Abstract
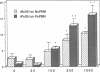
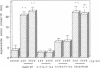
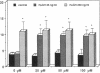
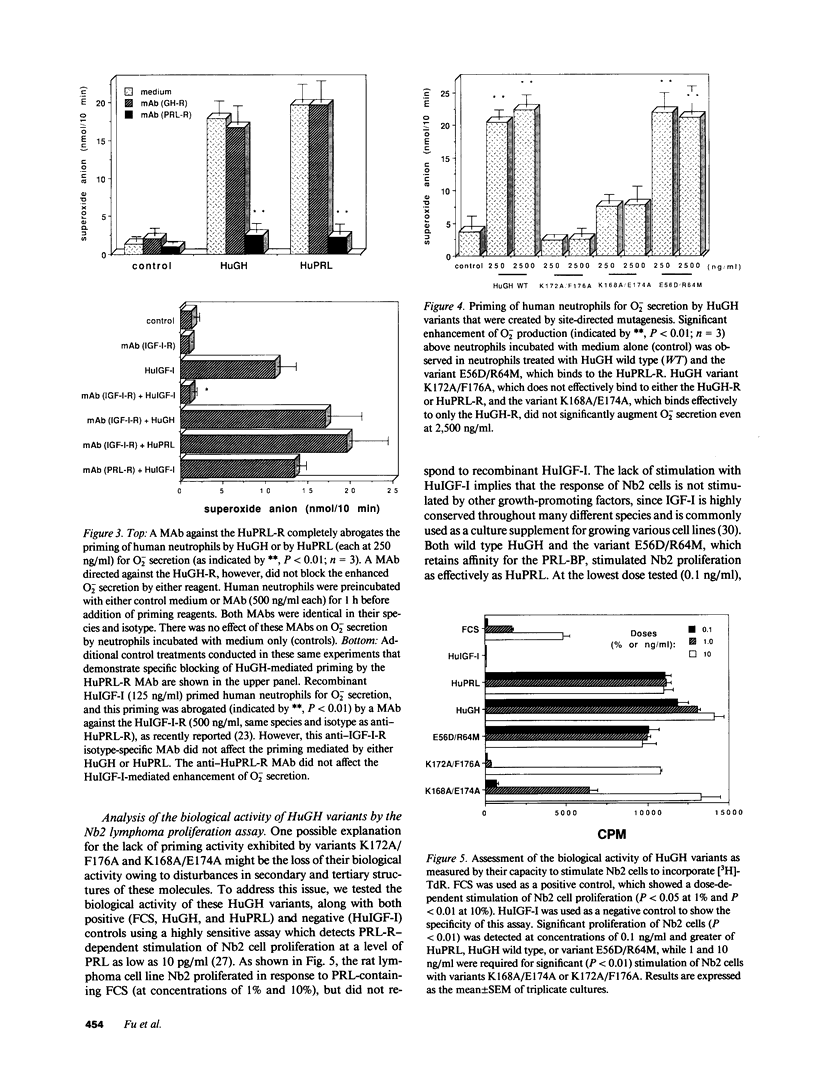
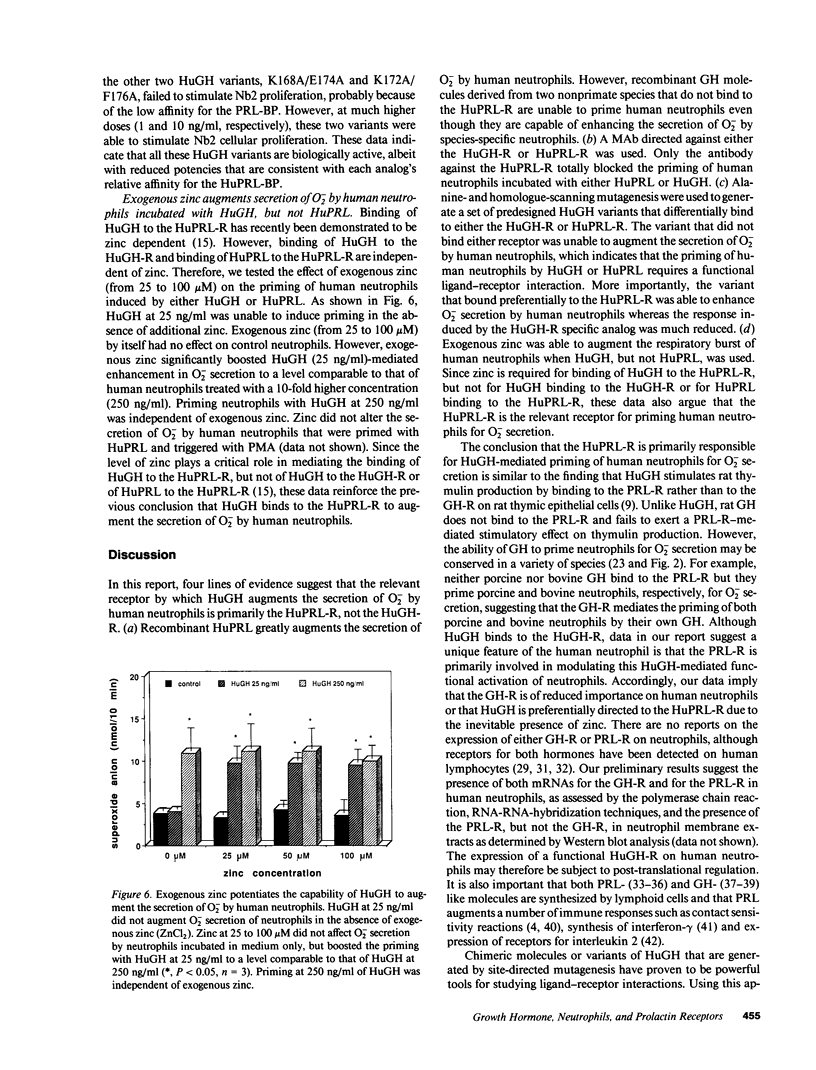
Recombinant human growth hormone (HuGH) and human prolactin (HuPRL), but not GH of bovine or porcine origin, prime human neutrophils for enhanced superoxide anion (O2-) secretion. Since HuGH, but not GH of other species, effectively binds to the HuPRL receptor (HuPRL-R), we used a group of HuGH variants created by site-directed mutagenesis to identify the receptor on human neutrophils responsible for HuGH priming. A monoclonal antibody (MAb) directed against the HuPRL-R completely abrogated O2- secretion by neutrophils incubated with either HuGH or HuPRL, whereas a MAb to the HuGH-R had no effect. The HuGH variant K172A/F176A, which has reduced affinity for both the HuGH-binding protein (BP) and the HuPRL-BP, was unable to prime human neutrophils. This indicates that priming is initiated by a ligand-receptor interaction, the affinity of which is near that defined for receptors for PRL and GH. Another HuGH variant, K168A/E174A, which has relatively low affinity for the HuPRL-BP but slightly increased affinity for the HuGH-BP, had much reduced ability to prime neutrophils. In contrast, HuGH variant E56D/R64M, which has a similar affinity as wild-type HuGH for the HuPRL-BP but a lower affinity for the HuGH-BP, primed neutrophils as effectively as the wild-type HuGH. Finally, binding of HuGH to the HuPRL-BP but not to the HuGH-BP has been shown to be zinc dependent, and priming of neutrophils by HuGH was also responsive to zinc. Collectively, these data directly couple the binding of HuGH to the HuPRL-R with one aspect of functional activation of human target cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984 Nov;115(5):1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berczi I., Nagy E., de Toledo S. M., Matusik R. J., Friesen H. G. Pituitary hormones regulate c-myc and DNA synthesis in lymphoid tissue. J Immunol. 1991 Apr 1;146(7):2201–2206. [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Edery M., Shirota M., Jolicoeur C., Lesueur L., Ali S., Gould D., Djiane J., Kelly P. A. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Byatt J. C., Welply J. K., Leimgruber R. M., Collier R. J. Characterization of glycosylated bovine placental lactogen and the effect of enzymatic deglycosylation on receptor binding and biological activity. Endocrinology. 1990 Sep;127(3):1041–1049. doi: 10.1210/endo-127-3-1041. [DOI] [PubMed] [Google Scholar]
