Abstract
Propofol is an anesthetic agent that gained wide use because of its fast induction of anesthesia and rapid recovery post-anesthesia. However, previous studies have reported immediate neurodegeneration and long-term impairment in spatial learning and memory from repeated neonatal propofol administration in animals. Yet, none of those studies has explored the sex-specific long-term physical changes and behavioral alterations such as social (sociability and social preference), emotional (anxiety), and other cognitive functions (spatial working, recognition, and avoidance memory) after neonatal propofol treatment. Seven-day-old Wistar-Kyoto (WKY) rats underwent repeated daily intraperitoneal injections of propofol or normal saline for 7 days. Starting fourth week of age and onwards, rats were subjected to behavior tests including open-field, elevated-plus-maze, Y-maze, 3-chamber social interaction, novel-object-recognition, passive-avoidance, and rotarod. Rats were sacrificed at 9 weeks and hippocampal protein expressions were analyzed by Western blot. Results revealed long-term body weight gain alterations in the growing rats and sex-specific impairments in spatial (female) and recognition (male) learning and memory paradigms. A markedly decreased expression of hippocampal NMDA receptor GluN1 subunit in female- and increased expression of AMPA GluR1 subunit protein expression in male rats were also found. Other aspects of behaviors such as locomotor activity and coordination, anxiety, sociability, social preference and avoidance learning and memory were not generally affected. These results suggest that neonatal repeated propofol administration disrupts normal growth and some aspects of neurodevelopment in rats in a sex-specific manner.
Keywords: Propofol, Anesthesia, Neurodevelopment, Sex-difference, Weight gain, Learning and memory
INTRODUCTION
A number of newborns and infants are inevitably prescribed with anesthesia for a much needed treatment outcome. Accordingly, concerns are raised about the developmental liability of exposed individuals to the long term effects of neonatal anesthesia with a much greater need to study its alleged impact in humans (Sun, 2010). The early postnatal age is thought to display the highest vulnerability to anesthetics, especially in an immature and rapidly developing brain. In humans, this so-called brain growth spurt period begins at mid-pregnancy and continues well until 2 years postnatal age (Dobbing and Sands, 1973). Some clinical studies offered hints of increased risk of neurodevelopmental disturbances in children exposed to multiple, but not single, administration of anesthesia during the earliest ages (Wilder et al., 2009; Yan et al., 2014). However, clinical reports are limited and inconclusive.
Preclinical studies provided a clearer outcome of neonatal anesthesia, especially in studying the immediate neurochemical and long-term behavioral or neurocognitive effects in animal models, as ethical concerns limit the application of some studies in humans. Using rat models, anesthesia can be induced during the brain growth spurt period, which had been previously determined to be during 2 weeks of age (Dobbing and Sands, 1973). Indeed, exposure of neonatal rodent models to a variety of clinical anesthetics being studied (e.g. ketamine, nitrous oxide, isoflurane, sevoflurane, and propofol) induced neuroapoptotic events with long term behavioral, social and neurocognitive consequences (Jevtovic-Todorovic et al., 2003; Yon et al., 2005; Cattano et al., 2008; Satomoto et al., 2009; Milanovic et al., 2010; Cui et al., 2011; Karen et al., 2013; Yu et al., 2013). In particular, our group has given interest in propofol which showed a rapidly increasing evidence of its deleterious effects in preclinical studies (Cattano et al., 2008; Milanovic et al., 2010; Cui et al., 2011; Karen et al., 2013; Yu et al., 2013).
The small molecule propofol (2, 6-diisopropylphenol) is an anesthetic or sedative that gained wide use in pediatrics because of its expedient pharmacokinetics for the fast onset of anesthesia and rapid recovery (Mallory et al., 2011). GABA-A receptors are thought to be the target of propofol (Feng et al., 2007) with observed lesser impact on excitatory amino acid receptors. Propofol is being prescribed to younger children, yet little is known about its long term outcome. Animal models of neonatal propofol induction have shown the appearance of neuroapoptosis (Cattano et al., 2008; Cui et al., 2011; Karen et al., 2013; Milanovic et al., 2010; Yu et al., 2013), impaired brain circuitry (Briner et al., 2011), and long term effects on cortical- and hippocampal-related learning and memory performance (Feng et al., 2007; Gao et al., 2014; Yu et al., 2013). However, none of these studies has attempted to evaluate whether propofol-induced impairments have sex-specific differences. Furthermore, physical growth, locomotor activity, anxiety, social behaviors, and other aspects of learning and memory are also substantially important areas of evaluation to elucidate the effects of propofol treatment in neonates, which we thus sought in the present study.
The main objective of this study is to explore the long-term physical, emotional, social and cognitive effects of repeated neonatal propofol exposure in rats. We also intended to assess if these long-term effects have sex-specificity. The outcome of this study will support and add more preclinical evidence to open new insights on the long-term effects of neonatal propofol administration that may possibly occur in humans.
