bind to PPARγ receptors (Bouaboula et al., 2005). It was reported that rimonabant, a CB1 receptor antagonist, also increases the adiponectin gene transcription in the adipose tissue of obese rats (Gary-Bobo et al., 2006; Tam et al., 2014). The information on CB receptors and endocannabinoids from the literature may be contradictory because both agonists and antagonists to CB receptors increase the adipogenesis in adipocytes or preadipocyte cell lines. However, due to the existence of the rimonabant-related inverse agonism for CB1 (Pertwee, 2005; Fong and Heymsfield, 2009; Pertwee et al., 2010), the effects of cannabinoid agonists like THC and AEA on adipogenesis in hBM-MSCs were phenotypically similar. The premise of an inverse agonist is the presence of constantly active receptors, even without the presence of their endogenous ligands (Milligan, 2003; Kenakin and Williams, 2014). If rimonabant promotes adipogenesis as an inverse agonist (Tam et al., 2012), the constant activity of CB1 is associated with the suppression of the adipocyte differentiation of hBM-MSCs. Endocannabinoids can affect multiple biological targets. AEA can bind to CB1, TRPV1 and PPARγ. In the present study, AEA significantly promoted adipogenesis (Fig. 2–4), suggesting that the effect of AEA on PPARγ overwhelms the AEA-activated CB1 signal transduction in hBM-MSCs. NADA, an endogeneous CB1 agonist, inhibited adipogenesis in hBM-MSCs. In contrast to AEA, NADA did not induce the PPARγ transactivation (Fig. 6B). In addition, the effect of NADA was antagonized by the CB1 receptor antagonist/inverse agonist rimonabant. In contrast, capsazepine, a TRPV1 antagonist, did not affect the NADA-induced inhibition during adipogenesis in hBM-MSCs. Therefore, CB1 has the intrinsic activity in hBM-MSCs associated with the suppression of adipogenesis. As a pure agonist, NADA may promote the effect of CB1 activation, leading to the inhibition of adipogenic differentiation. This study suggests that the differential effect of endocannabinoids on CB1 should be carefully considered to develop novel CB1 receptor modulators to treat metabolic diseases (D’Eon et al., 2008; Bajzer et al., 2011).
In summary, the endocannabinoid NADA significantly inhibits adipogenesis in hBM-MSCs. As an inverse agonist, rimonabant inhibited the constantly active CB1 in hBM-MSCs, which resulted in the promotion of adipocyte differentiation. Other endocannabinoids activating PPARγ like AEA may affect the intrinsic activity of CB1 in hBM-MSCs, resulting in the promotion of adipogenesis. Therefore, we conclude that the CB1 may constantly activate the cellular signal transduction pathway associated with the suppression of adipogenesis in hBM-MSCs.
Acknowledgments
This work was partly supported by Research Resettlement Fund for the new faculty of SNU, by the R&E program of Seoul Science High School in 2014 and by BK21 Plus Program in 2014.
Footnotes
CONFLICT OF INTEREST
No conflict of interest is reported.
REFERENCES
- Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT, Tschöp MH, Krawczewski-Carhuatanta KA, Cota D, Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Siva FJ, Serrano A, Diaz-Molina FJ, Sánchez Vera I, Juan-Pico P, Nadal A, Fuentes E, Rodríguez de Fonseca F. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol. 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Naylor RJ, Whittle BA, Javid FA. The effects of cannabidiolic acid and cannabidiol on contractility of the gastrointestinal tract of Suncus murinus. Arch Pharm Res. 2011;34:1509–1517. doi: 10.1007/s12272-011-0913-6. [DOI] [PubMed] [Google Scholar]
- D’Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes. 2008;57:1262–1268. doi: 10.2337/db07-1186. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food in-take and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Fong TM, Heymsfield SB. Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions. Int J Obes (Lond) 2009;33:947–955. doi: 10.1038/ijo.2009.132. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Scatton B, Le Fur G, Oury-Donat F, Bensaid M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol. 2006;69:471–478. doi: 10.1124/mol.105.015040. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Williams M. Defining and characterizing drug/compound function. Biochem Pharmacol. 2014;87:40–63. doi: 10.1016/j.bcp.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, Bataller R, O’Hare J, Scherer T, Buettner C, Kunos G. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol Pharmacol. 2003;64:1271–1276. doi: 10.1124/mol.64.6.1271. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DW, Kim SN, Lee SM, Lee W, Song MJ, Park SM, Lee TR, Baik JH, Kim HK, Hong JH, Noh M. (-)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPAR gamma transactivation. Biochem Pharmacol. 2009;77:125–133. doi: 10.1016/j.bcp.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Song D, Bandsma RH, Xiao C, Xi L, Shao W, Jin T, Lewis GF. Acute cannabinoid receptor type 1 (CB1R) modulation influences insulin sensitivity by an effect outside the central nervous system in mice. Diabetologia. 2011;54:1181–1189. doi: 10.1007/s00125-011-2082-z. [DOI] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF, Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Godlewski G, Earley BJ, Zhou L, Jourdan T, Szanda G, Cinar R, Kunos G. Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity. Am J Physiol Endocrinol Metab. 2014;306:457–468. doi: 10.1152/ajpendo.00489.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D, Pestana D, Faria A, Calhau C, Azevedo I, Monteiro R. Modulation of adipocyte biology by Δ9-tetrahydrocannabinol. Obesity. 2010;18:2077–2085. doi: 10.1038/oby.2010.100. [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Zhang Y, Fulp A, Maitra R, Thomas BF, Mahadevan A. Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice. Eur J Pharmacol. 2012;695:62–70. doi: 10.1016/j.ejphar.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]