- Carlson G. P., Kaneko J. J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973 Mar;142(3):853–856. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- Chawla R. K., Parks J. S., Rudman D. Structural variants of human growth hormone: biochemical, genetic, and clinical aspects. Annu Rev Med. 1983;34:519–547. doi: 10.1146/annurev.me.34.020183.002511. [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Wight D. C., Wagner T. E., Kopchick J. J. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5061–5065. doi: 10.1073/pnas.87.13.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C. V., Altmann S. W., Prystowsky M. B. Requirement of nuclear prolactin for interleukin-2--stimulated proliferation of T lymphocytes. Science. 1991 Jul 5;253(5015):77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Russell D. H., Appasamy P. M., Prystowsky M. B. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Bass S., Fuh G., Wells J. A. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science. 1990 Dec 21;250(4988):1709–1712. doi: 10.1126/science.2270485. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Henner D. J., Wells J. A. Engineering human prolactin to bind to the human growth hormone receptor. Science. 1990 Mar 23;247(4949 Pt 1):1461–1465. doi: 10.1126/science.247.4949.1461. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Jhurani P., Ng P., Wells J. A. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989 Mar 10;243(4896):1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. Rational design of receptor-specific variants of human growth hormone. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3407–3411. doi: 10.1073/pnas.88.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Ghiasuddin S. M., Schepper J. M., Yunger L. M., Kelley K. W. A newly defined property of somatotropin: priming of macrophages for production of superoxide anion. Science. 1988 Feb 12;239(4841 Pt 1):769–771. doi: 10.1126/science.2829357. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Lorence R. M., Dunham D. M., Arkins S., Yunger L. M., Greager J. A., Walter R. J., Dantzer R., Kelley K. W. Hypophysectomy inhibits the synthesis of tumor necrosis factor alpha by rat macrophages: partial restoration by exogenous growth hormone or interferon gamma. Endocrinology. 1991 Feb;128(2):989–986. doi: 10.1210/endo-128-2-989. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Schepper J. M., Yunger L. M., Kelley K. W. Somatotropin and prolactin enhance respiratory burst activity of macrophages. Ann N Y Acad Sci. 1988;540:698–699. doi: 10.1111/j.1749-6632.1988.tb27216.x. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Yunger L. M., Lorence R. M., Dantzer R., Kelley K. W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemark M., Comer M., Korner G., Handwerger S. A unique placental lactogen receptor: implications for fetal growth. Endocrinology. 1987 May;120(5):1865–1872. doi: 10.1210/endo-120-5-1865. [DOI] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Wang B. S., Kelley K. W. A novel role of growth hormone and insulin-like growth factor-I. Priming neutrophils for superoxide anion secretion. J Immunol. 1991 Mar 1;146(5):1602–1608. [PubMed] [Google Scholar]