MATERIALS AND METHODS
Animals and treatment conditions
All procedures and animal handling were in agreement with the approved Animal Care and Use Guidelines of Sahmyook University, Korea, and Konkuk University, Korea; and were carried out in compliance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). All subjects were maintained on a 12:12-h circadian cycle (lights on at 07:00 and off at 19:00), in a constant temperature (22 ± 2°C) and humidity (55 ± 5%). Forty-seven Wistar Kyoto rats (WKY) from 6 dams were used in this study. In each litter at P7, pups were divided into 4 groups by treatment and sex and were almost equally distributed per group. Each pup was marked and numbered with indelible ink according to the group it belongs. After weighing (averaging from 12–16 g), all pups were administered with either normal saline or propofol (10 mg/ml, Claris Lifesciences limited, India) through intraperitoneal injection for 7 days, in three divided doses per day with 90 min interval at 40, 20, 20 mg/kg dosages, respectively. Dams were removed from the cages during the administration period, then returned to each cage after the pups regained full consciousness. During administration period, pups were kept warm at body temperature by placing heating pad under and heating lamps above the cages. Previous experiments of higher dosages and longer series of injections of propofol confirmed that animals do not show respiratory, metabolic, circulatory, or glycemic alterations (Briner et al., 2011; Yu et al., 2013; Gao et al., 2014). Pups were closely monitored during the sedated state observing their appearance and respiration. Weights were continuously monitored until weaned at P24. Weaned rats were housed by group (3–4 per cage) and sex, and were given food and water ad libitum. All efforts were done to minimize the number of animals used in the experiments and to minimize their suffering. Animals were assigned according to sex and treatment condition in the following groups and number of animals: Control (male, n=8; female, n=13) and propofol-exposed (male, n=10; female, n=16).
Behavior experiments
All behavior experiments were conducted from 4 to 8 weeks of age and sufficient amount of rest intervals were given to the subjects in each experiment (Fig.1). Experimental procedures were carried out in designated testing rooms and animals were habituated in the area for an hour before the actual experiments began. Tests were conducted in the active phase (dark cycle) of the animals as necessary or in the light phase otherwise. Experiments were sequenced according to the complexity and averseness of the test with the most aversive at the end. For most of the tests, an overhead video recording apparatus (CCD camera) connected to a computer was installed and behaviors were automatically tracked by EthoVision software (EthoVision 3.1, Noldus Information Technology, the Netherlands). Floors of testing equipment were thoroughly cleaned with 70% ethanol at the end of each trial.
Fig. 1.
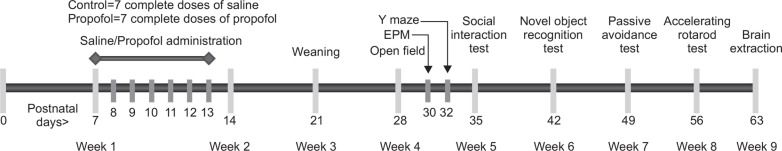
Propofol administration and behavioral experiment scheme.
Open field test
At P28, rats were allowed to explore the open field area for 15 minutes to assess the general locomotor activity of rats. Five black boxes (42×42×42[height] cm) made of polyvinylchloride (PVC) were available to accommodate 5 animals simultaneously. After introducing each animal to the center area as the test commenced, the distance moved and movement duration were automatically tracked by EthoVision software. Movement to the center area (20×20 cm2) was also assessed as a measure of anxiety behavior (Prut and Belzung, 2003).
Elevated plus maze (EPM) test
At P30, rats were tested in the EPM apparatus at 60 cm elevation. The maze was made of PVC forming a plus shape. Two open arms (50×10 cm) and 2 close arms (50×10×30 [height] cm) were interconnected forming the neutral area (10×10 cm) in the center. Subjects of each group were alternately tested in the maze for 8 minutes, initially placing each subject in the neutral area facing an open arm. Automatic recording of movement and placement was done through Etho-Vision software. The parameters of anxiety measured in this test, frequency of entry and time spent in the open arms, were analyzed by calculating their percentages from the total arm entries and time spent, respectively (Pellow et al., 1985).
Y maze test
Two days after EPM test, rats were subjected to the Y maze apparatus to assess the spatial learning and memory of propofol-exposed animals (Maurice et al., 1999). An equally angled Y maze made of PVC materials was used, measuring 50×10×20 cm each arm. Each rat, alternately tested in each group, was first introduced in one end of the Y maze facing the central intersection and allowed to explore for 8 minutes. An arm entry was counted when the four paws and tail of the rat enter an arm zone. A complete alternation was recorded when the rat enters different arms in three successive entries. Finally, total entries and spontaneous alternations of entries (total alternations/[total entries-2]×100) in the Y maze were calculated (Sarter et al., 1988).
Three-chamber sociability and social novelty preference tests
Sociability and social novelty preference tests are methods to evaluate the social ability of a subject rat to explore a stranger rat in a wire cage over the empty cage (phase 1), and preference to novel stranger rat over an already familiar one (phase 2), respectively. This method was first established by Moy and colleagues (Moy et al., 2004) and was slightly modified in our laboratory (Kim et al., 2011) to accommodate the rat subjects and the EthoVision software as the technology used to measure the parameters of the experiment. Here, we summarized the method in brief (please see (Kim et al., 2011), for details).
Sociability and social novelty preference tests were conducted from P35–38 age of rats and in each day of experiment, each group is evenly represented. The test was performed in a three-chamber rectangular cage (23×40×22 cm in each compartment) and two 10-cm2 openings provided access to the three chambers. The whole experiment was divided into two phases. Before phase 1, subject rats were habituated in the 3-chamber apparatus for 5 minutes. As phase one commenced, two wire cages were introduced containing either an unfamiliar rat inside or nothing (empty) in both side chambers with an alternation to which side of the chambers the stranger rat was placed in each trial. The subject rat was then allowed to explore each chamber and wire cages for 10 minutes. Phase two commenced immediately after sociability test wherein another novel stranger rat was placed in the previously empty cage to compare the preference for novelty. Another 10 minutes was allotted for the subject rat to explore the chambers in this test. Time spent in each chamber was measured automatically through EthoVision software. Sniffing duration towards the wire cages was also recorded through manual observation 2 meters from the testing apparatus.
Novel object recognition test (NORT)
The recognition aspect of learning and memory was assessed in subject rats on their 42nd–45th days of age. Using the same apparatus for the three chamber social tests, while removing the two center walls that divided the chambers, rats were once again allowed to explore this arena during the novel object recognition test. The social tests primarily served as the habituation phase for the NORT. The NORT has three phases: habituation, familiarization and novel object recognition as described previously with some modifications (Bevins and Besheer, 2006). In every phase, each rat was placed at the start area near the front wall.
After 5 minutes of habituation in the empty area, each tested rat was moved to an empty transport box while two identical objects (blue bottles) were placed in the testing box and taped on the floor for stabilization. The objects were placed in two opposite and equidistant locations; 50 cm from the front wall, 15 cm from the back wall, 10 cm from the sidewalls and 12 cm apart. Thereafter, as the familiarization phase began, each subject was allowed to explore the objects for 10 min. Object exploration was defined when the rat’s nose was in close proximity (<2 cm) to the object while the vibrissae were moving but not when the body of the rats touched the object but the head was in another direction.
Three hours after the familiarization trial, subject was reintroduced to the arena for novel object recognition test. This time, one object during the familiarization phase was alternately replaced with a novel object (Pororo character toy). Object exploration time was recorded for 5 min between the novel and the familiar object. Novel object preference index was also calculated as: novel object exploration time/(novel object exploration time+familiar object exploration time)×100 (%) (Wang et al., 2007). After each trial, the arena floor and the objects were wiped with 70% ethanol to eliminate odor cues for the next subject.
Passive avoidance test
At P49–50, subject rats were tested in the one-trial passive avoidance test as previously described (D’Agata and Cavallaro, 2003), with slight modifications. The test involved the exposure of the rat to the passive avoidance apparatus (Gemini avoidance apparatus, San Diego Instruments, San Diego, CA, USA). The apparatus has two chambers: one lighted and one dark compartment separated by a switch-operated guillotine door. During the conditioning phase, subjects were initially put in the light chamber for 10 s before the door was raised to allow access to the dark chamber. Once the rat was in the dark chamber, the door was closed and shock was delivered for 2 s (1 mA intensity) before rat was taken back to its home cage. Latency to enter the dark chamber up to 5 minutes maximum time was recorded. 24 h later, rats were tested again with the same procedure but no shock was given when a rat enters the dark chamber. Latency to enter the dark chamber was again recorded as an indicator of avoidance or training experience memory (D’Agata and Cavallaro, 2003).
Rotarod test
The final behavior test done for all rats was the rotating rod (Rota Rod, Ugo Basile S.R.L., Italy) in accelerating speed as described previously with some modifications (Morgan et al., 2008). The rats were given 2 training sessions to acclimate to the mechanics of the rotating corrugated drum by walking and balancing on it until they fall. The drums were set to rotate at an accelerating speed from 5 to 25 rpm during a 5-minute trial period. Latency to fall was recorded and the whole experiment was done for 3 successive days with one trial per day.
Brain dissection
A week after the last behavior experiment, about 8 to 10 rats each group were decapitated through rodent guillotine for brain dissection and molecular studies. Hippocampi were isolated and flash frozen in the liquid nitrogen for further molecular analysis.
Western blot analysis
Tissues were homogenized with 500 μl homogenization buffer (50 mM Tris-HCl [pH.7.4] 150 mM NaCl) including proteinase inhibitor cocktail. Homogenized samples were centrifuged at 13,200 rpm for 20 min at 4°C. After centrifugation, supernatants were harvested and added with 2x sample buffer then boiled using heat block at 100°C. The collected aliquot of proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk in TBS including 1% tween-20 for 1 h. Thereafter, the membranes were incubated with first antibody overnight at 4°C and then with peroxidase-conjugated secondary antibody (Santa Cruz, CA, USA) for 2 h at room temperature. Specific bands were detected using the ECL system (Amersham, Buckinghamshire, UK) and exposed to LAS3000.
Antibodies
Antibodies against PSD95 (AB9708), GAD65/67 (AB1511), NMDA receptor (AB9864), Glutamate receptor 1 (AB31232), Glutamate receptor 2 (AB20673), NMDA receptor 2A (AB1555p), NMDA receptor 2B (AB1557p) were obtained from Millipore (Billerica, MA, USA) and beta-actin (A5316) from Sigma Aldrich (St. Louis, MO, USA).
Statistical analysis
All data were expressed as mean ± SEM. Statistical analyses were conducted using GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego, CA, USA. Two way ANOVA was used to measure effects of sex and treatment, or time and treatment, and interaction between each two variables, then Bonferroni post test was used as post hoc analysis. Male and female data were separated according to the objective of this study. Unpaired t-test was also used to calculate the difference between control and propofol-exposed groups in each sex.
RESULTS
Long-term body weight alteration of offspring following neonatal repeated administration of propofol
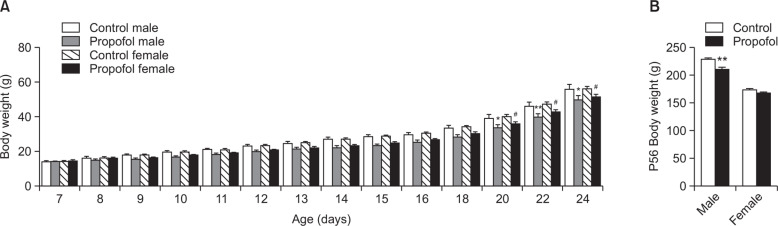
There were significant effects of neonatal repeated propofol administration on the weight profile of male and female offspring rats (F(3, 43)=3.838, p<0.05) (Fig. 2). An altered body weight gain was observed after the first day of propofol administration that differed significantly from the control during P22 to P24 weaning and grouping period (Fig. 2A). At P56 (Fig. 2B), propofol-treated males showed a persistent decrease in body weight than male controls (p<0.01) but no difference between females was found (p>0.05).
Fig. 2.