- Fuh G., Mulkerrin M. G., Bass S., McFarland N., Brochier M., Bourell J. H., Light D. R., Wells J. A. The human growth hormone receptor. Secretion from Escherichia coli and disulfide bonding pattern of the extracellular binding domain. J Biol Chem. 1990 Feb 25;265(6):3111–3115. [PubMed] [Google Scholar]
- Hesse G. W. Chronic zinc deficiency alters neuronal function of hippocampal mossy fibers. Science. 1979 Sep 7;205(4410):1005–1007. doi: 10.1126/science.224456. [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Friesen H. G. The nature and regulation of the receptors for pituitary growth hormone. Annu Rev Physiol. 1985;47:469–482. doi: 10.1146/annurev.ph.47.030185.002345. [DOI] [PubMed] [Google Scholar]
- Isaksson O. G., Edén S., Jansson J. O. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol. 1985;47:483–499. doi: 10.1146/annurev.ph.47.030185.002411. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Posner B. I., Tsushima T., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: ontogenesis, effects of sex and pregnancy. Endocrinology. 1974 Aug;95(2):532–539. doi: 10.1210/endo-95-2-532. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Härtl W., Jelkmann W., Zapf J., Bauer C. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest. 1985 Oct;76(4):1643–1648. doi: 10.1172/JCI112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J., Gavin J. R., 3rd Binding of 125I-human growth hormone to specific receptors in human cultured lymphocytes. Characterization of the interaction and a sensitive radioreceptor assay. J Biol Chem. 1974 Mar 25;249(6):1661–1667. [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Lowman H. B., Cunningham B. C., Wells J. A. Mutational analysis and protein engineering of receptor-binding determinants in human placental lactogen. J Biol Chem. 1991 Jun 15;266(17):10982–10988. [PubMed] [Google Scholar]
- Montgomery D. W., LeFevre J. A., Ulrich E. D., Adamson C. R., Zukoski C. F. Identification of prolactin-like proteins synthesized by normal murine lymphocytes. Endocrinology. 1990 Nov;127(5):2601–2603. doi: 10.1210/endo-127-5-2601. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F., Shah G. N., Buckley A. R., Pacholczyk T., Russell D. H. Concanavalin A-stimulated murine splenocytes produce a factor with prolactin-like bioactivity and immunoreactivity. Biochem Biophys Res Commun. 1987 Jun 15;145(2):692–698. doi: 10.1016/0006-291x(87)91020-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Mastro A. M., Hymer W. C. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990 Jan;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I. Hypophysectomized rats depend on residual prolactin for survival. Endocrinology. 1991 Jun;128(6):2776–2784. doi: 10.1210/endo-128-6-2776. [DOI] [PubMed] [Google Scholar]