Mean body weight gain alteration of rats after neonatal repeated administration of propofol from (A) P7 to P24, and (B) at P56. Bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). *p<0.05 and **p<0.01 vs control male, #p<0.05 vs control female.
Slightly increased locomotor activity in early exploration of female rats and no effect in anxiety related behaviors following neonatal repeated administration of propofol
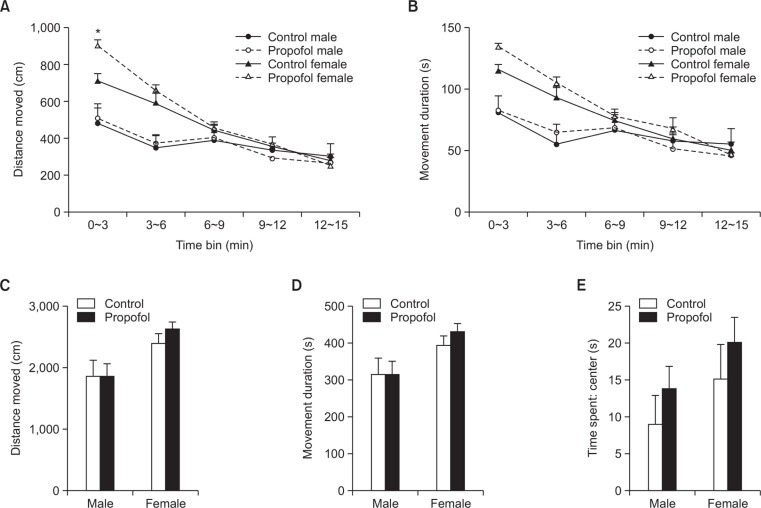
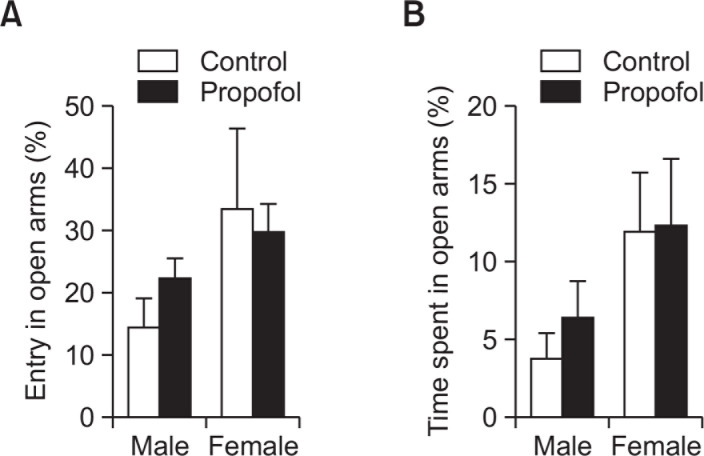
Neonatal repeated propofol administration did not show an effect on overall activity in the open field arena for the whole 15 min duration of exploration in either the distance moved (F(1, 43)=0.3545, p=0.55) (Fig. 3C) or the movement duration (F(1, 43)=0.2391, p=0.62) (Fig. 3D) parameters. However, a significant increase in distance moved during the first 3 minutes of exploration in the open field (Fig. 3A) was observed in the propofol-treated female versus the control female group (p<0.05) but not in between male groups (p>0.05). The succeeding minutes (time bins) of exploration did not show any more differences in locomotor activity of groups. Anxiety-related behaviors were not also evident in propofol-treated subjects as measured by time spent in the center area of the open field (Fig. 3E) or the parameters assessed in the elevated plus maze (Fig. 4).
Fig. 3.
Effects of neonatal repeated propofol administration on open field locomotor activity of rats at P28 by (A) distance moved (in time bins), (B) movement duration (in time bins), (C) total distance moved and (D) total movement duration. (E) Time spent in the center area (20×20 cm) was also measured as an initial measure of anxiety behavior. Symbols and bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and Propofol (male, n=10; female, n=16). *p<0.05 vs control female.
Fig. 4.
Effects of neonatal repeated propofol administration on anxiety-related behaviors and locomotor activity of rats in the elevated plus maze at P30 by (A) percentage of entry in open arms/total arm-entries and (B) percentage of time spent in open arms/total time spent in all arms. Bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). No significant difference was observed.
Impaired spontaneous alternation ability of female rats in the Y maze apparatus following neonatal repeated administration of propofol
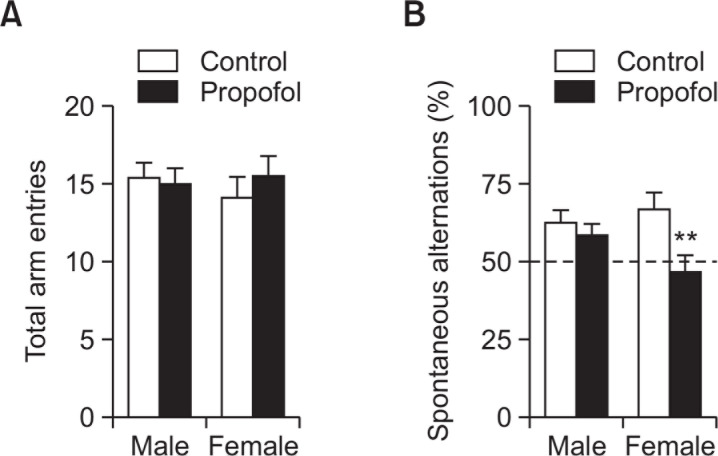
In Fig. 5A, the total arm entries were similar among groups. However, there was a significantly lower percentage of spontaneous alternation observed in the propofol treated female group as compared to female control (<50% chance level and p<0.01) (Fig. 5B). Propofol-treated male subjects were not affected in this parameter.
Fig. 5.
Effects of neonatal repeated propofol administration on spatial working memory of rats in the Y maze at P32 by measuring the (A) total arm entries and (B) percentage of spontaneous alternations from the total possible alternations. Bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). **p<0.01 vs control female.
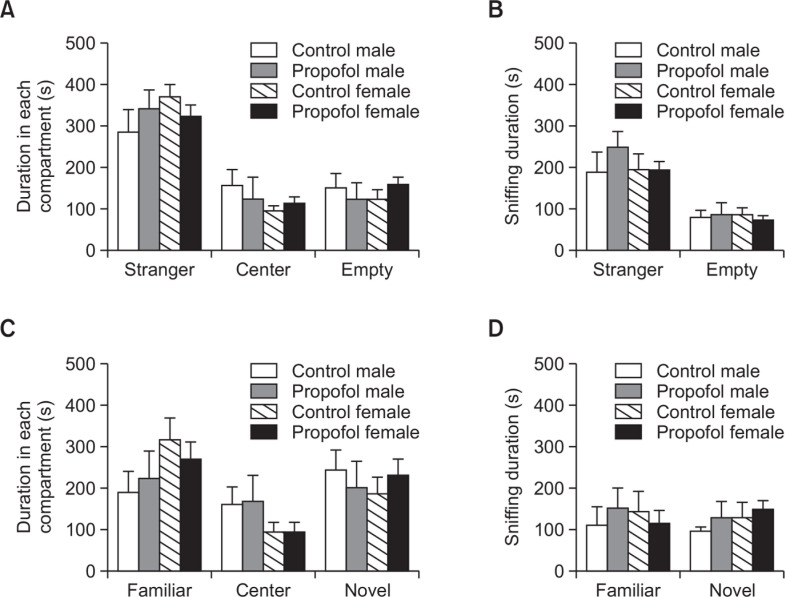
Unaffected social behavior of rats in the three-chamber social interaction test following neonatal repeated administration of propofol
The social behaviors of rats at P35–38 through sociability test and preference for novelty in the social preference test were not affected by repeated propofol treatment during neonatal period. As shown in Fig. 6, not any significant difference was observed in the social behavior parameters of the three-chamber paradigm between treated and control rats.
Fig. 6.
Effects of neonatal repeated propofol administration on (A, B) sociability and (C, D) social preference of rats in the 3-chamber apparatus at P35–38 by measuring the (A, C) duration in each compartment and (B, D) sniffing duration in the wire cages with or without stranger rats. Bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). No significant difference was observed.
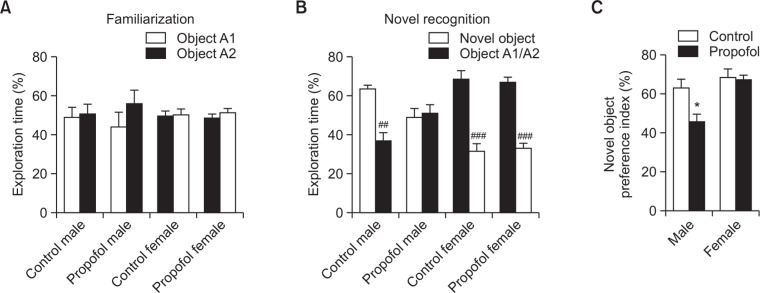
Impaired recognition memory of male rats in the novel object recognition test following neonatal repeated administration of propofol
Fig. 7A reveals the balance of exploration of animals to the two identical objects during the familiarization phase to ensure absence of object preference that may have affected the following novel recognition test. During the novel recognition phase (Fig. 7B), all groups displayed recognition to the familiar object thus significantly increased preference to the novel object, except the propofol treated male group. A significantly decreased novel object preference index (Fig. 7C) confirms this impairment of the propofol treated male rats in recognition memory (p<0.05).
Fig. 7.
Effects of neonatal repeated propofol administration on recognition learning and memory of rats in the novel object recognition test at P42–45. (A) Percentage of exploration time was measured between two identical objects during the familiarization phase. (B) Percentage of exploration time was measured between the novel object and the familiar object during the novel recognition phase. (C) Novel object preference index was calculated by the percentage of exploration time to the novel object from the total exploration time to both objects. Bars indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). *p<0.05 vs control male, ##p<0.01 and ###p< 0.001 vs novel object.
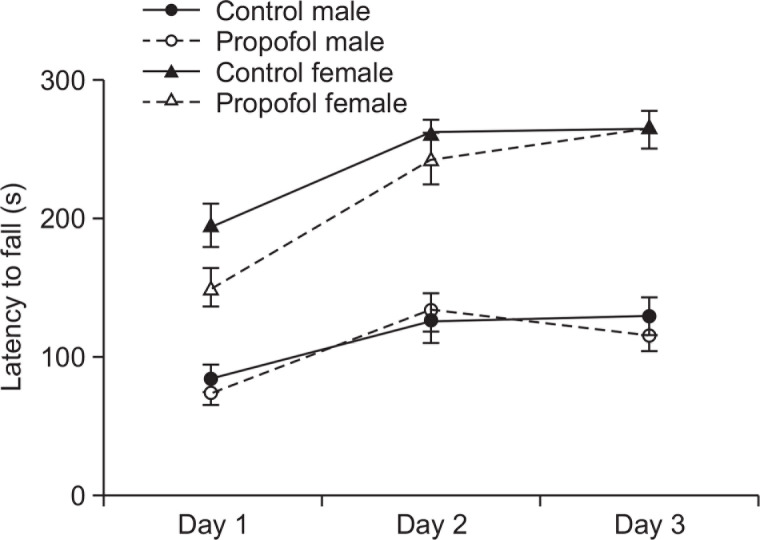
Unaffected avoidance memory retention and motor coordination in the passive avoidance and rotarod tests following neonatal repeated administration of propofol
Following an aversive stimulus induced by a mild foot shock in the dark chamber during the conditioning phase of the one-trial passive avoidance test, all rat groups showed retention to the aversive experience and avoided entering the dark chamber 24 h later during the test phase (data not shown). Repeated propofol administration in neonates did not show impairment in the avoidance learning and memory of rats. Furthermore, motor balance and coordination assessed in the accelerating rotarod did not show any difference between propofol treated rats and control groups (Fig. 8).