- O'Dell B. L., Conley-Harrison J., Besch-Williford C., Browning J. D., O'Brien D. Zinc status and peripheral nerve function in guinea pigs. FASEB J. 1990 Aug;4(11):2919–2922. doi: 10.1096/fasebj.4.11.2165949. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Matrisian L., Kibler R., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human lymphocytes and their modulation by cyclosporine. Biochem Biophys Res Commun. 1984 Jun 29;121(3):899–906. doi: 10.1016/0006-291x(84)90762-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Shiu R. P., Gout P. W., Beer C. T., Noble R. L., Friesen H. G. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab. 1980 Nov;51(5):1058–1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- Vruwink K. G., Fletcher M. P., Keen C. L., Golub M. S., Hendrickx A. G., Gershwin M. E. Moderate zinc deficiency in rhesus monkeys. An intrinsic defect of neutrophil chemotaxis corrected by zinc repletion. J Immunol. 1991 Jan 1;146(1):244–249. [PubMed] [Google Scholar]
- Weigent D. A., Baxter J. B., Wear W. E., Smith L. R., Bost K. L., Blalock J. E. Production of immunoreactive growth hormone by mononuclear leukocytes. FASEB J. 1988 Sep;2(12):2812–2818. doi: 10.1096/fasebj.2.12.3044906. [DOI] [PubMed] [Google Scholar]
- Weigent D. A., Blalock J. E., LeBoeuf R. D. An antisense oligodeoxynucleotide to growth hormone messenger ribonucleic acid inhibits lymphocyte proliferation. Endocrinology. 1991 Apr;128(4):2053–2057. doi: 10.1210/endo-128-4-2053. [DOI] [PubMed] [Google Scholar]
- Weigent D. A., Blalock J. E. The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol. 1991 Jun;135(1):55–65. doi: 10.1016/0008-8749(91)90253-8. [DOI] [PubMed] [Google Scholar]
- Winchurch R. A., Thomas D. J., Adler W. H., Lindsay T. J. Supplemental zinc restores antibody formation in cultures of aged spleen cells. J Immunol. 1984 Aug;133(2):569–571. [PubMed] [Google Scholar]
- Wirth J. J., Fraker P. J., Kierszenbaum F. Changes in the levels of marker expression by mononuclear phagocytes in zinc-deficient mice. J Nutr. 1984 Oct;114(10):1826–1833. doi: 10.1093/jn/114.10.1826. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Fraker P. J., Kierszenbaum F. Zinc requirement for macrophage function: effect of zinc deficiency on uptake and killing of a protozoan parasite. Immunology. 1989 Sep;68(1):114–119. [PMC free article] [PubMed] [Google Scholar]