Fig. 8.
Effects of neonatal repeated propofol administration on motor coordination of rats in the accelerating rotarod at P56–58 by measuring the latency to fall in three daily trials. Symbols indicate the mean ± SEM. Control (male, n=8; female, n=13) and propofol (male, n=10; female, n=16). No significant difference was observed.
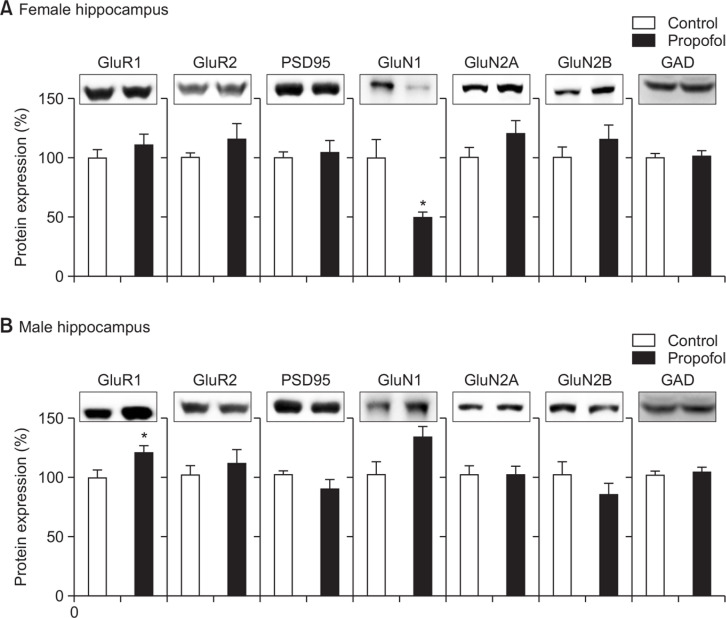
Female-specific decreased GluN1protein expression and male-specific increased GluR1 protein expression of rat hippocampus following neonatal repeated administration of propofol
Using Western blot analysis, hippocampal protein expression levels were measured including PSD95, NMDA and AMPA receptor subunits. Propofol treated female rats (Fig. 9A) showed a lower expression levels of GluN1 proteins than control females (p<0.05) while propofol treated male rats (Fig. 9B) had a higher expression levels of GluR1 proteins than control males (p<0.05). Other markers were not significantly different among all groups in each sex.
Fig. 9.
Effects of neonatal repeated propofol administration on hippocampal proteins expression levels. Western blot analysis was conducted after behavioral experiments. Expression levels of PSD95, and NMDA & AMPA receptor subunits were measured according to female (A) and male (B) sexes. GAD was used as loading control. Bars indicate the mean ± SEM. Control (male, n=8; female, n=10) and propofol (male, n=10; female, n=10). *p<0.05 vs control.
DISCUSSION
Altered physical growth is an important sign of malnutrition. The current data showed an altered body weight gain of rats immediately after the first propofol administration, which persisted until weaning period. However, during the late adolescent period at P56, the decreased weight was more persistent in the propofol-exposed male rats. The alteration in weight gain during and after propofol treatment might be related to poor feeding behaviors following repeated administration of anesthesia (Hayashi et al., 2002) or the anesthetic agent itself could have an impact on the nutrition of animals (Anand et al., 2004), which needs further investigations.
The generally unaffected locomotor behavior of neonatal propofol exposure is consistent with previous report (Karen et al., 2013). Motor coordination in the rotarod was not also affected by neonatal propofol treatment.
Furthermore, anxiety-related and social behaviors are as well important behavioral alteration markers in animal models of various disorders. Thus far, our study is the first to assess the long-term outcome of neonatal repeated propofol administration in anxiety and social behaviors in rats and we found no significant effect in this condition.
Impairments in learning and memory are the mostly reported consequences of neonatal propofol exposure in animal models. Inhibition of long term potentiation (LTP) in the hippocampus was observed (Feng et al., 2007; Gao et al., 2014), which could be related to the decreased levels of both Ca2+/calmodulin-dependent protein kinase II (CaMKII) and phosphorylated CaMKII (pCaMKII) proteins (Gao et al., 2014). Thus, the long-term outcomes in learning and memory could be partly explained by the immediate neuroapotosis and morphofunctional effects of propofol in the developing brain (Cattano et al., 2008; Milanovic et al., 2010; Briner et al., 2011; Cui et al., 2011; Karen et al., 2013; Yu et al., 2013). On the other hand, increased LTP may as well affect the normal learning and memory formation. For example, Han et al injected nano-zinc oxide (nano-ZnO) intraperitoneally in Wistar rats and observed an over-enhanced LTP in the hippocampus but decreased spatial learning and memory (Han et al., 2011). Thus, it is suggested that a balance within the normal range of function of LTP is important and that LTP alteration in either direction will similarly affect the cognitive function.
In the present study, we found that propofol exposed rats have sex-specific impairments in two aspects of learning and memory tests that we have done. However, the mechanisms of this sex differences are still unclear. With the increasing interest in sex differences in many health conditions, a lot of work needs to be done to elucidate the underlying mechanisms of these disparities. Hypothetically, sex differences occur possibly due to the sex-specific onsets of neural development processes, hormonal expressions and epigenetics (Brenhouse and Andersen, 2011). Thus, it would also be interesting to investigate the neurobiological mechanism of the sex-specific outcomes of neonatal propofol exposure.
Hippocampal NMDA receptors (NMDAR) are linked to spatial learning and memory (Boon et al., 2005) and regulation of synaptic plasticity (Nakazawa et al., 2004). Functional NMDARs are generally made up of a heterotetrameric assembly of typically two GluN1 plus two GluN2 or mixed GluN2 and GluN3 subunits (Paoletti et al., 2013). The present study showed that neonatal repeated propofol administration repressed the expression of GluN1 proteins, but not GluN2 or GluN3, of NMDARs in the hippocampus of female rats. GluN1 is a necessary component of functional NMDAR complexes (Chen et al., 2009); therefore, its decreased expression could affect the overall NMDAR-related learning and memory function in the hippocampal region. Indeed, disruption of GluN1 expression in mutant mouse models of a previous study revealed impairments in spatial learning and memory but not object recognition memory (Chen et al., 2009), which showed similar result to our current study.
AMPA receptors are involved in mediating synaptic plasticity; and their activation and insertion follows the NMDAR-induced activation of CaMKII after increased calcium influx into the cells during LTP (Lynch, 2004; Paoletti et al., 2013). Increased expression or modulation of AMPA receptor subunits was reported to improve recognition memory (Uslaner et al., 2009). However, our study showed an opposite finding where propofol-treated male rats have an increased GluR1 receptor subunit protein expression in the hippocampus but impaired recognition memory. This finding may further support the hypothesis that deviations of receptor expression from either direction may show the same cognitive dysfunction. In another perspective, the increased GluR1 expression may be a compensatory outcome from the neurodegenerative consequences of repeated neonatal propofol exposure in which GluR1 levels could have been initially reduced. However, the Western blot analysis adopted in this study does not discriminate the synaptic and extra-synaptic levels of GluR1, which is essential for the determination of synaptic activity. Nevertheless, the result gives us a viewpoint of the impaired recognition memory in propofol treated male rats. Follow up studies are needed in evaluating the hippocampus same period as during the behavior experiment to determine time-relevant changes.
The drug carrier of propofol used in this study is composed of soybean oil, glycerol, egg lecithin and sodium hydroxide (to adjust pH). Intriguingly, it was previously suggested that the lipid emulsion drug carrier could have an independent NMDAR activation properties (Weigt et al., 2002), aside from the specific action of the incorporated drugs. However, in all the lipid emulsions in that study, only 3 out of 9 preparations showed activation on NMDAR while the others did not. Furthermore, the experiments were conducted in vitro, making them difficult to compare to the in vivo situation as to whether the lipid emulsion could have direct access to the neurons from intraperitoneal injection. There were two studies which used intralipid as a vehicle control for the examination of the effects of prenatal exposure to propofol (Li et al., 2014; Xiong et al., 2014). However, both studies showed that intralipid administration did not affect the cleaved caspase levels and physical development of offspring rats. Therefore, it is less likely that the use of drug carrier in the current study may induce neural changes when given intraperitoneal to neonatal rats.
In the current study, we focused on the general expression of receptors in the hippocampus without dividing the subregions, which are suggested to be differently related to spatial and recognition memories. In the previous study, it was found that the hippocampus is more prominent for spatial memory performance than recognition memory, which shares a lesser portion from this brain location (Broadbent et al., 2004). Thus, histochemical analysis of hippocampal subregions may provide additional information of the role of region-specific receptor regulation in the modulation of cognitive function by repeated propofol exposure to neonates, which needs to be carefully examined in the future study.
Another interesting area of investigation related to learning and memory is the role of acetylcholine in the toxic effects of profopol during the neonatal period since acetylcholine is important for encoding and memory acquisition (Hasselmo, 2006). A previous study had investigated the effect of propofol on the hippocampal acetylcholine levels in adult rats (Kikuchi et al., 1998). Using in vivo microdialysis, it was shown that I.P. propofol injection (25 and 50 mg/kg) dose-dependently reduced the basal acetylcholine levels in the hippocampus by 47% and 72 %, respectively. Whereas the experimental conditions differ in the previous and current study, it could give us a hint of the deleterious effects of propofol on acetylcholine regulation. This point should be investigated in the future, especially focusing on the cholinergic system.
Translating the present findings to the clinical area would be challenging due to differences in the duration of brain growth spurt period between humans and rats (Dobbing and Sands, 1973). It is reasonable to assume that the longer duration of human brain growth spurt period could have more complex processes than that of rodents. In clinical studies, findings revealed that multiple exposures to anesthesia during the neonatal or toddler period in humans have higher incidences of learning disabilities growing up (Wilder et al., 2009; Flick et al., 2011; Yan et al., 2014). More clinical studies in larger populations of children or adolescents with single or multiple exposures to anesthesia, such as propofol, during their infancy period will help in comparing with the preclinical findings. Nevertheless, our ultimate goals of studying the long-term behavioral and molecular effects of repeated neonatal administration of propofol were met including the finding of sex differences. From here, further elucidation of the mechanistic pathways will be the next step for these known behavioral outcomes. In conclusion, neonatal repeated propofol administration affects growth and neurodevelopment of rats through altered weight gain and sex-specific impairments in spatial (in females) and recognition (in males) aspects of learning and memory during the adolescent period. The female-specific decrement of GluN1 NMDAR subunit and male-specific increment of GluR1 AMPA receptor subunit protein expression in the hippocampus may be involved. Other aspects of behavior such as locomotor activity, motor coordination and balance, anxiety, sociability and social novelty preference, and avoidance learning and memory were not generally affected.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A120029) and Yonsei Faculty Research Fund 6-2013-0145 (Koo, B-N). The authors have no conflict of interests to declare.
REFERENCES
- Anand KJS, Phil D, Soriano SG. Anesthetic agents and the immature brain: Are these toxic or therapeutic? Anesthesiology. 2004;101:527–530. doi: 10.1097/00000542-200408000-00033. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Boon WC, Diepstraten J, van der Burg J, Jones ME, Simpson ER, van den Buuse M. Hippocampal NMDA receptor subunit expression and watermaze learning in estrogen deficient female mice. Brain Res. 2005;140:127–132. doi: 10.1016/j.molbrainres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattano D, Young C, Straiko MM, Olney JW. Sub-anesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- Chen PE, Errington ML, Kneussel M, Chen G, Annala AJ, Rudhard YH, Rast GF, Specht CG, Tigaret CM, Nassar MA, Morris RG, Bliss TV, Schoepfer R. Behavioral deficits and subregion-specific suppression of LTP in mice expressing a population of mutant NMDA receptors throughout the hippocampus. Learn Mem. 2009;16:635–644. doi: 10.1101/lm.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Ling-Shan G, Yi L, Xing-Qi W, Xue-Mei Z, Xiao-Xing Y. Repeated administration of propofol upregulated the expression of c-Fos and cleaved-caspase-3 proteins in the developing mouse brain. Indian J Pharmacol. 2011;43:648–651. doi: 10.4103/0253-7613.89819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata V, Cavallaro S. Hippocampal gene expression profiles in passive avoidance conditioning. Eur J Neurosci. 2003;18:2835–2841. doi: 10.1111/j.1460-9568.2003.03025.x. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CS, Qiu JP, Ma HC, Yue Y. Effect of propofol on synaptic long-term potentiation in hippocampal slices of rats. Chi J Prev Med. 2007;87:763–767. [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Peng S, Xiang S, Huang J, Chen P. Repeated exposure to propofol impairs spatial learning, inhibits LTP and reduces CaMKIIα in young rats. Neurosci Lett. 2014;560:62–66. doi: 10.1016/j.neulet.2013.11.061. [DOI] [PubMed] [Google Scholar]
- Han D, Tian Y, Zhang T, Ren G, Yang Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nano-medicine. 2011;6:1453–1461. doi: 10.2147/IJN.S18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Pediatr Anaesth. 2002;12:770–774. doi: 10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen T, Schlager GW, Bendix I, Sifringer M, Herrmann R, Pantazis C, Enot D, Keller M, Kerner T, Felderhoff-Mueser U. Effect of propofol in the immature rat brain on short- and long-term neurodevelopmental outcome. PloS One. 2013;8:e64480. doi: 10.1371/journal.pone.0064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Wang Y, Sato K, Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br J Anaesth. 1998;80:644–648. doi: 10.1093/bja/80.5.644. [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim P, Go HS, Choi CS, Yang S-I, Cheong JH, Shin CY, Ko KH. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol Lett. 2011;201:137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Li J, Xiong M, Alhashem HM, Zhang Y, Tilak V, Patel A, Siegel A, Ye JH, Bekker A. Effects of prenatal propofol exposure on postnatal development in rats. Neurotixicol Teratol. 2014;43:51–58. doi: 10.1016/j.ntt.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Mallory MD, Baxter AL, Yanosky DJ, Cravero JP, Pediatric Sedation Research, C Emergency physician-administered propofol sedation: a report on 25,433 sedations from the pediatric sedation research consortium. Ann Emerg Med. 2011;57:462–468. doi: 10.1016/j.annemergmed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Noda Y, Yamada K, Privat A, Nabeshima T. The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (σ) receptor ligands involves both σ1 and σ2 sites. Br J Pharmacol. 1999;127:335–342. doi: 10.1038/sj.bjp.0702553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic D, Popic J, Pesic V, Loncarevic-Vasiljkovic N, Kanazir S, Jevtovic-Todorovic V, Ruzdijic S. Regional and temporal profiles of calpain and caspase-3 activities in postnatal rat brain following repeated propofol administration. Dev Neurosci. 2010;32:288–301. doi: 10.1159/000316970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Munireddy S, Alamed J, DeLeon J, Diamond DM, Bickford P, Hutton M, Lewis J, McGowan E, Gordon MN. Apparent behavioral benefits of tau overexpression in P301L tau transgenic mice. J Alzheimers Dis. 2008;15:605–614. doi: 10.3233/jad-2008-15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alternation behaviour by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology. 1988;94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl 1):i61–68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Mouri A, Mizoguchi H, Nitta A, Chen W, Nabeshima T. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine ameliorates the cognitive dysfunction in beta amyloid25-35 icv-injected mice: involvement of dopaminergic systems. Neuropsychopharmacol. 2007;32:1261–1271. doi: 10.1038/sj.npp.1301256. [DOI] [PubMed] [Google Scholar]
- Weigt HU, Georgieff M, Beyer C, Föhr KJ. Activation of neuronal N-methyl-D-aspartate receptor channels by lipid emulsions. Anesth Analg. 2002;94:331–337. doi: 10.1097/00000539-200202000-00018. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Li J, Alhashem HM, Tilak V, Patel A, Pisklakov S, Siegel A, Ye JH, Bekker A. Propofol exposure in pregnant rats induces neurotoxicity and persistent learning deficit in the offspring. Brain Sci. 2014;4:356–375. doi: 10.3390/brainsci4020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li YR, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol. 2014;29:1333–1338. doi: 10.1177/0883073813517508. [DOI] [PubMed] [Google Scholar]
- Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Yu D, Jiang Y, Gao J, Liu B, Chen P. Repeated exposure to propofol potentiates neuroapoptosis and long-term behavioral deficits in neonatal rats. Neurosci Lett. 2013;534:41–46. doi: 10.1016/j.neulet.2012.12.033. [DOI] [PubMed] [Google Scholar]